Comparative larval development of three amphidromous Rhinogobius species, making reference to their habitat preferences and migration biology
Masashi Kondo A E , Ken Maeda B , Kentarou Hirashima C and Katsunori Tachihara DA Graduate School of Engineering and Science, University of the Ryukyus, 1 Senbaru, Nishihara, Okinawa 903-0213, Japan.
B Marine Genomics Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Okinawa 904-0495, Japan.
C Wakayama Prefectural Museum of Natural History, 370-1 Funao, Kainan, Wakayama 642-0001, Japan.
D Laboratory of Fisheries Biology and Coral Reef Studies, Faculty of Science, University of the Ryukyus, 1 Senbaru, Nishihara, Okinawa 903-0213, Japan.
E Corresponding author. Email: mimizu5b@yahoo.co.jp
Marine and Freshwater Research 64(3) 249-266 https://doi.org/10.1071/MF12234
Submitted: 28 August 2012 Accepted: 3 December 2012 Published: 18 March 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Eggs and larvae of three amphidromous species of Rhinogobius goby (Rhinogobius brunneus, Rhinogobius sp. MO and Rhinogobius sp. CB) from Okinawa Island, Japan, were reared under uniform conditions to describe and compare their larval development. Although the larval morphologies of the three species were very similar, some differences were observed in the timing of ontogenetic events among them. R. brunneus had the largest yolk and saved it for a longer period of time, whereas Rhinogobius sp. MO had the smallest yolk, which was exhausted earlier. The period until yolk exhaustion is thought to restrict the distance that migrating larvae can drift, which determines the specific adult distribution. Each of these two amphidromous species are close relatives of different fluvial resident species. Evolution of the fluvial residents could be explained by different scenarios based on the larval traits of R. brunneus and Rhinogobius sp. MO. Rhinogobius sp. CB hatched at a smaller size and grew slower than the other two species. No fluvial species have derived from Rhinogobius sp. CB. One possible explanation is that the smaller and slower-growing larvae of Rhinogobius sp. CB find it more difficult to remain within streams.
Additional keywords: egg size, Gobiidae, Gobioidei, insular stream, pool, Ryukyu Archipelago, speciation, waterfall.
Introduction
Rhinogobius Gill, 1859 is a very species-rich freshwater gobiid genus in river drainages, lakes and ponds along the continental coast from the Russian Far East to Thailand, and around islands in Japan, Taiwan and the Philippines, comprising more than 80 valid species (Oijen et al. 2011; Suzuki and Chen 2011). At least 15 distinct species are found in Japan, but the specific names of more than half of the species are unclear because of complications with their taxonomy, although recent studies have started to resolve these problems (Oijen et al. 2011; Suzuki and Chen 2011; Suzuki et al. 2011). Hereafter, the species that have not been given reliable specific names before Suzuki et al. (2011) are referred to by tentative names using two-letter codes defined by Akihito et al. (2002), Takahashi and Okazaki (2002) and Suzuki and Sakamoto (2005).
Highly diversified migration patterns are found among Rhinogobius species. For example, R. flumineus (Mizuno, 1960) is a fluvial direct development species that lacks a pelagic larval phase (Mizuno 1960). Rhinogobius sp. BB and Rhinogobius sp. YB are also fluvial species, although they spend a short period of their pelagic larval life in the middle or upper reaches of insular streams (Hirashima and Tachihara 2000). Rhinogobius sp. BW and Rhinogobius sp. TO are lentic species, the former being endemic to an ancient lake, Lake Biwa (Takahashi and Okazaki 2002), and the latter inhabiting ponds and creeks (Suzuki and Sakamoto 2005; Tsunagawa et al. 2010). Rhinogobius sp. CB and R. fluviatilis Tanaka, 1925 are amphidromous species; the adults inhabit and spawn in freshwater after spending their pelagic larval life in the sea, although they are sometimes land-locked where dams obstruct their migration (Tsunagawa and Arai 2008, 2011). R. giurinus (Rutter, 1897) also migrates between streams and the sea, but the adults are found both in freshwater and brackish areas in streams (Hirashima and Tachihara 2006; Inui et al. 2010). This variation in migration patterns has often been discussed with reference to the remarkable differences in egg size and developmental stage at hatching between three fluvial species and some of the amphidromous species; R. flumineus produces the largest eggs and its larvae are the most developed at hatching, whereas the eggs and larvae of Rhinogobius sp. BB and Rhinogobius sp. YB are also larger and more developed than those of amphidromous species (Nishida 1994, 2001). However, no study has compared early developmental stages among amphidromous species or discussed them with reference to migration biology, although egg and larval morphologies have been described for some amphidromous species (e.g. Sakai and Yasuda 1978; Yokoi and Hosoya 2006).
In the present study, the eggs and larvae of three amphidromous Rhinogobius species, R. brunneus (Temminck & Schlegel, 1845) (formerly Rhinogobius sp. DA; see Oijen et al. 2011), Rhinogobius sp. MO and Rhinogobius sp. CB, collected from Genka Stream on Okinawa Island, southern Japan, were reared under uniform conditions to describe their larval development and to compare the timing of major ontogenetic events among the species. Although the three subject species co-occur in Genka Stream with R. giurinus and Rhinogobius sp. BB, all five species have different in-stream distributions and habitat preferences. R. giurinus is the only species that inhabits brackish areas, and it also occurs in the lower reaches of the freshwater area. Rhinogobius sp. CB is mainly found in rapids, from the lower to the upper reaches. The remaining three species mainly inhabit pools or slower-flowing courses, but Rhinogobius sp. MO is found only in the middle reaches and R. brunneus and Rhinogobius sp. BB occur widely from the middle to the uppermost reaches (Hirashima and Tachihara 2006). Because R. giurinus adults are found in brackish water as well as freshwater, and Rhinogobius sp. BB is a fluvial species without a marine larval phase, only R. brunneus, Rhinogobius sp. MO and Rhinogobius sp. CB are amphidromous species that migrate between freshwater and the sea. Therefore, in the present study, we discuss the larval development of these three amphidromous Rhinogobius species, making reference to habitat preferences and migration biology.
Materials and methods
Egg masses of R. brunneus, Rhinogobius sp. MO and Rhinogobius sp. CB were collected from Genka Stream, Okinawa Island, on 2 March 2008. Genka Stream flows for 12.8 km into the East China Sea. It contains no barriers against migration, such as major waterfalls, within the main course; a weir and a sand-guard dam are located 1.2 and 6.0 km from the stream mouth, respectively, but both have functional fishways. Hirashima and Tachihara (2006) provided detailed distributions of Rhinogobius spp. in this stream. The R. brunneus egg mass that was used in the present study was collected from the upper reaches of the stream, at a site that was ~10 km from the stream mouth and at an altitude of ~65 m, whereas the egg masses of the other two species were collected from the middle reaches, ~2 km from the stream mouth and at an altitude of less than 5 m. All of the egg masses were formed of eggs that were densely attached in a single layer to the underside of stones and were accompanied by solitary males. An egg mass could be identified after the male was caught using a hand net and identified because the egg masses of most gobioid species are cared for by the male parent (Miller 1984). Because the larvae of Rhinogobius sp. MO and Rhinogobius sp. CB started hatching out from the collected clutches immediately after they were transferred into buckets, we brought those larvae to the laboratory to describe their larval development. We were able to take a subsample of Rhinogobius sp. CB eggs from the clutch and fix it in 5% formalin diluted with seawater; these eggs were used to describe the egg morphology of this species. Because all of the eggs within a reared clutch of Rhinogobius sp. MO had already hatched before we could take a subsample, additional eggs were collected from another clutch that was under the care of the same male parent and was in an earlier developmental stage to allow the description of egg morphology. An egg mass of R. brunneus was brought to the laboratory after a subsample of eggs was removed and fixed in 5% formalin diluted with seawater. The egg mass was incubated in a plastic case containing ~5 L of freshwater (19.0–19.4°C) and exposed to weak water flow, produced by aeration, until hatching. All eggs hatched by 5 days after egg-mass collection, although a portion of the eggs had hatched before this date, probably owing to stimulation during handling. Our unpublished observations on 16 clutches of the three Rhinogobius species showed that eggs in each clutch always hatched simultaneously during 3-h period by the 8th day after collection, except for a case in which a portion of the eggs hatched as a result of handling on the day of collection, and the remaining eggs hatched simultaneously 2 days after collection. Moreover, we have never observed in streams an egg mass with some eggs remaining unhatched after most eggs have hatched. Therefore, in the present study, we began to describe the larval development of R. brunneus at 5 days after collection, using the larvae that hatched on this date (Day 0) because all of the eggs should have hatched simultaneously on this date if they had been left to develop under parental care in the natural stream. The egg samples from the three species were observed and sketched under an optical microscope and a stereomicroscope. The lengths and widths of the eggs were measured under a stereomicroscope, using an ocular micrometer.
The 700 larvae were transferred into 30-L clear polycarbonate tanks by species and reared to study larval development. The salinity in all of the tanks was regulated at 18 because half seawater proved better for maintaining food supplies than did full seawater. Water temperature in the R. brunneus tank was kept at 19.4–21.0°C and the other two tanks were kept at 19.0–21.0°C. The room lights were left on for ~12 h to approximate the natural photoperiod. Feeding with the super-small (i.e. SS) rotifers (Brachionus rotundiformis) began immediately after hatching and the rotifers were maintained in the tanks by feeding them algae (Nannochloropsis oculata) three or four times per day. Nauplii of Artemia salina were provided together with the rotifers from 7 days after hatching and onward. One-tenth to one-fifth of the water was replaced from the bottom of each tank every day after 5 days post-hatching.
In all, 3–28 larvae or juveniles of each species were randomly collected from the tanks every day until Day 30 (for R. brunneus and Rhinogobius sp. MO) or Day 32 (for Rhinogobius sp. CB), except on 29 March (Day 22 for R. brunneus; Day 27 for the other two species). In addition, larvae or juveniles were collected on Days 35 and 40 for all species (Table 1). Collected larvae and juveniles were anaesthetised with 2-phenoxyethanol and placed in cold storage before fixation in 5% formalin diluted with seawater; they were then observed under an optical microscope and/or a stereomicroscope. The larvae were sketched whenever major ontogenetic changes were observed. Body length (BL = notochord length for pre-flexion and flexion larvae or standard length after completion of notochord flexion), total length (TL), yolk-sac diameter (YD), pre-anal length (PAL), head length (HL), snout length (SnL), pectoral fin length (P1L) and pelvic fin length (P2L) were measured for all fixed specimens with an ocular micrometer; their notochord tip was checked and classified into three notochord flexion stages (pre-flexion, flexion and post-flexion) and the occurrence of the hypural plate was also recorded. In addition, eye diameter (ED), body depth at the pectoral fin base (BD) and body width at the pectoral fin base (BW) were also measured using an ocular micrometer, and other characters, including fin development and pigmentation, were observed with at least five specimens per species on 0, 5, 10, 15, 20, 25, 30, 35 and 40 days after hatching (Table 1). The terminology used for developmental stages, morphological characters and measurements follows that of Leis and Carson-Ewart (2000). All observed specimens were preserved in 70% ethyl alcohol and deposited at the General Research Center in the Ocean Exposition Commemorative Park Management Foundation, Okinawa, Japan, and catalogued as URM-P 47065–47367 (this collection was transferred from the Faculty of Science, University of the Ryukyus, in 2011).
To compare BL among species, one-way factorial analysis of variance (ANOVA) was carried out on Days 0, 5, 10, 15, 20, 25, 30, 35 and 40. Multiple comparisons were conducted using Tukey’s HSD post hoc test to determine which species were significantly different. To compare the P2Ls among species, the same methods were used on Days 15, 20, 25, 30, 35 and 40, except for Rhinogobius sp. CB on Day 15, because its pelvic fins had not yet appeared. We estimated yolk exhaustion by applying Broken Stick regression, which is a non-asymptotic growth model. Broken Stick regression fits two linear models to the data. A breakpoint is determined as a point at which two lines are connected. In the present study (x = age, y = YD), a line for ages older than the breakpoint was determined using the equation y = 0 to represent YD after the yolk was completely exhausted, and the other line was expressed by the equation y = a + bx, which was fitted to the decrease in YD before the age of yolk exhaustion (determined as the breakpoint defined by x = –a/b). To measure the goodness of fit, the coefficient of determination (r2) was also calculated for the line before the breakpoint. The intercept and slope of the line were compared among the three species, by using a general linear model. The Broken Stick regression was fitted to YD at age data, using the least-squares method with the Solver function in Microsoft Excel 2007 and all other statistical analyses were carried using R version 2.13.1 (R Development Core Team 2011). Significant differences were determined at the 0.01 probability level.
Results
Rhinogobius brunneus
Egg morphology (Fig. 1a)

