Comparative habitat use by large riverine fishes
John D. Koehn A C and Simon J. Nicol A BA Freshwater Ecology, Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
B Present Address: Oceanic Fisheries Programme, Secretariat of the Pacific Community, BP D5, 98848 Noumea Cedex, New Caledonia.
C Corresponding author. Email: John.Koehn@dse.vic.gov.au
Marine and Freshwater Research 65(2) 164-174 https://doi.org/10.1071/MF13011
Submitted: 11 January 2013 Accepted: 1 July 2013 Published: 14 October 2013
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
The present radio-tracking study compared adult daytime microhabitat use by three large Australian native freshwater fishes (Murray cod, Maccullochella peelii, trout cod, M. macquariensis, golden perch, Maquaria ambigua) and introduced carp, Cyprinus carpio, in the Murray River, south-eastern Australia. The paper describes habitat patches used by all species and quantifies differences among species. All species were strongly associated with structural woody habitat (>68% cover), deeper (>2.4 m), slower water (<0.2 m s–1) closer to the river bank, with variations in substrate. Murray cod and trout cod used deeper habitats (2.8 m and 2.9 m, respectively), with higher surface water velocities (0.37 m s–1 and 0.49 m s–1, respectively) and further from the bank than the habitats of golden perch (2.6 m; 0.31 m s–1) or carp (2.4 m; 0.20 m s–1), the latter species using wood higher in the water column than did cod species. Trout cod used habitats furthest from the bank and carp those closest. These data provide support and direction for reintroduction of structural woody habitat patches for rehabilitation which, in general, should have >70% cover, be >1.5 m high, located <15% of the river channel (width) closest to the bank, with surface water velocities of 0.3–0.6 m s–1.
Additional keywords: Australia, carp competition, Maccullochella, Macquaria, radio tracking, rehabilitation, telemetry, threatened species.
Introduction
Riverine fish habitats consist of many variables relating to water quality and quantity and in-stream structure, including depth and velocity, channel form, substratum, other physical structures and stream position (Kramer et al. 1997; Rosenfeld and Hatfield 2006). These habitat variables, or combinations of them, occur in patches that vary in size, quantity and quality, and are located heterogeneously across the ‘riverscape’ (Fausch et al. 2002; Wiens 2002). Fishes are expected to select preferred variables that will maximise their fitness (Krebs and Kacelnik 1991) and, hence, their abundances tend to be clustered around more suitable habitat patches, depending on the needs of each species (Inoue and Nakano 1998). Habitat quality and the selection of optimal habitats can affect fish condition (Oliva-Paterno et al. 2003). Habitats may change both temporally and spatially (Naiman and Latterell 2005) and use can be influenced by habitat availability (Grossman and Freeman 1987), and inter- and intra-specific interactions (Natsumeda 1998).
Globally, lowland riverine fishes face many threats and conservation challenges (Dudgeon et al. 2006; Cooke et al. 2012), especially in semi-arid systems (Filipe et al. 2002) and habitat degradation has contributed to the decline of freshwater fish populations (Cadwallader 1978; Cowx and Welcomme 1998; Cooke et al. 2012). The importance of structural habitat for fish has been widely recognised (Matthews 1998) and is a cornerstone central to the management of fishes (Rice 2005), especially keystone species (Naiman and Latterell 2005). Protecting and rehabilitating habitats is hence critical for conservation management and threatened-species recovery (Freeman and Freeman 1994; Peterson and Rabeni 2001) and this involves understanding the habitat needs of fish species (Rice 2005; Rosenfeld and Hatfield 2006). Detailed habitat requirements of species can be used to develop models to predict priority areas for their conservation or rehabilitation (Filipe et al. 2002). Because habitat patches occur at differing scales, understanding fish–habitat patch relationships is essential (Wiens 2002), as is the importance of habitat patches to multiple species in the ‘riverscape’ (Fausch et al. 2002; Welcomme et al. 2006; Winemiller et al. 2010).
Rivers of the Murray–Darling Basin (MDB), south-eastern Australia, are now considered to be in a degraded ecological state (Davies et al. 2010). The native fishes (~46 species; Lintermans 2007), have undergone significant reductions in distribution and abundance and population levels are now estimated to be at ~10% of those before European settlement (~1800s; Murray–Darling Basin Commission 2004; Koehn and Lintermans 2012). The rehabilitation of Murray–Darling Basin fish populations is now the focus of a multi-State Government initiative with a key rehabilitation measure being the protection and restoration of habitats (Murray–Darling Basin Commission 2004; Koehn and Lintermans 2012). The three native species studied here, Murray cod, Maccullochella peelii (Mitchell), trout cod, M. macquariensis (Cuvier) and golden perch, Macquaria ambigua (Richardson), are iconic, large-bodied fish that co-occur at reaches of the Murray–Darling Basin (Lintermans 2007). All three species are considered to be opportunistic predators (Ebner 2006; Baumgartner 2007; Lintermans 2007) and are popular angling species. Whereas the populations of all native species have suffered substantial declines, Murray cod and trout cod are listed as nationally threatened (www.environment.gov.au/biodiversity/threatened). Take of trout cod by anglers is prohibited and Murray cod is managed as a species for both conservation and for the recreational fishery (Koehn and Todd 2012). Previous studies have described Murray cod as a demersal (Koehn 2009b) main channel specialist, on the basis of its early life history (King 2004; Koehn and Harrington 2005; 2006) and macro-habitat selection (Koehn 2009a). Both Murray cod and trout cod have strong preferences for structural woody habitats (Growns et al. 2004; Nicol et al. 2007; Koehn 2009a). Trout cod habitats have often been considered to be similar to those of Murray cod, perhaps preferring faster waters (see Koehn and O’Connor 1990). Although populations of golden perch have declined, this species remains widespread and relatively abundant (Lintermans 2007).
Carp Cyprinus carpio (L.) is an introduced species that has dominated many Australian fish communities in lowland rivers of the Murray–Darling Basin, both in numbers and biomass, and has received much attention as a pest species (Koehn et al. 2000). Comparison with Australian native fish species, including those from the Murray–Darling Basin, indicates considerable ecological differences (Koehn 2004). Carp is a mid-water schooling species and an omnivore that feeds by sifting through the substrate (see Koehn et al. 2000). The use of wetlands by adult carp, especially for spawning, is well documented (Stuart and Jones 2006a, 2006b), but carp also uses habitats in the main river channel. High carp abundances have been suggested to be a potential contributor to the decline in native fishes and a factor that may inhibit rehabilitation of native fish populations (Koehn et al. 2000; Murray–Darling Basin Commission 2004). There is the potential for competition by carp for physical habitat space used by native species at both local (Crook et al. 2001) and larger scales (Boys and Thoms 2006) and, although habitats of juvenile carp have been determined to be different from those of juvenile Murray cod (Jones and Stuart 2007), interactions with adult native species have not been quantified.
