Particle aggregation, pH changes and metal behaviour during estuarine mixing: review and integration
Luke M. Mosley A C and Peter S. Liss BA School of Biological Sciences, University of Adelaide Waite Campus, PMB 1, Glen Osmond, SA 5064, Australia.
B Centre for Ocean and Atmospheric Sciences, School of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK.
C Corresponding author. Email: luke.mosley@adelaide.edu.au
Marine and Freshwater Research 71(3) 300-310 https://doi.org/10.1071/MF19195
Submitted: 26 May 2019 Accepted: 30 June 2019 Published: 4 October 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Estuaries are dynamic mixing zones where river water interacts with seawater, resulting in large and complex geochemical changes. How two key factors, particle aggregation and pH, affect metal behaviour in estuaries is reviewed and integrated in this paper. Riverine particles are coated with organic matter and electrostatic repulsive forces restrict aggregation. In estuaries, increased concentrations of divalent cations reduce the repulsive forces between particles at low salinities, resulting in their rapid coagulation and removal of particulate-associated metals (e.g. Fe and Pb). However, truly dissolved metals may mix conservatively, and metals associated more with colloidal and dissolved organic material (e.g. Cu and Zn) can show variable behaviour. In many field studies and modelling of river inputs with different compositions, pH decreases slightly at low salinity. Geochemical model simulations of dissolved metal speciation indicated that Zn would be desorbed from iron oxide binding surfaces due to these pH and cation concentration changes, with Cu also showing less binding to dissolved organic matter (DOM). DOM, pH and particle surfaces can influence individual metal behaviour at various spatial and temporal scales. Further integrated field and laboratory research in estuaries where key geochemical processes affecting metal concentrations are measured and modelled is needed.
Introduction
Estuaries are complex geochemical reactors that can greatly modify the delivery of dissolved and suspended material from rivers to the ocean. Understanding how pollutants behave in estuarine environments is important because they have very high environmental and socioeconomic value (e.g. culturally, aesthetically, fisheries and recreation). Although there have been many field and laboratory studies on estuaries over the past 40–50 years, there are gaps remaining in our understanding of geochemical processes and how they interact. The aim of this paper is to review and integrate how two fundamental processes, particle aggregation and pH changes, influence metal dynamics in estuaries.
The degree to which metals sorb or desorb to particles in estuaries, and how those particles behave, is a very important driver of their fate and transport in estuaries (Burton and Liss 1976; Benoit et al. 1994; Dai and Martin 1995; Waeles et al. 2008; Illuminati et al. 2019). Metals adsorbed to particles greater than ~1 μm in size can be removed from the water column by sedimentation. However, at low salinity, particles <1 μm (colloids) will not settle out of the water column even over long periods of time because their gravitational settling rate is less than their random displacement by the Brownian motion of the surrounding water molecules. In estuaries, the destabilising influence of the seawater cations can lead to aggregation of colloidal material (Sholkovitz 1976, 1978; Boyle et al. 1977; Mosley et al. 2003). This also provides a mechanism for the removal of other metals that have a tendency to be adsorbed to colloid surfaces (e.g. Pb, Zn). In estuaries affected by pollution, significant amounts of anthropogenic metal inputs may end up in the sediment (Liu et al. 2019). Following deposition, mixing and redistribution of sediment phases and associated metals can occur (Reese et al. 2019). Flux of metals to the water column from the sediment arises due to diffusion or resuspension and oxidation of sulfide phases (Richards et al. 2018; Duan et al. 2019).
Binding of some metals (e.g. Cu) to colloidal and dissolved organic matter (DOM) is also very important in estuaries because a quite different geochemistry can be observed for these metals compared with the predominantly particle-associated metals (Santschi et al. 1997; Tang et al. 2002; Illuminati et al. 2019). The binding behaviour of individual metals to DOM, and the functional composition of the DOM itself, can also vary with salinity in estuaries (Zhou et al. 2016; Ksionzek et al. 2018). In addition, pH and inorganic carbon speciation may vary markedly in an estuary as river water, with typically lower pH and alkalinity (except in some calcareous drainage basins), mixes with seawater. The partial pressure of carbon dioxide (pCO2) is often higher in rivers due to system respiration, driven by microbial degradation of organic matter, exceeding net primary production (Cole and Caraco 2001). Typically, pH increases towards seawater values in a non-linear way in estuaries, and decreasing pH has also been observed at low salinity (Mook and Koene 1975; Mosley et al. 2004, 2010). pH is a ‘master variable’ controlling metal sorption to particles surfaces (Dzombak and Morel 1990), so it could be expected that pH changes in estuaries will also greatly affect metal behaviour. The increased ionic strength, and changing inorganic and organic ligand concentration, in estuaries could also result in altered dissolved metal speciation and binding compared with river water.
Processes affecting trace metal behaviour in an estuary can be inferred from field studies, but it is often difficult to separate the effects of physicochemical processes occurring in the water column from the effects of biological activity or input of material from the underlying sediment. Controlled laboratory experiments in which river water and seawater are mixed in varying amounts to simulate the likely salinity gradient in an estuary have been widely used to provide information on processes occurring in the water column generally on short time scales (Hunter 1983). By comparing the laboratory results to the results from field studies for the same estuary, it may be possible to identify other processes that affect the behaviour of trace metals (Sholkovitz et al. 1978; Shiller and Boyle 1991). The speciation and partitioning of trace metals in estuaries has also been calculated using a knowledge of the equilibrium constants for the interaction of metal ions with various ligands and particle surfaces (Mantoura et al. 1978; Bourg 1987; Tipping et al. 1998; Hamilton-Taylor et al. 2002; Pearson et al. 2018). However, to the best of our knowledge, all the potential factors affecting metal behaviour (pH, ionic strength, metal oxide surfaces, inorganic and organic complexation) have not been integrated.
To achieve the aim of this review, the relevant international peer-reviewed literature in this area was synthesised. Geochemical model simulations were also performed to help elucidate why individual metal behaviour may vary in estuaries.
Particle aggregation due to increasing salinity in estuaries
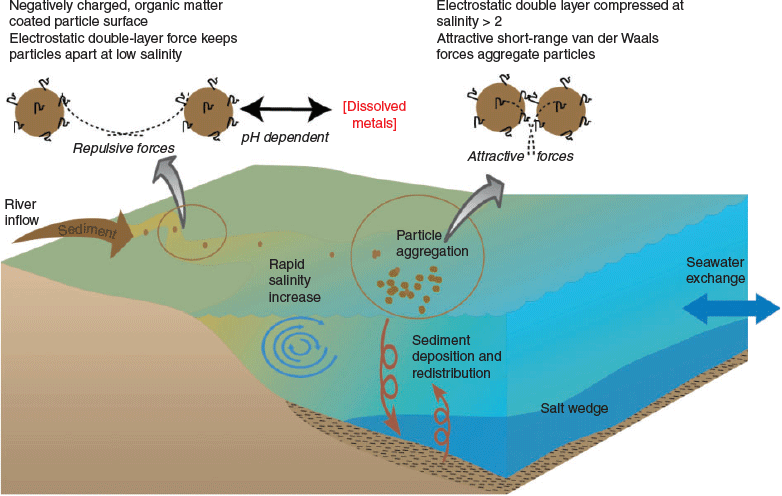
River-borne particles entering estuaries can be very diverse in terms of their origin, mineralogy, hydrous metal (Fe, Mn) oxide concentration, elemental composition and size distribution. However, the surface properties of particles are usually rendered quite uniform by the ubiquitous surface film of natural organic matter that envelops the particles (Hunter and Liss 1979, 1982). Exceptions to this general rule may occur in rivers that are very rich in Fe particulates and relatively low in organic matter (Newton and Liss 1987). Almost all particles are found to be negatively charged, which is attributed to the presence of carboxyl acid groups (pKa = ~4.5) on the organic matter dominating the surface chemistry (Hunter 1980). The negative charge on the particle–organic matter surface creates an electrostatic ‘double layer’ that extends from the particle surface. The first layer in the double layer is the charged surface that originates from the dissociated functional groups (e.g. COO–) and tightly absorbed or substituted ions. The second layer corresponds to the diffuse layer, which contains the neutralising charge consisting of accumulated counter ions (cations in the case of negatively charged surfaces in natural waters) and depleted co-ions (i.e. different concentration compared with the bulk solution). Atomic force microscopy (AFM) measurements have shown that the double layer distance controls the range of interparticle repulsive forces and is sufficient to prevent particle aggregation in fresh water (Mosley et al. 2003; Sander et al. 2004). However, coagulation may occur in cation-rich rivers before they reach their estuaries.
