Resolving the early life history of King George whiting (Sillaginodes punctatus: Perciformes) using otolith microstructure and trace element chemistry
Troy A. Rogers A C , Anthony J. Fowler B , Michael A. Steer B and Bronwyn M. Gillanders
A C , Anthony J. Fowler B , Michael A. Steer B and Bronwyn M. Gillanders  A
A
A Southern Seas Ecology Laboratories, School of Biological Sciences, University of Adelaide, Adelaide, SA 5005, Australia.
B South Australian Research and Development Institute, PO Box 120, Henley Beach, SA 5022, Australia.
C Corresponding author. Email: troy.rogers@adelaide.edu.au
Marine and Freshwater Research 70(12) 1659-1674 https://doi.org/10.1071/MF18280
Submitted: 1 August 2018 Accepted: 4 December 2018 Published: 5 February 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Understanding the early life history processes of fish that lead to recruitment is critical for understanding population dynamics. This study explored the early life history of King George whiting (Sillaginodes punctatus) that recruited to an important nursery area in South Australia in 2016 and 2017. The early life history was reconstructed based on the retrospective analysis of otolith microstructure and chemistry for settlement-stage larvae collected fortnightly from July to November. These fish hatched between March and July, but a 3-week period in May led to 52–71% of recruitment. Recruits from successive sampling occasions differed in age, size and growth rate, potentially related to seasonal changes in water temperature and larval food availability. During both years, there were significant changes in otolith elemental chemistry among the groups of recruits that primarily related to changes in Sr : Ca. There are two hypotheses to account for the differences in otolith chemistry: either (1) a single, primary spawning source and within-season environmental change; or (2) multiple spawning sources. Further investigation with oceanographic models of larval dispersal will help differentiate between these. The retrospective analysis of otoliths has improved the understanding of early life history for this important species, with implications for fishery management.
Additional keywords: LA-ICP-MS, laser ablation–inductively coupled plasma–mass spectrometry, recruitment, strontium, temporal.
Introduction
Understanding the early life history processes of fish that contribute to recruitment is critical for interpreting changes in adult populations (Chambers and Trippel 1997; Cowen and Sponaugle 2009). For marine species with spatially distinct spawning and nursery areas, recruitment can be directly related to larval survivorship during dispersal (Leggett and Deblois 1994; Cowen and Sponaugle 2009). Survival rates can be highly variable because they are affected by environmental factors, including food availability, predator abundance and the prevailing abiotic conditions (Pepin 1991; Leggett and Deblois 1994; Sponaugle et al. 2006). Even though larvae are small and their behavioural and sensory abilities are poorly understood, their dispersal is not simply controlled by physical oceanographic processes (Jones et al. 2009; Leis et al. 2011), and biological factors play an important role in larval survival and subsequent recruitment (Houde 1989; Chambers and Trippel 1997; Rankin and Sponaugle 2014).
Many fish species are batch spawners that produce offspring repeatedly throughout an extended spawning season (Brown-Peterson et al. 2011). The larvae that originate from an extensive range of hatch dates can be exposed to different environments throughout their early development (Cargnelli and Gross 1996; Radtke et al. 2001). Physical and ecological conditions are dynamic and can vary greatly at different spatial and temporal scales. As such, there is the potential for recruits within a single spawning season, and between years, to experience different environments and have considerably different early life history characteristics (Cargnelli and Gross 1996; Radtke et al. 2001; Rankin and Sponaugle 2014). For example, because larval growth is highly correlated with temperature, changes in water temperature during the spawning season may affect larval development rates and their subsequent survival (Pepin 1991; Green and Fisher 2004). Therefore, understanding temporal variation in the biological characteristics of early stage fish may contribute considerably to understanding variation in recruitment.
The biological information stored in calcified structures provides unique opportunities to retrospectively investigate the life history of fishes (Campana 1999; Elsdon et al. 2008). Otoliths are paired crystalline structures that function for hearing and orientation and form continuously throughout the lives of bony fishes (Campana and Neilson 1985). The continual accretion of carbonate material at variable rates relative to somatic growth is reflected in the otolith structure as alternating increments that can be used to estimate age (Campana and Neilson 1985). Daily growth increments in the otolith microstructure of early stage fish provide highly resolved temporal information on age that can be interpreted to inform larval growth, presettlement duration and hatch date (Campana and Jones 1992). In addition to microstructure analysis, otolith chemistry is a powerful tool that can be used to discriminate between groups of fish that have occupied different physio-chemical environments throughout their lives (Campana 1999; Elsdon et al. 2008). The material used for otolith formation is derived from the aquatic environment occupied by the fish. Water chemistry is influenced by extrinsic factors, including temperature and salinity, which vary at different spatial and temporal scales and can affect otolith elemental composition (Campana 1999; Elsdon et al. 2008). The incorporation of elements into the otolith matrix does not simply reflect the concentrations present in the surrounding environment (Izzo et al. 2018), but is regulated by a complex suite of physiological processes that are not yet fully understood (Sturrock et al. 2012; 2014). Nevertheless, otolith elemental composition relates to the aquatic environments experienced by a fish (Sturrock et al. 2012) that, when interpreted concurrently with age information, describes a chronological record of environmental history (Campana and Thorrold 2001; Elsdon et al. 2008). In the context of early life history, otolith chemistry has been particularly useful for understanding habitat use (Dorval et al. 2005; Hogan et al. 2017), establishing connectivity between life stages (Gillanders 2002; Hamer et al. 2003) and delineating natal sources (Swearer et al. 1999; Thorrold et al. 2001).
King George whiting (Sillaginodes punctatus; Perciformes) is a demersal finfish species endemic to temperate coastal waters of southern Australia, and is one of the most important fishery species of this region (Kailola et al. 1993; Fowler and Jones 2008). South Australia (SA) is in the centre of this distribution, and supports the highest abundances and most significant fishery for King George whiting (Steer et al. 2018). However, catches and the estimated biomass of King George whiting in SA have declined over recent years. In particular, catches from Gulf St Vincent, one of the important fishery regions of SA, have declined to record lows. Despite a large body of research over the past 30 years to improve the understanding of King George whiting life history (Jenkins and May 1994; Fowler and Short 1996; Fowler et al. 2000a; Fowler and Jones 2008; Jenkins et al. 2015), there remains considerable uncertainty about the spawning sources, population connectivity and early life history processes that ultimately culminate in recruitment.
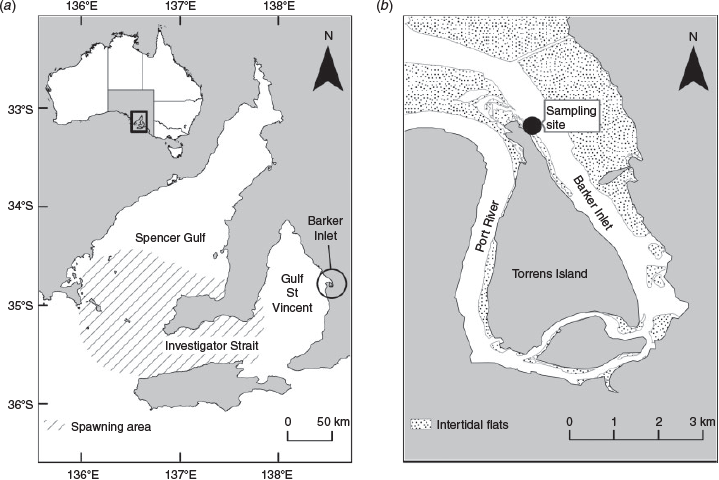
The understanding of life history for King George whiting was that adult fish spawn between March and May in the offshore waters of Investigator Strait and southern Spencer Gulf (Fig. 1; Fowler et al. 2000b). This is the only area where adult fish with hydrated oocytes, fertilised eggs and developing larvae have been found in south-eastern Australia (Fowler and Jones 2008; Jenkins et al. 2015). Even though previous research suggests that there are other spawning areas, their locations remain unknown (Fowler et al. 2000a; Jenkins et al. 2000, 2015). The developing larvae are subject to a long advection phase of 3–5 months before they settle to protected bays within the gulfs (Jenkins and May 1994; Fowler and Short 1996). Juveniles develop within the nursery for 12–18 months before moving into adjacent deeper water, and eventually migrate southwards as young adults to replenish the offshore spawning population (Fowler et al. 2000b). In Gulf St Vincent there is one specific nursery area that is particularly significant for the regional population. Barker Inlet is the largest recognised nursery area in Gulf St Vincent and has a multidecadal history of annual recruitment (Fig. 1; Fowler and Jones 2008). However, the source of recruits to Barker Inlet and the early life history processes they have experienced before settlement remain poorly understood.