|
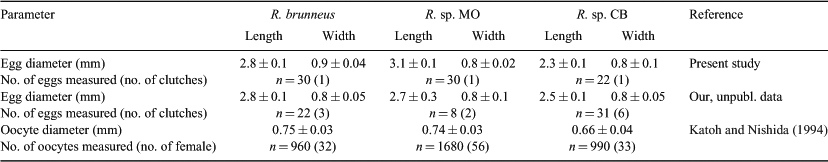
Eggs were elliptical and slightly narrowed just around the tip, measuring 2.5–3.0 (mean ± s.d. = 2.8 ± 0.1, n = 30) mm in length and 0.8–1.0 (0.9 ± 0.04) mm in width, with adhesive filaments at the micropylar end. The embryos had a yolk sac, partially pigmented eyes and melanophores dorsally and ventrally on the mid-tail, ventrally near the notochord tip and around the yolk and gut.

|
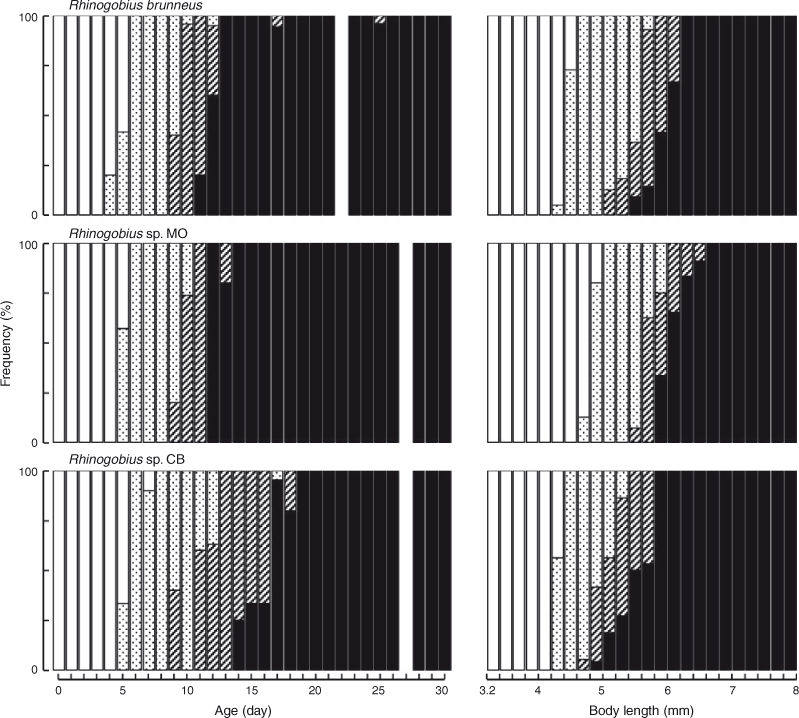
The BL of newly hatched larvae ranged from 3.4 to 4.0 (mean ± s.d. = 3.7 ± 0.2, n = 20) mm, and the TL ranged from 3.5 to 4.2 (3.8 ± 0.2) mm; BL exceeded 5.0 mm on Days 6–10 and exceeded 10.0 mm on Days 25–29 (Fig. 3). The body was elongated to moderate (BD = 13–21% of BL, n = 59) and initially compressed (BW = 48–65% of BD, n = 19), with 27 myomeres; the body began to thicken from Day 25 and became 83–100% of BD on Day 40 (n = 5). Newly hatched larvae had a yolk sac (YD = 0.3–0.5, mean ± s.d. = 0.4 ± 0.1 mm, n = 20) (Fig. 1b). The yolk was completely absorbed at 3.7–5.4-mm BL (Days 5–8) (Figs 1d, 4). Newly hatched larvae had pigmented eyes, a mouth and an opened anus (Fig. 1b). The rudiments of the hypural plates were formed at 4.3–4.7-mm BL (Days 4–6) (Figs 1c, d, 5). Notochord flexion began at 5.0–5.6-mm BL (Days 9–13) (Fig. 1e) and was completed at 5.5–6.2-mm BL (Days 11–25) (Figs 2a, 5). The gut was short to moderate (PAL = 42–62% of BL, n = 386). The head was small to large (HL = 16–30% of BL, n = 386). SnL was 3–9% of BL (n = 386). The eyes were small to large (ED = 21–48% of HL, n = 59).