The flocculation of riverine colloids in estuaries is driven by the increase in the amount of seawater cations such as Ca2+ and Mg2+ (Eckert and Sholkovitz 1976; Boyle et al. 1977; Nowostawska et al. 2008). The multivalent ions Ca2+ and Mg2+ are more effective than singly charged cations (Na+, K+) at screening the electrostatic repulsive force in the diffuse double layer, which then compresses closer to the surface of the particles (Fig. 1). The distance the double layer extends is called the Debye length (δD; nm) and can be approximated in estuaries by the following relationship (Stumm and Morgan 1996):

where I is ionic strength. In fresh water with a low ionic strength (I = ~0.0001 M), δD extends ~30 nm, which prevents particles from aggregating. By a salinity of ~1 (I = ~0.02 M), the electric double layer is compressed to ~2 nm, which is close enough for attractive short-range van der Waals forces to occur (Fig. 1; Mosley et al. 2003). These forces are insensitive to salinity, and aggregate and stick together particles that come into close contact. Interparticle forces in river–estuarine water have been measured directly and predicted quantitatively (Zhou et al. 1994; Mosley et al. 2003; Sander et al. 2004) using classical DLVO theory (Verwey and Overbeek 1948). The organic matter on the surface can introduce additional steric forces but, based on AFM measurements, these do not appear to provide a barrier to aggregation except at short interparticle separations (Mosley et al. 2003; Sander et al. 2004). Conformational changes may also occur on the natural organic matter due to increased salinity that assist in coiling it or compressing it closer to the particle surface (Kruger et al. 2011). Organic matter coatings and divalent cations may create additional bridging mechanisms that influence particle aggregation and separation forces (Mosley et al. 2003). Different organic matter amounts and sources relative to the particulate load may result in different behaviour in this regard (Furukawa et al. 2014). The role of transparent exopolymeric particles (TEP), released by phytoplankton and other microorganisms, has also been shown to enhance aggregation in estuaries, particularly at mid to high salinity ranges (Wetz et al. 2009; Mari et al. 2012).
The removal of colloidal particles by coagulation in estuaries was elucidated by the early laboratory studies of Sholkovitz (1976), who used a batch mixing approach (a portion of river water mixed with a portion of seawater to create a particular salinity). Continuous mixing approaches may more accurately simulate the mixing processes in an estuary, where the mixing occurs gradually and in a continuous and sequential manner (Hunter et al. 1997). Using such approaches, the kinetics of the particle coagulation process have been found to be fast, with a significant amount of aggregation occurring within seconds to minutes of salinity increasing in estuaries (Hunter and Leonard 1988; Nowostawska et al. 2008; Gigault et al. 2018). The aggregation rate has been found to be dependent on salinity but independent of the mixing rate and temperature (Hunter et al. 1997). The presence of larger (>1 µm) particles has been found to enhance the aggregation process in laboratory experiments due to increasing interparticle collisions (Hunter and Leonard 1988). However, it is important to note that particle dynamics in real estuaries are much more complex than in laboratory experimental settings (e.g. Wang et al. 2013). For example, redistribution of particles can occur very dynamically in response to riverine, tidal and wind-driven currents and, along with salinity stratification, create what is known as the estuarine turbidity maxima (McLachlan et al. 2017). Hence, it can be difficult to understand and separate the different particle aggregation and dynamic processes occurring in estuaries, although there has been some success in simulating these (Trento and Vinzon 2014; Ramírez-Mendoza et al. 2014).
Metal behaviour during estuarine mixing
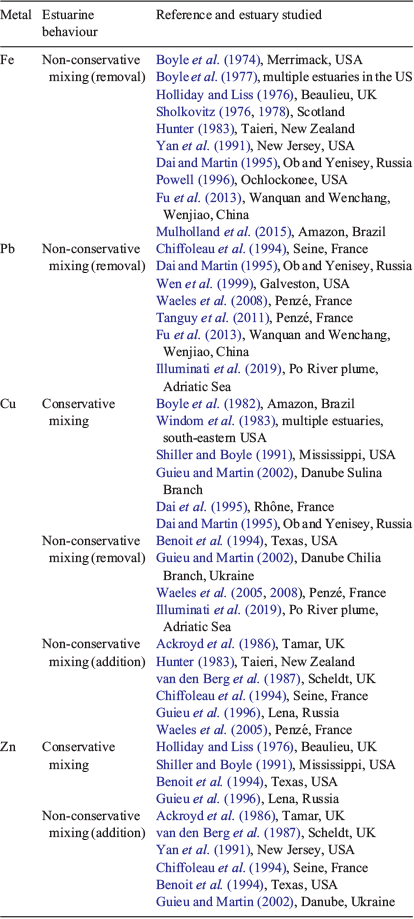
Metals in estuarine water are predominantly derived from riverine inputs, atmospheric deposition and fluxes from the sediment, all of which can have natural and anthropogenic sources. Metal behaviour in different estuaries is often interpreted by comparing concentrations that would be expected if conservative mixing (linear interpolation between freshwater and seawater end member metal concentrations and salinity) v. non-conservative mixing (removal or addition of metals above conservative mixing line) occurred (Liss 1976). Table 1 shows a collation of metal behaviour results from different studies for selected different metals that are commonly studied (Fe, Pb, Cu, Zn) and have different propensities for partitioning to particulate, organic matter or truly dissolved solution phases. As shown below, these differences can explain why these metals have quite different estuarine mixing behaviour. However, it should be noted that there are potential pitfalls of the conservative mixing line approach because it assumes it is possible to identify a unique freshwater end-member composition when the river water may come from several sources, which themselves are time variant or may enter within the estuarine mixing zone. External sources of metals can be significant in contaminated estuaries (Liu et al. 2019). Fixing the seawater end-member composition is often more straightforward due to its relative homogeneity. Other problems include the collection of sufficient samples to cover the whole salinity range, the effect of high suspended loads making pre-analysis filtration difficult and analytical difficulties arising from the wide salinity range of the samples. Further information and a discussion of issues arising in the interpretation of results can be found in Liss (1976).
|
Fe is found mainly in particulate (including colloidal) FeIII oxide form in rivers, which is its most stable form in air-saturated waters at near neutral pH values (e.g. Boyle et al. 1977; Hunter 1983; Dai and Martin 1995). River inputs of Fe to estuaries can show large seasonal variation (Fu et al. 2013). Fe has exhibited non-conservative removal behaviour in estuaries across a wide range of different studies (see Table 1). The aggregation of colloids by the mechanisms described above is the accepted reason for the observed removal of Fe in estuaries and, indeed, Fe concentration has been used as a proxy measure for colloid concentration in laboratory experiments (Hunter and Leonard 1988). However, with regard to the truly dissolved Fe fraction (typically a much lower concentration than the solid Fe phase), Dai and Martin (1995) found it behaved conservatively in the Ob and Yenisey estuaries. Some dissolved and colloidal Fe-binding constituents of aquatic humic substances also appear not to be subject to coagulation along the estuarine salinity gradient (Merschel et al. 2017; Muller 2018). Hence, although it seems likely much particulate Fe is deposited to estuarine sediments, a portion can still be transported through estuaries to the coastal ocean (Sanders et al. 2015). The application of advanced isotope techniques has been shown to be useful to trace riverine sediment–metal redistribution in estuaries (Reese et al. 2019). The redox state of the water column and sediment is also an important control of Fe speciation and flux. The formation of iron (hydr)oxides is effective for the removal of trace metals under oxidising conditions, whereas the formation of sulfides can be conducive to the removal of trace metals under reducing conditions (Duan et al. 2019). Resuspension and oxidation of solid FeS phases can also release dissolved Fe and other metals (Richards et al. 2018).
Pb has also been found predominantly in the particulate (including colloidal) fraction of rivers and estuaries (Erel et al. 1991; Benoit et al. 1994; Benoit 1995; Tanguy et al. 2011). Pb shows strong binding to iron oxide phases at typical estuarine, circumneutral, pH values (Dzombak and Morel 1990). Consistent with this partitioning, the behaviour of Pb has generally showed non-conservative removal in the low salinity (<5) zone across multiple studies (see studies in Table 1). Rare earth elements, which also strongly partition to iron oxides, also show strong removal behaviour in estuaries (Hoyle et al. 1984; Sholkovitz 1995; Merschel et al. 2017). This indicates the estuarine mixing profile of particle-reactive elements is dominantly controlled by the extent of partitioning to colloidal material (e.g. iron oxides) and the aggregation of the colloids typically removes these metals from solution. However, like Fe, the truly dissolved Pb fraction, which is much lower in concentration, may show conservative mixing (Dai and Martin 1995; Tanguy et al. 2011) and increase to form a large component of the total dissolved Pb approaching seawater values (Illuminati et al. 2019). The dynamics of Pb in estuaries can be disturbed by anthropogenic inputs and the activity of benthic organisms (Duan et al. 2019).
