PIT POP! Bursting the bubble on home-range bias with fine-scale PIT telemetry
Hugh Allan A * , Richard P. Duncan
A * , Richard P. Duncan  A , Peter Unmack A , Duanne White A and Mark Lintermans A
A , Peter Unmack A , Duanne White A and Mark Lintermans A
A Centre for Applied Water Science, Institute for Applied Ecology, University of Canberra, 11 Kirinari Street, Bruce, ACT 2617, Australia.
Marine and Freshwater Research 73(11) 1297-1309 https://doi.org/10.1071/MF22021
Submitted: 28 January 2022 Accepted: 28 June 2022 Published: 26 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Improved tracking technologies increase understanding of fish movement, but care is required when comparing studies of different design.
Aims: We used an approach that allowed fine-scale tracking to compare results from individual-tracking designs to simulated batch-marking designs.
Methods: Adult Galaxias tantangara (a small freshwater fish) individuals were tagged with 9-mm PIT tags in a small headwater stream and tracked with an accuracy of 1 lineal metre. To evaluate differences between common study designs, data were re-analysed to simulate both batch-marking section size and tracking resolution between 1 and 250 m.
Key results: Home-range estimates decreased with a smaller section size and tracking resolution. Batch-marking simulations differed in 99% of cases, whereas individual tracking simulations differed in only 17% of comparisons. Comparisons between different methods were rarely statistically equivalent, being so only when section size or resolution was less than 4 m.
Implications: Importantly, batch-marking studies are often likely to overestimate home-range size, and results from different studies may be comparable only when resolution is very fine or identical, even if the same method was used.
Keywords: batch marking, conservation, freshwater, home range, movement, passive integrated transponder, spatial ecology, threatened species.
Introduction
Understanding the spatial behaviour and requirements of fishes is critical to fish biology (Koehn and Crook 2013). Interactions between fish and their environment are imperative to feeding, sheltering and spawning, and in the case of threatened species, their recovery and conservation often hinges on improvements to spatial availability and connectivity (Carroll et al. 2015). Designing effective methods to observe fish movement is not a trivial task; the underwater world differs immensely from the terrestrial realm, and methods of visual surveillance, identification and tracking come with complex challenges for the fish biologist. Nonetheless, a proper understanding of spatial requirements of fish remains at the forefront of designing, implementing and evaluating effective conservation management strategies (Daly et al. 2021).
The various methods for analysing, interpreting and reporting movement data mean that study design, and how movement metrics are calculated, must be considered before findings can be confidently compared among studies. Inherent bias of different marking approaches is not always immediately obvious, and can lead to confusion and incorrect assumptions regarding differences among studies or species. As tracking technologies evolve, new insights into the ecology of fishes will arise and it is critically important that findings based on new methods can be considered alongside past and current literature (Gerber et al. 2017). Understanding differences and bias among different monitoring and tracking methods is particularly important when devising management plans, policies and priorities.
Monitoring spatial behaviour of fishes, namely movement and home-range characteristics, requires researchers to be able to repeatedly identify individuals or groups of fish. Some species have unique and complex pigmentation patterns which can be used as natural marks and used to track fish (David and Stoffels 2003; Town et al. 2013; Norman and Morgan 2016; McInnes et al. 2020), but many species require an artificial mark (Koehn and Crook 2013). Electronic tags such as radio or acoustic tags are useful in that they allow individual fish to be tracked and repeatedly identified, often without being captured; however, they are limited to fish sizes that can accommodate suitable tag-to-body weight ratios and are generally less suitable for small-bodied species (Klinard et al. 2018).
Batch marking may be used when electronic tags are not appropriate and it is common for marking small fish, where a group of fish are marked with the same ‘batch mark’. Popular marking techniques include fin clipping (Berra 1973; Lintermans 1998), injection of coloured dyes or elastomers (Cadwallader 1976a; Jungwirth et al. 2019) and coded wire tags (Rash et al. 2018; Lane et al. 2019). Sample size is limited by the number of possible combinations of fin clips or colour markings, or the cost of marking and retrieving fish. Groups receiving the same mark are generally determined by a series of arbitrary marking sections designated in the study area, with all fish in the same section receiving the same mark (Berra 1973; Cadwallader 1976a; Lintermans 1998). Movement and home range is estimated using the distance fish are later recaptured away from their initial marking section. A larger study area means that more fish may be marked, but often requires larger marking sections, which affects the resolution of movement observations (Lucas and Baras 2000); when large sections are used, estimated distances are similarly large and coarse-scale results are returned. So as to observe fine-scale movement, sections must be small and therefore sample size and study area are often reduced.
When individual fish are tracked with transmitters, two main metrics are often reported as home range, namely, total linear length of river or habitat, determined by the distance between the most up- and downstream observed positions (Crook 2004a; Broadhurst et al. 2012), or, alternatively, a narrower range over which an individual is located either more frequently (Koehn et al. 2009) or during certain time periods (Ebner and Thiem 2009). Differences between these metrics, and those often described in batch-marking studies, are important; unable to monitor individual fish, batch-marking metrics are often derived from movement distances rather than the area over which a fish ranges. Individual tracking offers many benefits over traditional batch-marking designs; individual fish can be monitored, facilitating attribution of behaviours and spatial patterns to individual characteristics such as fish size, sex or previous locations (Pillans et al. 2017; Daly et al. 2021). As technology advances and transmitters become smaller and cheaper, more studies are able to implement this design rather than traditional batch-marking approaches (Allan et al. 2018).
Stocky galaxias (Galaxias tantangara Raadik) was chosen for this study as its conservation predicament is representative of several closely related and recently described species in the mountain galaxias (Galaxias olidus Günther) complex (Raadik 2014); relictual populations of many species exist only above natural waterfall barriers, which provide a barrier to introduced predatory trout. Their recent description means that almost all of these species are largely unstudied, and fragmentation and contraction of their historical ranges by introduced trout means that many are of conservation concern (Lintermans et al. 2020). Effective conservation management plans are urgently required for these species, and the establishment of additional populations by ways of conservation translocation is needed to safeguard populations and species against localised losses from climate change, drought, bushfire and habitat destruction. Understanding spatial behaviour and requirements is critical for effective management of these species, especially when designing translocation plans and assessing potential translocation sites.
Observations from past studies indicate that G. tantangara in Tantangara Creek are largely benthic and cover-oriented during the day. Small fish (<50 mm) are occasionally observed swimming in small groups (<10 fish) in the few slower-moving sections of Tantangara Creek, but this is uncommon, especially for larger fish. The majority of the creek consists of shallow cobble-bottomed riffles and fast runs (mean 12 cm deep and 81 cm wide; see Allan et al. 2021), and fish have often been caught among this substrate as part of electrofishing surveys in past studies (Allan and Lintermans 2018; Allan et al. 2021). Its affinity for cover such as substrate interstices makes G. tantangara a good candidate for tracking with portable-passive integrated-transponder (PIT) telemetry; if spooked, it is assumed that fish will be more likely to ‘go to ground’ and take cover close to their original location rather than swim large distances away. As such, spooking by researchers should not create too much bias towards artificially large movements.
Adult G. tantangara individuals were tagged with 9-mm PIT tags and tracked using portable-PIT telemetry, with 1-m spatial resolution. Specific objectives of the study were to (1) examine differences in movement and home-range metrics between different simulated study designs and analytical approaches, (2) determine the suitability of novel portable-PIT telemetry for monitoring and tracking fish in small streams, and (3) investigate movement behaviour and home range of G. tantangara. We expected to see a difference in home-range estimates when derived from different simulated study designs and analytical approaches, specifically when different batch-marking section sizes were used. Additionally, based on small home ranges of closely related species (Berra 1973) and other small-stream fishes (Hesthagen 1990; Lintermans 1998; Bell 2001), we expected G. tantangara to undergo small movements and exhibit a small home range.