The importance of structural woody habitats to riverine fish has been widely recognised with positive relationships reported for many fish species in a range of different aquatic habitats (see Crook and Robertson 1999; Dolloff and Warren 2003; Nagayama et al. 2012). The reinstatement of structural woody habitats is seen as a key rehabilitation measure (Murray–Darling Basin Commission 2004; Koehn and Lintermans 2012), with the assumption that it will be used by most large-bodied native fish species. Information from individual studies on the three native species considered in the present study does suggest that they may occupy similar habitats in lowland rivers and also have strong associations with structural woody habitat (Crook et al. 2001; Growns et al. 2004; Koehn et al. 2004; Boys and Thoms 2006; Jones and Stuart 2007; Nicol et al. 2007; Koehn 2009a). There has, however, been no examination of microhabitat use by these species and carp simultaneously, with previous studies having been conducted at different times, in different locations, with different methods (e.g. radio-tracking, electrofishing collection) and at different scales, making comparisons of habitat use difficult. For example, the size of study sites has varied considerably from Lake Mulwala (4390 ha, maximum width 4 km, maximum depth 14.6 m; Koehn 2009a) to the Broken river (width 10–50 m, maximum depth 4 m; Crook et al. 2001). There is a growing trend towards the multi-species management (species assemblages or communities) to maximise benefits from expenditure on river rehabilitation measures (Minckley et al. 2003; Murray–Darling Basin Commission 2004; Koehn and Lintermans 2012). Hence, there is a need for an understanding of comparative habitat use by multiple fish species; do all species use the same habitats, particularly in-stream structural wood; or is there differentiation in use among the species?
The present study describes microhabitat use during daylight hours by three native (two threatened) and an introduced large-bodied fish species at the same temporal and spatial scale in the Murray River in south-eastern Australia. It quantifies the characteristics of the physical habitats used by each species compared with those available and each other species. It then uses these data to provide direction for the reconstruction of structural woody habitat patches.
Materials and methods
Study area
The study was conducted in a 40-km reach of the Murray River, downstream of Lake Mulwala (36°00′S, 146°00′E; see Koehn 2009a). The Murray River in this region is a large, lowland river situated on low-gradient riverine plains, with low energy, and is characterised by meandering bends (Mackay 1990; Rutherfurd 1990). The Murray River in the study reach is highly regulated because of upstream storages and flows have been affected by water storage in winter and spring and the delivery of water for irrigation in summer, leading to a pattern of seasonal flow reversal (i.e. high summer, low winter flows, Close 1990; Thoms et al. 2000). The average river width is 108 m, with an average depth of 2 m at low flows and a maximum depth of ~11 m under normal conditions. This reach of river has been considered to have good water quality, with conductivity ~50 EC and abundant structural woody habitat, with few records of removal in comparison with other sections of the river (Thoms et al. 2000; Koehn et al. 2004).
Data collection
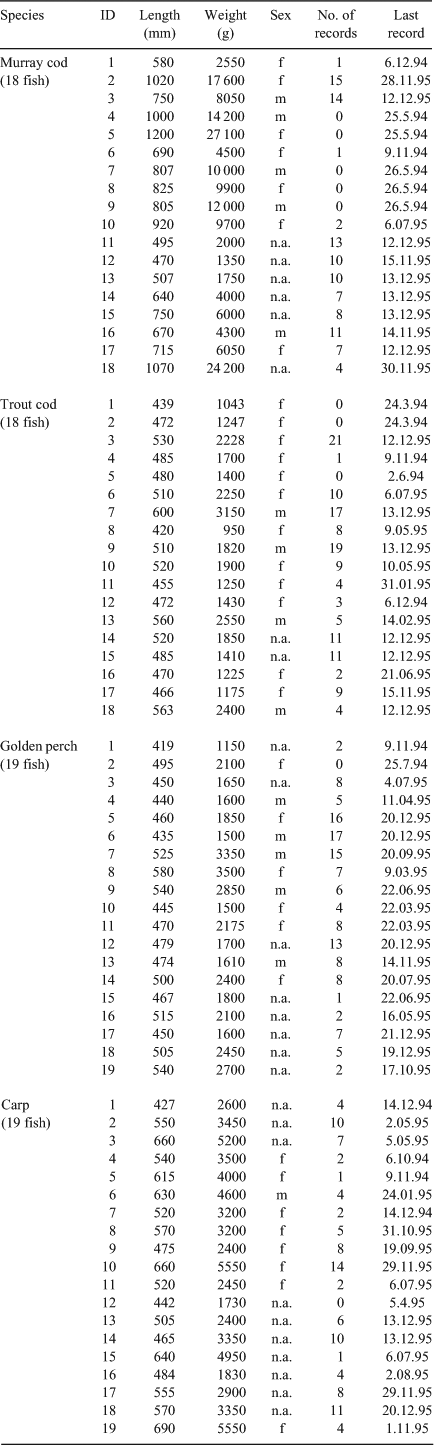
Habitat use was determined by radio-tracking fish implanted with radio-transmitters. Fish were caught with a boat-mounted electro-fisher (7.5 GPP, Smith Root, Portland, Washington, USA) and weighed and measured. Each fish to be tagged was anaesthetised using 5 mg L–1 of Maranil (Fort Collins, Colorado, USA) solution, and a 48–49 MHz transmitter (Advanced Telemetry Systems, Isanti, Minnesota, USA) was then surgically implanted into their abdominal cavity. The weight of the transmitter never exceeded 2% of the fish bodyweight (Knights and Lasee 1996) in air or 1.25% of the weight in water (Winter 1983). Fish were revived, released at the point of capture and tracking commenced only a minimum of one month later. Detailed descriptions of tag implantation, sterile procedures and antiseptics used to prevent infections, radio-tracking and habitat measurement methods are given in Nicol et al. (2007) and Koehn (2009a). Habitat data were collected only for fish located in a stationary position. Eighteen Murray cod, 18 trout cod, 19 golden perch and 19 carp individuals were radio-tagged for the study (Table 1). The transmitters contained a ‘mortality switch’ (a mercury motion sensor) that indicated a lack of movement and potential fish mortality. If a fish was missing during a tracking session (i.e. no live or mortality signals detected), it was assumed that the fish had migrated away from the study site, had been caught and kept by anglers or there had been transmitter failure.
|
Fish were tracked by boat during daylight hours by using a receiver and antenna, with care taken to avoid any disturbance, and could be located with an accuracy of 0.19 (±0.13 s.e.) (Koehn et al. 2012). Vertical location could not be determined, but a subset of tagged fish fitted with pressure transmitters had deemed Murray cod to be largely a demersal species (Koehn 2009b). Once a fish was located, its position was marked with a buoy. Habitat variables were then measured at the location of the fish and in the area immediately surrounding the location using the grid method outlined in Nicol et al. (2007). Briefly, a 12 m × 12 m grid was centred on the location of the fish and divided into sixteen 3 m × 3 m cells. In each of the grid cells, the presence/absence of structural woody habitat and overhanging vegetation was recorded and water depth measured. Water depth and the presence of structural woody habitat in the cell were determined using a Lowrance model X-16 paper trace echo sounder (Lowrance, Tulsa, Oklahoma, USA) mounted on the rear of a 4.3-m flat-bottom boat. Overhanging vegetation was recorded from visual observations looking upward. The water-depth readings in each cell were averaged across the grid (GMD). The coefficient of variation for this mean was calculated to provide an estimate of the bed heterogeneity within the grid (CVD). The proportion of cells with structural woody habitat and overhanging vegetation was calculated for each location to provide an estimate of the abundance of structural wood (GWD) and cover of overhanging vegetation (GOHV) within the grid.