Measurements of Cu speciation have indicated that most (>99%) of the Cu in rivers (Sunda and Hanson 1979) and estuaries (van den Berg et al. 1987; Paulson et al. 1994) is strongly bound by an unidentified organic ligand or group of ligands present at low concentrations. In terms of organic matter size fraction partitioning, some studies have found that Cu was predominantly found in the truly dissolved (<10 kDa) size fraction (Benoit et al. 1994; Paulson et al. 1994; Greenamoyer and Moran 1997), whereas others have found that the majority of the Cu was in the colloidal fraction (Dai and Martin 1995; Wen et al. 1996). During estuarine mixing, the behaviour of Cu has also been observed to be variable, with differing partitioning behaviour (conservative, addition, removal) found between different studies (Table 1). Laboratory mixing experiments have also shown a mixed behaviour with, for example, Sholkovitz (1978) finding a 40% removal of Cu during estuarine mixing (Water of Luce), whereas Shiller and Boyle (1991) found that Cu mixed conservatively (Mississippi River). This variable behaviour has been postulated to be dependent on whether Cu is bound to colloidal organic matter fractions (typically high molecular weight humic acids) that can flocculate out of solution and remove Cu or to DOM, which may behave conservatively and hence also lead to the conservative behaviour of Cu (Sholkovitz 1978; Dai and Martin 1995). Laboratory experiments have shown that the binding of Cu to organic ligands may be unaffected by increases in salinity in estuaries (Hamilton-Taylor et al. 2002). However, biological ligand production and metal uptake may affect Cu concentrations (Guieu and Martin 2002; Illuminati et al. 2019). Waeles et al. (2008) found that seasonal variation in Cu complexation, with the production of strongly complexing hydrophobic organic ligands by phytoplankton in summer, increased organic complexation. This also highlights a further limitation of the conservative mixing line approach, which assumes organic and other ligands that bind metals are not produced internally during estuarine mixing. In general, the observed variable behaviour of Cu is probably to be expected because it is too simplistic to treat all rivers as having the same properties (e.g. pH, organic matter amount and type, as well as the amount and type of suspended solids).
Zn has also been found to be strongly organically complexed (but to a lesser extent than Cu) to ligands present at low concentration (van den Berg et al. 1987; Bruland 1989). In terms of partitioning, Wen et al. (1999) found that on average 91% of Zn was present in the 1-kDa or 0.4-μm (colloidal) fraction, which is similar to the partitioning also found in Texan estuaries (Benoit et al. 1994). Similar to Cu, Zn has also exhibited variable behaviour in estuaries, with addition above the conservative mixing line in some studies and conservative behaviour in others (Table 1). Guieu and Martin (2002) postulated that Zn was behaving more similarly to inorganic solid or colloidal phases in estuaries, which could be expected if predominantly colloid partitioning occurred. Many studies have highlighted addition of Zn occurring at low salinity (Table 1), which would not be expected if the binding to colloid surfaces was maintained during the early stages of estuarine mixing. Church (1986) found Zn was desorbed when unfiltered river water was used in a mixing experiment, whereas conservative mixing was observed when filtered water was used. The potential role of pH changes in estuaries leading to desorption of Zn from particles (non-conservative addition) is discussed and modelled further below. There can also be dynamic production of different-sized colloids, sediment : water fluxes, resuspension and sorption–desorption reactions that can affect the concentration of Zn and other metals (Benoit et al. 1994; Wen et al. 1999; Richards et al. 2018). Duan et al. (2019) found that the dynamics of Cu and Zn were primarily controlled by Fe and Mn diagenesis, and that sediment fluxes were a significant source of trace metals in the water column.
Changes in pH in estuaries
pH measurements in estuaries were first made in the 1970s using the conventional hydrogen electrode (Mook and Koene 1975; Morris 1978). Subsequent studies found that there were serious difficulties in the accurate and reproducible measurement using this method because of the need to calibrate the electrode for different ionic strengths (Millero 1986), as well as variation in electrode responses with time and between different electrodes (Whitfield et al. 1985). Cai and Wang (1998) measured pH in several US estuaries but had only limited success with calibration of the pH electrodes using a series of Tris buffers of different salinities. Despite these earlier findings, glass electrode measurements have continued to be made in estuaries, but some results may be questionable because ionic strength-specific calibration has often not been reported.
Increasingly spectrophotometric methods are being used for pH measurement in seawater and were first applied to estuaries by Mosley et al. (2004). In these methods, a sulfonephthalein indicator dye (e.g. m-cresol purple or thymol blue) is added to the sample and, based on knowledge of the dye dissociation constant (salinity and temperature dependent) and molar absorptivity, pH can be calculated following spectrophotometric measurement of the absorption maxima of the acid and base forms of the dye. Recently the m-cresol purple indicator dye characterisation has been reassessed to enable accurate spectrophotometric pH measurements for the entire estuarine salinity range (Douglas and Byrne 2017).
Based on spectrophotometric pH measurements in three New Zealand estuaries, pH was observed to decrease slightly at low salinities (salinity <2), increase rapidly at intermediate salinities (salinity 2–15) and then stabilise at higher salinities (salinity >15; Mosley et al. 2010). Similar trends in estuarine pH measured using glass electrodes have been reported previously, including slight decreases in pH at low salinities (Mook and Koene 1975; Morris 1978; Cai and Wang 1998; Guo et al. 2008). Using measurements and modelling, Mosley et al. (2010) explained the drivers of the general pH patterns during mixing of a circumneutral river with seawater as follows: (1) bicarbonate is the dominant species in circumneutral river water and at all salinities in estuaries, and exhibits a generally linear increase in concentration with salinity; (2) due to the large increases in ionic strength at low salinities (<5), the inorganic carbon dissociation constants change markedly, which can result in a decline in pH in this zone; and (3) following the initial pH decline, pH increases rapidly and then stabilises as salinities rise above 15 and the relative effects of changes in ionic strength on the inorganic carbon system equilibrium constants becomes smaller.
pH can vary widely in rivers, ranging from high pH (≥8) in calcium carbonate-dominated systems (e.g. Edwards 1973) to low pH (≤4) in organic or mineral acid-affected systems (e.g. Raymond and Oh 2009). The pH and inorganic carbon concentrations in rivers can also vary seasonally and annually depending on discharge patterns and longer-term climatic and anthropogenic drivers (Edwards 1973; Raymond and Oh 2009; Biswas and Mosley 2019). Given the uncertainty in pH changes in many estuaries due to the complications noted above, we used the end-member mixing model approach validated by Mosley et al. (2010) to explore how pH may vary with four different river water input compositions (Fig. 2): Case 1, average river water alkalinity (500 µmol kg–1) with near atmospheric pCO2 (400 ppm); Case 2, average river water alkalinity with elevated pCO2 (4000 ppm); Case 3, higher alkalinity river water (2000 µmol kg–1) and near atmospheric pCO2; and Case 4, higher alkalinity river water with elevated pCO2. The salinity of the freshwater and seawater end-members in the model was 0.05 and 35 respectively. The model assumed equilibrium and used the salinity, alkalinity and pCO2 values in the four cases to calculate the total dissolved inorganic carbon (DIC) in the end-members. The alkalinity and DIC were then mixed conservatively between the end-members in the model and the pH calculated for different salinities between 0.05 and 35.

|
All cases showed a slight decrease in pH at low salinities before being relatively stable at values >15 (Fig. 2), which was generally consistent with the field and modelling studies described above. The average alkalinity and elevated pCO2 river water model (Case 2) exhibited the largest changes in pH throughout the estuarine salinity range. This case is likely most representative of a large number of rivers globally that have elevated pCO2 due to respiration exceeding net primary production (Cole and Caraco 2001). The higher alkalinity with atmospheric pCO2 model (Case 3) showed only a decrease and stabilisation rather than an increase in pH at salinities between 3 and 15, as in the other cases. This is likely because the alkalinity is similar to seawater, so the main pH changes are due to variation in inorganic carbon system equilibrium constants at low salinity. It should be noted that the assumption made in this model was that that dissolved organic carbon (DOC) and alkalinity behaved conservatively between the end-members. This is likely to be a valid assumption for estuaries with short residence times, but additions of DIC and alkalinity have been observed in estuaries that have been suggested to result from air–sea exchange of CO2 (for DIC because alkalinity is unaffected by this) and processes occurring in the underlying sediments such as denitrification, sulfate reduction, carbonate dissolution and the diffusion of organic acids (Cai and Wang 1998; Cai et al. 1998; Mosley et al. 2010; Shen et al. 2019). DIC isotope measurements have been found to be useful in tracing internal alkalinity sources (Atekwana et al. 2003), with calcium and strontium isotopes also useful for tracing carbonate precipitation and dissolution (Shao et al. 2018). Eutrophication, anoxia and elevated pCO2 due to climate change have been implicated in observations of increased acidification in some estuaries (Cai et al. 2017; Shen et al. 2019), as has resuspension of volatile sulfide phases (Richards et al. 2018). Spatial and temporal changes in pH in estuaries could greatly affect the sorption of trace metals and ligands to particle surfaces (Dzombak and Morel 1990), which is assessed further below.