In order to better inform fishery management, the aim of this study was to investigate the early life history of King George whiting that recruited to Barker Inlet. This was achieved through the retrospective interpretation of otolith microstructure and elemental chemistry for new recruits collected throughout two complete settlement seasons. The specific objectives addressed were: (1) to identify when spawning that resulted in settlement occurred, particularly for the peak settlement period; (2) to examine temporal variation in the early life history characteristics of recruits that settled throughout the season; and (3) to interpret the otolith chemistry of recruits collected at different times through the settlement season in terms of potential spawning sources and larval advection pathways.
Materials and methods
Sample collection
Recently settled King George whiting were collected from Barker Inlet, a semi-enclosed, protected system near Adelaide in Gulf St Vincent, SA (Fig. 1). Sampling was done at one site at the northern end of Torrens Island that supported a shallow, subtidal seagrass bed of Zostera spp. In each of 2016 and 2017, samples were collected on nine occasions at fortnightly intervals throughout the austral winter–spring settlement period (July–November). The day of sampling was determined by the lowest tide for each fortnightly cycle. Sampling was done using a small beach seine net (mouth width 5 m, semicircular perimeter 7 m, drop 2 m, mesh size 1 mm2) that was hauled over 40 m by two people through shallow water (0.5 m). King George whiting were removed from the net, stored in resealable plastic bags on ice immediately after capture and frozen for later analysis. The total sample area for each seine was 200 m2. On each occasion six seines were sampled, giving a total area sampled of 1200 m2. Sample collection and processing for this study was approved by The University of Adelaide Animal Ethics Committee (S-2016-133).
Sample processing
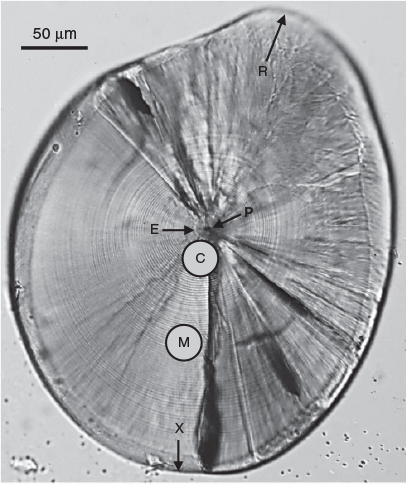
King George whiting collected on each occasion generally contained a mix of recently settled larvae and developing juveniles. No distinctive settlement check was routinely visible in the otoliths of these fish. This prevented the exact day of settlement from being identified, which was consistent with previous studies of this species (Jenkins and May 1994; Fowler and Short 1996). Settlement for King George whiting is more related to size than age (Fowler and Short 1996). Therefore, to best investigate the early life history characteristics of the newest recruits at different times throughout the settlement season, we considered the smallest fish on each sample occasion to have most recently undergone settlement. As such, each fish collected was measured for standard length (SL) to the nearest 0.1 mm using digital callipers, and the 20 smallest fish from each sample occasion were used for otolith analyses. The sagittal otoliths were removed from these fish under a dissecting microscope (Olympus SZX7, Tokyo, Japan) using stainless steel needles. Otoliths were rinsed in three successive drops of ultrapure water, adhering tissue was removed, the otoliths were allowed to dry and were then transferred to individual Eppendorf microcentrifuge tubes for storage.
We followed the widely accepted definitions of early life history stages for demersal and benthic fishes described by Neira et al. (1998) and Leis and Carson-Ewart (2004). The approximate size range for each stage followed those for S. punctatus described by Bruce (1995) and Hamer and Jenkins (1997). Briefly, these were: (1) ‘larva’, development stage between hatching and attainment of full meristic complements (fins and scales), with a size range of 2.0–15.0 mm SL; (2) ‘settlement-stage larva’, development stage during which a larva transitions from the pelagic to benthic environment (settlement), often associated with a morphologic transition from larva to juvenile, with a size range of 15.0–20.5 mm SL; and (3) ‘juvenile’, development stage from attainment of full meristic complements to sexual maturity, with a size range >20.5 mm SL.
Otolith microstructure
For each fish, one otolith was randomly chosen for microstructure interpretation and the other was used for trace element chemistry analysis. The former was mounted proximal surface upward on a glass microscope slide using thermoplastic glue (CrystalBond 509, ProSciTech, Townsville, Qld, Australia), then ground and polished through the sagittal plane to the level of the primordium using three grades (9, 3 and 1 µm) of aluminium oxide lapping film (AusOptic, Sydney, NSW, Australia). For interpretation, a live digital image of each polished otolith section was viewed on a computer screen using an image analysis system, which consisted of an Olympus DP73 video camera mounted on an Olympus BX51 compound microscope and used Olympus Stream software (ver. 1.9.1, Tokyo, Japan). Otolith sections were viewed through a 100× objective using immersion oil. The daily periodicity of increment formation for S. punctatus otoliths has previously been validated based on reared larvae of known age (B. D. Bruce and D. A. Short, unpubl. data). Daily increments were counted from the primordium to the posterior margin (longest axis; Fig. 2), with two successive counts made for each otolith. When these counts differed by less than 5%, their mean was considered the estimated age. If they differed by more than 5%, additional counts were done until an acceptable estimate of the number of increments was achieved. If this was not achieved, the otolith was rejected (n = 9). The date of hatch for each fish was calculated by subtracting the estimated age from the date of capture. Average growth rate (mm day–1) was calculated as:
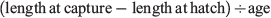
where length at hatch was 2.1 mm SL (Bruce 1995).
|
Trace element chemistry
The method used to prepare otolith sections for trace element chemistry allowed them to be individually polished and relocated to a new slide without altering the orientation of the polished section. Each otolith (n = 326) was embedded proximal surface upward in thermoplastic glue (CrystalBond 509) on top of an epoxy resin disc (diameter 5 mm, height 1 mm). The thermoplastic glue was spiked with indium (115In) at ~200 ppm to aid discrimination between otolith material and glue during analysis. Otoliths were polished through the sagittal plane to the level of the primordium using three grades of aluminium oxide lapping film (9, 3 and 1 µm), rinsed with ultrapure water and allowed to dry. Then, 24 randomly selected polished sections comprising two to three otoliths from each of the nine sample occasions within each year were fixed to a new ‘analysis’ slide using thermoplastic glue. This resulted in 14 ‘analysis’ slides that were each triple rinsed with ultrapure water, air dried under a laminar flow hood and stored individually in sealed plastic bags.
Otoliths were analysed for trace element chemistry by laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS). The system consisted of a New Wave Research (Fremont, CA, USA) 213-nm high-performance (Nd:YAG) ultraviolet probe laser ablation system coupled to an Agilent (Santa Clara, CA, USA) 7900 quadrupole ICP-MS located at Adelaide Microscopy (University of Adelaide, Adelaide, SA, Australia). Two ‘analysis slides’ were placed in the sealed chamber at one time and viewed remotely by an image analysis system. Each otolith was sampled at two places using a 30-µm diameter ‘spot’ ablation (Fig. 2). These places were: (1) ‘core’, posterior to the exogenous feeding check incorporating the first 20 days or so of planktonic larval life, representing the ‘natal origin’; and (2) ‘mid’, 100–130 µm from the primordium towards the posterior margin, representing a period of the ‘larval advection’, ~60–70 days post hatch.