Materials and methods
The care and use of experimental animals complied with animal welfare laws, guidelines and policies as approved by the University of Canberra Animal Ethics Committee (CEAE 17-04). This study forms one of the few emerging studies investigating the ecology of this newly described species, of which ecological understanding is limited. Findings from this study will help inform critical conservation management plans for this species, and other closely related threatened species by aiding understanding of spatial requirements of the species. Management actions such as protecting habitat and stream connectivity, to ensure suitable spatial extents that facilitate effective feeding, sheltering and spawning behaviours will draw on this information when developing plans.
Study site
The study was conducted in Tantangara Creek in the upper Murrumbidgee catchment, Nw South Wales. G. tantangara is known only from Tantangara Creek above a natural waterfall (Fig. 1, Raadik 2014; Allan et al. 2021) and is believed to have been eliminated from over 90% of its historical range because of introduced predatory Salmonidae, brown trout Salmo trutta L., and rainbow trout Oncorhynchus mykiss (Walbaum; NSW Fisheries Scientific Committee 2016; Lintermans and Allan 2019). Salmonids are abundant in the stream immediately below the waterfall, but G. tantangara is absent. G. tantangara is found between the waterfall barrier and ∼3 km upstream of the barrier, with fish being most abundant in the first 2 km upstream of the waterfall (Lintermans and Allan 2019; Allan and Lintermans 2021; Allan et al. 2021).
A 250-m section of Tantangara Creek was the focal reach for the movement study. The focal reach was located 200 m upstream of the main waterfall barrier on Tantangara Creek, and was chosen as it contained a variety of habitat types present in the upper Tantangara Creek. Mean stream width was ∼60–80 cm, and depth 12 cm. Surveys from previous studies indicated that fish density was representative of the wider population (average 1.18 fish m−1; see Allan et al. 2018). Labelled stakes were installed every 5 m along the stream bank to mark locations within the focal reach. Additional markers were installed 30 m up- and downstream of the focal reach.
Tagging
Fish were tagged over two separate occasions, namely, 17–18 January 2018 and 10–11 January 2019. Fish were captured by two-pass backpack electrofishing (Smith-Root LR-24, typical settings 900 V, 60 Hz, 4.2 millisecond pulse width, 25% duty cycle) on both occasions. In 2018, fish for tagging were captured within a 200 m reach, separated into nine separate 20–50 m-long sections. In 2019, fish for tagging were captured within a 250-m reach, that is, the same 200-m reach as sampled in 2018, plus an additional 50 m immediately upstream, separated into 10-m sections. After capture each fish was measured (length to caudal fork, LCF), weighed (to the nearest 0.1 g) and the section where the fish was captured was recorded. All fish 69 mm or greater were retained for tagging, on the basis of findings from an aquaria pilot study on the closely related G. olidus (Allan et al. 2018). In total, 69 fish were tagged with 9-mm PIT tags (HPT9; Biomark, Inc.), by using the same procedure as outlined in Allan et al. (2018). Once tagged, fish were recovered in aerated buckets in the shade before being released into the section where they were captured.
Tracking
Eight tracking surveys were conducted between 24 January 2018 and 2 June 2018, and following tagging in 2019, five tracking surveys were undertaken between 26 January 2019 and 14 May 2019. All tracking surveys were conducted during daylight hours. Weather events such as snow and heavy rain limit access to the field site at the tail end of the monitoring seasons, and the presence of spawning fish limits the commencement of marking activities (Allan et al. 2021). A BP Lite antenna (Biomark, Inc.) was used to locate PIT tags during tracking surveys, and had a read range of ∼20 cm. Tracking surveys involved a researcher scanning the stream and substrate with the antenna throughout the study area. Tracking surveys began at the downstream end of the study area, and the operator moved in an upstream direction, ensuring that all wetted areas and in-stream substrates were scanned.
When a tag was detected, the position of the tag was recorded with a labelled marker. The location of the marker was recorded to the nearest metre, using the distance to the nearest marker peg on the side of the stream, along with the serial number of the tag. The operator then continued upstream until the entire study area had been surveyed. The 310-m survey area took ∼60–80 min to survey. Immediately following the first pass of the study area, a second pass was undertaken, using the same method as for the first pass.
If during the second pass a tag was located in the same position as recorded in the first pass, the area was disturbed to determine whether a fish was present and had not moved, if a fish was present but was deceased or if a tag had been expelled in that position. The same location was rescanned with the antenna after disturbance, to see whether the tag still remained in the same position. Sometimes a fish was observed swimming away from the spot during disturbance, whereas other times the tag remained in the same location. If the tag was still present after disturbance, a magnet was scanned over the area to collect a tag if it had been expelled. Tag retention in the aquaria trial showed a very high tag retention rate, although this may differ in a wild setting (Allan et al. 2018). Longevity and growth rate of G. tantangara is not known, but fish 69 mm and greater are estimated to be 3–4 years of age, and it is possible that some fish may have died of old age or natural causes during the study (Allan et al. 2021).
The probability that a particular fish was detected during a given survey was calculated by dividing the total number of surveys after a fish was tagged, by the number of surveys during which a fish was detected.
Detection efficiency
Before each tracking survey, a second researcher placed up to five ‘dummy’ PIT tags in the stream within the study reach. These tags were placed among in-stream substrate in places where fish were expected, and were not visible to the tracking operator. The location of these tags was not known by the researcher operating the PIT antenna. Detection of these hidden tags allowed the effectiveness of the PIT tracking method for each survey to be estimated, on the basis of how many hidden tags were detected. The hidden tags were retrieved after each survey and different hiding places were used each time, ensuring that their location was not known by the operator throughout the study.
Data analysis
In total, 40 fish were detected two times or more, and were used for home-range and movement analysis, and minimum number of detections did not significantly affect final home-range estimates; see Results for details. Movement was calculated as the distance between an observed location and the previous known location of that fish. Home range was defined as the total linear range occupied by an individual fish, determined by the distance between furthest up- and downstream locations (Crook 2004a; Broadhurst et al. 2012). This was chosen as the home-range metric, because this study was based on a high number of tagged individuals, each with a low number of locations. Unlike studies with a small number of individuals and large number of locations (such as some continuous or fixed-station methods, such as acoustic or radio-tracking), it was unsuitable to calculate density-based metrics for individual fish. All data analysis was performed using R (ver. 4.0.0, R Foundation for Statistical Computing, Vienna, Austria).
Length and weight were positively correlated for the group of tagged fish used in home-range analysis (n = 40, Spearman’s rho = 0.904, P < 0.001), so length was used as a measure of size when determining relationships with home range, distance moved between observed locations and detection probability by using linear models (lm() function in R), and when relating size to probability of detection with a logistic regression model (glm() function in R). The relationship between detection probability between 2018 and 2019 surveys, for fish tagged in 2018, was determined using a linear model (lm() function in R).