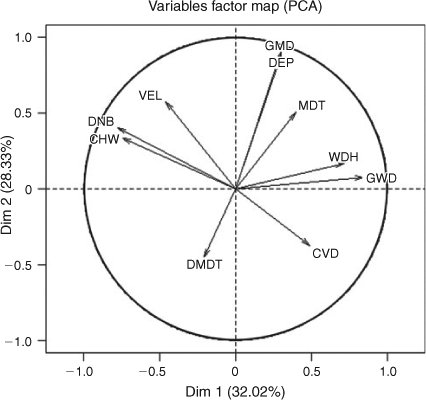
At the location of each fish, water depth (DEP) and the height of any structural wood (WDH) up into the water column was measured using the echo sounder. To quantify lateral position of the fish within the river channel, the channel width, distance from each fish to the nearest bank (DNB) and to the maximum cross-sectional depth of the river (DMDT) were measured using a forester’s hip chain. The maximum depth of the cross-sectional transect was recorded with the echo sounder (MDT). The variable DMDT indicated whether the fish location was in the low-flow channel of the river or in the medium- to high-flow areas. DNB was divided by river width to provide an estimate of lateral position corrected for variation in river width (CHW). Surface water velocity was measured over a 20-s interval using an OSS-B1 velocity meter (OSS, Sydney, Australia). Variables recorded at each location are listed in Table 2.
To provide a measure of habitat availability, random habitat locations were selected and habitat measurements recorded using the same methods as outlined above. The locations of these sites were pre-defined at the commencement of the study, with longitudinal (i.e. how far along the river bank), and lateral (i.e. how far from the bank) positions determined using a random-number generator. One of these sites was then selected within 800 m upstream or downstream of the location of a radio-tagged fish (regardless of whether the site was new or had been previously occupied). After an alternative habitat site was measured, it was deleted from the list so that it could be measured only once.
Statistical analysis
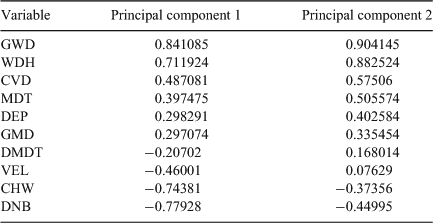
We used generalised linear mixed modelling (GLMM) in combination with principal components analysis (PCA) to estimate the fixed effect of species and season on habitat use by each species, and the extent to which individuals varied in their use of habitat (random effect). GLMM with Laplace approximation was used because the dataset was unbalanced because of all individuals not being located on all sampling occasions. We followed the guidelines from Bolker et al. (2009) to build the GLMMs and interpret the analysis using the lme4 package (Bates et al. 2013) in the R language and environment version 2.15.2 (R Development Core Team 2012). The small number of observations per individual meant that there was an insufficient number of replicates to estimate the variance components of all habitat variables measured and their interactions. Careful selection of model terms, based on both biology and the data, was warranted and rather than excluding some habitat variables because of lack of statistical power, we kept all habitat variables by using PCA. PCA summarises a multi-variate dataset to reduce its dimensionality. It generates orthogonal linear combinations (principal components) of the habitat variables and we used these principal components in the GLMM as the response variable. Significant correlations between the principal components and the original habitat variables were used to describe each dimension. To determine the number of PCA dimensions to include in the GLMM, we examined a scree plot to evaluate the variance explained by each component (Quinn and Keough 2002). All habitat variables were standardised to a mean of 0 and variance of 1 before the PCA was performed in the R add-in package ‘FactoMineR’ (Husson et al. 2009). We checked that the variances across categories and groups were homogeneous by using box and whisker plots and that the random effects were normally distributed in the GLMM using quantile–quantile plots. We also checked the GLMM for correlation between different parameters in the random effects and observed perfect correlation between some parameters. The GLMM was constrained to modelling the intercept only (1|fish) as the random effect and the main effects without an interaction term to avoid this over-fitting. A likelihood ratio test was used to ascertain support for inclusion of each fixed effect.
To evaluate how significant fixed effects from the GLMM related to the principal components, we added species and season as supplementary categorical variables to estimate the correlation between each principal component and the species in the PCA. We computed 95% confidence ellipses for each of the categories of the species and season groups to visualise the significant differences between species and season using FactoMineR (Husson et al. 2009).
Results
The number of habitat measurements recorded for each species was as follows: Murray cod 103, trout cod 134, golden perch 134, carp 103, with a corresponding 409 random sites (Table 1). Five Murray cod individuals, three trout cod individuals, one golden perch individual and one carp individual were never located by radio-tracking (Table 1), despite additional tracking by aircraft being undertaken over a river length of 250 km from the centre of the study site (see Koehn et al. 2008), or had mortality switches activated.
All species were strongly associated with structural woody habitat (>68 ± 0.05% grid cover) and had positive association to deeper (>2.4 ± 0.2 m), slower water (<0.2 ± 0.04 m s–1 surface velocity), closer to the river bank, with variations in substrate (Table 2). There were, however, considerable differences among species for many variables. Murray cod (2.9 ± 0.2 m) and trout cod (2.8 ± 0.2 m) used deeper habitats than did golden perch (2.6 ± 0.2 m) or carp (2.4 ± 0.2 m). Trout cod used habitats with faster surface waters (0.49 ± 0.05 m s–1) than those of Murray cod (0.37 ± 0.04 m s–1), golden perch (0.31 ± 0.03 m s–1) or carp (0.20 ± 0.04 m s–1) habitats in locations further from the bank (0.15 ± 0.02 CHW) and closer to the low-flow channel than those of the other species (0.9–0.10 ± 0.01 CHW). Carp had the greatest habitat overlap with golden perch and both used structural wood higher in the water column (1.5 ± 0.2 m) than did the cod species (1.2 ± 0.2 m).