Each otolith spot was sampled at a pulse rate of 5 Hz and a fluence of 11 J cm–2. Otoliths were pre-ablated using the described settings for 3 s to eliminate possible surface contamination. Ablation occurred in a helium-flushed chamber that was mixed with argon for injection into the plasma. The elemental isotopes sampled were 7Li, 25Mg, 55Mn, 65Cu, 66Zn, 88Sr, 138Ba and 208Pb, as well as43Ca, which was used as the internal standard, and 115In, which was used as an indicator to discriminate between otolith material and thermoplastic glue. The concentration of 43Ca in the otolith was assumed to be constant at 38.8% by weight (Yoshinaga et al. 2000). Element concentrations were calibrated against the National Institute of Standards (NIST) 612 glass reference pallet (Lahaye et al. 1997). Trace element measurements of the blank sample gases were recorded for 30 s before each sample ablation of 40 s, with the concentration of each mass recorded every 0.30 s. Data reduction, including background subtractions, minimum limits of detection (LOD) and mass count data conversion to concentrations (ppm), was done using Iolite software (ver. 2.5, see https://iolite-software.com; Paton et al. 2011). Elemental data were then converted to molar concentrations and standardised to calcium (element : Ca, µmol mol–1).
Internal precision and accuracy were assessed by analysing the NIST 612 as an unknown sample against the actual concentrations, and external precision was assessed by measurements of MACS-3 (United States Geological Survey, Reston, VA, USA) calcium carbonate reference material. The NIST 612 and MACS-3 standards were analysed twice at the beginning and end of each sampling session, as well as after every 12 ablations to correct for short-term instrumental drift. Mean recovery for the NIST 612 as an unknown ranged from 100.0 to 100.2% for all elements. Mean relative standard deviations (RSD; %) for NIST 612 were: 7Li 1.7, 25Mg 0.8, 55Mn 1.0, 65Cu 1.7, 66Zn 2.0, 88Sr 0.6, 138Ba 0.7 and 208Pb 1.9. External precision (RSD; %) assessed by measurements of the MACS-3 reference material was: 7Li 2.6, 25Mg 2.3, 55Mn 2.4, 65Cu 5.3, 66Zn 12.8, 88Sr 1.7, 138Ba 3.2 and 208Pb 12.8. Average LOD (µmol mol–1) based on three times the standard deviation of the blank gases adjusted for ablation yield (Lahaye et al. 1997), was: 7Li 0.60, 25Mg 0.45, 55Mn 0.26, 65Cu 0.04, 66Zn 0.19, 88Sr 0.01, 138Ba 0.01 and 208Pb 0.01.
Data analysis
Microstructure
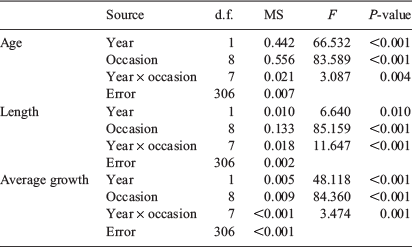
Size, age and average growth rate of the 20 smallest fish from each sample occasion were compared between sample occasions and years by two-way analyses of variance (ANOVA) with both factors fixed. Normality was assessed by visual examination of histograms, quantile–quantile (Q–Q) plots and the Shapiro–Wilk goodness of fit statistic, and equality of group variances was assessed using Levene’s test. Parametric assumptions were violated for mean age and length, and the data were transformed to natural logarithms. When significant differences were found, Tukey post hoc comparisons were done to identify differences among means.
Trace element chemistry
Each element considered, aside from 65Cu, 66Zn and 208Pb, consistently exceeded the detection limits of the ICP-MS. The concentrations of the remaining five elemental isotopes (i.e. 7Li, 25Mg, 55Mn, 88Sr and 138Ba) conformed to parametric assumptions following fourth-root transformation and were considered for analyses. Individual element : Ca ratios were compared between sample occasions and years by two-way ANOVA, and significant differences were identified by Tukey post hoc comparisons. For each analysis, sample occasion and year were fixed factors within the full factorial model.
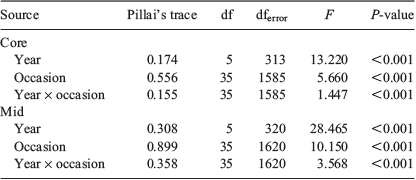
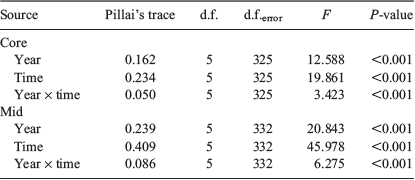
Multi-elemental chemistry was compared between sample occasions and years by two-factor multivariate analysis of variance (MANOVA). Pillai’s trace statistic was used because it is considered the most robust to any deviations from multivariate normality. Equivalence of covariance matrices was tested by Box’s M. Discriminant function analysis (DFA) determined whether fish could be allocated into groups based on the multi-elemental signals of their otoliths and whether such groups conformed to the different sample occasions. Step-wise DFA determined the most important elements to discriminate between groups. The entry of predictors into the analysis was determined using Wilks’ lambda (λ) with Pentry = 0.05 and Pexit = 0.10. Leave-one-out, jack-knifed cross-validation calculated whether individual fish could be classified back to their time of capture based on the elemental signals of the remaining samples. Multi-elemental data were visualised through canonical discriminant function plots showing 95% confidence ellipses around group centroids. Statistical analyses were conducted using SPSS Statistics (ver. 24, IBM Corp., Armonk, NY, USA) and figures were produced using SigmaPlot (ver. 14.0, SYSTAT Software Inc., San Jose, CA, USA). The sample size for 11 July 2017 was small (n = 4) and therefore excluded from analyses.
Results
Temporal analysis of fish size
Length–frequency distribution
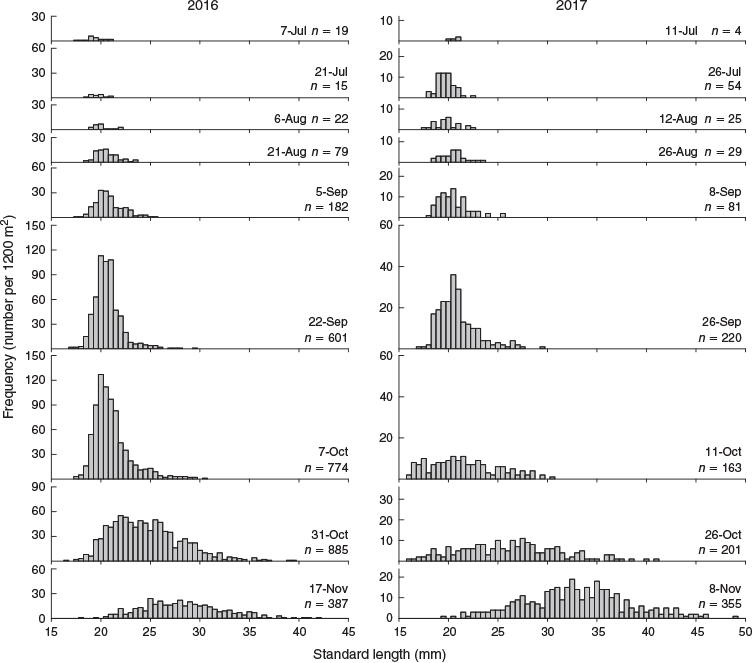
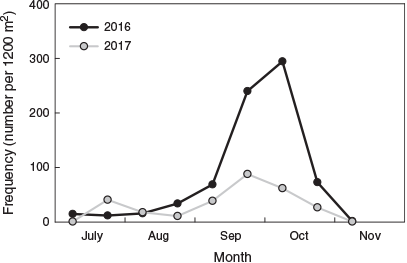
In all, 4096 settlement-stage larvae and juveniles were captured throughout the study (2964 and 1132 in 2016 and 2017 respectively). The temporal trend in catch rate was consistent between years, with the lowest numbers collected in July and August and the highest collected in late September to November (Fig. 3). Length–frequency distributions of fish collected at different times were similar between years (Fig. 3). Samples from July and August were characterised by fish of 19–23 mm SL, and the length range systematically increased from early September to November. An influx of 18- to 22-mm SL fish in late September and early October represented the highest frequency of any size. Only three fish <20 mm SL were collected in November across both years.