Insights and integration using geochemical modelling
A geochemical model was developed in the Visual MINTEQ program (G. P. Gustaffson, see https://vminteq.lwr.kth.se, accessed 22 August 2019) to help integrate and understand the key processes affecting the estuarine behaviour of trace metals (Cu, Zn and Pb in this case). The model included competitive metal binding to dissolved inorganic ligands, hydrous ferric oxide (HFO) particle surfaces (Dzombak and Morel 1990) and DOM by the Non-Ideal Competitive Adsorption (NICA)–Donnan model (Kinniburgh et al. 1999; Mosley et al. 2015). The pH variation in the initial model was based on the average alkalinity, high pCO2 scenario above (Case 2; Fig. 2). Metal concentrations in the river water end-member were Zn 1 µM, Cu 1 µM and Pb 0.1 µM, compared with seawater end-member concentrations of 1, 1 and 0.1 nM for Zn, Cu and Pb respectively. The HFO concentration was maintained at 0.1 g L–1 throughout the estuarine salinity range. Further details of the model setup and input parameters are described in the ‘Modelling Description and Input File Parameters’ section of the Supplementary material.
The model results for Zn, Cu and Pb are shown in Fig. 3. More Pb and Zn is predicted to be bound to the Fe oxide phase than Cu, which is generally consistent with previous measurements of metal partitioning (see Table 1 and the above discussion). For Zn, the model results show an addition of dissolved Zn above the conservative mixing line at low to moderate salinities (0–10; Fig. 3). This can be explained by desorption from HFO surfaces due to decreasing pH (Fig. 2), increased competitive binding of Ca and Mg to these surfaces and to DOM for Mg (see Fig. S1). The model predictions of desorption of Zn at low salinities appear consistent with those found in many previous studies (Holliday and Liss 1976; Ackroyd et al. 1986; Yan et al. 1991; Chiffoleau et al. 1994). With regard to the pH-driven desorption, the sorption isotherm for Zn binding to HFO is in the range of pH 7–8, which is in the range of pH changes, at least in the seaward part, of most estuaries (Dzombak and Morel 1990). However, the desorbed Zn may become readsorbed as pH increases towards mid to high salinities according to the model results (Fig. 3). Standring et al. (2002) found, by isotope labelling experiments, that Zn–sediment interactions could be broadly defined as a two-step process, namely a short period of relatively fast surface sorption followed by a longer period of redistribution between different binding sites within the sediment until equilibrium is attained. Sediment characteristics, such as cation exchange capacity and the amount of organic material present, were proposed to affect these interactions, as would the prior distribution of Zn in suspended sediment in river water entering an estuary.

|
With regard to cation interactions, the model results show Mg binding to DOM increasing at low salinities, whereas Ca binding decreases. This suggests Mg is competitively replacing Ca initially, and also likely Cu, from DOM. The model suggests released Cu then binds to HFO (Fig. 3). Ksionzek et al. (2018) measured a reduction in Cu–DOM binding with increasing salinity, which is consistent with our modelling results. Hamilton-Taylor et al. (2002) also predicted that Cu–humic binding decreased substantially with increasing ionic strength under typical estuarine conditions, but they did not postulate Ca and Mg binding as a significant factor. Waeles et al. (2005) found desorption of Cu occurred at low salinities for a large part of the year in the Penzé Estuary. Chiffoleau et al. (1994) also found additions of Cu to the dissolved phase during estuarine mixing, but suggested this was desorption from particles, whereas van den Berg et al. (1987) suggested Cu and Zn will remain bound to the organic ligands throughout the salinity range of an estuary. The NICA–Donnan model suggests metal binding to DOM will change in response to increasing divalent cation concentrations (Kinniburgh et al. 1999). More integrated laboratory and field experimental research and modelling on competitive binding interactions would be useful to try and better determine the nature of Cu and Zn complexation during estuarine mixing. This should be coupled with spectrophotometric pH measurements in order to accurately quantify the pH changes. In contrast with Cu and Zn, Pb was predicted to remain strongly bound to HFO in the river water and throughout the estuarine salinity range (Fig. 3), which is generally consistent with field observations (Table 1).
To help explore how different river–estuarine water compositions may affect metal behaviour, the HFO, DOC and pH were varied in the geochemical model (Fig. 4). As the HFO increased, more Zn and Cu became bound to this phase, with the dissolved inorganic Zn and dissolved organic Cu fractions decreasing respectively. A similar general pattern was seen for Pb, but Pb was predominantly bound to HFO, except at the lowest concentration assessed (1 mg L–1). Varying the DOC had little effect on Zn, but Cu binding to the dissolved organic fraction increased greatly as DOC increased. In the NICA–Donnan model, Cu is predicted to be a stronger competitor for organic binding sites than Zn and Pb when at equimolar concentrations. For Pb, the proportion bound to DOM increased only at very high DOC concentrations (>100 mg L–1), with it remaining strongly bound to the HFO phase. Decreasing the pH markedly reduced Zn binding to HFO, with little effect on Cu and Pb binding. Again, this is consistent with pH decreases in the early stages of estuarine mixing. Taken as a whole, these model sensitivity results help explain why metal behaviour could vary between different estuaries (e.g. particle- or Fe-rich v. dissolved organic-rich riverine inputs), as well as at different temporal and spatial scales in the same estuary. Over the longer term, hydrological alterations to rivers or climate change may also alter the flux of metals, pH and other key geochemical processes (e.g. anoxia) affecting metal behaviour in estuaries.

|
Conclusion
The results integrate and provide insight into the complex geochemical processes affecting metals in estuaries. The behaviour of a particular trace metal was found to be at least partially dependent on its partitioning in the river water end-member. The metals found in the colloidal fraction (e.g. Fe and Pb) in the river water end-member were removed non-conservatively during the early stages of estuarine mixing as a result of the aggregation of colloidal material. Modelling indicated Pb would remain bound to iron oxide surfaces during the large ionic strength increases and minor pH decreases during estuarine mixing. Hence, metals strongly bound to particles such as Pb should be rapidly deposited to the sediments and their distributions dominated by particle-phase dynamics. In contrast, other metals, such as Cu and Zn, that are less strongly bound to particle surfaces or predominantly associated with colloidal and DOM can behave quite differently, with variable behaviour between different estuaries and even seasonally within the same estuary. During the early stages of estuarine mixing, pH decreases occur and can result in, at least temporarily, desorption of metals such as Zn for particle surfaces. In contrast, Cu may remain strongly bound to dissolved organic ligands, although cation exchange may potentially result in less binding on DOM. Based on these findings, the distribution of metals such as Cu and Zn is observed and predicted to be much more complex and variable in estuaries, influenced dynamically by ionic strength, pH, sediment and metal oxide concentration, as well as the amount and nature of organic ligands. Integrated studies linking metal partitioning and speciation, particle aggregation and transport dynamics, biological uptake, sediment flux and pH changes would be beneficial. An increasing use of state-of-the-art isotope tracing and DOM and metal speciation techniques, as well as geochemical models incorporating multi-assemblage metal binding mechanisms, should help improve our understanding of the drivers of variable metal behaviour in different estuaries.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgments
This review is dedicated to the memory of the late Professor Keith Hunter. Keith, together with his students and collaborators, made several seminal contributions to estuarine chemistry that are incorporated in this review. This includes his early research as a Ph.D. student that organic matter coatings on particles in natural waters are ubiquitous and determine their surface properties and stability, his research on the kinetics of Fe colloid aggregation and metal behaviour in estuaries using laboratory and field measurements, the use of atomic force microscopy for the first direct measurement of interparticle forces in seawater and estuarine water, and the development of new methods for the measurement and modelling of pH in estuaries. Keith was a passionate and highly talented scientist who was widely respected internationally. He was a strong advocate for the environment, in particular the effects of climate change on aquatic systems. He is sorely missed but leaves an enduring influence in his high-quality body of published works and the hearts and mind of the people who were fortunate enough to have worked with him.