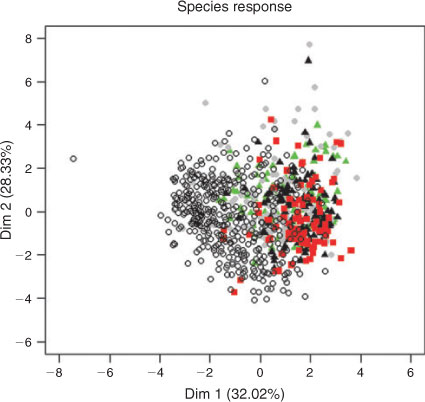
The GLMM and PCA supported these observations. The first two dimensions of the PCA explained the majority of the variance (32% and 28% for Components 1 and 2, respectively) and we considered this sufficient to use only these two components for further analyses. The remaining components explained 11%, 8%, 6%, 5%, 4%, 3%, 2% and 1% respectively. The first principal component was characterised by positive correlations with structural woody habitats (GWD and WDH) and deeper sections of the river (MDT) and negative correlations with longer distances to the bank (DNB and CHW) and higher surface velocities (VEL) (Table 3). The second principal component was characterised by positive correlations with deeper water (GMD and DEP) and higher surface water velocities (VEL) and negative correlations with larger distances to the low-flow channel (DMDT) and bed heterogeneity (CVD) (Table 3). A plot of the first and second component axes shows that fish were using only a subset of the available habitat (Fig. 1). This corresponded to habitat with deeper water that has high amounts of structural woody habitat close to the bank (Fig. 2).
|
|
|
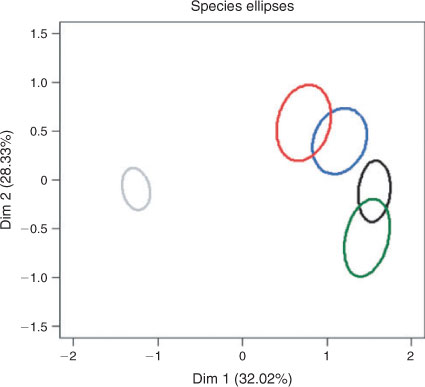
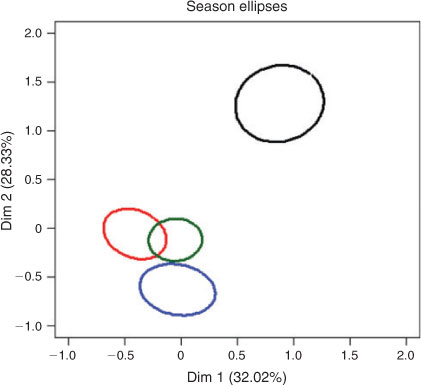
The variance range for the predictions of the intercept for the random effect in the GLMMs included zero for all individuals except for three trout cod and two golden perch individuals, and one Murray cod and one carp individual. There was support in the GLMMs for the inclusion of species effects on Principal component 1 and a season effects on Principal component 2 (Table 4). The correlations between Principal component 1 and carp, golden perch and Murray cod were positive and statistically significant and negative and statistically significant for available habitats (Table 5). The correlations between Principal component 2 and trout cod and Murray cod were positive and statistically significant and negative and statistically significant for carp (Table 5). The 95% confidence ellipse confirmed statistically significant differences between the habitats used by the four species and available habitat and differences among the species (Fig. 3). The habitats of Murray cod and trout cod were significantly different from the habitats of carp and golden perch (Fig. 3). Similarly, the 95% confidence ellipses demonstrated statistically significant differences between summer habitats and those of spring, autumn and winter (Fig. 4). A statistically significant difference was also detected between winter and spring and autumn habitats (Fig. 4).

|

|
|
|
Discussion
There is a need for detailed knowledge of the habitat requirements of riverine fishes, especially threatened species, for conservation management and habitat rehabilitation (Rosenfeld and Hatfield 2006; Cooke et al. 2012). Understanding habitat patch dynamics (Winemiller et al. 2010) can help predict priority areas (Filipe et al. 2002) that can cater for multiple species (Welcomme et al. 2006). The present study of adults of four large Australian freshwater fishes in the Murray River has provided quantification of their comparative daytime microhabitat use within the same river reach, under the same environmental conditions. Results showed that fish were not randomly distributed, but rather selected microhabitats that could be described by structural parameters. All species used only a subset of available habitats, generally selecting similar microhabitats with a strong association with structural wood. In addition, all species typically used habitats that, on average, were deeper, had lower surface water velocities and a heterogeneous river bed, and were closer to the river bank than were the habitats randomly available. This supports the contention that fish respond to habitat patchiness within rivers (Schlosser 1991) by selecting patches of suitable habitat (Inoue and Nakano 1998), often with broad overlap in habitat use among species (Gido and Propst 1999).
All species were positively associated with structural woody habitat, supporting individual observations reported by other individual studies, including adult, juvenile and Age-0 Murray cod in the nearby Ovens River and Lake Mulwala (Koehn 2009a) and trout cod (Growns et al. 2004; Nicol et al. 2007). Wood has also been shown to be an important habitat attribute for the closely related Mary River cod, Maccullochella mariensis (Simpson and Mapleston 2002). The observations of all of these Maccullochella species utilising structural woody habitats suggest that this may be an important habitat predictor for this genus where structural woody habitats are a natural feature of the rivers throughout their range. There was a strong positive association with both the height of this wood in the water column and its abundance (>68% cover). The occurrence of structural woody habitat in this reach is largely determined by trees falling from eroding banks, creating patches where the structural woody habitat load is high and areas where it is low (Koehn et al. 2004). Although the overall loads of structural woody habitat have been considered to be high in comparison with other reaches of the Murray River (Thoms et al. 2000), our results indicated that areas of high density are still relatively rare in this river reach (average 20% cover). Crook et al. (2001) reported a positive association between golden perch and structural woody habitat in pools, but an unexpected negative association with structural woody habitat at larger scales, possibly because of peculiarities with wood distribution at their study site. Murray cod, golden perch and carp were all associated with structural woody habitat more than any other mesohabitat type in the Barwon–Darling River system in Australia (Boys and Thoms 2006) and Murray cod was consistently associated with wood at all sites.
Structural woody habitat that extends higher into the water column is used more by golden perch and carp than by Murray cod and trout cod and this may be explained by differences in habits of these species. Murray cod is known to be largely demersal (Koehn 2009b), whereas golden perch and carp have been described as mid-water species (Lintermans 2007). These latter two species may use tree branches that extend up into the water column, an observation also made while collecting fish for the present study, whereas Murray cod and trout cod use the trunks and larger branches lying on the river bed. Branching complexity of structural woody habitat has been considered important in meeting the habitat needs of a range of fish species (Newbrey et al. 2005).
All species used habitats with greater bed heterogeneity than was generally available and it is likely that this is strongly associated with wood being present at the site. Structural woody habitat can alter the local hydraulics in rivers by deflecting the flow which in turn generates scour, ultimately resulting in pool and bar formation in large alluvial rivers (Lisle 1986; Abbe and Montgomery 1996). These scour pools can provide additional areas of zero or low velocities and, consequently, provide further shelter from river currents (Abbe and Montgomery 1996), while allowing fish to remain close to faster velocities for feeding (Lehtinen et al. 1997).