Settlement pattern
The total number of fish captured and the length–frequency distributions for the different sampling occasions do not represent the temporal settlement pattern because they included juvenile fish that had previously settled. To investigate the temporal settlement pattern, only fish <20.5 mm SL were considered to represent the most recent recruits (Hamer and Jenkins 1997). The settlement pattern was similar between years (Fig. 4). The number of new recruits was lowest in July and August, then increased exponentially during early September and peaked in early October. Fish that settled in late September and early October were the smallest and represented 70.7 and 51.7% of total recruits for 2016 and 2017 respectively. The number of new recruits decreased rapidly by late October, and there were almost none by mid-November.
|
Otolith microstructure
Age and growth
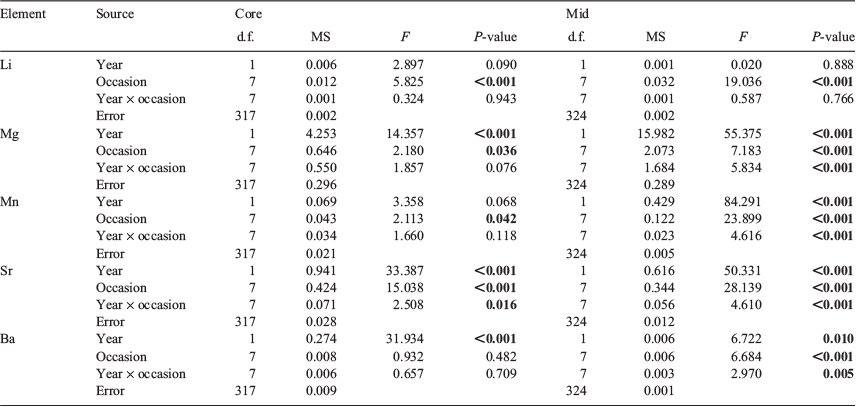
Of the 4096 fish collected, 326 were aged (n = 163 for each year). Age estimates ranged from 93 to 184 days for 2016 and from 92 to 179 days for 2017 (Fig. 5a). Estimated ages were significantly different among sampling occasions and between years, and a significant interaction indicated that the pattern of variation among sampling occasions differed between years (Table 1). For 2016, mean age increased successively from 108 to 132 days between July and early September, then remained at 128 days until early October before increasing considerably to 151 and 164 days in late October and November respectively. In 2017, mean age followed a similar trend, although fish were consistently 5–15 days younger at the same time of year (see Table S1, available as Supplementary material to this paper).
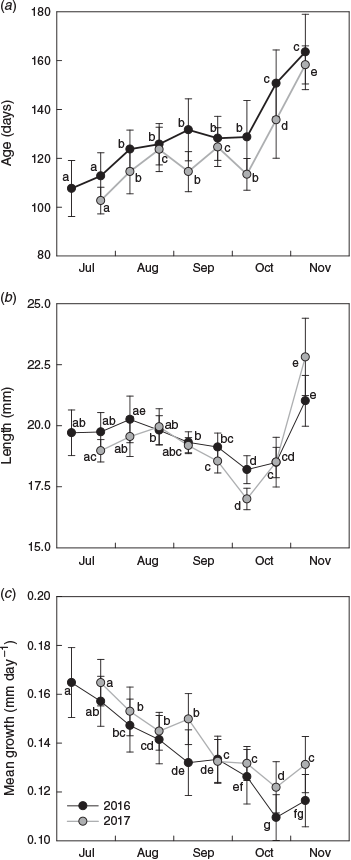
|
|
The patterns of variation in size of the newest recruits also differed between years (Table 1). Mean length ranged from 18.2 to 20.0 mm SL for 2016 and from 17.0 to 22.8 mm SL for 2017 (Fig. 5b). Size was similar in July and August, then decreased during September to a minimum in early October. Mean length increased in late October and the largest fish were collected in November. Average growth rate decreased systematically throughout the settlement seasons in both years. It was highest in early July and lowest in late October, and declined from 0.16 to 0.11 mm day–1 in 2016 and from 0.18 to 0.12 mm day–1 in 2017 (Fig. 5c).
Hatch date
Calculated hatch dates ranged from 26 February to 6 July for 2016, and from 21 March to 13 July for 2017 (Fig. 6). As such, the duration of spawning that resulted in recruitment was 131 days for 2016 and 114 days for 2017. Fish sampled at a similar time of the settlement season had hatched earlier in 2016. Mean hatch date generally increased from July to early October, then remained similar for late October and November. There were large differences in mean hatch dates between consecutive sample occasions for early and late September in 2016 (21 days), and for late August to early September in 2017 (22 days). The largest number of new recruits was collected during late September and early October, and their mean hatch dates were from 17 to 31 May for 2016, and from 24 May to 20 June for 2017.
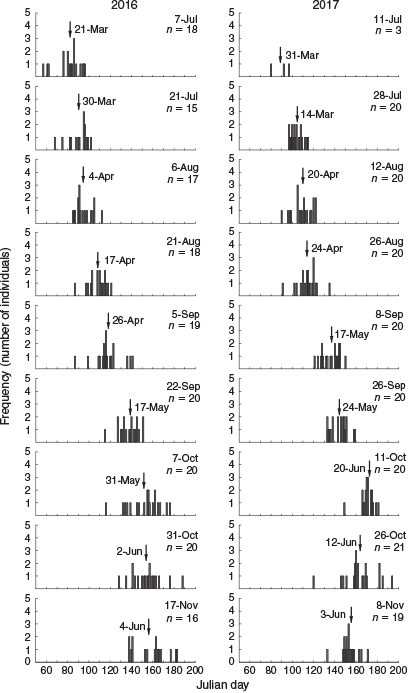
|
Otolith chemistry
Individual elements: natal origin
Trace element chemistry for the otolith core varied among sample occasions and between years (Table 2). For Li, differences were among sample occasions, but not between years. Li increased during July to a maximum in late August, then declined to a low in late October and November (Fig. 7a). Significant differences were detected between early and late August for 2016, and between late August and late October for 2017. Mg differed among occasions and between years. Mean Mg was higher in July and August for 2016 than 2017, but similar between years from late September to November (Fig. 7b). Differences were found for Mn among occasions, but not between years. For 2016, mean Mn peaked in late July, then decreased to a minimum by late August and remained low until November (Fig. 7c). In 2017, there were no differences among occasions. For Sr, there were significant differences among occasions and between years, and the pattern of variation between years. Mean Sr increased between fish sampled in July and August and those sampled later, and was consistently higher for 2017 (Fig. 7d). Ba was consistently higher for 2017 than 2016 (Fig. 7e; Table S2).
Individual elements: larval advection
Elemental differences related to the larval advection life stage varied between elements and years (Table 2). The within-season trend for Li was markedly similar between years. Li was highest in late July, then decreased progressively to a minimum in November (Fig. 7f). Differences were found between late July and November for both years. Significant interactions between occasion and year were detected for Mg, Mn, Sr and Ba, indicating that in each case the pattern of variation among sample occasions differed between years. Mg : Ca ratios between July and early September were higher for 2016 than 2017 (Fig. 7g). Within-season differences were found between August and late September for 2016, and between August and November for 2017. For Mn, there was considerable variation within and between years. Mean Mn was consistently higher for 2016 than 2017, particularly during July and early August (Fig. 7h). Differences were found between July–August and September–October for each year. Sr was lowest in early July, increased to a maximum near the start of October and then remained stable through to November (Fig. 7i). Mean Sr differed between July to August, and late September to November. There were differences in Ba among occasions and between years. Mean Ba differed between the maximum concentration in late July and the minimum in September (2016) and October (2017; Table S3).