|

|
Newly hatched larvae had pectoral fin buds (Fig. 1b); pectoral fin rays appeared at 8.1–9.5-mm BL (Days 20–25) (Fig. 2b) and all rays were visible at 9.8–10.0-mm BL (Days 25–30) (Fig. 2c). Pectoral fin rays were branched and segmented at 12.3–13.0-mm BL (Days 30–35) (Fig. 2c). P1L was initially 8–11% of BL (n = 20) and became longer (P1L = 21–25% of BL at Day 40, n = 9). Pelvic fin buds appeared at 6.4-mm BL (Days 15–20) (Fig. 2a); pelvic fin rays and a frenum appeared at 9.5-mm BL (Day 25), and all pelvic fin rays (one spine and five soft-rays) were visible at 10.9-mm BL (Day 30); the pelvic fin became a sucking disc at 11.4-mm BL (Days 30–35). The P2L became longer with growth (P2L = 16–17% of BL at Day 40, n = 9) (Fig. 6). The anlagen of the caudal fin rays appeared near the tip of the notochord at 5.7-mm BL (Day 10); caudal fin rays became parallel to the body-axis with notochord flexion (Fig. 2a) and were segmented at 5.7-mm BL (Day 10) and branched at 6.2-mm BL (Day 15). The second dorsal fin rays first appeared at 5.7-mm BL (Day 10) (Fig. 1e) and were segmented and branched at 6.2-mm BL (Day 15); all second dorsal fin rays were present at 6.2-mm BL (Day 15). The anal fin rays first appeared at 5.7-mm BL (Day 10) and were segmented at 5.8–5.9-mm BL (Days 10–15) and branched at 6.2-mm BL (Day 15); all anal fin rays were present at 5.7–6.2-mm BL (Days 10–15). The anlagen of the first dorsal fin appeared at 6.2-mm BL (Day 15); the first dorsal fin rays appeared at 7.0–8.1-mm BL (Days 20–25) (Fig. 2b); all first dorsal fin rays were present at 11.3–13.0-mm BL (Days 30–35). The dorsal and ventral finfolds disappeared at 6.8–7.2-mm BL (Days 20–30) (Fig. 2b).

|
Larvae initially had melanophores dorsally on the gas bladder and dorsally and ventrally on the mid-tail, 3–7 melanophores along the ventral mid-line of the gut and one melanophore dorsally on the hindgut, just above the anus (Fig. 1b). Some specimens had a small melanophore ventrally on the anterior part of the tail (4 of 19 specimens), on the angle of the lower jaw (5 of 19) and ventrally around the notochord tip (13 of 19). The frequency of occurrence of melanophore on the angle of the lower jaw increased with growth and all specimens had it by 5.7-mm BL (Day 10). The melanophores that were located ventrally on the tail increased and expanded with growth and came to cover the majority of the ventral mid-line on the tail after completion of notochord flexion. The small melanophore that was located ventrally around the notochord tip became melanophores along the caudal fin base after the completion of notochord flexion (Fig. 2a). Melanophores appeared along the caudal fin rays at 5.7-mm BL (Day 10). The ventral melanophores on the gut expanded anteriorly to the cleithral symphysis at 4.0–5.3-mm BL (Days 5–10); one melanophore on the cleithral symphysis, a Y-shaped melanophore around the pelvic fin base and 1–6 small melanophores located ventrally on the gut were arranged along the ventral mid-line of the head and trunk at 7.0-mm BL (Day 20). Melanophores that were located ventrally on the gut become invisible at 13.0-mm BL (Day 35). Melanophores that were located ventrally on the otolith cavity appeared at 5.7–6.2-mm BL (Days 10–15). Two specimens (6.2- and 6.7-mm BL, Day 15) had a melanophore between the otolith cavity and the gas bladder. Five specimens (3.8–4.1-mm BL, Days 0–5; 11.3- and 12.3-mm BL, Day 30) had a melanophore dorsally on the trunk. Melanophores occurred internally in the operculum (1 of 5), externally on the operculum (2 of 5), posteriorly on the upper jaw (2 of 5) and externally on pectoral fin base (1 of 5) on Day 30. Melanophores expanded over the body and head surface at 13.0-mm BL (Day 35); they appeared on the second dorsal, anal and pectoral fin rays at 13.0-mm BL (Day 35), and the first dorsal fin rays at 13.3-mm BL (Day 35).
Rhinogobius sp. MO
Egg morphology (Fig. 7a)

|
Eggs were elliptical and slightly narrowed just around the tip, measuring 2.8–3.3 (mean ± s.d. = 3.1 ± 0.1, n = 30) mm in length and 0.8–0.9 (0.8 ± 0.02) mm in width, with adhesive filaments at the micropylar end. The embryos had a yolk sac, pigmented eyes and melanophores dorsally on the gas bladder, dorsally and ventrally on the mid-tail, ventrally near the notochord tip and around the yolk and gut.