Although there was considerable overlap, there were also significant differences in habitat use by each species. The greatest similarity in habitat use was shown for Murray cod and trout cod, with golden perch and carp also using habitats more similar to those of each other. Trout cod used faster water, further from bank and closer to the low-flow channel than did Murray cod. These habitats were deeper, had higher surface water velocity and were further from the bank than were those used by golden perch and carp, with carp using slower water and habitats more distant from the low-flow channel than did golden perch. Carp has also been observed to inhabit off-stream waters with slow or zero velocity (Koehn et al. 2000; Stuart and Jones 2006a; J. Koehn, pers. obs.). Carp has been shown to have ecological characteristics different from those of many other Australian native species, including fish species of the Murray–Darling Basin; however, this mainly relates to their diet and feeding (Koehn 2004). The habitats used by carp in the present study were similar to those of the native species, in particular golden perch, but differed by generally having a slower flow and by being shallower and closer to the river bank. The similarity in habitat use between carp and golden perch was supported by observations of capture of both species from the same location when electrofishing during the present study. The differences in microhabitat use among species in our study were consistent with the hypothesis that resource partitioning facilitates the coexistence of species (van Snik Gray and Stauffer 1999).
It is recognised that the present study was limited to daylight hours because of the difficulties of tracking these fish in these habitats, and hence it was not possible to obtain equivalent night-time habitat data. Observations from the present and other studies (Crook et al. 2001; Thiem et al. 2008) have suggested that at least the three native species move more widely at night, using a greater range of habitats. Crook et al. (2001) reported golden perch and carp to use habitats with similar water velocities during the day, but that golden perch used higher-velocity areas at night. Nevertheless, the high levels of fidelity shown to these sites (Crook et al. 2001; Koehn et al. 2008, 2009) indicated their importance as home-habitat areas from which other local movements occur. The preference by trout cod for faster waters reflects the distribution of this species, which was once more widespread, particularly in higher-gradient upland streams (Trueman 2011). Interestingly, the re-establishment of trout cod populations has been limited in those smaller, upland habitats, whereas several populations have been established in lowland reaches (Koehn et al. 2013).
Seasonal differences in habitat use may be partly due to increased water velocities, river widths and depths from higher flows. In this case, this is largely due to constant high irrigation flows that occur during summer and autumn in this regulated river, rather than the natural winter–spring flooding (Close 1990). The impact of such changes on populations, distances moved and variations in habitat use by individual fish over time, comparison of habitat use between the sexes, and intra- and inter-specific variations in use by season, sex or fish size are all interesting questions for future research, but were beyond the scope of the present study.
Structural woody habitat may provide a range of benefits to fishes, including velocity refuges, territorial markers (Crook and Robertson 1999) and attachment sites for many invertebrates (O’Connor 1991) that may be used as prey (Ebner 2006; Baumgartner 2007). The accumulation of detritus in low-flow areas may attract the benthic-feeding carp to the associated invertebrate community (Malmqvist et al. 1978). By experimentally adding or removing structural woody habitats from stream sections, it has been shown that sections with structural woody habitats supported more and larger fish than did cleared sections (Angermeier and Karr 1984; Nagayama et al. 2012). Removal of structural woody habitats from the Murray River and other rivers in south-eastern Australia has been widespread (see Koehn et al. 2004), and this loss of fish habitat has often been cited as a reason for the decline of native fish populations (Cadwallader 1978). The identification of structural woody habitats as an important habitat for the three native fish species supports the reintroduction of structural woody habitat as a habitat-restoration measure to rehabilitate native fish populations (Murray–Darling Basin Commission 2004; Nicol et al. 2004).
Data from the present study can be used in the development of the best design and configuration of in-stream wood construction sites and to assist in predicting outcomes and setting measures for its success (Boavida et al. 2012). This is particularly relevant to the reconstruction of woody habitats (Nicol et al. 2004) as a recovery action for threatened species such as Murray cod and trout cod, especially where they may co-occur (Trout Cod Recovery Team 2008; National Murray Cod Recovery Team 2010). Although it is recognised that, the measures (Table 2) will not be absolutely applicable to other sites (e.g. because of differences in size and depths), the present paper has provided general guidance to assist such habitat reconstruction. For example, to benefit the native fishes, patches of structural woody habitat should have cover of >70%, be located within the 15% of the river channel (width) closest to the bank, in waters with surface velocities of 0.3–0.6 m s–1 and extend >1.5 m into the water column. Wood in faster areas may give trout cod a competitive advantage over Murray cod or carp. The study also highlighted the importance of substrate undulations that are caused by water scour associated with in-stream wood.
The present paper has described the comparative microhabitat use by four large-bodied fish species in the Murray River. Importantly, the study was conducted at the same temporal and spatial scale, allowing a ‘real time’ comparison. It showed that each species uses only a small proportion of the available habitats and indicated that the use was heavily influenced by the presence of structural woody habitat. It quantified the habitat variables used by each species and examined inter-specific differentiation. The use of habitat patches indicated that reconstructed habitats may be used by multiple native species, hence maximising environmental benefits. This can be used to increase the positive perceptions by both the public and managers of the beneficial outcomes from woody-habitat reconstruction as a rehabilitation action (Chin et al. 2012). The differences in habitat use shown can be used to design habitats that are more suited to the native species (e.g. comparatively faster waters) than for the introduced carp. The study has highlighted the importance of structural woody habitats for all species, hence supporting its reintroduction as a rehabilitation measure.
Acknowledgements
The authors thank all those who originally helped develop radio-tracking techniques: John McKenzie, Damien O’Mahony, Justin O’Connor and Bill O’Connor. We thank Peter Fairbrother, Des Harrington, John Mahoney, John McKenzie, Justin O’Connor, Damien O’Mahony, Russell (Gus) Strongman and others for technical and field support. Jo Potts and Dave Ramsey provided advice on statistical methods and Des Harrington and Ruth Lennie assisted with data collation. Comments on the draft manuscript were kindly provided by Jeremy Hindell, Dave Ramsey, Dave Crook and the anonymous reviewers. This research was funded by the Murray–Darling Basin Commission and the Department of Sustainability and Environment, Victoria. It was conducted in accordance with conditions outlined under Victorian Fisheries Scientific Permit No. FSP/CW/020(5), Flora and Fauna Guarantee Act Licence No. F/1994/003, NSW Fisheries Permit No. F93/158 and the University of Melbourne Animal Experimentation Ethics Committee Register No. 98089. This paper is dedicated to the late Dave Arnett (1949–2009) of Yarrawonga.