Simulation study
For the simulation study, tracking data were restructured to emulate a batch-marking study design. Rather than tracking individual fish movement to the nearest metre, movement was categorised as number of ‘sections’ moved from the initial ‘marking’ location. Simulated marking section sizes were systematically determined by dividing the focal study area (250 m) by between 1 and 250 equal-sized sections. For example, in the instance of 1 equal-sized section, section size was 250 m; when number of sections was 10, section size was 25 m; when number of sections was 250, section size was 1 m. The possible section sizes were rounded to the nearest metre before retaining unique numbers, resulting in 31 simulated section sizes: 1–19, 21, 23, 25, 28, 31, 36, 42, 50, 62, 83, 125 and 250 m. Section sizes encompassed those often observed in other studies on small stream fishes (e.g. 12.5 m, Hesthagen 1990; 13 m, Berra 1973; 20 m, Bell 2001; 3.3–26.7 m, Hill and Grossman 1987; 30–39 m, Berra and Gunning 1972), and larger sizes not used as often, but included to emphasise the difference between small and large sections. Section boundaries started at the marker peg of 0 m, and were arranged in an upstream direction. For example, when section size was 10 m, sections included 0–9, 10–19 and 20–29 m pegs etc., and included sections downstream of peg 0: −1 to −10, −11 to −20 and −21 to −30 m pegs, etc.
To simulate initial marking locations, the post-tagging release location of each fish was removed from analysis; instead, the first subsequent detection was considered the marking location. This distributed marking locations throughout the study area rather than restricting the simulation to only a small number of release locations, and lessened effects of capture, electrofishing and tagging on fish location.
Individual fish movement was determined as the number of sections away from its marking location. For example, if a fish was initially marked in Section 1 and repeatedly located in Section 3, movement was repeatedly recorded as two sections, even though the fish was in its same previous location; this reflects a limitation of batch-marking designs. Number of sections moved paired with section size was then used to calculate movement distances and home-range estimates of tagged fish.
As described by Berra (1973), a movement of one section may be only a short distance and less than one whole section, or just under the length of two sections depending on which end of each section a fish is located. The same goes for any number of sections moved. As such, movement and home-range estimates in batch-marking studies are typically described as a range rather than a single value, with distances being representative of the number of sections moved, plus one (Berra 1973; Hesthagen 1990). Accordingly, home range was reported as a range of values for batch-marking simulations, whereas the maximum value of the range was used for statistical comparison between simulations (below).
Individual tracking data were also restructured to emulate an individual tracking study with different spatial resolutions, other than the 1 m originally used in this study. Simulated spatial resolutions mimicked the section sizes used for ‘marking’ in the batch-marking simulation, namely, 1–19, 21, 23, 25, 28, 31, 36, 42, 50, 62, 83, 125 and 250 m. Individual fish locations were rounded to the nearest multiple of the simulated section size. For example, if resolution was 9 m and a fish was located at 7 m, the location was rounded to 9 m; if resolution was 5 m and a fish was located at 7 m, the location was rounded to 5 m. Data were then processed in the same manner as in the original home-range study; home range was defined as the total linear range occupied by an individual fish, determined by distance between most up- and downstream locations. Uncertainty around estimates is equal to one unit of spatial resolution above and below the estimate. However, like for other individual tracking studies, home range was reported as a single value rather than a range (Crook 2004b; e.g. Broadhurst et al. 2011, 2012).
Home-range estimates of the same section size or spatial resolution were compared between batch-marking and individual tracking methods by using Mann–Whitney tests (wilcox.text()). Estimates from the same method but different spatial resolutions were compared using Kruskal–Wallis tests (kruskal.test()), and pairwise Wilcoxon rank sum tests (pairwise.wilcox.test()) post hoc, to determine which groups were statistically different from each other.
Ethics and permits
The study was conducted under NSW NPWS Scientific Licence (SL101755) and NSW Scientific Collection Permit (P07/0007-5.4).
Results
Tracking
Of 69 tagged fish (2018: n = 38; 2019: n = 31), 49 (2018: n = 28; 2019: n = 21) were detected once or more across 13 separate tracking surveys. Seven tags were recovered, found among in-stream substrate with the magnet and were likely to be a result of fish mortality or tag expulsion, whereas 13 were never detected. Fish with tags that were either recovered or never detected were grouped together and compared to those fish whose tags were detected during surveys. Size was not a significant determinant in modelling probability of being recovered or never detected (logistic regression model, z = 0.9, n = 69, P = 0.3). All 20 tags recovered or not detected were omitted from further analysis.
Fish tagged in 2018 were subjected to all 13 tracking surveys and were detected between one and nine times each (mean ± s.e.; 4.39 ± 0.42 detections per fish), and fish tagged in 2019 were subjected to five surveys and were detected between one and four times each (mean ± s.e.; 2.19 ± 0.24). The second pass of PIT tracking detected an average of an additional 33% of individual tags not detected on the first pass (min. 0%, max. 75%, n = 13).
The probability that a particular fish would be detected during a given survey ranged from 0.07 to 0.60 (mean ± s.e.; 0.30 ± 0.02), and was not significantly different between fish tagged in 2018 and those tagged in 2019 (linear model, t = −0.07, n = 49, P = 0.95), or associated with fish size (linear model, t = −0.8, n = 49, P = 0.44). However, fish tagged in 2018 were 14% less likely to be detected in 2019 than they were in 2018 (linear model, t = −2.5, n = 56, P = 0.02).
Detection efficiency of dummy tags was high on all but one survey. In most instances, detection probability was 1.00. Mean efficiency of dummy tag detection was 0.86 ± 0.07 (mean ± s.e.) across all surveys. The PIT tracking antenna malfunctioned on one of the surveys, resulting in decreased efficiency on this survey (0.40).
Home range
Fish typically occupied a small home range during the study, ranging from 0 to 173 m (median 5.5 m; mean ± s.e., 19.1 ± 5.6 m, n = 40; Fig. 2; Supplementary material Table S1). Home range was less than or equal to 5, 10 and 20 linear metres for 48, 68 and 81% of fish respectively. Two fish had a home range greater than 100 m, with one of these fish being the second-largest fish tagged in the study (Supplementary material Table S1). However, home range was not significantly associated with either fish size (linear models, t = 1.3, n = 40, P = 0.2), or year of tagging (linear model, t = −1.6, n = 40, P = 0.1). No significant relationships were observed between number of detections and home-range size for individual fish (linear model, t = 1.9, n = 40, sample size, P = 0.07), or minimum number of detections required per fish to be included in the calculation of home range for all fish (linear model, t = 0.9, n = 119, P = 0.4). No definitive home-range shifts were observed, although a small number of fish moved large distances before returning close to their previous location (Supplementary material Fig. S1). Home range of multiple individuals overlapped.
|
|
Movement
Distances moved by individual fish between tracking surveys ranged from 0 to 173 m, although most were small (median 2.0 m; mean ± s.e., 10.3 ± 2.4 m; n = 119; Fig. 3; Supplementary material Fig. S1). Movement distance was less than or equal to 5, 10 and 20 lineal metres in 71, 83 and 89% of instances. Two movements greater than 100 m were observed in the study, both by the second-largest fish (fish 306236: 173 and 167 m, Supplementary material Fig. S1), although fish size was not significantly associated with movement distances (linear model, t = −0.5, n = 119, P = 0.6). No significant relationships were observed between the number of detections and movement distance for individual fish (linear model, t = −0.1, n = 119, P = 0.9), or minimum number of detections required per fish to be included in movement distances for all fish (linear model, t = −1.1, n = 499, P = 0.3).
|
|
Simulation study
The simulation study showed significant variability in home-range estimates between different spatial resolutions, and between different methods (Table 1; Supplementary material Table S2). In both batch-marking and individual tracking simulations, home-range size and uncertainty decreased with finer spatial resolution. The smallest home-range estimates came from the smallest batch-marking sections of 1 and 2 m and individual tracking resolution of 1 m, whereas the largest estimates came from the largest simulated section size or tracking resolution of 250 m (Supplementary material Table S2).