|
The BL of newly hatched larvae ranged from 3.3 to 4.1 (mean ± s.d. = 3.8 ± 0.2, n = 26) mm, and the TL ranged from 3.4 to 4.2 (4.0 ± 0.2) mm; BL exceeded 5.0 mm on Days 5–11 and exceeded 10.0 mm on Days 26–29 (Fig. 3). The body was elongated to moderate (BD = 13–21% of BL, n = 112) and initially compressed (BW = 52–74% of BD, n = 26), with 27 myomeres; the body began to thicken from Day 35 and became 81–89% of BD on Day 40 (n = 5). Newly hatched larvae had a yolk sac (YD = 0.2–0.5, mean ± s.d. = 0.3 ± 0.1 mm, n = 26) (Fig. 7b). The yolk was completely absorbed at 4.3–4.9-mm BL (Days 3–6) (Figs 4, 7c). Newly hatched larvae had pigmented eyes, a mouth and an opened anus (Fig. 7b). The rudiments of the hypural plates were formed at 4.7–5.0-mm BL (Days 5–6) (Figs 5, 7d). Notochord flexion began at 5.5–5.9-mm BL (Days 9–11) (Fig. 7e) and was completed at 5.9–6.5-mm BL (Days 12–14) (Figs 5, 8a). The gut was short to moderate (PAL = 44–60% of BL, n = 378). The head was small to large (HL = 15–29% of BL, n = 378). SnL was 2–8% of BL (n = 378). The eyes were small to large (ED = 22–55% of HL, n = 112).
Newly hatched larvae had pectoral fin buds (Fig. 7b); pectoral fin rays appeared at 8.5-mm BL (Days 20–25) (Fig. 8c) and all rays were visible at 8.5–10.4-mm BL (Days 20–30) (Fig. 8d). Pectoral fin rays were branched at 10.4-mm BL (Day 30) and were segmented at 12.1-mm BL (Day 35). The P1L was initially 6–12% of BL (n = 26) and became longer (P1L = 20–23% of BL on Day 40, n = 9). Pelvic fin buds appeared at 6.0–6.4-mm BL (Days 15–20); pelvic fin rays and a frenum appeared at 8.5-mm BL (Days 20–25), and all pelvic fin rays (one spine and five soft-rays) were visible at 8.5–9.3-mm BL (Days 25–30); the pelvic fin became a sucking disc at 10.4-mm BL (Day 30). The P2L became longer with growth (P2L = 16–19% of BL on Day 40, n = 11) (Fig. 6). The anlagen of the caudal fin rays appeared near the tip of the notochord at 5.2–5.5-mm BL (Days 5–15); caudal fin rays became parallel to the body-axis with notochord flexion (Fig. 8a) and were segmented at 5.7–6.2-mm BL (Days 10–15) and branched at 6.0–6.7-mm BL (Day 15) (Fig. 8a). The anlagen of the second dorsal fin appeared at 4.9–5.5-mm BL (Day 10); second dorsal fin rays first appeared at 5.5–5.8-mm BL (Days 10–15) (Fig. 7e) and were segmented and branched at 6.0–6.4-mm BL (Day 15) (Fig. 8a); all second dorsal fin rays were present at 6.0–6.4-mm BL (Day 15). The anlagen of the anal fin appeared at 4.9–5.5-mm BL (Day 10); anal fin rays first appeared at 5.5-mm BL (Day 10) (Fig. 7e) and were segmented and branched at 6.0–6.4-mm BL (Day 15); all anal fin rays were present at 6.0–6.4-mm BL (Day 15). The anlagen of the first dorsal fin appeared at 6.0–6.4-mm BL (Days 15–20) (Fig. 8b); the first dorsal fin rays appeared at 8.5–8.8-mm BL (Day 25); all first dorsal fin rays were present at 12.1-mm BL (Day 35). The dorsal and ventral finfolds disappeared at 7.2–7.4-mm BL (Days 20–25) (Fig. 8c).
Larvae initially had melanophores dorsally on the gas bladder and dorsally and ventrally on the mid-tail, two to five melanophores along the ventral mid-line of the gut and one melanophore dorsally on the hindgut, just above the anus (Fig. 7b). Some specimens had a small melanophore ventrally on the anterior part of the tail (4 of 26 specimens), on the angle of the lower jaw (3 of 26) and ventrally around the notochord tip (16 of 26). The frequency of occurrence of melanophore on the angle of the lower jaw increased with growth, and all specimens had it by 10.4-mm BL (Day 30). The melanophores that were located ventrally on the tail increased and expanded with growth and came to cover the majority of the ventral mid-line on the tail after completion of notochord flexion. The small melanophore that was located ventrally around the notochord tip became melanophores along the caudal fin base after the completion of notochord flexion (Fig. 8a). Melanophores appeared along the caudal fin rays at 6.0–8.0-mm BL (Days 15–20). The ventral melanophores on the gut expanded anteriorly to the cleithral symphysis at 4.0–5.3-mm BL (Days 5–10); one melanophore on the cleithral symphysis, a Y-shaped melanophore around the pelvic fin base and one to five small melanophores located ventrally on the gut were arranged along the ventral mid-line of the head and trunk at 8.2–8.5-mm BL (Days 20–25). Melanophores located ventrally on the gut became invisible at 12.1–12.5-mm BL (Days 35–40). Melanophores began to expand over the body and head surface at 12.1–13.1-mm BL (Days 35–40). They appeared on the second dorsal fin rays at 8.6–11.2-mm BL (Days 20–35), the anal fin rays at 7.3–11.2-mm BL (Days 15–35), the pectoral fin rays at 13.0-mm BL (Days 35–40) and the first dorsal fin rays at 12.7–13.1-mm BL (Day 40). One or two pairs of melanophores occurred dorsally on the head (2 of 5), internally in the operculum (1 of 5) and dorsally on the upper jaw (5 of 5) at Day 30.
Rhinogobius sp. CB
Egg morphology (Fig. 9a)

|
Eggs were elliptical and slightly narrowed just around the tip, measuring 2.1–2.7 (mean ± s.d. = 2.3 ± 0.1, n = 22) mm in length and 0.7–0.9 (0.8 ± 0.1) mm in width, with adhesive filaments at the micropylar end. The embryos had a yolk sac, pigmented eyes and melanophores located ventrally on the post-trunk, ventrally and dorsally on the mid-tail, dorsally on the gas bladder, ventrally near the notochord tip and around the yolk and gut.