References
Abbe, T. B., and Montgomery, D. R. (1996). Large woody debris jams, channel hydraulics and habitat formation in large rivers. Regulated Rivers: Research and Management 12, 201–221.| Large woody debris jams, channel hydraulics and habitat formation in large rivers.Crossref | GoogleScholarGoogle Scholar |
Angermeier, P. L., and Karr, J. R. (1984). Relationships between woody debris and fish habitat in a small warmwater stream. Transactions of the American Fisheries Society 113, 716–726.
| Relationships between woody debris and fish habitat in a small warmwater stream.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Meaechlar, M., and Bolker, B. (2013). ‘Package lme4.’ Available at www.cran.r-project.org [Accessed 15 April 2013].
Baumgartner, L. J. (2007). Diet and feeding habits of predatory fishes upstream and downstream of a low-level weir. Journal of Fish Biology 70, 879–894.
| Diet and feeding habits of predatory fishes upstream and downstream of a low-level weir.Crossref | GoogleScholarGoogle Scholar |
Boavida, I., Santos, J. M., Cortes, R., Pinheiro, A., and Ferreira, M. T. (2012). Benchmarking river habitat improvement. River Research and Applications 28, 1768–1779.
| Benchmarking river habitat improvement.Crossref | GoogleScholarGoogle Scholar |
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H., and White, J. S. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology & Evolution 24, 127–135.
| Generalized linear mixed models: a practical guide for ecology and evolution.Crossref | GoogleScholarGoogle Scholar |
Boys, C. A., and Thoms, M. C. (2006). A large-scale, hierarchical approach for assessing habitat associations of fish assemblages in large dryland rivers. Hydrobiologia 572, 11–31.
| A large-scale, hierarchical approach for assessing habitat associations of fish assemblages in large dryland rivers.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, P. L. (1978). Some causes of the decline in range and abundance of native fish in the Murray–Darling River system. Proceedings of the Royal Society of Victoria 90, 211–224.
Chin, A., Laurence, L. R., Daniels, M. D., Wohl, E., Urban, M. A., Boyer, K. L., Butt, A., Piegay, H., and Gregory, K. J. (2012). The significance of perceptions and feedbacks for effectively managing wood in rivers. Rivers Research and Applications , .
| The significance of perceptions and feedbacks for effectively managing wood in rivers.Crossref | GoogleScholarGoogle Scholar |
Close, A. (1990). The impact of man on the natural flow. In ‘The Murray’. (Eds N. Mackay and D. Eastburn.) pp. 61–77. (Murray–Darling Basin Commission: Canberra.)
Cooke, S. J., Paukert, C., and Hogan, Z. (2012). Endangered river fish: factors hindering conservation and restoration. Endangered Species Research 17, 179–191.
| Endangered river fish: factors hindering conservation and restoration.Crossref | GoogleScholarGoogle Scholar |
Cowx, I. G., and Welcomme, R. L. (Eds) (1998). ‘Rehabilitation of Rivers for Fish.’ (Fishing News Books, Blackwell Science: Oxford, UK.)
Crook, D. A., and Robertson, A. I. (1999). Relationships between riverine fish and woody debris: implications for lowland rivers. Marine and Freshwater Research 50, 941–953.
| Relationships between riverine fish and woody debris: implications for lowland rivers.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Robertson, A. I., King, A. J., and Humphries, P. (2001). The influence of spatial scale and habitat arrangement on diel patterns of habitat use by two lowland river fishes. Oecologia 129, 525–533.
Davies, P. E., Harris, J. H., Hillman, T. J., and Walker, K. F. (2010). The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia. Marine and Freshwater Research 61, 764–777.
| The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFGrs7Y%3D&md5=a99a766e4786d007d65cf72ebff92a22CAS |
Dolloff, C. A., and Warren, M. L., Jr (2003). Fish relationships with large wood in streams. In ‘The Ecology and Management of Wood in World Rivers. American Fisheries Society Symposium 37, International Conference on Wood in World Rivers held at Oregon State University, Corvallis, Oregon 23–27 October 2000’. (Eds S. V. Gregory, K. L. Boyer, and A. M. Gurnell.) pp. 179–193. (American Fisheries Society: Bethesda, MD.)
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar | 16336747PubMed |
Ebner, B. (2006). Murray cod an apex predator in the Murray River, Australia. Ecology Freshwater Fish 15, 510–520.
| Murray cod an apex predator in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Fausch, K. D., Torgersen, C. E., Baxter, C. E., and Li, H. W. (2002). Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. Bioscience 52, 483–498.
| Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes.Crossref | GoogleScholarGoogle Scholar |
Filipe, A. F., Cowx, I. G., and Collares-Pereira, M. J. (2002). Spatial modelling of freshwater fish in semi-arid systems: a tool for conservation. River Research and Applications 18, 123–136.
| Spatial modelling of freshwater fish in semi-arid systems: a tool for conservation.Crossref | GoogleScholarGoogle Scholar |
Freeman, B. J., and Freeman, M. C. (1994). Habitat use by an endangered riverine fish and implications for species protection. Ecology Freshwater Fish 3, 49–58.
| Habitat use by an endangered riverine fish and implications for species protection.Crossref | GoogleScholarGoogle Scholar |
Gido, K. B., and Propst, D. L. (1999). Habitat use and association of native and nonnative fishes in the San Juan River, New Mexico and Utah. Copeia 1999, 321–332.
| Habitat use and association of native and nonnative fishes in the San Juan River, New Mexico and Utah.Crossref | GoogleScholarGoogle Scholar |
Grossman, G. D., and Freeman, M. C. (1987). Microhabitat use in a stream fish assemblage. Journal of Zoology 212, 151–176.
| Microhabitat use in a stream fish assemblage.Crossref | GoogleScholarGoogle Scholar |
Growns, I., Wooden, I., and Schiller, C. (2004). Use of instream woody habitat by trout cod Maccullochella macquariensis (Cuvier) in the Murrumbidgee River. Pacific Conservation Biology 10, 261–265.
Husson, F., Josse, J., Le, S., and Mazet, J. (2009). ‘FactoMineR: Multivariate Exploratory Data Analysis and Data Mining with R.’ Available at www.cran.r-project.org
Inoue, M., and Nakano, S. (1998). Effects of woody debris on the habitat of juvenile masou salmon (Oncorhynchus masou) in northern Japanese streams. Freshwater Biology 40, 1–16.
| Effects of woody debris on the habitat of juvenile masou salmon (Oncorhynchus masou) in northern Japanese streams.Crossref | GoogleScholarGoogle Scholar |
Jones, M. J., and Stuart, I. G. (2007). Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australia river. Ecology Freshwater Fish 16, 210–220.