Multi-element: natal origin
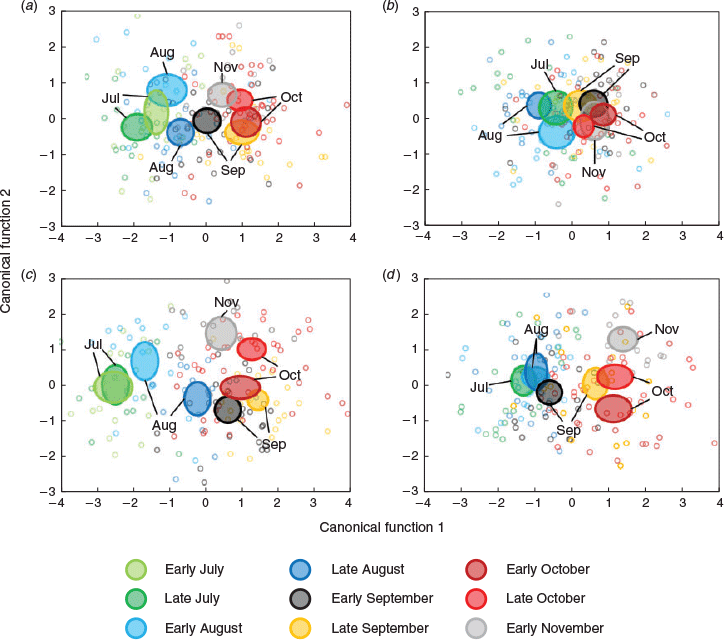
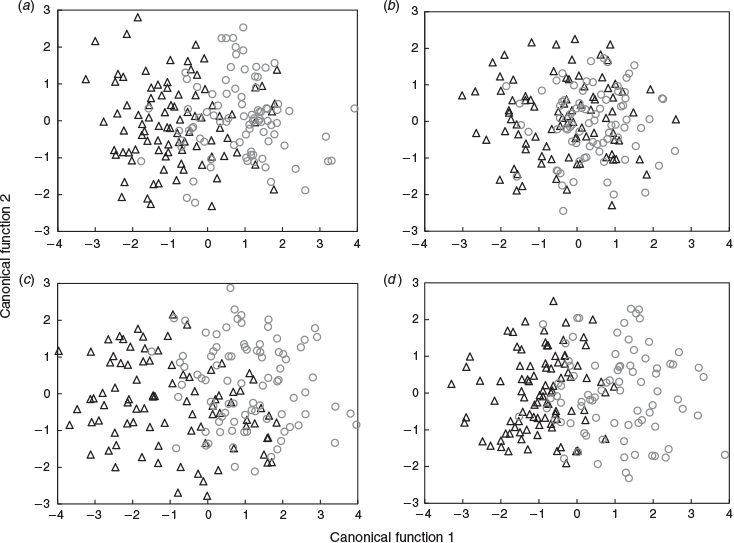
MANOVA identified significant differences in the multi-elemental chemistry related to the natal origin among sample occasions and between years (Table 3). For 2016, within-season differences were explained by four discriminant functions (λ = 0.377, d.f. = 32, P < 0.001), with the first two describing 95.5% of total variance. Sr and Mn were primarily responsible for group separation along the first discriminant axis (81.2% of total variance), and Li was responsible for group separation along the second axis (14.3%). Sr and Mn broadly separated the data into two clusters along the first axis: larvae sampled in July and August, and those sampled from late September to November (Fig. 8a). Variation within these groups was driven by Li, although overlapping 95% confidence ellipses suggested that multi-element chemistry was similar. Classification of individuals back to their respective sample occasions was low at 25.0%, which indicated that although temporal differences were significant, there was considerable variability among samples (Table S4). Misclassification was highest among successive occasions.
|
The temporal distribution of sample occasions was similar for 2017, although group separation was lower. Within-season differences were explained by two discriminant functions (λ = 0.689, d.f. = 40, P < 0.001), with Sr predominantly responsible for separation along the first axis (79.0%) and Li along the second (21.0%; Fig. 7b). Sr drove separation between fish sampled in July and August from those sampled in September–November (Fig. 8b). However, higher overlap in confidence ellipses and overlap between some samples collected months apart indicated that otolith chemistry was similar between them. Classification was marginally lower than in 2016 at 24.1% (Table S4).
Multi-element: larval advection
Multi-element chemistry related to the larval advection differed among occasions and between years (Table 3). These differences were larger than those identified for the natal origin. For 2016, within-season differences were explained by five discriminant functions (λ = 0.157, d.f. = 40, P < 0.001), the first two describing 90.8% of total variance. Sr and Mn were responsible for separation along the first axis (72.3%), and Li was responsible for separation along the second axis (18.5%). Fish from July and early August had the lowest Sr and highest Mn, and were clearly separated along the first axis (Fig. 8c). Late August to early October samples grouped together, although there was minimal overlap between confidence ellipses, and late October and November samples were separated from all others. Classification success was 33.1% and misclassification was highest to adjacent occasions (Table S5).
Within-season differences for 2017 were explained by four discriminant functions (λ = 0.328, d.f. = 28, P < 0.001), the first two describing 92.9% of total variation. Like 2016, Sr and Mn drove separation along the first axis (76.7%), whereas Li drove separation on the second axis (16.1%). Fish from late July to early September were separated from those sampled between late September and November (Fig. 8d). Overlapping ellipses among the July–early September samples indicated similarity in multi-element chemistry. Fish sampled from late September to November grouped together along the first axis, but separated along the second. Classification success was low at 34.7% (Table S5).
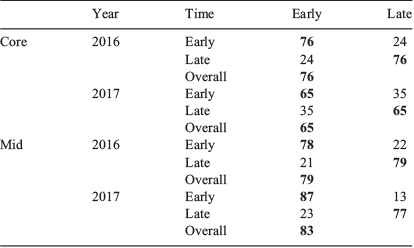
Multi-element comparison between the start and end of the season
The distribution of sample occasions based on multi-element chemistry related to the natal origin and larval advection stages were broadly separated into two groups: those fish sampled ‘early’ in the season (from July to mid-September) and those sampled ‘late’ (from mid-September to November). MANOVAs that compared the multi-element chemistry between these two groups identified significant differences for both life stages (Table 4). Regardless of life stage or year, Sr was foremost responsible for group separation, although separation improved when Mn was influential. For the otolith core, within-season differences were larger for 2016 than 2017 (Fig. 9a, b). Classification success was 76.1% for 2016 and 65.3% for 2017 (Table 5). Comparatively, between-group differences for the larval advection were larger. Fish that settled early or late in the settlement season were divided into two groups based on their otolith chemistry (Fig. 9c, d). Within-season differences were greater for 2017 than 2016 (Table 4), supported by classification success of 82.5 and 78.5% respectively.
|
|
Discussion
This study investigated the early life history of King George whiting larvae that recruited to an important nursery area over two complete settlement seasons, based on the retrospective analysis of their otoliths. We identified significant temporal variation in early life history characteristics of new recruits both within and between years. Otolith chemistry related to the physiochemical environment occupied during the natal origin and larval advection stages also demonstrated considerable temporal variation at multiple scales. When individual elements were combined, they described a significant within-season change in multi-element chemistry that related to recruits that hatched at different times.
Defining the spawning season
Throughout the 4-month settlement season, 52–71% of new recruits were collected in late September and early October, which identified this as the time of peak recruitment. These larvae were spawned between mid-May and early June. As such, spawning during this short 3- to 4-week period made the most significant contribution to annual recruitment. Several other batch-spawning species with protracted spawning seasons have also exhibited a short reproductive window responsible for the majority of recruitment (Cargnelli and Gross 1996; Rankin and Sponaugle 2014; Beveren et al. 2016). However, in the present study, the bulk of recruitment corresponded to spawning towards the end of the reproductive season when recruits were smallest and relatively old, rather than at the beginning when they were larger and younger. This seems counterintuitive, because the older and slower-growing larvae are likely to have experienced higher mortality during an extended critical period, and subsequently have lower survivorship (May 1974). There are several possibilities to explain this. One is that changes in the plankton community resulted in lower predation on King George whiting eggs and larvae that subsequently improved survivorship. Another option is that spawning success improved, either through higher gamete production or increased fertilisation rates, which culminated in higher recruitment (Leggett and Deblois 1994; Chambers and Trippel 1997). Alternatively, a third possibility is that the larval dispersal pathways changed throughout the spawning season and became more favourable for settlement to Barker Inlet later on. It is likely that larval dispersal would be considerably affected by seasonal changes in larval duration and prevailing oceanographic conditions (Fowler et al. 2000b; Jenkins et al. 2000).
There was some similarity between the settlement pattern to Barker Inlet in 2017 and that for 1993 (i.e. 24 years earlier; Fowler and Short 1996). In 1993, settlement was characterised by two distinct cohorts: the first that settled in June and July, and the second that settled in late September and October. For 2017, it appears that a second, less abundant, cohort appeared in late September and October. The settlement pattern for 2016 aligned with the second phase of recruitment, corresponding with the timing of settlement for King George whiting in other regions of its distribution (Jenkins and May 1994; Hyndes et al. 1996). Higher settlement during late September and October is likely associated with either improved larval survivorship or higher reproductive output later in the season (Leggett and Deblois 1994). It would be unlikely for survivorship to have improved, based on the longer presettlement durations and slower growth rates of the late-season recruits (Pepin 1991; Chambers and Trippel 1997). Furthermore, the timing and duration of spawning that resulted in recruitment to Barker Inlet does not completely align with the recognised spawning season for Investigator Strait. Estimated hatch dates indicated that spawning occurred from March until July, although adult reproductive activity is highest between March and May (Fowler et al. 1999). This period of spawning corresponds to the first cohort of recruits from July to mid-September, but does not account for the high abundance of new settlers in late September and October that were responsible for the majority of recruitment.