References
Ackroyd, D. R., Bale, A. J., Howland, R. J. M., Knox, S., Millward, G. E., and Morris, A. W. (1986). Distributions and behaviour of dissolved Cu, Zn and Mn in the Tamar estuary. Estuarine, Coastal and Shelf Science 23, 621–640.| Distributions and behaviour of dissolved Cu, Zn and Mn in the Tamar estuary.Crossref | GoogleScholarGoogle Scholar |
Atekwana, E. A., Tedesco, L. P., and Jackson, L. R. (2003). Dissolved inorganic carbon (DIC) and hydrologic mixing in a subtropical riverine estuary, southwest Florida, USA. Estuaries 26, 1391–1400.
| Dissolved inorganic carbon (DIC) and hydrologic mixing in a subtropical riverine estuary, southwest Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Benoit, G. (1995). Evidence of the particle concentration effect for lead and other metals in freshwaters based on ultraclean technique analysis. Geochimica et Cosmochimica Acta 59, 2677–2687.
| Evidence of the particle concentration effect for lead and other metals in freshwaters based on ultraclean technique analysis.Crossref | GoogleScholarGoogle Scholar |
Benoit, G., Oktay-Marshall, S. D., Cantu, A., Hood, E. M., Coleman, C. H., Corapcioglu, M. O., and Santschi, P. H. (1994). Partitioning of Cu, Pb, Ag, Zn, Al, and Mn between filter retained particles, colloids, and solution in six Texas estuaries. Marine Chemistry 45, 307–336.
| Partitioning of Cu, Pb, Ag, Zn, Al, and Mn between filter retained particles, colloids, and solution in six Texas estuaries.Crossref | GoogleScholarGoogle Scholar |
Biswas, T. K., and Mosley, L. M. (2019). From mountain ranges to sweeping plains, in droughts and flooding rains; River Murray water quality over the last four decades. Water Resources Management 33, 1087–1101.
| From mountain ranges to sweeping plains, in droughts and flooding rains; River Murray water quality over the last four decades.Crossref | GoogleScholarGoogle Scholar |
Bourg, A. C. M. (1987). Trace metal adsorption modelling and particle-water interactions in estuarine environments. Continental Shelf Research 7, 1319–1332.
| Trace metal adsorption modelling and particle-water interactions in estuarine environments.Crossref | GoogleScholarGoogle Scholar |
Boyle, E., Collier, R., Dengler, A. T., Edmond, J. M., Ng, A. C., and Stallard, R. F. (1974). On the chemical mass-balance in estuaries. Geochimica et Cosmochimica Acta 38, 1719–1728.
| On the chemical mass-balance in estuaries.Crossref | GoogleScholarGoogle Scholar |
Boyle, E. A., Edmond, J. M., and Sholkovitz, E. R. (1977). The mechanism of iron removal in estuaries. Geochimica et Cosmochimica Acta 41, 1313–1324.
| The mechanism of iron removal in estuaries.Crossref | GoogleScholarGoogle Scholar |
Boyle, E. A., Huested, S. S., and Grant, B. (1982). The chemical mass balance of the Amazon plume – II. Copper, nickel, and cadmium. Deep-Sea Research 29, 1355–1364.
| The chemical mass balance of the Amazon plume – II. Copper, nickel, and cadmium.Crossref | GoogleScholarGoogle Scholar |
Bruland, K. W. (1989). Complexation of zinc by natural organic ligands in the central North Pacific. Limnology and Oceanography 34, 269–285.
| Complexation of zinc by natural organic ligands in the central North Pacific.Crossref | GoogleScholarGoogle Scholar |
Burton, J. D., and Liss, P. S. (1976). ‘Estuarine Chemistry.’ (Academic Press: London, UK.)
Cai, W.-J., and Wang, Y. (1998). The chemistry, fluxes, and sources of CO2 in the estuarine waters of the Satilla and Altamaha rivers, Georgia. Limnology and Oceanography 43, 657–668.
| The chemistry, fluxes, and sources of CO2 in the estuarine waters of the Satilla and Altamaha rivers, Georgia.Crossref | GoogleScholarGoogle Scholar |
Cai, W.-J., Wang, Y., and Hodson, R. E. (1998). Acid–base properties of dissolved organic matter in the estuarine waters of Georgia, USA. Geochimica et Cosmochimica Acta 62, 473–483.
| Acid–base properties of dissolved organic matter in the estuarine waters of Georgia, USA.Crossref | GoogleScholarGoogle Scholar |
Cai, W. J., Huang, W. J., Luther, G. W., Pierrot, D., Li, M., Testa, J., Xue, M., Joesoef, A., Mann, R., Brodeur, J., Xu, Y. Y., Chen, B., Hussain, N., Waldbusser, G. G., Cornwell, J., and Kemp, W. M. (2017). Redox reactions and weak buffering capacity lead to acidification in the Chesapeake Bay. Nature Communications 8, 369.
| Redox reactions and weak buffering capacity lead to acidification in the Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar | 28848240PubMed |
Chiffoleau, J.-F., Cossa, D., Auger, D., and Truquet, I. (1994). Trace metal distribution, partition and fluxes in the Seine estuary (France) in low discharge regime. Marine Chemistry 47, 145–158.
| Trace metal distribution, partition and fluxes in the Seine estuary (France) in low discharge regime.Crossref | GoogleScholarGoogle Scholar |
Church, T. M. (1986). Biogeochemical factors influencing the residence time of microconstituents in a large tidal estuary, Delaware Bay. Marine Chemistry 18, 393–406.
| Biogeochemical factors influencing the residence time of microconstituents in a large tidal estuary, Delaware Bay.Crossref | GoogleScholarGoogle Scholar |
Cole, J. J., and Caraco, N. F. (2001). Carbon in catchments: connecting terrestrial carbon losses with aquatic metabolism. Marine and Freshwater Research 52, 101–110.
| Carbon in catchments: connecting terrestrial carbon losses with aquatic metabolism.Crossref | GoogleScholarGoogle Scholar |
Dai, M.-H., and Martin, J.-M. (1995). First data on trace metal level and behaviour in two major Arctic river–estuarine systems (Ob and Yenisey) and in the adjacent Kara Sea, Russia. Earth and Planetary Science Letters 131, 127–141.
| First data on trace metal level and behaviour in two major Arctic river–estuarine systems (Ob and Yenisey) and in the adjacent Kara Sea, Russia.Crossref | GoogleScholarGoogle Scholar |
Dai, M., Martin, J.-M., and Cauwet, G. (1995). The significant role of colloids in the transport and transformation of organic carbon and associated trace metals (Cd, Cu and Ni) in the Rhône delta (France). Marine Chemistry 51, 159–175.
| The significant role of colloids in the transport and transformation of organic carbon and associated trace metals (Cd, Cu and Ni) in the Rhône delta (France).Crossref | GoogleScholarGoogle Scholar |
Douglas, N. K., and Byrne, R. H. (2017). Spectrophotometric pH measurements from river to sea: Calibration of mCP for 0 ≤ S ≤ 40 and 278.15 ≤ T ≤ 308.15K. Marine Chemistry 197, 64–69.
| Spectrophotometric pH measurements from river to sea: Calibration of mCP for 0 ≤ S ≤ 40 and 278.15 ≤ T ≤ 308.15K.Crossref | GoogleScholarGoogle Scholar |
Duan, L., Song, J., Liang, X., Yin, M., Yuan, H., Li, X., Ren, C., Zhou, B., Kang, X., and Yin, X. (2019). Dynamics and diagenesis of trace metals in sediments of the Changjiang Estuary. The Science of the Total Environment 675, 247–259.
| Dynamics and diagenesis of trace metals in sediments of the Changjiang Estuary.Crossref | GoogleScholarGoogle Scholar | 31030132PubMed |
Dzombak, D. A., and Morel, F. M. M. (1990). ‘Surface Complexation Modeling Hydrous Ferric Oxide.’ (Wiley: New York, NY, USA.)