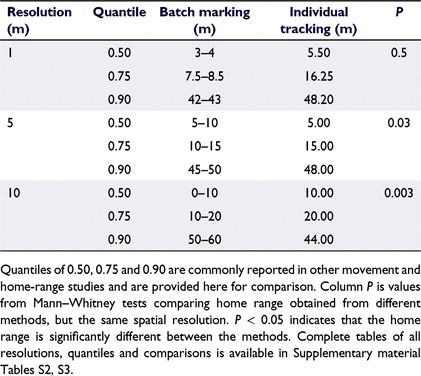
|
When batch-marking section size and individual tracking resolution were equal, home range was significantly different between the methods in all instances when section size or spatial resolution was greater than 4 m (Supplementary material Table S3).
Home ranges derived from batch-marking were significantly different among all simulated section sizes except for 2 and 3 m (P = 0.077), whereas individual tracking home ranges differed significantly in only 17% (n = 78) of instances (Supplementary material Fig. S2, S3.
Discussion
Simulated results indicate that home-range estimates can be significantly influenced by both tracking technique and its spatial resolution, with a wide range of final estimates possible depending on the chosen study design. Moreover, results from studies with different resolutions were rarely statistically comparable for batch-marking (differed in 99% of cases), but not as bad for individual tracking (differed in only 17% of cases). Results were statistically equivalent between the two different techniques only when resolution was less than 4 m, which is uncommon in batch-marking studies. Portable-PIT telemetry equipment was shown to be an effective and reliable method to detect small-bodied fish in a small headwater stream. G. tantangara exhibited small linear home ranges, equal to 5.5, 16.2 and 48.2 m in 50, 75 and 90% of fish, although some individuals did undergo large movements (>100 m). Home range at the finest resolution (1 m, individual tracking) was smaller than reported for other small galaxiids (e.g. 13–26 m, Berra 1973; 10–20 m Allibone et al. 2003); we suggest that this is more influenced by the fine resolution and tracking technique used in our study, rather than inter-specific differences alone. As such, current understanding of home range for other species, particularly those derived from batch marking, may be substantially overinflated because of large section sizes.
Simulation study
By tracking individual fish and collecting data with 1 m accuracy, this study provided a unique opportunity to compare home-range estimates from alternative monitoring methods and analytical approaches. Simulations highlighted the tendency for home ranges to contract as marking section sizes decrease; home ranges from batch marking decreased across all simulations until marking area was reduced to 1–2 m. For home ranges at the 50th percentile, fish had moved less than or equal to only one marking section in all instances where marking sections were larger than 1 m; the 75th percentile increased this threshold to 5 m. This means that if reporting home range on the basis of the 50th or 75th percentile, once the marking section is greater than 1 or 5 m respectively, home range is equivalent to the marking section size and would be easily overestimated. Given that most studies utilise marking sections greater than 5 m (e.g. 13 m, Berra 1973; 12.5 m, Hesthagen 1990; 20 m, Bell 2001), it is possible that overestimation of home range is not uncommon.
Another main difference is highlighted when inspecting home range for different methods (individual tracking v. batch marking) using the same spatial resolution or marking section size. Despite equal spatial resolution, home-range estimates were significantly different between methods except when sections were smaller than 4 m (Supplementary material Table S3). It is likely that deriving home range from movement distances away from marking locations (as is common in batch-marking studies), rather than distance between furthest upstream and downstream fixes, causes this discrepancy. This will be important as individual telemetry-based methods become more popular than batch marking.
Individual tracking simulations were not as susceptible to the effects of varying spatial resolution as were batch-marking studies, with only 17% of tracking simulations with different resolution being significantly different, compared with all but one comparison for batch marking. For example, when 1-, 5- and 10-m resolution was used in individual tracking simulations, home range at the 90th percentile was equal to 48, 48 and 44 m respectively; the same spatial resolution in batch-marking simulations resulted in three different home ranges, namely, 10–20, 20–30 and 30–40 m. In other words, if home range was calculated for this set of data, using batch marking and two different section sizes, there would be a 99.8% chance home-range estimates would be statistically different. By individual tracking rather than batch marking, this probability is reduced to only 17%, and is even less likely when spatial resolutions are similar (see Supplementary material Fig. S2 and S3).
The simulation study is subject to the same limitations as was this tracking study and many other tracking studies; findings do not reflect diel patterns, spawning migrations, exploratory movements outside the study area or seasonal changes and the fate of undetected fish is unknown. The whole study is of course possibly susceptible to the idea of the restricted-movement paradigm, involving fish which were never detected, the ability of fish to move out of the study area, and often weeks between tracking surveys. Simulations are similarly susceptible, being based on the same data, but, nonetheless, this does not affect comparison between methods, because differences can arise only from differing approaches to tracking, study design and data analysis. Given the seemingly sedentary behaviour of many fish in these data, comparisons between methods and spatial resolutions may differ for data on more mobile fish.
Tracking
Detection rate of tagged fish in the study was good, with 71% of all tagged fish being detected at least once during surveys, and 20% being detected five times or more. When fish were detected, researchers could mark the location where the fish were found with confidence, because the short read-range of the antenna (∼20 cm) facilitated high tracking accuracy. This was also confirmed by in situ dummy tags hidden by a second operator.
Fate of tags that were never detected after tagging is unknown; fish were able to move out of the study reach, so these tags may represent fish that emigrated out of the study area. This is a limitation of PIT telemetry and most conventional tagging and marking techniques, compared with the ability of radio telemetry to track down specific transmitters. Stationary PIT antennae at the top and bottom of the focal reach would have been useful in determining emigration out of the study area, but were logisitcally unfeasible on the limited budget of the project. This study encompassed a 310-m reach of a small upland stream; mean stream width was ∼60–80 cm, and depth 12 cm. Field researchers were generally able to survey the area in 60–80 min per pass. In-stream habitat in Tantangara Creek mainly comprises rocky substrates and overhanging vegetation. Being a small stream with simple habitat types, the stream was easy to survey with portable-PIT telemetry equipment. Larger, deeper streams with complex habitats may be more difficult to survey (Enders et al. 2007), although manageable providing operators can effectively scan all in-stream habitat with tracking equipment. Without some form of telemetry tag, reliable identification of small fish among complex cobble and boulder habitat would be difficult.
Uniquely coded PIT tags are beneficial over conventional batch marking because individual fish could be tracked throughout the study. This means that movement metrics could be compared across fish size and, if known, fish sex, rather than overall findings for a batch of fish. This level of detail can provide greater understanding of a species life-history spatial requirements.
Movement and home range
At finer scales of resolution, the majority of tagged fish moved small distances and occupied a small home range, whereas a small portion of fish exhibited larger ranges and underwent large movements. Freshwater fish in the same population do not necessarily exhibit similar spatial behaviours; Gerking (1953) and Funk (1957) described fish displaying sedentary behaviour around a home, and also those with a tendency to be more mobile and undergo larger movements. This concept has been reported in both riverine and reservoir environments, with both behaviour types being observed in different individuals simultaneously (Crook 2004a; Broadhurst et al. 2012; Koster et al. 2020), and by the same individuals but at different times of year (Ebner and Thiem 2009; Koehn et al. 2009). Magnitude of large movements and the exhibition of varying spatial tendencies will be dictated by habitat connectivity, and may be less pronounced for small-bodied species in small upland streams where both natural and man-made barriers may prevent upstream passage. Whether a species undergoes spawning-related movements or migrations will of course influence temporal movement patterns, with homing ability and site fidelity determining longer-term patterns (Koehn and Crook 2013). A combination of behaviours is important in a population for dispersal, resilience to environmental changes and genetic mixing (Broadhurst et al. 2011; Davis 2017), and must be considered when developing species management plans. This is especially important when considering conservation strategies for range-restricted threatened species, with small population sizes and potential threats of genetic bottlenecking, if managed incorrectly (Hilderbrand and Kershner 2000; DeHaan et al. 2017; Fluker et al. 2019).