Temporal variation in early life history
Larval King George whiting that hatched at different times throughout the long autumn–winter spawning season exhibited differences in early life history characteristics in response to changes in environmental conditions (Pepin 1991; Cargnelli and Gross 1996; Radtke et al. 2001). Estimates of size at age decreased successively throughout the settlement season, with those larvae sampled at the time of peak recruitment being smaller and having experienced a longer presettlement duration than those that settled earlier. The difference in presettlement duration between those that settled in July and October was 24 days, representing a 20% increase. In addition, over the same period, average growth rate declined by 23–26%. As such, larvae that settled later in the season not only had longer larval durations, but also developed at considerably slower rates. The larval phase is when fish are most vulnerable, and therefore any prolongation of presettlement duration is likely to be reflected in survivorship and subsequent recruitment (Houde 1989; Leggett and Deblois 1994; Jenkins and May 1994). However, here settlement was highest later in the season when recruits were smaller and had experienced longer larval durations. Larval growth is strongly correlated with temperature, such that even small changes in water temperature can affect growth and development (Houde 1989; Green and Fisher 2004). The systematic decline in average growth rate throughout the season is consistent with the progressively decreasing water temperatures during the autumn–winter period (Fowler and Short 1996). Furthermore, decreasing water temperature may also affect primary productivity and plankton density, which has implications for larval development. It is possible that the decline in average growth rate may also relate to changes in prey availability, as well as the direct effect of water temperature on growth (Leggett and Deblois 1994; Meekan et al. 2003). It also needs to be recognised that this study was focused at a single site, and therefore the results may not be representative of recruitment for this species at a broader scale. As such, differences in temporal recruitment patterns to other nursery areas could have considerable implications for management recommendations.
Trace element chemistry
Fish that settled early in the season had significantly lower Sr than those that settled later, which broadly separated the samples into two groups. Sr is one of the most widely used elements in otolith chemistry analysis to reconstruct environmental histories and differentiate between groups of fish that have occupied different environments (Walther and Thorrold 2006; Izzo et al. 2018). Laboratory experiments and field studies have demonstrated positive relationships between the Sr concentration in otoliths and ambient concentrations in the aquatic environment (Bath et al. 2000; Elsdon and Gillanders 2003; Izzo et al. 2018). This is likely to be associated with the ability of Sr2+ to directly substitute for Ca2+ at the accreting surface of the otolith (Doubleday et al. 2014). However, in this study it is difficult to disentangle the physiochemical influences responsible for the changes in otolith chemistry. Regardless, the observed differences either directly relate to physical environmental conditions or are mediated by their effects on the physiology of the fish (Sturrock et al. 2012, 2014).
Even though Sr was primarily responsible for within-season differences, changes in Li, Mg, Mn and Ba also contributed to group separation and significant multi-element differences between sample occasions. The distribution of sample occasions in multivariate space was similar between years, with successive occasions generally showing the greatest similarity in elemental composition. However, there was considerable variation in otolith chemistry within and between sample occasions. For both years, within-season differences in elemental composition were larger for the larval advection life stage than the natal origin. It is difficult to determine whether this related to greater environmental heterogeneity during larval dispersal compared with the spawning source or whether ontogenetic influences during early development compromised environmental signals (Ruttenberg et al. 2005). Several studies have identified changes in otolith chemistry associated with the primordium of otoliths for marine and freshwater fish, which may mask environmental influences and affect the ability to delineate natal origins (Brophy et al. 2004; Ruttenberg et al. 2005; Lazartigues et al. 2014). However, we specifically sampled otoliths outside the exogenous feeding check to reduce such influence.
Daily age information from otolith microstructure provided a highly resolved temporal scale to assist the interpretation of otolith chemistry (Campana 1999; Campana and Thorrold 2001). Although we identified variation in multi-element chemistry among successive sample occasions, the largest differences were between the two broad groups of recruits that were sampled at the beginning and end of the settlement season. Daily increment counts determined that recruits collected from July to early September hatched from March until the end of April, whereas those collected from mid-September to November hatched from mid-May into June. The differences in otolith chemistry between these two groups of recruits corresponded to a 3-week difference in mean hatch date at the beginning of May. Only a handful of fish for each year overlapped in hatch date between these groups, the transition of which was between early and late September in 2016, and from August to September in 2017. The corresponding changes in otolith chemistry suggest that recruits collected at different stages of the settlement season developed in different physiochemical environments.
Ecological interpretation and fishery implications
The recruits that settled to Barker Inlet between July and early September were spawned in March and April, whereas those that settled in mid-September and October were spawned from mid-May into June. Therefore, different groups of developing larvae were moved towards Barker Inlet between March and October. The two groups of recruits had significantly different early life history characteristics. Larvae that settled early were larger, faster growing, had shorter presettlement durations and had significantly different otolith chemistry than those that settled in mid-September and October. However, the latter contributed most to annual recruitment. There are two hypotheses to account for these within-season changes in early life history characteristics and otolith chemistry. The first is that all recruits to Barker Inlet throughout the long settlement season originated from a primary spawning source and followed a similar larval advection pathway. Here, the differences in otolith chemistry would reflect a temporal change in the physiochemical environment experienced by the larvae between the time of hatch and throughout the period of larval development. The second possibility is that the different multi-elemental signals represent different spawning sources with different physiochemical conditions, and that these sources contributed recruits to Barker Inlet at different stages of the settlement season. In this scenario, the geographic source that produced the larvae that settled from mid-September to November made the greatest contribution to annual recruitment. Furthermore, interannual variation in reproductive success and larval survival for each spawning source would be reflected in recruitment to Barker Inlet, and would help explain the interannual variation in settlement (Fowler and Short 1996).
Two approaches are being used to differentiate between these hypotheses. To understand the spatial distribution of spawning that leads to recruitment, we will use a high-resolution oceanographic model seeded with early life history information derived from otolith analyses to reverse simulate larval dispersal and identify potential spawning sources for larvae that recruited throughout the settlement season. Second, ichthyoplankton surveys throughout the currently recognised spawning area will be undertaken to improve the spatial understanding of spawning activity. The larvae from these surveys could also be used to investigate the hypotheses from this study. These two approaches will provide complementary spatial information to be interpreted along with the information on otolith microstructure and chemistry.
Understanding the spatial and temporal variation in early life history characteristics will help develop the most appropriate fishery management strategies. Most recruitment to Barker Inlet occurred in late September and early October, corresponding to spawning between mid-May and June. These recruits had significantly different early life history characteristics and otolith chemistry than those that settled earlier. Since 2017, a seasonal closure has been imposed throughout an extensive area in Investigator Strait and southern Spencer Gulf for the month of May to protect aggregations of spawning whiting (Steer et al. 2018). Nevertheless, there may be temporal and spatial issues associated with this closure. Based on the timing of peak settlement and the retrospective hatch dates of these recruits, the closure does not completely encompass the period of spawning responsible for the majority of recruitment to Barker Inlet. The discrepancy in the timing of spawning that resulted in peak recruitment to Barker Inlet and the current seasonal spawning closure has potential implications for management. Resolving the underlying cause of the significant change in otolith chemistry throughout the reproductive season should produce better spatial information regarding where King George whiting originate. This could lead to refinement of the current seasonal spawning closure. Combining otolith microstructure and trace element chemistry has improved our understanding of early life history for this important fishery species.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declarations of funding
Operating costs were partly funded by the Fisheries Research and Development Corporation (Project number 2016/003) and logistical support was provided by the South Australian Research and Development Institute (Aquatic Sciences). This study was done as part of a Ph.D. by Troy Rogers, who was supported by an Australian Postgraduate Award (APA) and a Playford Trust Ph.D. Scholarship. Part of this study was presented at the 6th International Otolith Symposium in Taiwan (15–20 April 2018), with funding from the Holsworth Wildlife Research Endowment and the Ecological Society of Australia.