|
The BL of newly hatched larvae ranged from 3.3 to 4.1 (mean ± s.d. = 3.6 ± 0.1, n = 23) mm, and the TL ranged from 3.4 to 4.2 (3.7 ± 0.1) mm; BL exceeded 5.0 mm on Days 10–31 and exceeded 10.0 mm on Days 30–40 (Fig. 3). The body was elongated (BD = 14–19% of BL, n = 45) and initially compressed (BW = 57–65% of BD, n = 5), with 27 myomeres; the body began to thicken from Day 25 and became 72–82% of BD on Day 40 (n = 5). Newly hatched larvae had a yolk sac (YD = 0.3–0.5, mean ± s.d. = 0.4 ± 0.04 mm, n = 23) (Fig. 9b). The yolk was completely absorbed at 3.3–4.7-mm BL (Days 5–7) (Figs 4, 9c). Newly hatched larvae had pigmented eyes, a mouth and an opened anus (Fig. 9b). The rudiments of the hypural plates were formed at 4.3–4.4-mm BL (Days 5–8) (Figs 5, 9c). Notochord flexion began at 4.8–5.5-mm BL (Days 9–18) (Fig. 9d) and was completed at 5.2–5.9-mm BL (Days 14–19) (Figs 5, 10a). The gut was short to moderate (PAL = 44–60% of BL, n = 392). The head was small to large (HL = 17–30% of BL, n = 392). SnL was 3–8% of BL (n = 392). The eyes were small to large (ED = 21–46% of HL, n = 45).
Newly hatched larvae had pectoral fin buds (Fig. 9b); pectoral fin rays appeared at 9.6-mm BL (Day 30) (Fig. 10d) and all rays had appeared and were branched at 10.5–10.9-mm BL (Day 35). The P1L was initially 8–10% of BL (n = 23) and became longer (P1L = 12–19% of BL at Day 40, n = 10). Pelvic fin buds appeared at 5.8–6.2-mm BL (Days 20–25) (Fig. 10b); pelvic fin rays and a frenum appeared at 8.5–8.8-mm BL (Days 20–25), with all pelvic fin rays (one spine and five soft-rays) visible at 8.5–8.8-mm BL (Days 20–30); the pelvic fin became a sucking disc at 10.5–10.9-mm BL (Day 35). The P2L became longer with growth (P2L = 9–15% of BL on Day 40, n = 10) (Fig. 6). The anlagen of the caudal fin rays appeared near the tip of the notochord at 4.6-mm BL (Day 10); caudal fin rays became parallel to the body-axis with notochord flexion and were segmented at 5.3–6.2-mm BL (Days 15–20) and branched at 6.5-mm BL (Day 20). The second dorsal fin rays first appeared at 5.0-mm BL (Day 15) (Fig. 9d) and were segmented and branched at 6.2-mm BL (Day 20). All second dorsal fin rays were present at 5.0–6.2-mm BL (Days 15–20). The anlagen of the anal fin appeared at 4.6–5.2-mm BL (Days 10–15); anal fin rays first appeared at 5.0–5.2-mm BL (Day 15) (Fig. 9d) and were segmented and branched at 6.2-mm BL (Day 20); all anal fin rays were present at 5.0-mm BL (Day 15). The anlagen of the first dorsal fin appeared at 6.2–8.5-mm BL (Days 20–25) (Fig. 10b); first dorsal fin rays appeared at 8.5–8.8-mm BL (Days 25–30); all first dorsal fin rays were present from 12.2-mm BL (Day 40). The dorsal and ventral finfolds disappeared at 7.0–7.4-mm BL (Days 20–35) (Fig. 10c).
Larvae initially had melanophores dorsally on the gas bladder, dorsally and ventrally on the mid-tail, three to seven melanophores along the ventral mid-line of the gut and one melanophore dorsally on the hindgut just above the anus (Fig. 9b). Some specimens had a small melanophore ventrally on the anterior part of the tail (4 of 5 specimens) and ventrally around the notochord tip (4 of 5). The frequency of occurrence of the melanophore on the angle of the lower jaw increased with growth, and all specimens had it by 4.6-mm BL (Day 15). The melanophores that were located ventrally on the tail increased and expanded with growth and came to cover the majority of the ventral mid-line on the tail after the completion of notochord flexion. The small melanophore that was located ventrally around the notochord tip became melanophores along the caudal fin base after the completion of notochord flexion. Melanophores appeared along the caudal fin rays at 5.0–5.2-mm BL (Day 15). The ventral melanophores on the gut expanded anteriorly to the cleithral symphysis at 4.0–4.6-mm BL (Days 5–10); one melanophore on the cleithral symphysis, a Y-shaped melanophore around the pelvic fin base and one to seven small melanophores located ventrally on the gut were arranged along the ventral mid-line of the head and trunk at 8.8-mm BL (Days 25–30). Melanophores located ventrally on the otolith appeared at 5.2–6.2-mm BL (Days 15–20). One specimen (7.6-mm BL, Day 20) had an internal melanophore between the otolith cavity and the gas bladder. An internal melanophore anterior of the proximal part of the caudal fin appeared at 9.8-mm BL, Days 30–35). Melanophores began to expand posteriorly on the body surface at 12.2-mm BL (Day 40); they appeared on the second dorsal fin rays at 10.8–11.9-mm BL (Days 35–40), the anal fin rays at 10.7–11.6-mm BL (Days 30–40) and the first dorsal fin rays at 12.2-mm BL (Day 40). Some of the specimens had melanophores on the lower jaw (6 of 20 specimens in Days 25–40, 8.5–12.3-mm BL) and the upper jaw (3 of 10 specimens in Days 35–40, 11.6–12.3-mm BL).
Behaviour
Some rotifers were observed in the gut of Day-1 larvae of all three species before the yolk sac had been completely absorbed. The larvae showed positive phototaxis and swam against the slow water circulation caused by the tank aeration from just after hatching. The first R. brunneus and Rhinogobius sp. MO individuals at the bottom of the tank were observed on Day 24 and the first individuals of Rhinogobius sp. CB were observed on Day 29. At that time, their pelvic fins had not formed a complete sucker and the larvae were easily moved, even by a gentle current, and still usually swam in the water column. They could completely settle at the bottom with functional sucker-like pelvic disc on Days 30, 29 and 32 for R. brunneus, Rhinogobius sp. MO and Rhinogobius sp. CB, respectively. Within 5 days after this point, almost all of the fish from all three species had settled at the bottom.
Comparison of growth among the three species
The mean BL of Rhinogobius sp. CB was the smallest at every age (Fig. 3). The mean BL of R. brunneus was almost always smaller than that of Rhinogobius sp. MO until about Day 20, after which the former became larger than the latter (Fig. 3). BL was significantly different among the species at all tested ages (one-way ANOVA; Table 2). The results of Tukey’s HSD post hoc tests (Table 2) showed that the BLs of Rhinogobius sp. MO (at Days 0 and 5), Rhinogobius sp. CB (at Days 10, 15, 25 and 30) or all three species (at Days 20, 35 and 40) were significantly different. Rhinogobius sp. MO was larger than the other two species before Day 10; however, the difference in BL between Rhinogobius sp. MO and R. brunneus was non-significant for Days 10–30, with R. brunneus then becoming larger after settlement. Rhinogobius sp. CB was significantly smaller than Rhinogobius sp. MO at all ages and was also smaller than R. brunneus from Day 10.

|
Mean YD was the smallest in Rhinogobius sp. MO at every age and the value for R. brunneus was the largest at every age, except on Day 0 when the mean YD of Rhinogobius sp. CB was slightly larger (Fig. 4). Rhinogobius sp. MO exhausted their yolk at the youngest age (Day 4.4; breakpoint of the Broken Stick model; Fig. 4), followed by Rhinogobius sp. CB (Day 5.9; Fig. 4). R. brunneus exhausted its yolk at the oldest age (Day 7.2; Fig. 4). The coefficients of determination (r2) for the linear regression analyses were 0.88 for R. brunneus, 0.72 for Rhinogobius sp. MO and 0.84 for Rhinogobius sp. CB (Table 3). Linear models for the three species had significantly different intercepts, which represent YD at hatching, and the slope differed between R. brunneus and Rhinogobius sp. MO (Table 4).

|

|
The hypural plates of R. brunneus always started forming while some yolk remained (Fig. 1c). The plates of Rhinogobius sp. CB started forming at a similar age and BL; however, some of the larvae had already exhausted their yolk by that time (Figs 5, 9c). In contrast, the hypural plates of Rhinogobius sp. MO started forming at a slightly larger BL and always after the yolk was completely exhausted (Figs 5, 7d). Notochord flexion in Rhinogobius sp. CB occurred at an older age and a smaller BL than in the other two species (Fig. 5).
The pelvic fin of Rhinogobius sp. CB occurred at an older age (Day 17) than in R. brunneus and Rhinogobius sp. MO (Days 12 and 14, respectively). Although the proportion of P2L in BL increased with age and size in all three species, the P2L of Rhinogobius sp. CB developed more slowly than that in the other two species (Fig. 6). P2L differed significantly among species on Days 20, 25, 30, 35 and 40 (one-way ANOVA; Table 5). Tukey’s HSD post hoc tests indicated that P2L did not differ significantly between R. brunneus and Rhinogobius sp. MO, except on Day 25, whereas that of Rhinogobius sp. CB differed significantly (i.e. was smaller) from those of the other two species at all tested ages (Table 5).