King, A. J. (2004). Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. Journal of Fish Biology 65, 1582–1603.
| Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river.Crossref | GoogleScholarGoogle Scholar |
Knights, B. C., and Lasee, B. A. (1996). Effects of implanted transmitters on adult bluegills at two temperatures. Transactions of the American Fisheries Society 125, 440–449.
| Effects of implanted transmitters on adult bluegills at two temperatures.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2004). Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshwater Biology 49, 882–894.
| Carp (Cyprinus carpio) as a powerful invader in Australian waterways.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2009a). Multi-scale habitat selection by Murray cod (Maccullochella peelii peelii) in two lowland rivers. Journal of Fish Biology 75, 113–129.
| Multi-scale habitat selection by Murray cod (Maccullochella peelii peelii) in two lowland rivers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjnsVOrtg%3D%3D&md5=5f1a28930e42f5462216e23e44386708CAS | 20738486PubMed |
Koehn, J. D. (2009b). Using radio telemetry to evaluate depths inhabited by Murray cod Maccullochella peelii peelii. Marine and Freshwater Research 60, 317–320.
| Using radio telemetry to evaluate depths inhabited by Murray cod Maccullochella peelii peelii.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Harrington, D. J. (2005). Collection and distribution of early life stages the Murray cod (Maccullochella peelii peelii) in components of a regulated river system. Australian Journal of Zoology 53, 137–144.
| Collection and distribution of early life stages the Murray cod (Maccullochella peelii peelii) in components of a regulated river system.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Harrington, D. J. (2006). Conditions and timing of the spawning of Murray cod (Maccullochella peelii peellii) and the endangered trout cod (M. macquariensis) in regulated and unregulated rivers. River Research and Applications 22, 327–342.
| Conditions and timing of the spawning of Murray cod (Maccullochella peelii peellii) and the endangered trout cod (M. macquariensis) in regulated and unregulated rivers.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Lintermans, M. (2012). A strategy to rehabilitate fishes of the Murray–Darling Basin, south-eastern Australia. Endangered Species Research 16, 165–181.
| A strategy to rehabilitate fishes of the Murray–Darling Basin, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and O'Connor, W. G. (1990). ‘Biological Information for Management of Native Freshwater Fish in Victoria.’ (Government Printer: Melbourne.)
Koehn, J. D., and Todd, C. R. (2012). Balancing conservation and recreational fishery objectives for a threatened species, the Murray cod, Maccullochella peelii. Fisheries Management and Ecology 19, 410–425.
| Balancing conservation and recreational fishery objectives for a threatened species, the Murray cod, Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Brumley, A. R., and Gehrke, P. C. (2000). ‘Managing the Impacts of Carp.’ (Bureau of Resource Sciences: Canberra.)
Koehn, J. D., Nicol, S. J., and Fairbrother, P. S. (2004). Spatial arrangements and physical characteristics of structural woody habitat in a lowland river in south-eastern Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 14, 457–464.
| Spatial arrangements and physical characteristics of structural woody habitat in a lowland river in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Nicol, S. J., Leischke, J. A., Lyon, J. P., and Pomorin, K. (2008). Spatial ecology of an endangered native Australian percichthyid fish, the trout cod Maccullochella macquariensis. Endangered Species Research 4, 219–225.
| Spatial ecology of an endangered native Australian percichthyid fish, the trout cod Maccullochella macquariensis.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., McKenzie, J. A., O’Mahony, D. J., Nicol, S. J., O’Connor, J. P., and O’Connor, W. G. (2009). Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river. Ecology Freshwater Fish 18, 594–602.
| Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Eiler, J. H., McKenzie, J. A., and O’Connor, W. G. (2012). An improved method for obtaining fine-scale location of radio tags when tracking by boat. In ‘Proceedings of the 2nd International Symposium on Advances in Fish Tagging and Marking Technology, Auckland, New Zealand’. (Eds J. R., McKenzie, B. Parsons, A. C. Seitz, R. K. Kopf, M. G.., Mesa and Q. Phelps.) pp. 379–384. (American Fisheries Society Symposium 76, American Fisheries Society: Bethesda, MD.)
Koehn, J. D., Lintermans, M., Lyon, J. P., Ingram, B. A., Gilligan, D. M., Todd, C. R., and Douglas, J. W. (2013). Recovery of the endangered trout cod Maccullochella macquariensis: what have we achieved in over 25 years? Marine and Freshwater Research 64, 822–837.
| Recovery of the endangered trout cod Maccullochella macquariensis: what have we achieved in over 25 years?Crossref | GoogleScholarGoogle Scholar |
Kramer, D. L., Rangeley, R. W., and Chapman, L. J. (1997). Habitat selection: patterns of spatial distribution from behavioural decisions. In ‘Behavioural Ecology of Teleost Fishes’. (Ed J. J. Godin.) pp. 37–80. (Oxford University Press: Oxford, UK.)
Krebs, J. R., and Kacelnik, A. (1991). Decision-making. In ‘Behavioural Ecology: an Evolutionary Approach’. 3rd edn. (Eds J. D. Krebs and N. B. Davies.) pp. 105–136. (Blackwell Scientific Publications: Oxford, UK.)
Lehtinen, R. M., Mundahl, N. D., and Madejczyk, J. C. (1997). Autumn use of woody snags by fishes in backwater and channel border habitats of a large river. Environmental Biology of Fishes 49, 7–19.
| Autumn use of woody snags by fishes in backwater and channel border habitats of a large river.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: an Introductory Guide’. (Murray–Darling Basin Commission: Canberra.)
Lisle, T. E. (1986). Effects of woody debris on anadromous salmonid habitat, Prince of Wales Island, southeast Alaska. North American Journal of Fisheries Management 6, 538–550.
| Effects of woody debris on anadromous salmonid habitat, Prince of Wales Island, southeast Alaska.Crossref | GoogleScholarGoogle Scholar |
Mackay, N. (1990). Understanding the Murray. In ‘The Murray’. (Eds N. Mackay and D. Eastburn.) pp. ix–xii. (Murray–Darling Basin Commission: Canberra.)
Malmqvist, B. L., Nilsson, L. M., and Svesnsson, B. S. (1978). Dynamics of detritus in a small stream in southern Sweden and its influence on the distribution of the bottom animal communities. Oikos 31, 3–16.
| Dynamics of detritus in a small stream in southern Sweden and its influence on the distribution of the bottom animal communities.Crossref | GoogleScholarGoogle Scholar |
Matthews, W. J. (1998). ‘Patterns in Freshwater Fish Ecology.’ (Kluwer Academic Publishers: Norwell, MA.)
Minckley, W. L., Marsh, P. C., Deacon, J. E., Dowling, T. E., Hedrick, P. W., Matthews, W. J., and Mueller, G. (2003). A conservation plan for native fishes of the lower Colorado River. Bioscience 53, 219–234.
| A conservation plan for native fishes of the lower Colorado River.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Commission (2004). ‘Native Fish Strategy for the Murray–Darling Basin 2003–2013.’ (Murray–Darling Basin Commission: Canberra.) Available at www.mdbc.gov.au [Accessed 11 July 2008].