Acknowledgements
The authors thank Michael Drew and the numerous others who assisted with fieldwork. The LA-ICP-MS was done at Adelaide Microscopy and the authors thank Sarah Gilbert for her assistance. Damian Matthews prepared the map. The authors thank three anonymous reviewers whose feedback improved a previous version of the manuscript.
References
Bath, G. E., Thorrold, S. R., Jones, C. M., Campana, S. E., McLaren, J. W., and Lam, J. W. H. (2000). Strontium and barium uptake in aragonitic otoliths of marine fish Geochimica et Cosmochimica Acta 64, 1705–1714.| Strontium and barium uptake in aragonitic otoliths of marine fishCrossref | GoogleScholarGoogle Scholar |
Beveren, E. V., Klein, M., Serrao, E. A., Goncalves, E. J., and Borges, R. (2016). Early life history of larvae and early juvenile Atlantic horse mackerel Trachurus trachurus off the Portuguese west coast Fisheries Research 183, 111–118.
| Early life history of larvae and early juvenile Atlantic horse mackerel Trachurus trachurus off the Portuguese west coastCrossref | GoogleScholarGoogle Scholar |
Brophy, D., Jeffries, T. E., and Danilowicz, B. S. (2004). Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins Marine Biology 144, 779–786.
| Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural originsCrossref | GoogleScholarGoogle Scholar |
Brown-Peterson, N. J., Wyanski, D. M., Saborido-Rey, F., Macewicz, B. J., and Lowerre-Barbieri, S. K. (2011). A standardized terminology for describing reproductive development in fishes Marine and Coastal Fisheries 3, 52–70.
| A standardized terminology for describing reproductive development in fishesCrossref | GoogleScholarGoogle Scholar |
Bruce, B. (1995). Larval development of King George whiting, Sillaginodes punctata, school whiting, Sillago bassensis, and yellowfin whiting, Sillago schomburgkii (Percoidei: Sillaginidae), from South Australian waters Fishery Bulletin 93, 27–43.
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applicationsCrossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Jones, C. M. (1992). Analysis of otolith microstructure data. In ‘Otolith Microstructure Examination and Analysis’. (Eds D. K. Stevenson and S. E. Campana.) Canadian Special Publication of Fisheries and Aquatic Sciences 117, pp. 73–100. (Canada Communication Group – Publishing: Ottawa, ON, Canada.)
Campana, S. E., and Neilson, J. D. (1985). Microstructure of fish otoliths Canadian Journal of Fisheries and Aquatic Sciences 42, 1014–1032.
| Microstructure of fish otolithsCrossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58, 30–38.
| Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?Crossref | GoogleScholarGoogle Scholar |
Cargnelli, L. M., and Gross, M. R. (1996). The temporal dimension in fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrohirus) Canadian Journal of Fisheries and Aquatic Sciences 53, 360–367.
| The temporal dimension in fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrohirus)Crossref | GoogleScholarGoogle Scholar |
Chambers, R. C., and Trippel, E. A. (1997). ‘Early Life History and Recruitment in Fish Populations’, 1st edn. (Chapman and Hall: New York, NY, USA.)
Cowen, R. K., and Sponaugle, S. (2009). Larval dispersal and marine population connectivity Annual Review of Marine Science 1, 443–466.
| Larval dispersal and marine population connectivityCrossref | GoogleScholarGoogle Scholar | 21141044PubMed |
Dorval, E., Jones, C. M., Hannigan, R., and van Montfrans, J. (2005). Can otolith chemistry be used for identifying essential seagrass habitats for juvenile spotted seatrout, Cynoscion nebulosus, in Chesapeake Bay? Marine and Freshwater Research 56, 645–653.
| Can otolith chemistry be used for identifying essential seagrass habitats for juvenile spotted seatrout, Cynoscion nebulosus, in Chesapeake Bay?Crossref | GoogleScholarGoogle Scholar |
Doubleday, Z. A., Harris, H. H., Izzo, C., and Gillanders, B. M. (2014). Strontium randomly substituting for calcium in fish otoliths Analytical Chemistry 86, 865–869.
| Strontium randomly substituting for calcium in fish otolithsCrossref | GoogleScholarGoogle Scholar | 24299165PubMed |
Elsdon, T. S., and Gillanders, B. M. (2003). Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri Marine Ecology Progress Series 260, 263–272.
| Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheriCrossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillanders, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life history parameters of fishes: hypotheses, assumptions, limitation and inferences Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life history parameters of fishes: hypotheses, assumptions, limitation and inferencesCrossref | GoogleScholarGoogle Scholar |
Fowler, A. J., and Jones, G. K. (2008). The population biology of King George whiting (Sillaginodes punctata) in Gulf St Vincent. In ‘Natural History of Gulf St Vincent’. (Eds S. A. Shepherd, S. Bryars, I. Kirkegaard, P. Harbison, and J. T. Jennings.) pp. 399–414. (Royal Society of South Australia: Adelaide, SA, Australia.)
Fowler, A. J., and Short, D. A. (1996). Temporal variation in the early life-history characteristics of the King George whiting (Sillaginodes punctata) from analysis of otolith microstructure Marine and Freshwater Research 47, 809–818.
| Temporal variation in the early life-history characteristics of the King George whiting (Sillaginodes punctata) from analysis of otolith microstructureCrossref | GoogleScholarGoogle Scholar |
Fowler, A. J., McLeay, L., and Short, D. A. (1999). Reproductive mode and spawning information based on gonad analysis for the King George whiting (Percoidei: Sillaginidae) from South Australia Marine and Freshwater Research 50, 1–14.
| Reproductive mode and spawning information based on gonad analysis for the King George whiting (Percoidei: Sillaginidae) from South AustraliaCrossref | GoogleScholarGoogle Scholar |
Fowler, A. J., Black, K. P., and Jenkins, G. P. (2000a). Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. II. South Australia Marine Ecology Progress Series 199, 243–254.
| Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. II. South AustraliaCrossref | GoogleScholarGoogle Scholar |
Fowler, A. J., McLeay, L., and Short, D. A. (2000b). Spatial variation in size and age structures and reproductive characteristics of the King George whiting (Percoidei: Sillaginidae) in South Australian waters Marine and Freshwater Research 51, 11–22.
| Spatial variation in size and age structures and reproductive characteristics of the King George whiting (Percoidei: Sillaginidae) in South Australian watersCrossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (2002). Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries? Marine Ecology Progress Series 240, 215–223.
| Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries?Crossref | GoogleScholarGoogle Scholar |
Green, B. S., and Fisher, R. (2004). Temperature influences swimming speed, growth and larval duration in coral reef fish larvae Journal of Experimental Marine Biology and Ecology 299, 115–132.
| Temperature influences swimming speed, growth and larval duration in coral reef fish larvaeCrossref | GoogleScholarGoogle Scholar |
Hamer, P. A., and Jenkins, G. P. (1997). Larval supply and short-term recruitment of a temperate zone demersal fish, the King George whiting, Sillaginodes punctata Cuvier and Valenciennes, to an embayment in south-eastern Australia Journal of Experimental Marine Biology and Ecology 208, 197–214.
| Larval supply and short-term recruitment of a temperate zone demersal fish, the King George whiting, Sillaginodes punctata Cuvier and Valenciennes, to an embayment in south-eastern AustraliaCrossref | GoogleScholarGoogle Scholar |
Hamer, P. A., Jenkins, G. P., and Gillanders, B. M. (2003). Otolith chemistry of juvenile snapper Pagrus auratus in Victorian waters: natural chemical tags and their temporal variation Marine Ecology Progress Series 263, 261–273.
| Otolith chemistry of juvenile snapper Pagrus auratus in Victorian waters: natural chemical tags and their temporal variationCrossref | GoogleScholarGoogle Scholar |
Hogan, J. D., Kozdon, R., Blum, M. J., Gilliam, J. F., Valley, J. W., and McIntyre, P. B. (2017). Reconstructing larval growth and habitat use in an amphidromous goby using otolith increments and microchemistry Journal of Fish Biology 90, 1338–1355.
| Reconstructing larval growth and habitat use in an amphidromous goby using otolith increments and microchemistryCrossref | GoogleScholarGoogle Scholar | 27990639PubMed |
Houde, E. D. (1989). Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects Fishery Bulletin 87, 471–495.