|
Discussion
Because the larval morphologies of the three Rhinogobius species described in the present study were very similar to each other, we could find no definite morphological characteristics that could be used to identify wild larvae of unknown ages. However, the comparison showed some differences in the timing of ontogenetic events among the three species. In the present study, we compared the early development of three species, using one clutch per species. Because intraspecific variations in egg size have been shown for some Rhinogobius species (e.g. Tamada 2009), the timing of ontogenetic events may exhibit some variation. However, some additional information has indicated that interspecific trends in the egg sizes of the three species generally correspond with the results of the present study; i.e. the eggs of R. brunneus and Rhinogobius sp. MO are similar in size, whereas those of both of them are larger than those of Rhinogobius sp. CB (Table 6). Therefore, we consider that we can discuss differences in the early development of the three species on the basis of the results of the present study, although a study using multiple clutches reared simultaneously would provide more precise information. The three species co-occur in Genka Stream, but they show specific in-stream distributions and habitat preferences (Hirashima and Tachihara 2006). How is their early development influenced by differences in their distributions and habitats? What can we conclude about the diversification of this genus on the basis of our current knowledge of the early development of these species?
|
First, we discuss our results in relation to downstream migration. Hatched larvae of amphidromous fishes start to migrate downstream to the sea, where they grow as pelagic larvae, before returning to freshwater for further growth and reproduction (Myers 1949; McDowall 1997). Indeed, abundant newly hatched drifting Rhinogobius larvae are collected in plankton nets set along the course of the stream on Okinawa Island, and these larvae occur predominantly during the hours after dusk (Maeda and Tachihara 2010; Yamasaki et al. 2011). Although adults of several amphidromous fish species, such as ayu (Plecoglossus altivelis altivelis), common whitebait (Galaxias maculatus), river sculpin (Cottus hangiongensis) and the gobies (Awaous guamensis and Luciogobius ryukyuensis), migrate downstream along the courses of rivers or streams to spawn (Benzie 1968; Goto 1986, 1988; Ha and Kinzie 1996; Iguchi et al. 1998; Kondo et al. 2012), such pre-spawning migrations are not known to occur in the three Rhinogobius species studied here. In addition to our unpublished observations that the egg masses of the three species are commonly found in their usual adult habitats, the fact that the spawned egg mass of R. brunneus was found in the upper reaches of Genka Stream suggests that Rhinogobius species spawn in their usual adult habitats without undergoing pre-spawning migrations. Therefore, the larvae of species that inhabit the upper reaches must drift longer distances to reach the sea. Downstream migration over longer distances increases the risk of starvation for drifting larvae, before they arrive at the sea where they can access rich food resources (Iguchi and Mizuno 1999; McDowall 2009). The results of the present study have indicated that R. brunneus, which inhabits the upper reaches among the three species, had the largest yolk and saved its yolk for the longest period of time, which may help reduce the risk of starvation during migration. Moriyama et al. (1998) suggested that the estimated ages of drifting Rhinogobius larvae collected from short and steep streams (0.5–12.0 km long) in Shikoku, Japan, varied from 0 to 7 days after hatching. Although the larvae of R. brunneus on Okinawa Island seem able to finish their drifting migration before they have consumed all of the yolk, because streams on this island are generally short (e.g. Genka Stream is 12.8 km), larvae hatched in the upper reaches still incur some starvation risk. McDowall (2009) concluded that ‘early hatch’ is one of the tactics that can be used to minimise starvation risk in amphidromous fish larvae. Despite the fact that the larvae of R. brunneus were not as undeveloped as those of Sicydiinae, Awaous, Stenogobius and Eleotris spp. (e.g. Maeda et al. 2008; Yamasaki et al. 2011), they are thought to be adapted to longer downstream migrations by having a somewhat ‘early hatch’ and a larger yolk. Rhinogobius sp. CB is also distributed in the upper reaches of the stream as well as in the lower reaches (Hirashima and Tachihara 2006), and its larvae also have larger yolks and keep their yolk for a longer period than does Rhinogobius sp. MO, which is not found in the upper reaches. Rhinogobius sp. MO is believed to not actively ascend to the upper reaches of the stream, partially because it is restricted by the shorter period before yolk exhaustion.
According to the observation by Oshiro and Nishizima (1978), after arriving at the sea, amphidromous Rhinogobius larvae grow in schools near the bottom of the bay off the stream mouth in the daytime, although they are distributed throughout the water column in the bay near the shoreline at night. Oshiro and Nishizima (1978) also observed that some of the larger larvae settled at the bottom of the bay at night. After their marine phase, the larvae recruit into the stream and shift to a benthic lifestyle. According to our unpublished observations from tidally influenced areas of streams on Okinawa Island, newly recruited Rhinogobius larvae ascend to the freshwater area along the bank of the stream, swimming in the water column and sometimes settling at the bottom or on bank vegetation. The larvae of R. brunneus, Rhinogobius sp. MO and Rhinogobius sp. CB reared in the present study started settling at the bottom on Days 24, 24 and 29, respectively, and had completely settled with a sucker-like pelvic disc on Days 30, 29 and 32, respectively. Thus, the marine larval duration of R. brunneus and Rhinogobius sp. MO appears to be slightly shorter than 1 month, whereas the period for Rhinogobius sp. CB is about 1 month under the conditions that were provided during study because the larvae are thought to recruit into streams just before or after their first settlement and to migrate upstream while developing their settling ability, specifically the development of the sucker-like pelvic disc. In the present study, we also found that the pelvic fin of Rhinogobius sp. CB developed more slowly than that in the other two species, together with the slower somatic growth of this species. The P2L of Rhinogobius sp. CB was only half as large as that of the other two species on Day 35, which corresponds to the recruitment phase under wild conditions. The sucker-like pelvic fin is required for individuals to ascend steep courses against strong water flows. Because Rhinogobius sp. CB prefers to inhabit rapids (Hirashima and Tachihara 2006), the slower development of its pelvic fin seems to conflict with its habitat preference. However, this could be explained by the species-specific timing of upstream migration; active upstream movement may begin later in Rhinogobius sp. CB than in the other two species.
Two fluvial species, Rhinogobius sp. YB and Rhinogobius sp. BB, inhabit streams on Okinawa Island. They are generally categorised as ‘medium-egg type’ on the basis of their intermediate egg size (4.3 mm in length for both species; Hirashima and Tachihara 2000) between the smaller eggs of amphidromous species (e.g. 2.1–3.3 mm; present study) and the larger egg of fluvial R. flumineus (6.0–6.5 mm; Mizuno 1961) (Fig. 11). Genetic studies have indicated that these two fluvial species of medium-egg type evolved from different amphidromous species; Rhinogobius sp. YB was derived from a common ancestor with R. brunneus, whereas Rhinogobius sp. BB was derived from a common ancestor with Rhinogobius sp. MO (Katoh and Nishida 1994; Nishida 1994). Nishida (2001) proposed the following model to explain the speciation of these two species of medium-egg type. Streams in the Ryukyu Archipelago often have major waterfalls. Because most of these waterfalls are located along the coastal terrace scarps of the islands where large-scale diastrophisms have occurred, and numerous waterfalls at the current reef slopes are likely to have existed when the coastlines of the islands regressed during the glacial period, these waterfalls should have appeared and disappeared by upheaval and subsidence or global sea-level changes throughout geologic time. For example, when a new waterfall formed within the course of a stream at a former reef slope because of regression, the habitat of an amphidromous Rhinogobius species within this stream could have been divided into the upper and lower reaches by the waterfall (Fig. 12). If this waterfall was so high that it prevented individuals from climbing, the egg size of the population that lived above the waterfall would become enlarged through natural selection for larger and more developed larvae that could reliably stay in the upper reaches; the upper population would eventually evolve to become a species of medium-egg type. The model of Nishida (2001) could explain the speciation of one of the two species of medium-egg type, namely Rhinogobius sp. YB; however, it cannot explain the speciation of the other species (Rhinogobius sp. BB) because it does not refer to an important difference between the two species of medium-egg type. The difference is that Rhinogobius sp. BB usually inhabits streams that lack major waterfalls, in contrast to Rhinogobius sp. YB, which is usually restricted to reaches above major waterfalls and does not co-occur with any congeners (Tachihara 2009). In fact, Genka Stream, where the present study was conducted, does not have a major waterfall and Rhinogobius sp. BB is distributed widely, from the middle to the uppermost reaches, together with R. brunneus. These two species co-exist, even with Rhinogobius sp. MO and Rhinogobius sp. CB in the middle reaches (Hirashima and Tachihara 2006).