Movement and home range were not significantly associated with fish size in this study. However, tagged fish were no smaller than 69 mm, and were all assumed to be adult fish (Allan et al. 2021). Riverine freshwater fishes may exhibit different spatial behaviours at different life-history stages (Akbaripasand et al. 2011; Fletcher et al. 2019; Herrera et al. 2019); so, fish smaller than those tagged in this study may possibly display different spatial behaviours than reported here.
Tracking surveys were not undertaken over the winter months because the study site was inaccessible (snow-covered), or during spawning season (November–December; see Allan et al. 2021) to minimise disturbance to this critically endangered species. Consequently, the results do not necessarily represent fish-movement behaviours for all seasons. However, many tagged fish were located close to their same positions after the 6 months between June 2018 and January 2019 (e.g fish 395226; Supplementary material Fig. S1). This suggests that fish may exhibit some site fidelity, and if significant movements are undertaken over winter or in relation to spawning, fish may return to their previous location. Spawning-related movements are recorded for small-stream galaxiids in New Zealand (Cadwallader 1976a; Allibone and Townsend 1997; Moore et al. 1999), although generally only to nearby riffles that contain suitable spawning habitat. Diel period can affect movement and activity of freshwater fishes and home-range estimates can therefore change depending on timing of tracking data (Broadhurst et al. 2012; Milano et al. 2013; Thiem et al. 2013; Dawson and Koster 2018). All tracking surveys in this study were conducted during daylight hours; so, if fish exhibit increased activity and movement during night hours then they are likely to return to a similar location each day.
Definitive home-range estimates for other species of freshwater Galaxiidae are scarce; several studies have included tagged or marked fish in Australia and New Zealand, although often without specific intention to determine home range. Without particular focus on home-range determination, studies have reported that individual fish often use the same pool or pools (Akbaripasand et al. 2011), or observed high recapture rates within relatively large study areas (350 m for Galaxias fasciatus, Akbaripasand et al. 2011; 300 m for Galaxias truttaceus, Crook and White 1995). Cadwallader 1976a specifically investigated the home range of Galaxias vulgaris and reported 87 and 97% of recaptures within 100 and 200 m of the marking section, although mentioned that some fish were caught within the same 24-m marking area 10–11 months after marking. Radio-tracking of a single Galaxias argenteus suggested that it stayed within a single 30-m pool across summer and winter months (David and Closs 2001). Berra (1973) and Allibone et al. (2003) batch-marked G. olidus and Galaxias postvectis and found that most recaptured individuals resided in 13–26- and 10–20-m reaches.
However, we suggest that home-range estimates obtained by batch marking are strongly influenced by the size of marking sections used, especially when the majority of fish remain within one to two sections of where they were marked. For example, Berra (1973) used 13-m sections and reported home range of 13–26 m, Hesthagen (1990) used 12.5–25-m sections and reported the same for home range, Bell (2001) recaptured all marked fish within the same 20-m marking section, and several other authors have reported similar recapture results (Berra and Gunning 1972; Cadwallader 1976a; Hill and Grossman 1987; Mundahl and Ingersoll 1989; Lintermans 1998). Estimated home range cannot be smaller than the marking section of course, because this is the effective resolution. In instances where the majority of fish were located in or adjacent to their marking section, authors suggest that it would be wise to treat findings as a conservative maximum.
Benefits of individual tracking with 1-m resolution become obvious when comparing findings with studies using marking sections of 10–20 m or greater. Although home ranges at the finest scales in this study may be smaller than in other studies, authors surmise that the difference is largely due to the use of a novel tracking technology and analysis approach, rather than a species- or environment-specific difference. This study design, particularly the portable-PIT telemetry, facilitated the collection of data at a much finer scale than in traditional mark–recapture studies, and allowed individual fish to be tracked rather than marking batches of fish. The accuracy of the portable PIT antenna and labelled marker pegs throughout the study area meant that fish were confidently located to within 1 m of their true position.
Importance of understanding study-method bias
Accurate understanding of fish spatial behaviours is critical for effective conservation and management, especially when working with threatened species (Cooke et al. 2016; Allan et al. 2018). However, misleading conclusions may be drawn from unsuitable comparisons among studies, such as when studies use different spatial resolutions or monitoring methods. For example, simulations have shown that statistical differences can arise among home-range estimates when batch-marking section size differs, except in one instance of 2 and 3 m. Similarly, only when the finest spatial resolutions of 4 m or less were compared, were results statistically equivalent between batch-marking and individual tracking methods. Spatial ecology and behaviour of the study species, and the structure of the study site are important too. In this study, G. tantangara in Tantangara Creek is more of a benthic rather than a pelagic fish; when tracking pelagic fish in streams with larger pools or deeper water where tagged individuals may spook and evade researchers, tracking technique may need to be modified or changed, so as to avoid bias introduced from tracking operations.
The tendency for some individuals to exhibit very small ranges may not be evident by monitoring using traditional batch-marking methods and commonly used section sizes, and likewise, the roaming nature of other individuals. If batch-marking section size is equal to or larger than the home range of a fish, then home range will be reported as the same size as the marking areas. This may lead to assumptions that disturbance to habitat patches smaller than this arbitrarily chosen size may have lesser or little impact on fish. Tracking technology with individual identification and high resolution may show that fish utilise much smaller areas of stream, and even small amounts of disturbance could cause disruption. This is especially relevant because conservation-related infrastructure interventions are often constructed in waterways to protect threatened species or habitats (Broadhurst et al. 2013; Bowie et al. 2018; Tamario et al. 2019; Waltham and Schaffer 2019), and such interventions involve some level of in-stream disturbance and permanent habitat modification or loss (Altenritter et al. 2019). A sound understanding of fish movement and home range is critical when considering acceptable levels of in-stream disturbance, and to ensure that appropriate measures are taken to reduce disturbance and minimise fish loss. In instances where large portions or even an entire home range of fish may be affected by works, it may be appropriate to conduct detailed investigations into behaviours of affected fish, and potentially translocate individuals likely to be affected or retain them for captive breeding, especially for fish in small populations. Accurate information is also important when considering conservation plans such as captive breeding and translocations, because both captive and translocated populations should have sufficient spatial extent over which to roam (Hilderbrand and Kershner 2000; Pittman 2011).
Implications for G. tantangara
Construction of an in-stream barrier has been suggested to prevent future alien fish invasion from downstream reaches of Tantangara Creek, augmenting the existing natural waterfall barrier (Pygas et al. 2019). Construction of such a barrier will cause in-stream disturbance and some permanent habitat loss, albeit being worthwhile long term. Fish in these construction zones may be translocated out of the affected areas, especially given the critically endangered status of the species and the small population size and distribution. When considering reintroduction strategies for conservation of G. tantangara, sites must be spatially adequate for fish to undertake regular movement patterns at appropriate in-stream population densities. Additional research on diel and seasonal movement patterns, including spawning-related movements, will be extremely beneficial to understanding movement behaviours and requirements of G. tantangara, and help inform the unknown ecology of many closely related threatened species. Virtually nothing is known of the movement ecology of the 14 newly described galaxiids (Raadik 2014). Nonetheless, although the findings of the current study are limited to adult fish during daylight hours and do not cover the entire year, they remain critically important for current conservation and management of this and other closely related species.