Hyndes, G. A., Potter, I. C., and Lenanton, R. C. J. (1996). Habitat partitioning by whiting species (Sillaginidae) in coastal waters Environmental Biology of Fishes 45, 21–40.
| Habitat partitioning by whiting species (Sillaginidae) in coastal watersCrossref | GoogleScholarGoogle Scholar |
Izzo, C., Reis-Santos, P., and Gillanders, B. M. (2018). Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation Fish and Fisheries 19, 441–454.
| Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluationCrossref | GoogleScholarGoogle Scholar |
Jenkins, G. P., and May, H. M. A. (1994). Variation in settlement and larval duration of King George whiting, Sillaginodes punctata (Sillaginidae), in Swan Bay, Victoria, Australia Bulletin of Marine Science 54, 281–296.
Jenkins, G. P., Black, K. P., and Hamer, P. A. (2000). Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. I. Victoria Marine Ecology Progress Series 199, 231–242.
| Determination of spawning areas and larval advection pathways for King George whiting in southeastern Australia using otolith microstructure and hydrodynamic modelling. I. VictoriaCrossref | GoogleScholarGoogle Scholar |
Jenkins, G. P., Hamer, P. A., Kent, J. A., Kemp, J., and Fowler, A. J. (2015). Spawning sources, movement patterns, and nursery area replenishment of spawning populations of King George whiting in south-eastern Australia – closing the life history loop. Final Report, Fisheries Research and Development Corporation, Canberra, ACT, Australia.
Jones, G. P., Almany, G. R., Russ, G. R., Sale, P. F., Steneck, R. S., van Oppen, J. H., and Willis, B. L. (2009). Larval retention and connectivity among populations of coral and reef fishes: history, advances and challenges Coral Reefs 28, 307–325.
| Larval retention and connectivity among populations of coral and reef fishes: history, advances and challengesCrossref | GoogleScholarGoogle Scholar |
Kailola, P. J., William, M. J., Stewart, P. C., Reichelt, R. E., McNee, A., and Grieve, C. (1993). ‘Australian Fisheries Resources. Bureau of Resource Sciences and the Fisheries Research and Development Corporation Canberra, Australia.’ (Imprint Limited: Brisbane, Qld, Australia.)
Lahaye, Y., Lambert, D., and Walters, S. (1997). Ultraviolet laser sampling and high resolution inductively coupled plasma mass spectrometry of NIST and BCR-2G glass reference materials. Geostandards Newsletter 21, 205–214.
| Ultraviolet laser sampling and high resolution inductively coupled plasma mass spectrometry of NIST and BCR-2G glass reference materials.Crossref | GoogleScholarGoogle Scholar |
Lazartigues, A. V., Sirois, P., and Savard, D. (2014). LA-ICP-MS analysis of small samples: carbonate reference materials and larval fish otoliths Geostandards and Geoanalytical Research 38, 225–240.
Leggett, W. C., and Deblois, E. (1994). Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Netherlands Journal of Sea Research 32, 119–134.
| Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages?Crossref | GoogleScholarGoogle Scholar |
Leis, J. M., and Carson-Ewart, B. M. (Eds) (2004). The larvae of Indo-Pacific Coastal Fishes: A Guide to Identification. Fauna Malesiana Handbook 2’, 2nd edn. (Brill: Leiden, Netherlands.)
Leis, J. M., Siebeck, U., and Dixson, D. L. (2011). How Nemo finds home: the neuroecology of dispersal and population connectivity in larvae of marine fishes Integrative and Comparative Biology 51, 826–843.
| How Nemo finds home: the neuroecology of dispersal and population connectivity in larvae of marine fishesCrossref | GoogleScholarGoogle Scholar | 21562025PubMed |
May, R. C. (1974). Larval mortality in marine fishes and the critical period concept. In ‘The Early Life History of Fish’. (Ed. J. H. S. Blaxter.) pp. 3–19. (Springer: Berlin, Germany.)
Meekan, M. G., Carleton, J. H., McKinnon, A. D., Flynn, K., and Furnas, M. (2003). What determines the growth of tropical reef fish larvae in the plankton: food or temperature? Marine Ecology Progress Series 256, 193–204.
| What determines the growth of tropical reef fish larvae in the plankton: food or temperature?Crossref | GoogleScholarGoogle Scholar |
Neira, F. J., Miskiewicz, A. G., and Trnski, T. (Eds) (1998). ‘Larvae of Temperate Australian Fishes: Laboratory Guide for Larval Fish Identification.’ (University of Western Australia Press: Perth, WA, Australia.)
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J. (2011). Iolite: freeware for the visualisation and processing of mass spectrometric data Journal of Analytical Atomic Spectrometry 26, 2508–2518.
| Iolite: freeware for the visualisation and processing of mass spectrometric dataCrossref | GoogleScholarGoogle Scholar |
Pepin, P. (1991). Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish Canadian Journal of Fisheries and Aquatic Sciences 48, 503–518.
| Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fishCrossref | GoogleScholarGoogle Scholar |
Radtke, R. L., Kinzie, R. A., and Shafer, D. J. (2001). Temporal and spatial variation in length of larval life and size at settlement of the Hawaiian amphidromous goby Lentipes concolor Journal of Fish Biology 59, 928–938.
Rankin, T. L., and Sponaugle, S. (2014). Characteristics of settling coral reef fish are related to recruitment timing and success PLoS One 9, e108871.
| Characteristics of settling coral reef fish are related to recruitment timing and successCrossref | GoogleScholarGoogle Scholar | 25250964PubMed |
Ruttenberg, B. I., Hamilton, S. L., Hickford, M. J. H., Paradis, G. L., Sheehy, M. S., Standish, J. D., Ben-Tzvi, O., and Warner, R. R. (2005). Elevated levels of trace elements in the cores of otoliths and their potential for use as natural tags Marine Ecology Progress Series 297, 273–281.
| Elevated levels of trace elements in the cores of otoliths and their potential for use as natural tagsCrossref | GoogleScholarGoogle Scholar |
Sponaugle, S., Grorud-Colvert, K., and Pinkard, D. (2006). Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys Marine Ecology Progress Series 308, 1–15.
| Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida KeysCrossref | GoogleScholarGoogle Scholar |
Steer, M. A., Fowler, A. J., McGarvey, R., Feenstra, J., Westlake, E. L., Matthews, D., Drew, M., Rogers, P. J., and Earl, J. (2018). Assessment of the South Australian Marine Scalefish Fishery in 2016. Report to PIRSA Fisheries and Aquaculture, SARDI Publication number F2017/000427-1, SARDI Research Report Series number 974, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Sturrock, A. M., Trueman, C. N., Darnaude, A. M., and Hunter, E. (2012). Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81, 766–795.
| Can otolith elemental chemistry retrospectively track migrations in fully marine fishes?Crossref | GoogleScholarGoogle Scholar | 22803735PubMed |
Sturrock, A. M., Trueman, C. N., Milton, J. A., Waring, C. P., Cooper, M. J., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research Marine Ecology Progress Series 500, 245–264.
| Physiological influences can outweigh environmental signals in otolith microchemistry researchCrossref | GoogleScholarGoogle Scholar |
Swearer, S. E., Caselle, J. E., Lea, D. W., and Warner, R. R. (1999). Larval retention and recruitment in an island population of a coral-reef fish Nature 402, 799–802.
| Larval retention and recruitment in an island population of a coral-reef fishCrossref | GoogleScholarGoogle Scholar |
Thorrold, S. R., Latkoczy, C., Swart, P. K., and Jones, C. M. (2001). Natal homing in a marine fish metapopulation Science 291, 297–299.
| Natal homing in a marine fish metapopulationCrossref | GoogleScholarGoogle Scholar | 11209078PubMed |
Walther, B. D., and Thorrold, S. R. (2006). Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish Marine Ecology Progress Series 311, 125–130.
| Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fishCrossref | GoogleScholarGoogle Scholar |
Yoshinaga, J., Atsuko, N., Masatoshi, M., and Edmonds, J. S. (2000). Fish otolith reference material for quality assurance of chemical analysis Marine Chemistry 69, 91–97.
| Fish otolith reference material for quality assurance of chemical analysisCrossref | GoogleScholarGoogle Scholar |