|

|
The differences in the timing of ontogenetic events between R. brunneus and Rhinogobius sp. MO described in the present study may provide a clue to understanding the speciation of species of medium-egg type, including both Rhinogobius sp. YB and Rhinogobius sp. BB. In contrast to R. brunneus, which can delay the exhaustion of its yolk for long-distance drifting, the early life strategy of Rhinogobius sp. MO is characterised by faster growth with quick yolk absorption, which increases the risks from long-distance drifting. Although Rhinogobius sp. YB and Rhinogobius sp. BB have similar egg sizes, these two species have significant differences in larval development. Newly hatched larvae of Rhinogobius sp. YB are larger than those of the amphidromous species, and upward flexion of their notochord does not start before hatching (Fig. 11). They absorb their larger yolk over 7 days after hatching and the pelagic larvae grow in the reaches above major waterfalls for 21–30 days (Hirashima and Tachihara 2000). Although the swimming ability of their pre-flexion and flexion larvae should be lower, they can survive because reaches above major waterfalls have few predators and competitors. This lower predation risk also permits a longer pelagic larval life. In contrast, notochord flexion in the larvae of Rhinogobius sp. BB starts before hatching (Fig. 11). They consume their yolk over only 3 days after hatching and have a shorter pelagic larval life (11–13 days) (Hirashima and Tachihara 2000). They are thought to develop further in their egg capsule under parental care so that they have better swimming abilities and grow faster after hatching, to shorten their relatively risky larval life because the larval habitat of Rhinogobius sp. BB is pools in middle to upper reaches of streams, where diadromous predators such as Kuhlia marginata and Tridentiger kuroiwae occur. It would be important for the survival of the pelagic larvae of both Rhinogobius sp. YB and Rhinogobius sp. BB that few cyprinid predators occur in Okinawan streams, whereas abundant cyprinids usually occupy rivers on the Japanese mainland, which are inhabited by a direct-development species, R. flumineus, instead of the species of medium-egg type.
On the basis of the nature of R. brunneus, the common ancestor of R. brunneus and Rhinogobius sp. YB is thought to have been amphidromous and it may have tended to conserve its yolk for longer periods and ascend streams to the uppermost reaches. Therefore, many opportunities would have occurred for the upper part of their habitat to become separated from lower reaches because of waterfalls. As Nishida (2001) proposed, egg size would have increased above the waterfall through natural selection for larger larvae with larger yolks that allow the larvae to reliably remain there, and the population would have evolved as a species of medium-egg type, Rhinogobius sp. YB. Their slower growth may not have represented a serious disadvantage under conditions with lower predation risks. In other words, the local populations would have been extinguished if the waterfall that prevented the invasion of other species had disappeared.
The amphidromous common ancestor of Rhinogobius sp. MO and Rhinogobius sp. BB is thought to have inhabited the middle reaches of streams. They could grow faster with quick yolk absorption, although this would make reproducing successfully in upper reaches more difficult. Rhinogobius sp. BB tends to be found in relatively longer and gentler streams. Because the sea around the mouth of Genka Stream is shallow, a longer stream may have existed when the coastline of the island had regressed during the glacial period. The ancestors of Rhinogobius sp. BB inhabited such long and gentle streams and would sometimes have ascended to relatively upper reaches and spawned there. Although the success of the downstream migrations of the species would have been impaired, the fish would have occasionally remained in the stream and successfully grown into juveniles, without experiencing marine life. Larvae from larger eggs would have had a higher potential to survive. We propose the following new hypothesis to explain the mechanism for the speciation of Rhinogobius sp. MO and Rhinogobius sp. BB. A large pool had existed in an upper reach that was within the range of their ancestral species (Fig. 13). Larvae from relatively larger eggs among the population had a chance to remain and grow in the pool. Even if a few individuals recruited to the pool after undergoing a pelagic marine phase, the egg size would have increased because it was difficult for recruits from the sea (i.e. from smaller eggs) to contribute to successful reproduction, owing to the great distance between the sea and the pool. After the opportunity for recruitment from the sea decreased as a result of further regression, which significantly extended the migration distance, land-locked population in the pool and amphidromous population inhabit in the lower reaches developed reproductive isolation. Consequently, Rhinogobius sp. BB speciated and expanded its range into upper and lower reaches and even into other tributaries after its eggs became large enough to survive outside of the large pool. The current distribution was formed after that transgression. The expected faster growth of its ancestor may have helped the species to be retained within the stream.
Rhinogobius sp. BB may also have evolved above the waterfall. In that case, however, the waterfall that caused its evolution should have been located in the lower reaches such as at the current reef slope during the regression, rather than in the upper reaches where the evolution of Rhonogobius sp. YB took place. Unlike Rhinogobius sp. YB, Rhinogobius sp. BB could survive after the waterfall disappeared because it hatches at a more developed stage with better swimming ability and settles after a shorter pelagic life. Because the waterfall, rather than the long distance, functioned as a barrier, the story is regarded as a variation of the above hypothesis. In any case, the evolution of these two fluvial species should be explained by different scenarios based on the different larval traits of R. brunneus and Rhinogobius sp. MO.
Rhinogobius sp. CB hatched at a smaller size and grew slower than did R. brunneus and Rhinogobius sp. MO. No fluvial species has been derived from Rhinogobius sp. CB, unlike R. brunneus and Rhinogobius sp. MO. This may be the result of the greater difficulty that the smaller, slower-growing larvae of Rhinogobius sp. CB had to face to stay within streams.
In the present study, we proposed a hypothesis to explain the speciation of two fluvial species on the basis of a comparison of larval development among three amphidromous species. To further develop the discussion, the current distributions of these species and their genetic structure need to be studied and we need to improve our knowledge of palaeogeography. We do not have precise data on their distributions yet because the fluvial species have often been misidentified in previous reports and they sometimes inhabit inaccessible military bases. On Okinawa Island, some populations of fluvial Rhinogobius species have already disappeared because of dam construction, and other populations are threatened by planned damming activities (Tachihara 2009). The original distributions of the fluvial species are also difficult to understand because they could have colonised different streams through artificial water tunnels that connect some of the reservoirs in the northern part of Okinawa Island (Tachihara 2009). To understand the mechanism of evolution in this diversified genus, precise surveys of the distributions of these fluvial species are urgently required. Studies on the early development of this genus are also needed. In comparison with the higher variation in life history that has been observed in this genus, available descriptions of early development are insufficient. If early development is studied in more species, including lentic species and populations along the continental coast, Taiwan, Hainan and the Philippines, our hypothesis can be verified in the future.
Acknowledgements
This study was partially supported by the 21st century COE program of the University of the Ryukyus. We are grateful to the members of the Laboratory of Fisheries Biology and Coral Reef Studies, University of the Ryukyus, who supported our surveys and provided advice on our manuscript, especially N. Ogata, T. Ishikawa, M. Kawahira, H. Hirasaka, N. Yamasaki-Hanahara and M. Iida.
References
Akihito, S. K., Ikeda, Y., and Sugiyama, K. (2002). Rhinogobius. In ‘Fishes of Japan with Pictorial Keys to the Species’. English edn. (Ed. T. Nakabo.) pp. 1251–1255. (Tokai University Press: Tokyo.)Benzie, V. (1968). Some ecological aspects of the spawning behaviour and early development of the common whitebait, Galaxias maculatus attenuatus (Jenyns). Proceedings of the New Zealand Ecological Society 15, 31–39.