Conclusions
This study has shown that fish home range may be significantly overestimated using popular tracking methods, and results are often not statistically comparable among different studies. Because range-restricted threatened species continue to be discovered and studied, it is important that appropriate tracking technique and spatial resolution is considered, given the possible sedentary nature of many small stream fishes and urgent requirement to establish additional populations in captivity and the wild. When interpreting findings from past and future movement studies, it is important to understand differences among monitoring methods and their bias on final results. The authors recommend that expected-movement and home-range results are considered when designing future studies, specifically the monitoring technique and spatial resolution. Similarly, final results must be presented with respect to their spatial resolution, including an honest perspective on its suitability.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Peter Unmack is an editor for Marine and Freshwater Research but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
Funding was provided through a New South Wales Fisheries Scientific Committee Student Research Grant, and CAWS/IAE student research funding.
Acknowledgements
This study was undertaken as part of a PhD degree by Hugh Allan at the University of Canberra and was conducted at the Centre for Applied Water Science (CAWS) and Institute for Applied Ecology (IAE). The authors are outstandingly grateful for the assistance and support of volunteers in the field, particularly Liam Allan, Kieran Allan, Glenn Allan, Gretel Allan, Ben Broadhurst, Harvey Broadhurst, Karl Moy and Robert Melodica. We thank Peter Zahr of Cooma, for catering late night travel into and from the field sites. Thanks go to the fish research teams at Broadlab and Rat Industries for professional support, assistance and advice on project design. The authors also thank Elouise Peach and Steve Cathcart from National Parks and Wildlife Service NSW Tumut, for their assistance and support for site access. Parts of this paper were conceptualised and prepared at the Tantangara Mountain Research Station, Witzes Hut.
References
Akbaripasand, A, Nichol, EC, Lokman, PM, and Closs, GP (2011). Microhabitat use of a native New Zealand galaxiid fish, Galaxias fasciatus. New Zealand Journal of Marine and Freshwater Research 45, 135–144.| Microhabitat use of a native New Zealand galaxiid fish, Galaxias fasciatus.Crossref | GoogleScholarGoogle Scholar |
Allan H, Lintermans M (2018) Ecology of the critically endangered stocky galaxias Galaxias tantangara. A progress report for the NSW Fisheries Scientific Committee. University of Canberra, ACT, Australia.
Allan H, Lintermans M (2021) Investigating the utility of drones to identify potential fish refugia. Report to the Australian Society for Fish Biology Threatened Fishes Committee.
| Crossref |
Allan, H, Unmack, P, Duncan, RP, and Lintermans, M (2018). Potential impacts of PIT tagging on a critically endangered small-bodied fish: a trial on the surrogate Mountain Galaxias. Transactions of the American Fisheries Society 147, 1078–1084.
| Potential impacts of PIT tagging on a critically endangered small-bodied fish: a trial on the surrogate Mountain Galaxias.Crossref | GoogleScholarGoogle Scholar |
Allan, H, Duncan, RP, Unmack, P, White, D, and Lintermans, M (2021). Reproductive ecology of a critically endangered alpine galaxiid. Journal of Fish Biology 98, 622–633.
| Reproductive ecology of a critically endangered alpine galaxiid.Crossref | GoogleScholarGoogle Scholar |
Allibone, RM, and Townsend, CR (1997). Reproductive biology, species status and taxonomic relationships of four recently discovered galaxiid fishes in a New Zealand river. Journal of Fish Biology 51, 1247–1261.
| Reproductive biology, species status and taxonomic relationships of four recently discovered galaxiid fishes in a New Zealand river.Crossref | GoogleScholarGoogle Scholar |
Allibone, R, Caskey, D, and Miller, R (2003). Population structure, individual movement, and growth rate of shortjaw kokopu (Galaxias postvectis) in two North Island, New Zealand streams. New Zealand Journal of Marine and Freshwater Research 37, 473–483.
| Population structure, individual movement, and growth rate of shortjaw kokopu (Galaxias postvectis) in two North Island, New Zealand streams.Crossref | GoogleScholarGoogle Scholar |
Altenritter, ME, Pescitelli, SM, Whitten, AL, and Casper, AF (2019). Implications of an invasive fish barrier for the long-term recovery of native fish assemblages in a previously degraded northeastern Illinois River system. River Research and Applications 35, 1044–1052.
| Implications of an invasive fish barrier for the long-term recovery of native fish assemblages in a previously degraded northeastern Illinois River system.Crossref | GoogleScholarGoogle Scholar |
Bell CP (2001) The ecology of Koaro (Galaxias brevipinnis) in Manson Creek, North Canterbury. MSc (Zoology) thesis, University of Canterbury, Christchurch, New Zealand. Available at https://ir.canterbury.ac.nz/handle/10092/4309Please%20check%20the%20edits%20made%20to%20Ref.%20(Bell%202001)
Berra, TM (1973). A home range study of Galaxias bongbong in Australia. Copeia 1973, 363–366.
| A home range study of Galaxias bongbong in Australia.Crossref | GoogleScholarGoogle Scholar |
Berra, TM, and Gunning, GE (1972). Seasonal movement and home range of the longear sunfish, Lepomis megalotis (Rafinesque) in Louisiana. The American Midland Naturalist 88, 368–375.
| Seasonal movement and home range of the longear sunfish, Lepomis megalotis (Rafinesque) in Louisiana.Crossref | GoogleScholarGoogle Scholar |
Bowie S, Jack D, Nelson D (2018) Use of built barriers in New Zealand streams as a conservation management tool. In ‘International conference on engineering and ecohydrology for fish passage’, 10–14 December 2018, Albury, NSW, Australia. (University of Massachusetts—Amherst: Amherst, MA, USA). Available at https://scholarworks.umass.edu/fishpassage_conference/2018/December13/25
Broadhurst, BT, Dyer, JG, Ebner, BC, Thiem, JD, and Pridmore, PA (2011). Response of two-spined blackfish Gadopsis bispinosus to short-term flow fluctuations in an upland Australian stream. Hydrobiologia 673, 63–77.
| Response of two-spined blackfish Gadopsis bispinosus to short-term flow fluctuations in an upland Australian stream.Crossref | GoogleScholarGoogle Scholar |
Broadhurst, BT, Lintermans, M, Thiem, JD, Ebner, BC, Wright, DW, and Clear, RC (2012). Spatial ecology and habitat use of two-spined blackfish Gadopsis bispinosus in an upland reservoir. Aquatic Ecology 46, 297–309.
| Spatial ecology and habitat use of two-spined blackfish Gadopsis bispinosus in an upland reservoir.Crossref | GoogleScholarGoogle Scholar |
Broadhurst, BT, Ebner, BC, Lintermans, M, Thiem, JD, and Clear, RC (2013). Jailbreak: a fishway releases the endangered Macquarie perch from confinement below an anthropogenic barrier. Marine and Freshwater Research 64, 900–908.
| Jailbreak: a fishway releases the endangered Macquarie perch from confinement below an anthropogenic barrier.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, PL (1976a). Home range and movements of the common river galaxies, Galaxias vulgaris Stokell (Pisces: Salmoniformes), in the Glentui River, New Zealand. Marine and Freshwater Research 27, 23–33.
| Home range and movements of the common river galaxies, Galaxias vulgaris Stokell (Pisces: Salmoniformes), in the Glentui River, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, PL (1976b). Breeding biology of a non-diadromous galaxiid, Galaxias vulgaris Stokell, in a New Zealand river. Journal of Fish Biology 8, 157–177.
| Breeding biology of a non-diadromous galaxiid, Galaxias vulgaris Stokell, in a New Zealand river.Crossref | GoogleScholarGoogle Scholar |
Carroll, C, Rohlf, DJ, Li, Y-W, Hartl, B, Phillips, MK, and Noss, RF (2015). Connectivity conservation and endangered species recovery: a study in the challenges of defining conservation-reliant species. Conservation Letters 8, 132–138.