Eckert, J. M., and Sholkovitz, E. R. (1976). The flocculation of iron, aluminium and humates from river water by electrolytes. Geochimica et Cosmochimica Acta 40, 847–848.
| The flocculation of iron, aluminium and humates from river water by electrolytes.Crossref | GoogleScholarGoogle Scholar |
Edwards, A. M. C. (1973). Dissolved load and tentative solute budgets of some Norfolk catchments. Journal of Hydrology 18, 201–217.
| Dissolved load and tentative solute budgets of some Norfolk catchments.Crossref | GoogleScholarGoogle Scholar |
Erel, Y., Morgan, J. J., and Paterson, C. C. (1991). Natural levels of lead and cadmium in a remote mountain stream. Geochimica et Cosmochimica Acta 55, 707–719.
| Natural levels of lead and cadmium in a remote mountain stream.Crossref | GoogleScholarGoogle Scholar |
Fu, J., Tang, X.-L., Zhang, J., and Balzer, W. (2013). Estuarine modification of dissolved and particulate trace metals in major rivers of East-Hainan, China. Continental Shelf Research 57, 59–72.
| Estuarine modification of dissolved and particulate trace metals in major rivers of East-Hainan, China.Crossref | GoogleScholarGoogle Scholar |
Furukawa, Y., Reed, A. H., and Zhang, G. (2014). Effect of organic matter on estuarine flocculation: a laboratory study using montmorillonite, humic acid, xanthan gum, guar gum and natural estuarine flocs. Geochemical Transactions 15, 1.
| Effect of organic matter on estuarine flocculation: a laboratory study using montmorillonite, humic acid, xanthan gum, guar gum and natural estuarine flocs.Crossref | GoogleScholarGoogle Scholar | 24386944PubMed |
Gigault, J., Balaresquea, M., and Tabuteau, H. (2018). Estuary-on-a-chip: unexpected results for the fate and transport of nanoparticles. Environmental Science. Nano 5, 1231–1236.
| Estuary-on-a-chip: unexpected results for the fate and transport of nanoparticles.Crossref | GoogleScholarGoogle Scholar |
Greenamoyer, J. M., and Moran, S. B. (1997). Investigation of Cd, Cu, Ni and 234Th in the colloidal size range in the Gulf of Maine. Marine Chemistry 57, 217–226.
| Investigation of Cd, Cu, Ni and 234Th in the colloidal size range in the Gulf of Maine.Crossref | GoogleScholarGoogle Scholar |
Guieu, C., and Martin, J. M. (2002). The level and fate of metals in the Danube River plume. Estuarine, Coastal and Shelf Science 54, 501–512.
| The level and fate of metals in the Danube River plume.Crossref | GoogleScholarGoogle Scholar |
Guieu, C., Huang, W. W., Martin, J.-M., and Yong, Y. Y. (1996). Outflow of trace metals into the Laptev Sea by the Lena River. Marine Chemistry 53, 255–267.
| Outflow of trace metals into the Laptev Sea by the Lena River.Crossref | GoogleScholarGoogle Scholar |
Guo, X., Cai, W.-J., Zhai, W., Dai, M., Wang, Y., and Chen, B. (2008). Seasonal variations in the inorganic carbon system in the Pearl River (Zhujiang) estuary. Continental Shelf Research 28, 1424–1434.
| Seasonal variations in the inorganic carbon system in the Pearl River (Zhujiang) estuary.Crossref | GoogleScholarGoogle Scholar |
Hamilton-Taylor, J., Postill, A. S., Tipping, E., and Harper, M. P. (2002). Laboratory measurements and modeling of metal–humic interactions under estuarine conditions. Geochimica et Cosmochimica Acta 66, 403–415.
| Laboratory measurements and modeling of metal–humic interactions under estuarine conditions.Crossref | GoogleScholarGoogle Scholar |
Holliday, L. M., and Liss, P. S. (1976). The behaviour of dissolved iron, manganese and zinc in the Beaulieu Estuary, S. England. Estuarine and Coastal Marine Science 4, 349–353.
| The behaviour of dissolved iron, manganese and zinc in the Beaulieu Estuary, S. England.Crossref | GoogleScholarGoogle Scholar |
Hoyle, J., Elderfield, H., Gledhill, A., and Greaves, M. (1984). The behaviour of the rare earth elements during mixing of river and sea waters. Geochimica et Cosmochimica Acta 48, 143–149.
| The behaviour of the rare earth elements during mixing of river and sea waters.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A. (1980). Microelectrophoretic properties of natural surface-active organic matter in coastal seawater. Limnology and Oceanography 25, 807–822.
| Microelectrophoretic properties of natural surface-active organic matter in coastal seawater.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A. (1983). On the estuarine mixing of dissolved substances in relation to colloid stability and surface properties. Geochimica et Cosmochimica Acta 47, 467–473.
| On the estuarine mixing of dissolved substances in relation to colloid stability and surface properties.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A., and Leonard, M. W. (1988). Colloid stability and aggregation in estuaries: 1. Aggregation kinetics of riverine dissolved iron after mixing with seawater. Geochimica et Cosmochimica Acta 52, 1123–1130.
| Colloid stability and aggregation in estuaries: 1. Aggregation kinetics of riverine dissolved iron after mixing with seawater.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A., and Liss, P. S. (1979). The surface charge of suspended particles in estuarine and coastal waters. Nature 282, 823–825.
| The surface charge of suspended particles in estuarine and coastal waters.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A., and Liss, P. S. (1982). Organic matter and the surface charge of suspended particles in estuarine waters. Limnology and Oceanography 27, 322–335.
| Organic matter and the surface charge of suspended particles in estuarine waters.Crossref | GoogleScholarGoogle Scholar |
Hunter, K. A., Leonard, M. R., Carpenter, P. D., and Smith, J. D. (1997). Aggregation of iron colloids in estuaries: a heterogeneous kinetics study using continuous mixing of river and sea waters. Colloids and Surfaces – A. Physicochemical and Engineering Aspects 120, 111–121.
| Aggregation of iron colloids in estuaries: a heterogeneous kinetics study using continuous mixing of river and sea waters.Crossref | GoogleScholarGoogle Scholar |
Illuminati, S., Annibaldi, A., Truzzi, C., Tercier-Waeber, M.-L., Nöel, S., Braungardt, C. B., Achterberg, E. P., Howell, K. A., Turner, D., Marini, M., Romagnoli, T., Totti, C., Confalonieri, F., Graziottin, F., Buffle, J., and Scarponi, G. (2019). In situ trace metal (Cd, Pb, Cu) speciation along the Po River plume (Northern Adriatic Sea) using submersible systems. Marine Chemistry 212, 47–63.
| In situ trace metal (Cd, Pb, Cu) speciation along the Po River plume (Northern Adriatic Sea) using submersible systems.Crossref | GoogleScholarGoogle Scholar |
Kinniburgh, D. G., van Riemsdijk, W. H., Koopal, L. K., Borkovec, M., Benedetti, M. F., and Avena, M. (1999). Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids and Surfaces – A. Physicochemical and Engineering Aspects 151, 147–166.
| Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency.Crossref | GoogleScholarGoogle Scholar |
Kruger, B. R., Dalzell, B. J., and Austin-Minor, E. C. (2011). Effect of organic matter source and salinity on dissolved organic matter isolation via ultrafiltration and solid phase extraction. Aquatic Sciences 73, 405–417.
| Effect of organic matter source and salinity on dissolved organic matter isolation via ultrafiltration and solid phase extraction.Crossref | GoogleScholarGoogle Scholar |
Ksionzek, K. B., Zhang, J., Ludwichowski, K.-U., Wilhelms-Dick, D., Trimborn, S., Jendrossek, T., Kattner, G., and Koch, B. P. (2018). Stoichiometry, polarity, and organometallics in solid-phase extracted dissolved organic matter of the Elbe–Weser estuary. PLoS One 13, e0203260.
| Stoichiometry, polarity, and organometallics in solid-phase extracted dissolved organic matter of the Elbe–Weser estuary.Crossref | GoogleScholarGoogle Scholar | 30183724PubMed |
Liss, P. S. (1976). Conservative and non-conservative behaviour of dissolved constituents during estuarine mixing. In ‘Estuarine Chemistry’. (Eds J. D. Burton and P. S. Liss.) pp. 93–130. (Academic Press: London, UK.)
Liu, Q., Jia, Z., Li, S., and Hu, J. (2019). Assessment of heavy metal pollution, distribution and quantitative source apportionment in surface sediments along a partially mixed estuary (Modaomen, China). Chemosphere 225, 829–838.
| Assessment of heavy metal pollution, distribution and quantitative source apportionment in surface sediments along a partially mixed estuary (Modaomen, China).Crossref | GoogleScholarGoogle Scholar | 30904763PubMed |
Mantoura, R. F. C., Dickson, A., and Riley, J. (1978). The complexation of metals with humic materials in natural waters. Estuarine and Coastal Marine Science 6, 387–408.
| The complexation of metals with humic materials in natural waters.Crossref | GoogleScholarGoogle Scholar |
Mari, X., Torréton, J.-P., Bich-Thuy Trinh, C., Bouvier, T., Van Thuoc, C., Lefebvre, J.-P., and Ouillon, S. (2012). Aggregation dynamics along a salinity gradient in the Bach Dang estuary, North Vietnam. Estuarine, Coastal and Shelf Science 96, 151–158.
| Aggregation dynamics along a salinity gradient in the Bach Dang estuary, North Vietnam.Crossref | GoogleScholarGoogle Scholar |
McLachlan, R. L., Ogston, A. S., and Allison, M. A. (2017). Implications of tidally varying bed stress and intermittent estuarine stratification on fine-sediment dynamics through the Mekong’s tidal river to estuarine reach. Continental Shelf Research 147, 27–37.