Nagayama, S., Nakamura, F., Kawaguchi, Y., and Nakano, D. (2012). Effects of configuration of instream wood on autumn and winter habitat use by fish in a large remeandering reach. Hydrobiologia 680, 159–170.
| Effects of configuration of instream wood on autumn and winter habitat use by fish in a large remeandering reach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFOht7bJ&md5=6c979c63e69162c35fda5b266e769b6fCAS |
Naiman, R. J., and Latterell, J. J. (2005). Principles for linking fish habitat to fisheries management and conservation. Journal of Fish Biology 67, 166–185.
| Principles for linking fish habitat to fisheries management and conservation.Crossref | GoogleScholarGoogle Scholar |
National Murray Cod Recovery Team (2010). ‘National Murray Cod Recovery Plan.’ (Victorian Government Department of Sustainability and Environment (DSE): Melbourne.)
Natsumeda, T. (1998). Size-assortative nest choice by the Japanese fluvial sculpin in the presence of male-male competition Journal of Fish Biology 53, 33–38.
Newbrey, M. G., Bozek, M. A., Jennings, M. A., and Cook, J. E. (2005). Branching complexity and morphological characteristics of coarse woody structure as lacustrine fish habitat. Canadian Journal of Fisheries and Aquatic Sciences 62, 2110–2123.
| Branching complexity and morphological characteristics of coarse woody structure as lacustrine fish habitat.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. J., Lieschke, J., Lyon, J., and Koehn, J. D. (2004). Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia. New Zealand Journal of Marine and Freshwater Research 38, 541–551.
| Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. J., Barker, R. J., Koehn, J. D., and Burgman, M. A. (2007). Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis Cuvier. Biological Conservation 138, 30–37.
| Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis Cuvier.Crossref | GoogleScholarGoogle Scholar |
O’Connor, N. A. (1991). The effects of habitat complexity on the macroinvertebrates colonising wood substrates in a lowland stream. Oecologia 85, 504–512.
| The effects of habitat complexity on the macroinvertebrates colonising wood substrates in a lowland stream.Crossref | GoogleScholarGoogle Scholar |
Oliva-Paterna, F. J., Minano, P. A., and Torralva, M. (2003). Habitat quality affects the condition of Barbus scalteri in Mediterranean semi-arid stream. Environmental Biology of Fishes 67, 13–22.
| Habitat quality affects the condition of Barbus scalteri in Mediterranean semi-arid stream.Crossref | GoogleScholarGoogle Scholar |
Peterson, J. T., and Rabeni, C. F. (2001). The relation of fish assemblages to channel units in an Ozark stream. Transactions of the American Fisheries Society 130, 911–926.
| The relation of fish assemblages to channel units in an Ozark stream.Crossref | GoogleScholarGoogle Scholar |
Quinn, G. G. P., and Keough, M. J. (2002). ‘Experimental Design and Data Analysis for Biologists.’ (Cambridge University Press: Cambridge, UK.)
R Development Core Team (2012). ‘R: a Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna.) Available at http://www.R-project.org [Accessed April 2013].
Rice, J. C. (2005). Understanding fish habitat ecology to achieve conservation. Journal of Fish Biology 67, 1–22.
| Understanding fish habitat ecology to achieve conservation.Crossref | GoogleScholarGoogle Scholar |
Rosenfeld, J. S., and Hatfield, T. (2006). Information needs for assessing critical habitats of freshwater fish. Canadian Journal of Fisheries and Aquatic Sciences 63, 683–698.
| Information needs for assessing critical habitats of freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Rutherfurd, I. (1990). Ancient river, young nation. In ‘The Murray’. (Eds N. Mackay D. Eastburn.) pp. 17–39. (Murray–Darling Basin Commission: Canberra.)
Schlosser, I. J. (1991). Stream fish ecology: a landscape perspective. Bioscience 41, 704–712.
| Stream fish ecology: a landscape perspective.Crossref | GoogleScholarGoogle Scholar |
Simpson, R. R., and Mapleston, A. J. (2002). Movements and habitat use of the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis. Environmental Biology of Fishes 65, 401–410.
| Movements and habitat use of the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Jones, M. J. (2006a). Movement of common carp, Cyprinus carpio, in a regulated lowland Australian river: implications for management. Fisheries Management and Ecology 13, 213–219.
| Movement of common carp, Cyprinus carpio, in a regulated lowland Australian river: implications for management.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Jones, M. J. (2006b). Large, regulated forest floodplain is an ideal recruitment zone for non-native common carp (Cyprinus carpio L). Marine and Freshwater Research 57, 337–347.
| Large, regulated forest floodplain is an ideal recruitment zone for non-native common carp (Cyprinus carpio L).Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Ebner, B. C., and Broadhurst, B. T. (2008). Diel activity of the endangered trout cod (Maccullochella macquariensis) in the Murrumbidgee River. Proceedings of the Linnean Society of New South Wales 129, 167–173.
Thoms, M., Suter, P., Roberts, J., Koehn, J., Jones, G., Hillman, T., and Close, A. (2000). ‘River Murray Scientific Panel on Environmental Flows – Dartmouth to Wellington and the Lower Darling River.’ (Murray–Darling Basin Commission: Canberra.)
Trout Cod Recovery Team (2008). ‘National Recovery Plan for Trout cod Maccullochella macquariensis.’ (Department of Sustainability and Environment: Victoria.)
Trueman, W. T. (2011). ‘True Tales of the Trout Cod: River Histories of the Murray–Darling Basin.’ (Murray–Darling Basin Authority: Canberra.) Available at www.environment.gov.au/biodiversity/threatened [Accessed 1 July 2012].
van Snik Gray, E., and Stauffer, J. R. (1999). Comparative microhabitat use of ecologically similar benthic fishes. Environmental Biology of Fishes 56, 443–453.
| Comparative microhabitat use of ecologically similar benthic fishes.Crossref | GoogleScholarGoogle Scholar |
Welcomme, R. L., Winemiller, K. O., and Cowx, I. G. (2006). Fish environmental guilds as a tool for assessment of ecological condition of rivers. River Research and Applications 22, 377–396.
| Fish environmental guilds as a tool for assessment of ecological condition of rivers.Crossref | GoogleScholarGoogle Scholar |
Wiens, J. A. (2002). Riverine landscapes: taking landscape ecology into the water. Freshwater Biology 47, 501–515.
| Riverine landscapes: taking landscape ecology into the water.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O., Flecker, A. S., and Hoeinghaus, D. J. (2010). Patch dynamics and environmental heterogeneity in lotic ecosystems. Journal of the North American Benthological Society 29, 84–99.
| Patch dynamics and environmental heterogeneity in lotic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Winter, J. D. (1983). Underwater biotelemetry. In ‘Fisheries Techniques’. (Eds L. A. Nielsen and D. L. Johnson.) pp. 371–395. (American Fisheries Society: Bethesda, MD.)