| Connectivity conservation and endangered species recovery: a study in the challenges of defining conservation-reliant species.Crossref | GoogleScholarGoogle Scholar |
Cooke, SJ, Martins, EG, Struthers, DP, et al. (2016). A moving target: incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environmental Monitoring and Assessment 188, 239.
| A moving target: incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations.Crossref | GoogleScholarGoogle Scholar |
Crook, DA (2004a). Movements associated with home-range establishment by two species of lowland river fish. Canadian Journal of Fisheries and Aquatic Sciences 61, 2183–2193.
| Movements associated with home-range establishment by two species of lowland river fish.Crossref | GoogleScholarGoogle Scholar |
Crook, DA (2004b). Is the home range concept compatible with the movements of two species of lowland river fish? Journal of Animal Ecology 73, 353–366.
| Is the home range concept compatible with the movements of two species of lowland river fish?Crossref | GoogleScholarGoogle Scholar |
Crook, DA, and White, RWG (1995). Evaluation of subcutaneously implanted visual implant tags and coded wire tags for marking and benign recovery in a small scaleless fish, Galaxias truttaceus (Pisces: Galaxiidae). Marine and Freshwater Research 46, 943–946.
| Evaluation of subcutaneously implanted visual implant tags and coded wire tags for marking and benign recovery in a small scaleless fish, Galaxias truttaceus (Pisces: Galaxiidae).Crossref | GoogleScholarGoogle Scholar |
Daly, R, Filmalter, JD, Peel, LR, Mann, BQ, Lea, JSE, Clarke, CR, and Cowley, PD (2021). Ontogenetic shifts in home range size of a top predatory reef-associated fish (Caranx ignobilis): implications for conservation. Marine Ecology Progress Series 664, 165–182.
| Ontogenetic shifts in home range size of a top predatory reef-associated fish (Caranx ignobilis): implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Davis A (2017) Investigating the role of long distance dispersal in the response of stream fishes to urbanization. MSc (Integrative Biology) thesis, Kennesaw State University. Available at https://digitalcommons.kennesaw.edu/integrbiol_etd/20
David, BO, and Closs, GP (2001). Continuous remote monitoring of fish activity with restricted home ranges using radiotelemetry. Journal of Fish Biology 59, 705–715.
| Continuous remote monitoring of fish activity with restricted home ranges using radiotelemetry.Crossref | GoogleScholarGoogle Scholar |
David, BO, and Stoffels, RJ (2003). Spatial organisation and behavioural interaction of giant kokopu (Galaxias argenteus) in two stream pools differing in fish density. New Zealand Journal of Marine and Freshwater Research 37, 315–322.
| Spatial organisation and behavioural interaction of giant kokopu (Galaxias argenteus) in two stream pools differing in fish density.Crossref | GoogleScholarGoogle Scholar |
Dawson, DR, and Koster, WM (2018). Habitat use and movements of Australian grayling (Prototroctes maraena) in a Victorian coastal stream. Marine and Freshwater Research 69, 1259–1267.
| Habitat use and movements of Australian grayling (Prototroctes maraena) in a Victorian coastal stream.Crossref | GoogleScholarGoogle Scholar |
DeHaan, PW, Von Bargen, J, and Scheerer, PD (2017). Genetic variation and the relationship between stream and lake ecotypes of a threatened desert Catostomid, the Warner sucker (Catostomus warnerensis). Ecology of Freshwater Fish 26, 609–620.
| Genetic variation and the relationship between stream and lake ecotypes of a threatened desert Catostomid, the Warner sucker (Catostomus warnerensis).Crossref | GoogleScholarGoogle Scholar |
Ebner, BC, and Thiem, JD (2009). Monitoring by telemetry reveals differences in movement and survival following hatchery or wild rearing of an endangered fish. Marine and Freshwater Research 60, 45–57.
| Monitoring by telemetry reveals differences in movement and survival following hatchery or wild rearing of an endangered fish.Crossref | GoogleScholarGoogle Scholar |
Enders, EC, Clarke, KD, Pennell, CJ, Ollerhead, LMN, and Scruton, DA (2007). Comparison between PIT and radio telemetry to evaluate winter habitat use and activity patterns of juvenile Atlantic salmon and brown trout. Hydrobiologia 582, 231–242.
| Comparison between PIT and radio telemetry to evaluate winter habitat use and activity patterns of juvenile Atlantic salmon and brown trout.Crossref | GoogleScholarGoogle Scholar |
Fletcher, CM, Collins, SF, Nannini, MA, and Wahl, DH (2019). Competition during early ontogeny: effects of native and invasive planktivores on the growth, survival, and habitat use of bluegill. Freshwater Biology 64, 697–707.
| Competition during early ontogeny: effects of native and invasive planktivores on the growth, survival, and habitat use of bluegill.Crossref | GoogleScholarGoogle Scholar |
Fluker, BL, Jones, KD, and Kuhajda, BR (2019). Genetic structure and diversity of the blueface darter Etheostoma cyanoprosopum, a microendemic freshwater fish in the southeastern USA. Endangered Species Research 40, 133–147.
| Genetic structure and diversity of the blueface darter Etheostoma cyanoprosopum, a microendemic freshwater fish in the southeastern USA.Crossref | GoogleScholarGoogle Scholar |
Funk, JL (1957). Movement of stream fishes in Missouri. Transactions of the American Fisheries Society 85, 39–57.
| Movement of stream fishes in Missouri.Crossref | GoogleScholarGoogle Scholar |
Gerber, KM, Mather, ME, and Smith, JM (2017). A suite of standard post-tagging evaluation metrics can help assess tag retention for field-based fish telemetry research. Reviews in Fish Biology and Fisheries 27, 651–664.
| A suite of standard post-tagging evaluation metrics can help assess tag retention for field-based fish telemetry research.Crossref | GoogleScholarGoogle Scholar |
Gerking, SD (1953). Evidence for the concepts of home range and territory in stream fishes. Ecology 34, 347–365.
| Evidence for the concepts of home range and territory in stream fishes.Crossref | GoogleScholarGoogle Scholar |
Herrera, M, Moreno-Valcárcel, R, De Miguel Rubio, R, and Fernández-Delgado, C (2019). From transient to sedentary? Changes in the home range size and environmental patterns of movements of European Eels (Anguilla anguilla) in a Mediterranean River. Fishes 4, 43.
| From transient to sedentary? Changes in the home range size and environmental patterns of movements of European Eels (Anguilla anguilla) in a Mediterranean River.Crossref | GoogleScholarGoogle Scholar |
Hesthagen, T (1990). Home range of juvenile Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, in a Norwegian stream. Freshwater Biology 24, 63–67.
| Home range of juvenile Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, in a Norwegian stream.Crossref | GoogleScholarGoogle Scholar |
Hilderbrand, RH, and Kershner, JL (2000). Conserving inland cutthroat trout in small streams: how much stream is enough? North American Journal of Fisheries Management 20, 513–520.
| Conserving inland cutthroat trout in small streams: how much stream is enough?Crossref | GoogleScholarGoogle Scholar |
Hill, J, and Grossman, GD (1987). Home range estimates for three north American stream fishes. Copeia 1987, 376–380.
| Home range estimates for three north American stream fishes.Crossref | GoogleScholarGoogle Scholar |
Jungwirth, A, Balzarini, V, Zöttl, M, Salzmann, A, Taborsky, M, and Frommen, JG (2019). Long-term individual marking of small freshwater fish: the utility of visual implant elastomer tags. Behavioral Ecology and Sociobiology 73, 49.
| Long-term individual marking of small freshwater fish: the utility of visual implant elastomer tags.Crossref | GoogleScholarGoogle Scholar |
Klinard, NV, Halfyard, EA, Fisk, AT, Stewart, TJ, and Johnson, TB (2018). Effects of surgically implanted acoustic tags on body condition, growth, and survival in a small, laterally compressed forage fish. Transactions of the American Fisheries Society 147, 749–757.