Goto, A. (1986). Movement and population size of the river sculpin Cottus hangiongensis in the Daitobetsu River of southern Hokkaido. Japanese Journal of Ichthyology 32, 421–430.
Goto, A. (1988). Reproductive behavior and homing after downstream spawning migration in the river sculpin, Cottus hangiongensis. Japanese Journal of Ichthyology 34, 488–496.
Ha, P. Y., and Kinzie, R. A. (1996). Reproductive biology of Awaous guamensis, an amphidromous Hawaiian goby. Environmental Biology of Fishes 45, 383–396.
| Reproductive biology of Awaous guamensis, an amphidromous Hawaiian goby.Crossref | GoogleScholarGoogle Scholar |
Hirashima, K., and Tachihara, K. (2000). Embryonic development and morphological changes in larvae and juveniles of two land-locked gobies, Rhinogobius spp. (Gobiidae), on Okinawa Island. Japanese Journal of Ichthyology 47, 29–41..
Hirashima, K., and Tachihara, K. (2006). Longitudinal distribution and dietary habits of the genus Rhinogobius in the Genka River of northern Okinawa Island, Japan. Japanese Journal of Ichthyology 53, 71–76..
Iguchi, K., and Mizuno, N. (1999). Early starvation limits survival in amphidromous fishes. Journal of Fish Biology 54, 705–712.
| Early starvation limits survival in amphidromous fishes.Crossref | GoogleScholarGoogle Scholar |
Iguchi, K., Ito, F., Yamaguchi, M., and Matsubara, N. (1998). Spawning downstream migration of ayu in the Chikuma River. Bulletin of the National Research Institute of Fisheries Science 11, 75–84..
Inui, R., Onikura, N., Kawagishi, M., Nakatani, M., Tomiyama, Y., and Oikawa, S. (2010). Selection of spawning habitat by several gobiid fishes in the subtidal zone of a small temperate estuary. Fisheries Science 76, 83–91.
| Selection of spawning habitat by several gobiid fishes in the subtidal zone of a small temperate estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1eqsg%3D%3D&md5=7fa274e66e3002f308ecb15a2a78561dCAS |
Katoh, M., and Nishida, M. (1994). Biochemical and egg size evolution of freshwater fishes in the Rhinogobius brunneus complex (Pisces, Gobiidae) in Okinawa, Japan. Biological Journal of the Linnean Society 51, 325–335.
| Biochemical and egg size evolution of freshwater fishes in the Rhinogobius brunneus complex (Pisces, Gobiidae) in Okinawa, Japan.Crossref | GoogleScholarGoogle Scholar |
Kondo, M., Maeda, K., Yamasaki, N., and Tachihara, K. (2012). Spawning habitat and early development of Luciogobius ryukyuensis (Gobiidae). Environmental Biology of Fishes 95, 291–300.
| Spawning habitat and early development of Luciogobius ryukyuensis (Gobiidae).Crossref | GoogleScholarGoogle Scholar |
Leis, J. M., and Carson-Ewart, B. M. (2000). ‘The Larvae of Indo-Pacific Coastal Fishes: an Identification Guide to Marine Fish Larvae.’ (Brill: Leiden, The Netherlands.)
Maeda, K., and Tachihara, K. (2010). Diel and seasonal occurrence patterns of drifting fish larvae in the Teima Stream, Okinawa Island. Pacific Science 64, 161–176.
| Diel and seasonal occurrence patterns of drifting fish larvae in the Teima Stream, Okinawa Island.Crossref | GoogleScholarGoogle Scholar |
Maeda, K., Yamasaki, N., Kondo, M., and Tachihara, K. (2008). Reproductive biology and early development of two species of sleeper, Eleotris acanthopoma and Eleotris fusca (Teleostei: Eleotridae). Pacific Science 62, 327–340.
| Reproductive biology and early development of two species of sleeper, Eleotris acanthopoma and Eleotris fusca (Teleostei: Eleotridae).Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (1997). Is there such a thing as amphidromy? Micronesica 30, 3–14.
McDowall, R. M. (2009). Early hatch: a strategy for safe downstream larval transport in amphidromous gobies. Reviews in Fish Biology and Fisheries 19, 1–8.
| Early hatch: a strategy for safe downstream larval transport in amphidromous gobies.Crossref | GoogleScholarGoogle Scholar |
Miller, P. J. (1984). The tokology of gobioid fishes. In ‘Fish Reproduction: Strategies and Tactics’. (Eds G. W. Potts and R. J. Wooton.) pp. 119–153. (Academic Press: London.)
Mizuno, N. (1960). Study on a freshwater goby, Rhinogobius similis Gill, with a proposition on the relationship between land-locking and speciation of some freshwater gobies in Japan. Memoirs of the College of Science, University of Kyoto, Series B 27, 97–115.
Mizuno, N. (1961). Study on the gobioid fish, ‘Yoshinobori’ Rhinogobius similis Gill – I. Comparison of life histories of three ecological types. Bulletin of Japanese Society of Scientific Fisheries 27, 6–11.
| Study on the gobioid fish, ‘Yoshinobori’ Rhinogobius similis Gill – I. Comparison of life histories of three ecological types.Crossref | GoogleScholarGoogle Scholar |
Moriyama, A., Yanagisawa, Y., Mizuno, N., and Omori, K. (1998). Starvation of drifting goby larvae due to retention of free embryos in upstream reaches. Environmental Biology of Fishes 52, 321–329.
| Starvation of drifting goby larvae due to retention of free embryos in upstream reaches.Crossref | GoogleScholarGoogle Scholar |
Myers, G. S. (1949). Usage of anadromous, catadromous and allied terms for migratory fishes. Copeia 1949, 89–97.
| Usage of anadromous, catadromous and allied terms for migratory fishes.Crossref | GoogleScholarGoogle Scholar |
Nishida, M. (1994). Life-history variation and speciation in Yoshinobori fishes. In ‘Migrating Freshwater Fishes Between Rivers and the Sea: Life History and Evolution’. (Eds A. Goto, K. Tsukamoto and K. Maekawa.) pp. 154–169. (Tokai University Press: Tokyo.) [In Japanese]
Nishida, M. (2001). Evolutionary process toward larger egg: Rhinogobius species. In ‘Evolutionary Biology of Egg Size in Aquatic Animals’. (Eds A. Goto and K. Iguchi.) pp. 149–170. (Kaiyusya: Tokyo.) [In Japanese]
Oijen, M. J. P., Suzuki, T., and Chen, I.-S. (2011). On the earliest published species of Rhinogobius. With a redescription of Gobius brunneus Temminck and Schlegel, 1845. Journal of the National Taiwan Museum 64, 1–17.
Oshiro, N., and Nishizima, S. (1978). An observation on the juveniles of ‘Yoshinobori’ (Gobiidae) in the sea-waters. Biological Magazine Okinawa 16, 17–22..
R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (last accessed 18 February 2013).
Sakai, H., and Yasuda, F. (1978). Development of eggs and larvae of the freshwater goby, Rhinogobius brunneus. Japanese Journal of Ichthyology 25, 92–100.
Suzuki, T., and Chen, I.-S. (2011). Redescriptions of three species of genus Rhinogobius (Perciformes, Gobiidae) described by Dr. Shigeho Tanaka. Bulletin of the Osaka Museum of Natural History 65, 9–24..
Suzuki, T., and Sakamoto, K. (2005). Record of a gobiid fish, Rhinogobius sp. TO (Perciformes, Gobiidae) from the Noubi and Okazaki plains, Japan. Bulletin of Biogeographical Society of Japan 60, 13–20..
Suzuki, T., Chen, I.-S., and Senou, H. (2011). A new species of Rhinogobius Gill, 1859 (Teleostei: Gobiidae) from the Bonin Islands, Japan. Journal of Marine Science and Technology 19, 693–701.
Tachihara, K. (2009). Two landlocked Rhinogobius species in the Ryukyu Archipelago: conservation and the future of gobies endemic to isolated rivers. Japanese Journal of Ichthyology 56, 70–74..
Takahashi, S., and Okazaki, T. (2002). A new lentic form of the ‘yoshinobori’ species complex, Rhinogobius spp. from Lake Biwa, Japan, compared with lake-river migrating Rhinogobius sp. OR. Ichthyological Research 49, 333–339.
| A new lentic form of the ‘yoshinobori’ species complex, Rhinogobius spp. from Lake Biwa, Japan, compared with lake-river migrating Rhinogobius sp. OR.Crossref | GoogleScholarGoogle Scholar |
Tamada, K. (2009). Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season. Ichthyological Research 56, 69–75.
| Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season.Crossref | GoogleScholarGoogle Scholar |
Tsunagawa, T., and Arai, T. (2008). Flexible migration of Japanese freshwater gobies Rhinogobius spp. as revealed by otolith Sr : Ca ratios. Journal of Fish Biology 73, 2421–2433.
| Flexible migration of Japanese freshwater gobies Rhinogobius spp. as revealed by otolith Sr : Ca ratios.Crossref | GoogleScholarGoogle Scholar |
Tsunagawa, T., and Arai, T. (2011). Migratory history of the freshwater goby Rhinogobius sp. CB in Japan. Ecology of Freshwater Fish 20, 33–41.
| Migratory history of the freshwater goby Rhinogobius sp. CB in Japan.Crossref | GoogleScholarGoogle Scholar |
Tsunagawa, T., Suzuki, T., and Arai, T. (2010). Otolith Sr : Ca ratios of freshwater goby Rhinogobius sp. TO indicating absence of sea migrating traits. Ichthyological Research 57, 319–322.
| Otolith Sr : Ca ratios of freshwater goby Rhinogobius sp. TO indicating absence of sea migrating traits.Crossref | GoogleScholarGoogle Scholar |
Yamasaki, N., Kondo, M., Maeda, K., and Tachihara, K. (2011). Reproductive biology of three amphidromous gobies, Sicyopterus japonicus, Awaous melanocephalus, and Stenogobius sp., on Okinawa Island. Cybium 35, 345–359.
Yokoi, K., and Hosoya, K. (2006). Early development of the endangered freshwater goby, Rhinogobius sp. BI (Gobiidae). Ichthyological Research 53, 160–165.
| Early development of the endangered freshwater goby, Rhinogobius sp. BI (Gobiidae).Crossref | GoogleScholarGoogle Scholar |