| Implications of tidally varying bed stress and intermittent estuarine stratification on fine-sediment dynamics through the Mekong’s tidal river to estuarine reach.Crossref | GoogleScholarGoogle Scholar |
Merschel, G., Bau, M., and Dantas, E. L. (2017). Contrasting impact of organic and inorganic nanoparticles and colloids on the behavior of particle-reactive elements in tropical estuaries: an experimental study. Geochimica et Cosmochimica Acta 197, 1–13.
| Contrasting impact of organic and inorganic nanoparticles and colloids on the behavior of particle-reactive elements in tropical estuaries: an experimental study.Crossref | GoogleScholarGoogle Scholar |
Millero, F. J. (1986). The pH of estuarine waters. Limnology and Oceanography 31, 839–847.
| The pH of estuarine waters.Crossref | GoogleScholarGoogle Scholar |
Mook, W. G., and Koene, B. K. S. (1975). Chemistry of dissolved inorganic carbon in estuarine and coastal brackish waters. Estuarine and Coastal Marine Science 3, 325–336.
| Chemistry of dissolved inorganic carbon in estuarine and coastal brackish waters.Crossref | GoogleScholarGoogle Scholar |
Morris, A. W. (1978). Chemical processes in estuaries: the importance of pH and its variability. In ‘The Aquatic Environment’. (Ed. W. E. Krumbein.) pp. 179–187. (Ann Arbor Science: Ann Arbor, MI, USA.)
Mosley, L. M., Hunter, K. A., and Ducker, W. A. (2003). Forces between particles in natural waters. Environmental Science & Technology 37, 3303–3308.
| Forces between particles in natural waters.Crossref | GoogleScholarGoogle Scholar |
Mosley, L., Hunter, K., and Husheer, S. (2004). Spectrophotometric pH measurement in estuaries using thymol blue and m-cresol purple. Marine Chemistry 91, 175–186.
| Spectrophotometric pH measurement in estuaries using thymol blue and m-cresol purple.Crossref | GoogleScholarGoogle Scholar |
Mosley, L. M., Peake, B. M., and Hunter, K. A. (2010). Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river and seawater end members. Environmental Modelling & Software 25, 1658–1663.
| Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river and seawater end members.Crossref | GoogleScholarGoogle Scholar |
Mosley, L. M., Daly, R., Palmer, D., Yeates, P., Dallimore, C., Biswas, T., and Simpson, S. L. (2015). Predictive modelling of pH and dissolved metal concentrations and speciation following mixing of acid drainage with river water. Applied Geochemistry 59, 1–10.
| Predictive modelling of pH and dissolved metal concentrations and speciation following mixing of acid drainage with river water.Crossref | GoogleScholarGoogle Scholar |
Mulholland, D. S., Poitrasson, F., Boaventura, G. R., Allard, T., Vieira, L. C., Santos, R. V., Mancini, L., and Seyler, P. (2015). Insights into iron sources and pathways in the Amazon River provided by isotopic and spectroscopic studies. Geochimica et Cosmochimica Acta 150, 142–159.
| Insights into iron sources and pathways in the Amazon River provided by isotopic and spectroscopic studies.Crossref | GoogleScholarGoogle Scholar |
Muller, F. L. L. (2018). Exploring the potential role of terrestrially derived humic substances in the marine biogeochemistry of iron. Frontiers of Earth Science 6, 159–169.
| Exploring the potential role of terrestrially derived humic substances in the marine biogeochemistry of iron.Crossref | GoogleScholarGoogle Scholar |
Newton, P. P., and Liss, P. S. (1987). Positively charged suspended particles: studies in an iron-rich river and its estuary. Limnology and Oceanography 32, 1267–1276.
| Positively charged suspended particles: studies in an iron-rich river and its estuary.Crossref | GoogleScholarGoogle Scholar |
Nowostawska, U., Kim, J. P., and Hunter, K. A. (2008). Aggregation of riverine colloidal iron in estuaries: a new kinetic study using stopped-flow mixing. Marine Chemistry 110, 205–210.
| Aggregation of riverine colloidal iron in estuaries: a new kinetic study using stopped-flow mixing.Crossref | GoogleScholarGoogle Scholar |
Paulson, A. J., Curl, H. C., and Gendron, J. F. (1994). Partitioning of Cu in estuarine waters, 1. Partitioning in a poisoned system. Marine Chemistry 45, 67–80.
| Partitioning of Cu in estuarine waters, 1. Partitioning in a poisoned system.Crossref | GoogleScholarGoogle Scholar |
Pearson, H., Comber, S. D. W., Braungardt, C. B., Worsfold, P., Stockdale, A., and Lofts, S. (2018). Determination and prediction of zinc speciation in estuaries. Environmental Science & Technology 52, 14245–14255.
| Determination and prediction of zinc speciation in estuaries.Crossref | GoogleScholarGoogle Scholar |
Powell, R. T. (1996). Colloidal trace metals, organic carbon and nitrogen in a southeastern U.S. estuary. Marine Chemistry 55, 165–176.
| Colloidal trace metals, organic carbon and nitrogen in a southeastern U.S. estuary.Crossref | GoogleScholarGoogle Scholar |
Ramírez-Mendoza, R., Souza, A. J., and Amoudry, L. O. (2014). Modeling flocculation in a hypertidal estuary. Ocean Dynamics 64, 301–313.
| Modeling flocculation in a hypertidal estuary.Crossref | GoogleScholarGoogle Scholar |
Raymond, P. A., and Oh, N. H. (2009). Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage: insights on the impact of coal mining on regional and global carbon and sulfur budgets. Earth and Planetary Science Letters 284, 50–56.
| Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage: insights on the impact of coal mining on regional and global carbon and sulfur budgets.Crossref | GoogleScholarGoogle Scholar |
Reese, A., Zimmermann, T., Pröfrock, D., and Irrgeher, J. (2019). Extreme spatial variation of Sr, Nd and Pb isotopic signatures and 48 element mass fractions in surface sediment of the Elbe River Estuary – suitable tracers for processes in dynamic environments? The Science of the Total Environment 668, 512–523.
| Extreme spatial variation of Sr, Nd and Pb isotopic signatures and 48 element mass fractions in surface sediment of the Elbe River Estuary – suitable tracers for processes in dynamic environments?Crossref | GoogleScholarGoogle Scholar | 30856563PubMed |
Richards, C. M., van Puffelen, J. L., and Pallud, C. (2018). Effects of sediment resuspension on the oxidation of acid-volatile sulfides and release of metals (iron, manganese, zinc) in Pescadero Estuary (CA, USA). Environmental Toxicology and Chemistry 37, 993–1006.
| Effects of sediment resuspension on the oxidation of acid-volatile sulfides and release of metals (iron, manganese, zinc) in Pescadero Estuary (CA, USA).Crossref | GoogleScholarGoogle Scholar | 29168891PubMed |
Sander, S., Mosley, L., and Hunter, K. (2004). Investigations of inter-particle forces in natural waters using AFM: effects of adsorbed humic acids on iron oxide and alumina surface properties. Environmental Science & Technology 38, 4791–4796.
| Investigations of inter-particle forces in natural waters using AFM: effects of adsorbed humic acids on iron oxide and alumina surface properties.Crossref | GoogleScholarGoogle Scholar |
Sanders, C. J., Santos, I. R., Maher, D. T., Sadat-Noori, M., Schnetger, B., and Brumsack, H. J. (2015). Dissolved iron exports from an estuary surrounded by coastal wetlands: can small estuaries be a significant source of Fe to the ocean? Marine Chemistry 176, 75–82.
| Dissolved iron exports from an estuary surrounded by coastal wetlands: can small estuaries be a significant source of Fe to the ocean?Crossref | GoogleScholarGoogle Scholar |
Santschi, P. H., Lenhart, J. J., and Honeyman, B. D. (1997). Heterogeneous processes affecting trace contaminant distribution in estuaries: the role of natural organic matter. Marine Chemistry 58, 99–125.
| Heterogeneous processes affecting trace contaminant distribution in estuaries: the role of natural organic matter.Crossref | GoogleScholarGoogle Scholar |
Shao, Y., Farkas, J., Holmden, C., Mosley, L., Kell-Duivestein, I., Izzo, C., Reis-Santos, P., Tyler, J., Torber, P., Fryda, J., Taylor, H., Haynes, D., Tibby, J., and Gillanders, B. M. (2018). Calcium and strontium isotope systematics in the lagoon estuarine environments of South Australia: implications for water source mixing, carbonate fluxes and fish migration. Geochimica et Cosmochimica Acta 239, 90–108.