| Effects of surgically implanted acoustic tags on body condition, growth, and survival in a small, laterally compressed forage fish.Crossref | GoogleScholarGoogle Scholar |
Koehn JD, Crook DA (2013) Movements and migration. In ‘Ecology of Australian Freshwater Fishes’. (Eds P Humphries, K Walker) pp. 105–129. (CSIRO Publishing: Melbourne, Vic., Australia)
Koehn, JD, McKenzie, JA, O’Mahony, DJ, Nicol, SJ, O’Connor, JP, and O’Connor, WG (2009). Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river. Ecology of Freshwater Fish 18, 594–602.
| Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Koster, WM, Dawson, DR, Kitchingman, A, Moloney, PD, and Hale, R (2020). Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool. Journal of Fish Biology 96, 782–794.
| Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool.Crossref | GoogleScholarGoogle Scholar |
Lane, AA, Kornis, MS, and Bronte, CR (2019). Evaluation of coded wire tag loss in brook trout tagged using automated methods. North American Journal of Fisheries Management 39, 29–35.
| Evaluation of coded wire tag loss in brook trout tagged using automated methods.Crossref | GoogleScholarGoogle Scholar |
Lintermans M (1998) The ecology of the two-spined blackfish Gadopsis bispinosus (Pisces: Gadopsidae). MSc thesis, Australian National University, Canberra, ACT, Australia. Available at https://openresearch-repository.anu.edu.au/handle/1885/10975
Lintermans M, Allan H (2019) Stocky galaxias Galaxias tantangara. In ‘IUCN Red List of Threatened Species 2019’. e.T122903246A123382161. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/122903246/123382161
Lintermans, M, Geyle, HM, Beatty, S, et al. (2020). Big trouble for little fish: identifying Australian freshwater fishes in imminent risk of extinction. Pacific Conservation Biology 26, 365–377.
| Big trouble for little fish: identifying Australian freshwater fishes in imminent risk of extinction.Crossref | GoogleScholarGoogle Scholar |
Lucas, MC, and Baras, E (2000). Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish and Fisheries 1, 283–316.
| Methods for studying spatial behaviour of freshwater fishes in the natural environment.Crossref | GoogleScholarGoogle Scholar |
McInnes, MG, Burns, NM, Hopkins, CR, Henderson, GP, McNeill, DC, and Bailey, DM (2020). A new model study species: high accuracy of discrimination between individual freckled hawkfish (Paracirrhites forsteri) using natural markings. Journal of Fish Biology 96, 831–834.
| A new model study species: high accuracy of discrimination between individual freckled hawkfish (Paracirrhites forsteri) using natural markings.Crossref | GoogleScholarGoogle Scholar |
Milano, D, Aigo, JC, and Macchi, PJ (2013). Diel patterns in space use, food and metabolic activity of Galaxias maculatus (Pisces: Galaxiidae) in the littoral zone of a shallow Patagonian lake. Aquatic Ecology 47, 277–290.
| Diel patterns in space use, food and metabolic activity of Galaxias maculatus (Pisces: Galaxiidae) in the littoral zone of a shallow Patagonian lake.Crossref | GoogleScholarGoogle Scholar |
Moore, SJ, Allibone, RM, and Townsend, CR (1999). Spawning site selection by two galaxiid fishes, Galaxias anomalus and G. depressiceps, in tributaries of the Taieri River, South Island, New Zealand. New Zealand Journal of Marine and Freshwater Research 33, 129–139.
| Spawning site selection by two galaxiid fishes, Galaxias anomalus and G. depressiceps, in tributaries of the Taieri River, South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Mundahl, ND, and Ingersoll, CG (1989). Home range, movements, and density of the central stoneroller, Campostoma anomalum, in a small Ohio stream. Environmental Biology of Fishes 24, 307–311.
| Home range, movements, and density of the central stoneroller, Campostoma anomalum, in a small Ohio stream.Crossref | GoogleScholarGoogle Scholar |
Norman, BM, and Morgan, DL (2016). The return of ‘Stumpy’ the whale shark: two decades and counting. Frontiers in Ecology and the Environment 14, 449–450.
| The return of ‘Stumpy’ the whale shark: two decades and counting.Crossref | GoogleScholarGoogle Scholar |
NSW Fisheries Scientific Committee (2016) ‘Final determination: Galaxias tantangara: Stocky Galaxias as a critically endangered species.’ (NSW Fisheries Scientific Committee)
Pillans, RD, Babcock, RC, Thomson, DP, et al. (2017). Habitat effects on home range and schooling behaviour in a herbivorous fish (Kyphosus bigibbus) revealed by acoustic tracking. Marine and Freshwater Research 68, 1454–1467.
| Habitat effects on home range and schooling behaviour in a herbivorous fish (Kyphosus bigibbus) revealed by acoustic tracking.Crossref | GoogleScholarGoogle Scholar |
Pittman RG (2011) Minimum stream length requirements for McCloud River redband trout (Oncorhynchus mykiss spp.) in Trout and Tate creeks, Siskiyou County, California. MSc thesis, Humboldt State University, Department of Fisheries, Arcata, CA, USA. Available at http://dspace.calstate.edu/handle/2148/733
Pygas D, Alderson B, Reeds K, Blount C (2019) Appendix M.2 aquatic ecology impact assessment, Snowy 2.0 main works, prepared for EMM Consulting by Cardno Pty Ltd. (EMM Consulting by Cardno Pty Ltd)
Raadik, TA (2014). Fifteen from one: a revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 3898, 1–198.
| Fifteen from one: a revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species.Crossref | GoogleScholarGoogle Scholar |
Rash, JM, Goodfred, DW, and Jones, EM (2018). Evaluation of coded wire tag retention in brown trout (Salmo trutta) fingerlings tagged at three anatomical locations. Journal of Freshwater Ecology 33, 503–509.
| Evaluation of coded wire tag retention in brown trout (Salmo trutta) fingerlings tagged at three anatomical locations.Crossref | GoogleScholarGoogle Scholar |
Tamario, C, Calles, O, Watz, J, Nilsson, PA, and Degerman, E (2019). Coastal river connectivity and the distribution of ascending juvenile European eel (Anguilla anguilla L.): implications for conservation strategies regarding fish-passage solutions. Aquatic Conservation: Marine and Freshwater Ecosystems 29, 612–622.
| Coastal river connectivity and the distribution of ascending juvenile European eel (Anguilla anguilla L.): implications for conservation strategies regarding fish-passage solutions.Crossref | GoogleScholarGoogle Scholar |
Thiem, JD, Broadhurst, BT, Lintermans, M, Ebner, BC, Clear, RC, and Wright, D (2013). Seasonal differences in the diel movements of Macquarie perch (Macquaria australasica) in an upland reservoir. Ecology of Freshwater Fish 22, 145–156.
| Seasonal differences in the diel movements of Macquarie perch (Macquaria australasica) in an upland reservoir.Crossref | GoogleScholarGoogle Scholar |
Town, C, Marshall, A, and Sethasathien, N (2013). Manta Matcher: automated photographic identification of manta rays using keypoint features. Ecology and Evolution 3, 1902–1914.
| Manta Matcher: automated photographic identification of manta rays using keypoint features.Crossref | GoogleScholarGoogle Scholar |
Waltham, NJ, and Schaffer, J (2019). Feral pig exclusion fencing provides limited fish conservation value on tropical floodplains. bioRxiv , 625053.
| Feral pig exclusion fencing provides limited fish conservation value on tropical floodplains.Crossref | GoogleScholarGoogle Scholar |