| Calcium and strontium isotope systematics in the lagoon estuarine environments of South Australia: implications for water source mixing, carbonate fluxes and fish migration.Crossref | GoogleScholarGoogle Scholar |
Shen, C., Testa, J. M., Li, M., Cai, W.-J., Waldbusser, G. G., Ni, W., Kemp, W. M., Cornwell, J., Chen, B., Brodeur, J., and Su, J. (2019). Controls on carbonate system dynamics in a coastal plain estuary: a modelling study. Journal of Geophysical Research – Biogeosciences 124, 61–78.
| Controls on carbonate system dynamics in a coastal plain estuary: a modelling study.Crossref | GoogleScholarGoogle Scholar |
Shiller, A. M., and Boyle, E. A. (1991). Trace elements in the Mississippi River Delta outflow region; behaviour at high discharge. Geochimica et Cosmochimica Acta 55, 3241–3251.
| Trace elements in the Mississippi River Delta outflow region; behaviour at high discharge.Crossref | GoogleScholarGoogle Scholar |
Sholkovitz, E. R. (1976). Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochimica et Cosmochimica Acta 40, 831–845.
| Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater.Crossref | GoogleScholarGoogle Scholar |
Sholkovitz, E. R. (1978). The flocculation of dissolved Fe, Mn, Al, Cu, Ni, Co, and Cd during estuarine mixing. Earth and Science Planetary Letters 41, 77–86.
| The flocculation of dissolved Fe, Mn, Al, Cu, Ni, Co, and Cd during estuarine mixing.Crossref | GoogleScholarGoogle Scholar |
Sholkovitz, E. R. (1995). The aquatic chemistry of rare earth elements in rivers and estuaries. Aquatic Geochemistry 1, 1–34.
| The aquatic chemistry of rare earth elements in rivers and estuaries.Crossref | GoogleScholarGoogle Scholar |
Sholkovitz, E. R., Boyle, E. A., and Price, N. B. (1978). The removal of dissolved humic acids and iron during estuarine mixing. Earth and Science Planetary Letters 40, 130–136.
| The removal of dissolved humic acids and iron during estuarine mixing.Crossref | GoogleScholarGoogle Scholar |
Standring, W. J. F., Oughton, D. H., and Salbu, B. (2002). Remobilisation of 109Cd, 65Zn and 54Mn from freshwater-labelled river sediments when mixed with seawater. Environment International 28, 185–195.
| Remobilisation of 109Cd, 65Zn and 54Mn from freshwater-labelled river sediments when mixed with seawater.Crossref | GoogleScholarGoogle Scholar |
Stumm, W., and Morgan, J. (1996). ‘Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters’, 3rd edn. (Wiley Interscience: New York, NY, USA.)
Sunda, W. G., and Hanson, P. J. (1979). Chemical speciation of copper in river water. In ‘Chemical Modeling in Aqueous Systems’. (Ed. E.A. Jenne.) pp. 147–180. (American Chemical Society: Washington, DC, USA.)
Tang, D., Warnken, K. W., and Santschi, P. H. (2002). Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters. Marine Chemistry 78, 29–45.
| Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters.Crossref | GoogleScholarGoogle Scholar |
Tanguy, V., Waeles, M., Gigault, J., Cabon, J.-Y., Quentel, F., and Riso, R. D. (2011). The removal of colloidal lead during estuarine mixing: seasonal variations and importance of iron oxides and humic substances. Marine and Freshwater Research 62, 329–341.
| The removal of colloidal lead during estuarine mixing: seasonal variations and importance of iron oxides and humic substances.Crossref | GoogleScholarGoogle Scholar |
Tipping, E., Lofts, S., and Lawlor, A. (1998). Modelling the chemical speciation of trace metals in the surface waters of the Humber system. The Science of the Total Environment 210–211, 63–77.
| Modelling the chemical speciation of trace metals in the surface waters of the Humber system.Crossref | GoogleScholarGoogle Scholar | 9573625PubMed |
Trento, A., and Vinzon, S. (2014). Experimental modelling of flocculation processes – the case of Paraiba do Sul Estuary. International Journal of Sediment Research 29, 378–390.
| Experimental modelling of flocculation processes – the case of Paraiba do Sul Estuary.Crossref | GoogleScholarGoogle Scholar |
van den Berg, C. M. G., Merks, A. G. A., and Duursma, E. K. (1987). Organic complexation and its control of the dissolved concentrations of copper and zinc in the Scheldt Estuary. Estuarine, Coastal and Shelf Science 24, 785–797.
| Organic complexation and its control of the dissolved concentrations of copper and zinc in the Scheldt Estuary.Crossref | GoogleScholarGoogle Scholar |
Verwey, E. J. W., and Overbeek, T. G. (1948). ‘Theory of the Stability of Lyphobic Colloids.’ (Elsevier: Amsterdam, Netherlands.)
Waeles, M., Riso, R. D., and Corre, P. L. (2005). Seasonal variations of dissolved and particulate copper species in estuarine waters. Estuarine, Coastal and Shelf Science 62, 313–323.
| Seasonal variations of dissolved and particulate copper species in estuarine waters.Crossref | GoogleScholarGoogle Scholar |
Waeles, M., Tanguy, V., Lespes, G., and Riso, R. D. (2008). Behaviour of colloidal trace metals (Cu, Pb and Cd) in estuarine waters: an approach using frontal ultrafiltration (UF) and stripping chronopotentiometric methods (SCP). Estuarine, Coastal and Shelf Science 80, 538–544.
| Behaviour of colloidal trace metals (Cu, Pb and Cd) in estuarine waters: an approach using frontal ultrafiltration (UF) and stripping chronopotentiometric methods (SCP).Crossref | GoogleScholarGoogle Scholar |
Wang, Y. P., Voulgaris, G., Li, Y., Yang, Y., Gao, J., Chen, J., and Gao, S. (2013). Sediment resuspension, flocculation, and settling in a macrotidal estuary. Journal of Geophysical Research. Oceans 118, 5591–5608.
| Sediment resuspension, flocculation, and settling in a macrotidal estuary.Crossref | GoogleScholarGoogle Scholar |
Wen, L.-S., Stordal, M. C., Tang, D., Gill, G. A., and Santschi, P. H. (1996). An ultraclean cross-flow ultrafiltration technique for the study of trace metal phase speciation in seawater. Marine Chemistry 55, 129–152.
| An ultraclean cross-flow ultrafiltration technique for the study of trace metal phase speciation in seawater.Crossref | GoogleScholarGoogle Scholar |
Wen, L.-S., Santschi, P., Gill, G., and Paternostro, C. (1999). Estuarine trace metal distributions in Galveston Bay: importance of colloidal forms in the speciation of the dissolved phase. Marine Chemistry 63, 185–212.
| Estuarine trace metal distributions in Galveston Bay: importance of colloidal forms in the speciation of the dissolved phase.Crossref | GoogleScholarGoogle Scholar |
Wetz, M. S., Robbins, M. C., and Paerl, H. W. (2009). Transparent exopolymer particles (TEP) in a river-dominated estuary: spatial–temporal distributions and an assessment of controls upon TEP formation. Estuaries and Coasts 32, 447–455.
| Transparent exopolymer particles (TEP) in a river-dominated estuary: spatial–temporal distributions and an assessment of controls upon TEP formation.Crossref | GoogleScholarGoogle Scholar |
Whitfield, M., Butler, R. A., and Covington, A. K. (1985). The determination of pH in estuarine waters I. Definition of pH scales and the selection of buffers. Oceanologica Acta 8, 423–432.
Windom, H., Wallace, G., Smith, R., Dudek, N., Maeda, M., Dulmage, R., and Storti, F. (1983). Behaviour of copper in southeastern United States estuaries. Marine Chemistry 12, 183–193.
| Behaviour of copper in southeastern United States estuaries.Crossref | GoogleScholarGoogle Scholar |
Yan, L., Stallard, R. F., Key, R. M., and Crerar, D. A. (1991). Trace metals and dissolved organic carbon in estuaries and offshore waters of New Jersey, USA. Geochimica et Cosmochimica Acta 55, 3647–3656.
| Trace metals and dissolved organic carbon in estuaries and offshore waters of New Jersey, USA.Crossref | GoogleScholarGoogle Scholar |
Zhou, J. L., Rowland, S., Fauzi, R., Mantoura, C., and Braven, J. (1994). The formation of humic coatings on mineral particles under simulated estuarine conditions – a mechanistic study. Water Research 28, 571–579.
| The formation of humic coatings on mineral particles under simulated estuarine conditions – a mechanistic study.Crossref | GoogleScholarGoogle Scholar |
Zhou, Z., Stolpe, B., Guo, L., and Shiller, A. M. (2016). Colloidal size spectra, composition and estuarine mixing behavior of DOM in river and estuarine waters of the northern Gulf of Mexico. Geochimica et Cosmochimica Acta 181, 1–17.
| Colloidal size spectra, composition and estuarine mixing behavior of DOM in river and estuarine waters of the northern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |