Addressing unmet needs following minor stroke (SUN study): a randomised controlled trial
Emma Finch A B C D * , Tegan Cruwys E , Jennifer Fleming B , Ian Williams F , Ashley Cameron A , Adele Coleman B , Philip Aitken G H , Katherine Jaques G and Darshan Shah G
A B C D * , Tegan Cruwys E , Jennifer Fleming B , Ian Williams F , Ashley Cameron A , Adele Coleman B , Philip Aitken G H , Katherine Jaques G and Darshan Shah G
A
B
C
D
E
F
G
H
Abstract
Growing evidence suggests that people with minor stroke experience persisting post‐stroke impairments across a range of domains. Our primary aim was to determine whether a new multicomponent intervention for people with minor stroke reduced unmet needs at 1 and 3 months post-hospital discharge compared with usual care. Secondary aims explored the efficacy of the intervention on functional outcomes, health-related quality of life, return to work and social group membership.
A parallel, randomised controlled trial design with 1:1 allocation to the intervention and control groups (n = 34 per group) was used. The intervention group received a multicomponent intervention (comprising information about minor stroke, checklist and group education sessions). The control group received usual care. Participants completed assessments at baseline (T1), 1 month (T2) and 3 months (T3) post-hospital discharge. The primary outcome measure was unmet need according to the Survey of Unmet Needs and Service Usage.
The intervention did not significantly reduce unmet need (P = 0.839); however, the control group reported a significant need for existing support to continue (P = 0.032). Participation in the intervention led to significant improvements in emotional wellbeing compared to the control group (P = 0.041). There was no difference between the groups according to social participation, health-related quality of life, return to work or social group membership (all P > 0.05). Usage of the three intervention components was lower than anticipated.
A suite of mixed format, evidence-based education and support tools did not fully meet the unmet needs of minor stroke survivors during their transition from hospital. Further research is required to refine the intervention.
Prospectively registered – Australian New Zealand Clinical Trials Registry (ACTRN12619000133134p).
Keywords: acquired brain injury, discharge, mental health, mild stroke, randomised controlled trial, social participation, stroke, transition, unmet need, wellbeing.
Introduction
Stroke is a major cause of death and disability worldwide (Johnson et al. 2016), with current estimates indicating that the burden of stroke will continue to increase into the future, particularly in low- and middle-income countries (Krishnamurthi et al. 2020). Beyond the physical and mental health impacts, stroke is also associated with negative social outcomes, including social isolation and reduced social participation (Hinojosa et al. 2011; Woodman et al. 2014). Medical advances in acute stroke management, such as dedicated stroke units and, in the case of ischaemic strokes, reperfusion therapies (such as thrombolysis and endovascular clot retrieval) have improved stroke care (Zerna et al. 2018). Due to the resultant lessening of stroke severity, more patients are leaving hospital with a minor stroke (also termed ‘mild stroke’ or ‘nondisabling stroke’). Over 50% of ischaemic strokes are classified as a minor stroke (defined by the authors of the study as a Scandinavian Stroke Scale Score, 45–58), with this number likely to increase in the future (von Weitzel-Mudersbach et al. 2013).
Although the growing proportion of strokes being classified as mild is good news, there is unfortunately growing evidence that the impairments experienced by people with minor stroke are neither minor nor short-lived. Instead, many experience long-term and significant stroke-related impairments, many of which do not become apparent until they leave the supported hospital environment. For example, Suda et al. (2020), in a study of 112 people with minor stroke (defined by the authors of the study as National Institutes of Stroke Scale (NIHSS) score <3), found that 63% of participants experienced a cognitive impairment within 5 days of stroke. Marsh et al. (2018) found that people with minor stroke (defined by the authors of the study as NIHSS < 4) at a mean of 83.6 days post-stroke reported difficulties with functional impairments, including activities of daily living, depression and fatigue. Similarly, recent research by our team found that patients with minor stroke reported unmet health, social and rehabilitation needs that remained unmet 2 months post-hospital discharge (Finch et al. 2020).
The enduring impact of these unmet needs was highlighted by Edwards et al. (2006) who found that 6 months after minor stroke (defined by the authors of the study as NIHSS < 5) 87% of participants reported persistent changes, which impacted on their driving, work, household management and recreation, despite being fully independent in the basic activities of daily living. These difficulties can persist for at least a year post-stroke. For example, 12 months post-minor stroke, 67% of people experience cognitive difficulties, 43% experience emotional difficulties, 28% experience both cognitive and emotional difficulties (Vlachos et al. 2021), and approximately one-third experience ongoing balance and gait issues (Hamre et al. 2020). Furthermore, evidence suggests that for a subset of stroke survivors, these difficulties remain long-term (McHutchison et al. 2019). These studies provide clear evidence that people with minor stroke have a critical need for support and services following hospital discharge that are largely unmet by current health services. This is important because having unmet health, rehabilitation and/or social needs is associated with adverse health consequences, lower life satisfaction and poorer psychological wellbeing (Allen and Mor 1997; Op Reimer et al. 1999; Heinemann et al. 2002). Furthermore, once a person has a stroke, there is a 43% risk of a subsequent stroke over the next 10 years (Hardie et al. 2004), placing an increased burden on the health system.
Despite growing evidence of persisting unmet need, people with minor stroke have reduced access to services (Turner et al. 2022). In their qualitative study in the UK, Turner and colleagues found that healthcare providers reported limited post-hospital services for people with minor stroke, with no standardised follow-up care pathway, variability of care between services and poor communication flow between services. In Australia, Grimley et al. (2020) found that 50% of people with minor stroke received no rehabilitation after their acute hospital admission. This is despite an increased risk of subsequent stroke and hospital readmissions following a minor stroke (Bushnell et al. 2021). Unfortunately, the optimal rehabilitation pathway for people with minor stroke is unknown. Indeed, ‘What is the most effective pathway for Transient Ischaemic Stroke (TIA)/minor stroke?’ was recently identified as the number one research priority by patients, clinicians and researchers in the area of TIA and minor stroke (Turner et al. 2018).
In seeking to answer this question about the most effective pathway for people with minor stroke, it is possible that self-management may be a critical feature. Indeed, self-management programs have been proven to be effective in stroke management (Eames et al. 2013; Fryer et al. 2016) and are recommended in stroke guidelines internationally (e.g. Powers et al. 2019; Stroke Foundation 2020; Intercollegiate Stroke Working Party 2023). Self-management typically involves the patient actively controlling the symptoms, consequences and treatment of their condition, with self-management programs designed to equip patients with the necessary skills and knowledge in this area (Lennon et al. 2013). It is possible that the minor stroke population may be ideally suited to a self-management program; however, further research is required to explore whether a self-management approach meets the needs of this patient population.
Finally, despite strong evidence of ongoing impairments (e.g. Edwards et al. 2006; Vlachos et al. 2021), it is difficult to have a clear understanding of the specific characteristics of minor stroke compared to other forms of events. In part this is due to contention about how to define minor stroke (Tellier and Rochette 2009; Finch et al. 2017), including which assessment measure or scale to use, and the tendency to combine TIA and minor stroke in research, despite evidence that minor stroke involves damage to neural tissue visible on imaging but TIA does not.
There is, therefore, an urgent need for more research to determine how to meet patient needs after minor stroke to improve outcomes and minimise morbidity. In particular, we sought to meet the need for an evidence-based pathway that facilitates the transition from hospital to community care for people with minor stroke. Our team has developed a multicomponent intervention called Stroke Unmet Needs (SUN) to meet the unmet needs of stroke survivors recently discharged from hospital following a minor stroke and living in the community (Finch et al. 2020), with self-management a key feature underpinning the program.
The primary aim of the current study was to trial the SUN intervention for minor stroke survivors to determine whether the intervention reduced unmet needs at 1 and 3 months post-hospital discharge compared with usual care.
The secondary aims were:
To assess the efficacy of SUN in improving participants’ functional outcomes (ability, adjustment, participation) and health-related quality of life 1- and 3-months post-hospital discharge compared to usual care
To examine the efficacy of SUN on participants’ ability to return to work compared to usual care
To assess the efficacy of SUN in supporting participants’ social group membership 1 and 3 months post-hospital discharge compared to usual care
To explore the acceptability of the intervention through participants’ perceptions of the new intervention.
It was hypothesised that the new intervention would reduce unmet needs at 1 and 3 months and improve functional outcomes, quality of life, return to work, social group membership and service access of people with minor stroke compared to usual care.
Method
Design
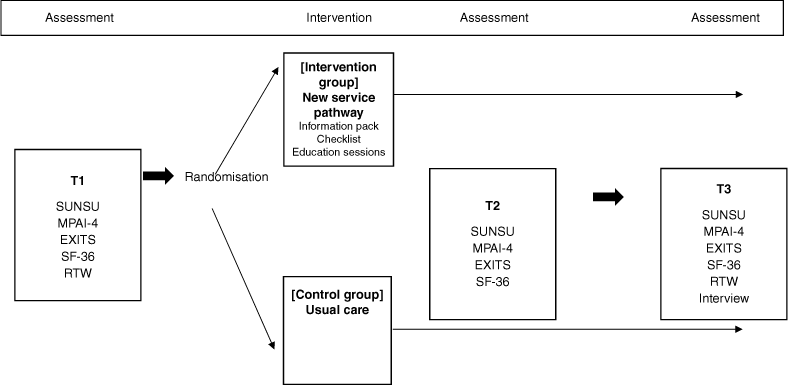
A parallel, randomised controlled trial (RCT) design with 1:1 allocation to the intervention and control groups was used. The RCT was designed and is reported according to Consolidated Standards of Reporting Trials (CONSORT) guidelines (Schulz et al. 2010) and the Template for Intervention Description and Replication (Hoffmann et al. 2014). The protocol has been published (Finch et al. 2019).
Participants
For the purposes of this study, we used the broad inclusive definition of minor stroke proposed by Green and King (2007): ‘Minor stroke is a cerebrovascular event that results in mild neurological impairment or disability’, potentially encompassing difficulties with motor function, sensation and/or communication; along with a NIHSS score of less than 5. Participants were recruited from a single quaternary hospital in Queensland, Australia. The study inclusion criteria were aged over 18 years, presenting with a confirmed new stroke with minor deficits at the time of discharge (i.e. NIHSS < 5) and having a total length of stay in hospital of less than 2 weeks inclusive of all episodes of care related to stroke. The exclusion criteria were other neurological conditions or previous stroke.
Our original planned sample size was 110 participants in total (Finch et al. 2019). Our calculation was for a medium effect size (0.5) with 80% power for the two groups, 51 participants were required per group (calculated with G Power × 2), increased to 55 to allow for dropout.
The control group received the usual care provided to minor stroke patients discharged from the recruiting hospital. This included an electronic discharge summary (about the patient’s stroke, previous medical history, treatment provided and ongoing health requirements) sent to the patient’s General Practitioner (GP) from the hospital treating team and, in some cases, an outpatient follow-up appointment was booked at the hospital. The need for outpatient follow-up was determined at discharge by the treating team. Patients may have also been referred to private allied health providers or community services at the discretion of the treating team, who were not part of the research team. A ‘My Stroke Journey’ booklet (Stroke Foundation 2019), a discharge planning pack, counselling about stroke, medications and follow-up, were usually provided to all patients with stroke, although the booklet was not specific to minor stroke.
In addition to usual care, participants in the new intervention received access to a multicomponent pathway comprising the three components outlined below. To promote self-management, participants were able to use all or any combination of the three components. Beyond self-selection of the intervention components, there was no further tailoring of the intervention components. The intervention was developed based on a multifaceted needs analysis involving a literature review (Finch et al. 2017), an unmet needs study comprising semi-structured interviews, and questionnaires completed by a cohort of people with minor stroke 2 weeks and 2 months post-hospital discharge about their post-stroke difficulties and health, service and social needs (Finch et al. 2020, 2021). The subsequent intervention components included:
Minor stroke written information (self-management kit): participants received a written information pack while admitted to the recruiting hospital. The self-management kit included written information about common issues specific to minor stroke, common issues to see a GP about post-discharge, medications, referral to allied health services, personalised issues to discuss with their GP (via the customised screening checklist described below) and a flyer about the education sessions (described below). A written format was used because the provision of written information about stroke on discharge from hospital has been found beneficial (Eames et al. 2013). The design and content of the written information was directly informed by the earlier needs analysis research by our team (Finch et al. 2020, 2021) and was refined through discussions with health professional clinical staff and management (directors of allied health departments) at the recruiting hospital, and a GP member of the research team. There was no restriction on how often or how long participants could use the information.
Minor stroke screening checklist: participants were provided with a checklist (during their hospital admission) designed to be shared with their GP to encourage self-management with their GP’s support. The one-page handout included information about when to book a GP appointment, what to take to the appointment and a checklist to guide conversations. The checklist was customised for people with minor stroke as a cohort and included questions about whether the GP could access the participant’s hospital discharge information, secondary stroke prevention, medications, driving, work assessments, services accessed/needed and residual difficulties/changes post-stroke. The checklist was developed in consultation with the GP member of the research team and in response to patient feedback during the needs analysis study. Specifically, participants in the needs analysis study described short, superficial sessions with medical professionals post-hospital discharge that did not delve into the post-stroke issues participants were experiencing. There was no restriction on how often or how long participants could use the checklist.
Minor stroke educational group sessions: Education sessions were included because they have been identified as a critical ingredient of successful self-management programs in chronic diseases (Lorig et al. 1999). Participants were offered a weekly group program of minor stroke community-based educational topics. A flyer was included as part of the minor stroke written information (self-management pack) and followed up with emails and/or text messages from a research assistant about the upcoming sessions. The six sessions were scheduled for up to 1.5 h weekly. The sessions were designed to be delivered at rotating community locations; however, due to the COVID-19 pandemic lockdowns, this then moved to a single location (a research centre meeting room opposite the recruiting hospital) and then to an online telehealth format (via Zoom videoteleconferencing) for some sessions. Each session included information about common issues following minor stroke, practical actions to take to address the issues, and how and where to seek help. Participants were provided with website links and/or written information to take home. Each session was presented by a qualified health professional experienced in stroke management (e.g. physiotherapy, occupational therapy), with extensive experience in the target area (e.g. the returning to driving topic was presented by a senior occupational therapist with expertise in driving assessments and education after stroke, the management medications after minor stroke session was delivered by an experienced hospital pharmacist, and the managing the emotional impact of stroke was delivered by a qualified psychologist experienced in stroke management). The sessions were also moderated by a member of the research team: one of two speech pathologists, each with over 10 years of experience in stroke management and a PhD in the field of speech pathology stroke management. The topics stemmed from areas of unmet need identified during the needs analysis study (Finch et al. 2020) and were modified through consultation with allied health members of the research team and our GP partners and through consultation with the recruiting hospital allied health staff and managers. The topics were:
Medications after minor stroke
Ddriving and transportation after minor stroke
Managing stroke fatigue and returning to everyday activities
Adopting (more) healthy habits and reducing risk for further strokes
Returning to exercise with fatigue
Managing the emotional impact of stroke and maintaining mental health.
An extra (7th) session was available to cover additional topics as specifically requested by participants if required. The sessions were run on a continuing cyclical basis. Participants were able to bring a support person if desired and completed a feedback form about the session at the end of each session.
Measures
Participants completed the following measures at three timepoints: T1 (baseline), T2 (approximately 1 month following hospital discharge) and T3 (approximately 3 months following hospital discharge) facilitated by a research assistant (either in person or via telephone).
Survey of Unmet Needs and Service Usage (SUNSU) (Heinemann et al. 2002): the SUNSU is a 27 item self-rating checklist used to determine needs across impairment, activities of daily living, occupational activities, psychological needs and community access (e.g. improving mood, travelling in the community). When completing the checklist, participants recorded whether they were receiving help and whether they needed help within each item. Three subscales were calculated: (1) unmet need (SUNSU unmet need, i.e. the sum of items for which a person indicated a need for support but were not receiving support), (2) support currently being received (SUNSU support received, i.e. the sum of the items for which a person indicated that they were currently receiving support), and (3) need for current support to be continued (SUNSU support continue, i.e. the sum of items for which a person indicated that they were currently receiving support and needed that support to continue). Participants received a score of 0–27 for each of these three elements. Higher scores represent higher need. The SUNSU has good reliability (Heinemann et al. 2002).
Six outcome measures were used to assess this study’s secondary aims:
Mayo-Portland Adaptability Inventory-4 (MPAI-4) (Malec and Lezak 2008): a self-report measure designed to explore the key, common consequences of acquired brain injury, including physical, cognitive, emotional and social impacts. The MPAI-4 consists of three subscales (ability index, adjustment index and participation index) and a full scale score, with raw scores converted to T scores for interpretation (M = 50, s.d. = 10). The typical T-score range for an individual receiving rehabilitation post-brain injury is 40–60 (Malec and Lezak 2008). The MPAI-4 performs well on measures of reliability and validity (Malec and Lezak 2008).
Exeter Identity Transition Scales (EXITS) (Haslam et al. 2008): a self-report measure with four subscales to explore changes to social group connections after the stroke. The scales explored four stages of group memberships: (1) group membership before the stroke (four items, e.g. ‘Before having a stroke, I belonged to lots of different groups’); (2) group membership after the stroke (four items, e.g. ‘After having a stroke, I belong to lots of different groups; (3) maintenance of group membership post-stroke (four items, e.g. ‘After my stroke, I still belong to the same groups I was a member of before my stroke’); and (4) new group memberships post-stroke (four items e.g. ‘After my stroke, I have joined one or more new groups’). For each item, participants responded on a scale from 1 (not at all true of me) to 7 (completely true of me), where higher scores represented greater social connection. The EXITS were measured at T2 and T3 only (as participants were in hospital at T1 and had not had a chance to resume social activities and friendships). The EXITS have strong evidence for reliability and validity (Cruwys et al. 2016).
Return to previous occupation (yes/no): recorded in dichotomous format in response to direct questioning with additional details obtained through MPAI-4 question 28 (28a paid employment or 28b other employment).
RAND 36-Item Health Survey 1.0 (SF-36) (www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html): a 36 item self-report measure exploring health-related quality of life. The SF-36 explores subjective health and quality of life across eight domains: physical functioning, pain, role limitations, emotional wellbeing, social functioning, energy, perceptions about general health and perceived change in health. Each subscale is scored 0–100, with higher scores denoting a more favourable health state. Individual items within the same scale (i.e. each of the domains listed above) are collated and averaged to give a score for the scale (health concept). The SF-36 performs well on measures of reliability, central tendency, and variability (www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html).
Perceptions of the intervention: attendance data and feedback ratings were obtained from participants in the intervention group only. Both quantitative and qualitative perceptions were collected. The 16 quantitative feedback ratings were scored 1 (strongly disagree) to 5 (strongly agree), and items included ‘The information was organised and easy to follow’, ‘The length of training was sufficient’, ‘The materials/handouts provided are helpful, I learned skills and/or identified habits I will be able to apply at home’, ‘After this session, I am more aware of where to get help’, and ‘There was sufficient time for questions’. Participants also rated the level of helpfulness of the session overall in an additional quantitative question from 1 (not at all helpful) to 5 (extremely helpful). There were two open-ended (qualitative) questions: What was most useful/helpful for you today? What was least useful/helpful for you today?
Additional information was collated from participants’ medical files, including age, date of stroke, length of stay in hospital, health insurance status, highest level of education and living situation.
Procedure
The study was approved by the Metro South Human Research Ethics Committee. All patients admitted to the recruiting hospital during the trial duration were screened for eligibility and recruited prior to discharge by a research assistant (i.e. consecutive recruitment). All participants provided written informed consent prior to study participation. Participants then completed the T1 (baseline) measures before being randomised to the SUN new intervention or usual care (see Fig. 1 for the study process). Randomisation was conducted in blocks of 10 using a computerised random number generator (by the first author) with the randomisation details in sealed, opaque envelopes. Participants in the SUN condition were then provided with the three intervention elements (described below). Participants in the usual care condition continued to receive usual care at the hospital and following discharge. All participants then completed T2 and T3 measures. The research assistant administering the assessments at each of the three timepoints was blinded to participant randomisation. Recruitment occurred from October 2019 to November 2020, with the final assessments completed by March 2021. Recruitment was ceased due to completion of the grant timeline and funded budget. The trial was impacted by the COVID-19 global pandemic. Due to the pandemic, recruitment temporarily paused due to COVID-19 research stoppages imposed by the hospital and university, the educations sessions were required to move to virtual delivery due to cessation of all face-to-face groups, and recruitment fell short of our target.
Data analysis
Quantitative data were initially analysed using descriptive analysis (counts, means, standard deviations where appropriate). Participant T1 (baseline) demographic information (age, gender, education) was compared between the two study groups using independent samples t-tests and Fisher exact tests in the case of work status (due to the categorical nature of the data and cell counts of less than five, which precluded the use of Chi square tests). Assessment measure data (SUNSU, EXITS, SF-36, MPAI-4) from each of the three timepoints (T1, T2, T3) were analysed using an Intention-To-Treat (ITT) approach. Consistent with the published protocol (Molenberghs et al. 2004), the analyses were conducted using a mixed model repeated measures (MMRM), which accounted for the non-independence of participants’ responses at each timepoint. Each model included timepoint and condition as categorical fixed effects and participant as a random intercept. The primary test of efficacy for each variable was whether adding the timepoint × condition interaction to the model led to a significant improvement in model fit. MMRM uses a full information maximisation likelihood estimation strategy that can model data even when some observations are missing to honour the ITT principle (Tellier and Rochette 2009). Follow-up comparisons were conducted using estimated marginal means calculated from the MMRM. All analyses were conducted blinded to group allocation. Return to work outcomes at T3 were analysed using Fisher exact tests. For the feedback data for the intervention group, the quantitative data were analysed descriptively (means). Due to the limited nature of the open-ended questions (e.g. ‘What did you find most useful/helpful today?’) further qualitative analysis was not conducted (instead the quotes were reported as-is for each question). To check the robustness of the effects found during the ITT analysis (Thabane et al. 2013), a sensitivity analysis was conducted using an As Treated (AT) analysis (the results of which are presented in Supplementary Appendix S1). This analysis was conducted to provide confidence that the effects were attributable to the content of our intervention rather than to spurious factors, such as expectancy bias. A P value of 0.05 was used to evaluate all analyses.
Results
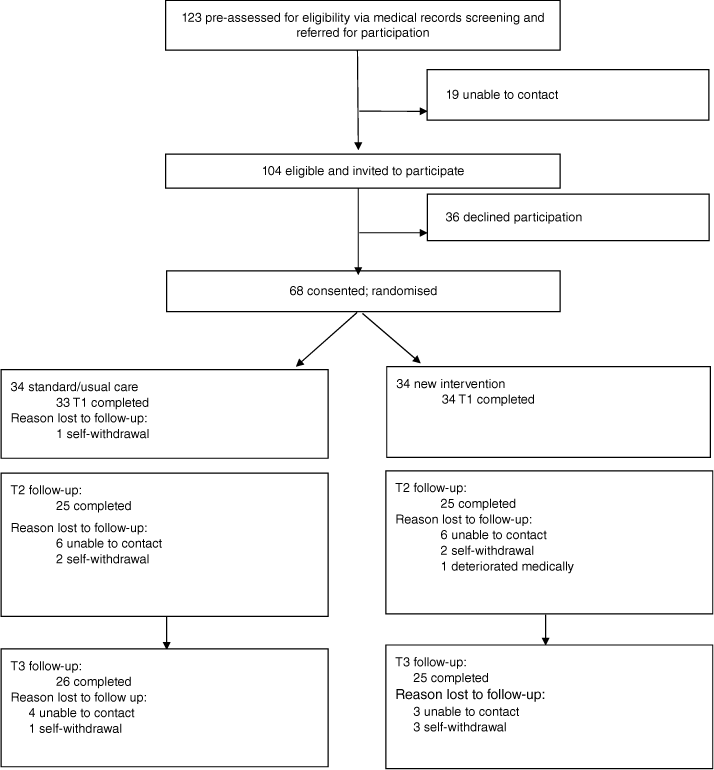
A total of 68 patients (37 M, 31 F) was recruited, which was below our target number due to COVID-19 and research cessation impacts (see Table 1 for demographic information). Following randomisation, 34 participants were allocated to each of the study conditions (see Fig. 2 for the CONSORT flowchart). Estimated marginal means are provided in Fig. 3.
| Demographic variable | Control group (n = 34) | Intervention group (n = 34) | |
|---|---|---|---|
| Age mean years (s.d., range) | 63 (15.44, 22–91) | 62 (14.52, 38–88) | |
| Gender n (%) | |||
| Female | 15 (44) | 16 (47) | |
| Male | 19 (56) | 18 (53) | |
| NIHSS score at T1 mean (s.d., range) | 1.64 (1.48, 0–5) | 1.48 (1.37, 0–4) | |
| Work status at T1 n (%) | |||
| Employed part-time or full time | 2 (6) | 3 (9) | |
| Not employed | 32 (94) | 31 (91) | |
| Work status at T3 n (%)A | |||
| Employed part-time or full time | 9 (34) | 11 (44) | |
| Not employed | 16 (62) | 13 (52) | |
| Highest level of education n (%) | |||
| Grade 10 or less | 9 (26) | 8 (24) | |
| Grade 12 | 9 (26) | 4 (12) | |
| Certificate/diploma/trade | 7 (21) | 16 (47) | |
| Bachelor degree | 8 (24) | 3 (9) | |
| Postgraduate study | 1 (3) | 3 (3) | |
| Health insurance status n (%) | |||
| Extras only | 2 (6) | 1 (3) | |
| Extras and hospital | 11 (32) | 13 (38) | |
| Hospital only | 3 (9) | 1 (3) | |
| None | 14 (4) | 16 (47) | |
| Unsure | 0 (0) | 1 (3) | |
| Living situation n (%) | |||
| Alone | 7 (21) | 5 (15) | |
| With family | 25 (74) | 28 (82) | |
| With friends | 1 (3) | 0 (0) | |
Note: percentages are rounded to the nearest whole number. NIHSS = National Institutes of Stroke Scale score. T1 = baseline, T3 = final assessment.
Consistent with their classification as minor stroke patients, none of the participants experienced severe communication or cognitive impairments. Eleven participants (16%) received speech pathology follow-up after hospital discharge, and nine participants (13%) received occupational therapy follow-up. All intervention materials were developed by a qualified speech pathologist, highly experienced in managing communication and cognitive difficulties after stroke, and were refined by the wider research team and by health professional clinical staff and management (directors of allied health departments) at the recruiting hospital and the GP member of the research team to ensure suitability for potential communication or cognitive difficulties after stroke.
Baseline
There were no significant differences between the control or intervention groups according to age, gender, highest level of education or work status at T1 (all P > 0.05). There were no significant differences at T1 between the two groups on any measure except for social functioning and physical functioning on the SF-36. By chance, the intervention group had poorer social functioning than the control group at T1; t(65) = 2.01, P = 0.048. In terms of physical functioning, the control group reported greater physical functioning at T1, t(89.8) = 1.99, P = 0.050.
SUNSU
The primary outcome measure of the trial (the SUNSU) was analysed using three variables: (1) unmet need (SUNSU unmet need), (2) support currently being received (SUNSU support received) and (3) need for current support to continue (SUNSU support continue). For SUNSU unmet need, the main effect of time significantly improved the model, χ2(2) = 23.82, P < 0.001. Scores in both groups decreased significantly over time, with medium–large effect sizes: Cohen’s dSUN = 0.78; 95% CI: 0.24, 1.32, P = 0.004. The main effect of condition did not significantly improve the model, χ2(3) = 2.21, P = 0.529. The interaction between time and condition was non-significant, χ2(2) = 0.35, P = 0.839.
For SUNSU support received, the amount of support currently being received, the main effects of time (χ2(2) = 1.42, P = 0.492) and condition (χ2(1) = 0.32, P = 0.571), as well as the interaction between time and condition (χ2(2) = 3.11, P = 0.212), were all non-significant. The effect size was small and non-significant in the intervention condition, t(113) = −0.43, P = 0.670 (dSUN = 0.09; CI: −0.47, 0.66).
For the SUNSU support continue, there was no main effect of time (χ2(2) = 1.39, P = 0.498), but a main effect of condition (χ2(1) = 4.56, P = 0.032), such that the control group reported a greater need for existing support to continue across timepoints. The addition of the interaction did not significantly improve the model, χ2(2) = 0.62, P = 0.734. Neither condition changed significantly over time, dSUN = −0.01; CI: −0.54, 0.52, P = 0.978.
MPAI-4
For MPAI-4 full scale score, there was a significant main effect of time, χ2(2) = 26.89, P < 0.001, with large effect sizes for improvement in both conditions, dSUN = 1.13; CI: 0.55, 1.72, P < 0.001. There was a non-significant main effect for condition, χ2(1) = 0.53, P = 0.467, and no significant time by group interaction, χ2(2) = 0.73, P = 0.694. The same pattern was replicated for the MPAI-4 ability, adjustment and participation subscales (all interaction ps > 0.05).
EXITS
For the EXITS current multiple group memberships subscale (EXITS Now), there was no significant main effect of time, χ2(1) = 0.35, P = 0.555, with small non-significant effect sizes, dSUN = 0.08; CI: −0.51, 0.67, P = 0.790. There was no significant main effect for condition, χ2(1) = 0.133, P = 0.716, and no significant interaction, χ2(1) = 0.04, P = 0.847. The same pattern was replicated for the group membership continuity subscale (EXITS Continue) and the new group memberships subscale (EXITS New) (all interaction ps > 0.05).
SF-36
For SF-36 emotional wellbeing, there was no main effect of time, χ2(2) = 3.99, P = 0.136, and no main effect of condition, χ2(1) = 0.567, P = 0.452. There was a significant linear interaction between time and condition, t(101.9) = 2.07, P = 0.041. Participants in the intervention group experienced a significant and large improvement in their emotional wellbeing over time, t(103.3), = −2.90, P = 0.013, dSUN = −0.80; CI: −1.37, −0.24, and people in the control group experienced no change, t(100.4) = −0.01, P > 0.999.
For SF-36 energy/fatigue, there was a main effect of time, χ2(2) = 15.22, P < 0.001, with medium–large effects in the intervention condition, dSUN = −0.71; CI: −1.27, −0.15, P = 0.012. There was no main effect of condition, χ2(1) = 1.71, P = 0.192 and no significant interaction, χ2(2) = 1.45, P = 0.485. The same pattern was seen for SF-36 role limitations due to emotional wellbeing (with a medium effect size) and SF-36 role limitations due to physical functioning (with a large effect size).
For SF-36 pain, there was no significant difference between the groups over time (P > 0.05).
SF-36 physical functioning showed a main effect for time, χ2(2) = 32.84, P < 0.001, with large and significant improvements in both conditions, dSUN = −1.32; CI: −1.91, −0.73, P < 0.001. There was a significant effect of condition, χ2(1) = 4.32, P = 0.038, such that the control condition reported greater physical functioning across timepoints. This difference was significant at T1, t(89.8) = 1.99, P = 0.050, and at T2, t(113.3) = 2.42, P = 0.017. By T3, this difference was no longer significant, t(107.8) = 1.06, P = 0.292. However, the interaction between time and condition was non-significant, χ2(2) = 2.79, P = 0.248.
SF-36 social functioning showed a similar pattern. There was a main effect for time, χ2(2) = 25.70, P < 0.001, with large and significant improvements in both conditions, dSUN = −1.09; CI: −1.66, −0.52, P < 0.001. There was a significant main effect of condition, χ2(1) = 4.69, P = 0.030, such that the control condition reported greater social functioning across timepoints. This difference was significant at T1, t(129) = 2.25, P = 0.026. Social functioning then increased significantly in the intervention group from T1 to T2, t(107) = 2.985, P = 0.006, but not in the control group, t(105) = −1.89, P = 0.111, and as a result, the differences between groups was no longer significant at T2 and T3 (P > 0.05). Nevertheless, the overall linear interaction between condition and time was non-significant, χ2(1) = 0.81, P = 0.669.
Return to work
There was no significant difference between the groups in terms of work status at T1 (Fisher exact test, P = 0.500, see Table 1). Although some participants had returned to work by T3, there was no significant difference between the two groups (Fisher exact test, P = 0.341). It is important to note, however, that the analysis was underpowered as a small number of participants were working pre-stroke.
Intervention feedback
No participants experienced adverse effects from the intervention. Overall, usage of the three intervention components was lower than anticipated. Sixteen participants in the intervention group attended at least one education session (attendance ranged from one to six sessions; Four participants attended six sessions, one participant attended five sessions, four participants attended four sessions, one participant attended three sessions, one participant attended two sessions and five participants attended one session). A further three participants responded to information about the sessions but then did not attend any sessions. The majority of participant responses to the session feedback criteria ranged between ‘agree’ and ‘strongly agree’ (see Table 2). Overall, participants rated the education sessions 3.84 out of 5 (which was between ‘somewhat helpful’ and ‘very helpful’). Participant positive comments about the usefulness/helpfulness of the sessions included ‘Really informative’, ‘Interesting and good to hear [the] other person[‘s] experience’, ‘Really useful information that is good for me and for my family to know too’ and ‘Feel safer to do exercise’. Less positive comments about the usefulness/helpfulness of the sessions included ‘I’ve made a lot of these changes already’ and ‘It’s all very common sense just need to get back into life’. Reasons listed by participants for not attending the education sessions when provided included ‘Didn’t have any problems and because of age’, ‘Was sent messages with subheadings but no details – where, when, what time, etc. I had asked to be emailed info or sent in mail as I would have found these quite helpful/very helpful’, ‘Didn’t hear anything’, ‘They were a double-up [with information received elsewhere]’, ‘Didn’t feel relevant to me’, and ‘Can’t remember’.
| Criteria | Rating (1 strongly disagree to 5 strongly agree) (s.d.) | |
|---|---|---|
| The information was organised and easy to follow | 4.55 (0.57) | |
| The presenters delivered the content well | 4.09 (1.33) | |
| The presenters were knowledgeable | 4.13 (1.29) | |
| The presenters were confident and prepared | 4.11 (1.28) | |
| The content was easy for me to understand | 4.55 (0.57) | |
| The length of training was sufficient | 4.47 (0.50) | |
| The information was delivered at an appropriate pace so I could understand it | 4.15 (1.32) | |
| The materials/handouts provided are/will be helpful | 4.06 (1.32) | |
| I learned skills and/or identified habits that I will be able to apply at home | 4.13 (1.30) | |
| After this session, I am more aware of where to get help | 4.49 (0.50) | |
| There was sufficient time for questions | 4.45 (0.80) | |
| The session answered many of my questions about this topic | 4.38 (0.49) | |
| I was offered the opportunity to share about my own experience/s with stroke | 4.47 (0.50) | |
| Hearing about other peoples’ experiences with minor stroke was helpful | 3.17 (1.92) | |
| Participation and interaction were encouraged | 4.28 (0.99) | |
| The venue was appropriate for this session | 2.92 (2.29) | |
| Overall, I would rate this educational session A | 3.83 (0.61) |
Four intervention group participants reported using the checklist provided as part of the intervention (with an additional five participants reporting that they used their own checklist, in some cases, based on the study checklist). Of the four participants who used the study GP checklist, one reported that the checklist was ‘extremely helpful’, and the remaining three participants described the checklist as ‘very helpful.’ Eleven participants reported referring to the minor stroke written information (self-management kit). These participants provided a mean rating of 3.85 out of 5 (between ‘somewhat helpful’ and ‘very helpful’, with seven of these participants describing the information as either ‘very helpful’ or ‘extremely helpful’.
AT analysis
For participants to be assigned to the intervention group during the AT analysis, they needed to have used at least one component of the intervention. This included attending at least one education session, or reported using the checklist with their GP, or reported reading the minor stroke information sheet. Physical impairments predicted whether participants participated in the intervention as allocated. Participants with higher physical functioning at baseline were less likely to get reassigned from the intervention group to the control group for the AT analysis, t(32) = −2.29, P = 0.029. This means that participants originally allocated to the intervention group and who had poorer physical functioning on the SF-36 physical functioning were more likely to not participate in any of the three intervention components, and therefore, were reassigned as control group participants in the AT analysis. The AT analysis involved conducting statistical analyses with group assignment based on participation in the intervention (SUN group) or non-participation (control group). The AT analysis (outlined in Supplementary Appendix S1) had the following key findings. The SUNSU, MPAI-4 and EXITS AT results replicated the ITT results. For the SF-36, change in emotional wellbeing was no longer significantly different between the two groups over time, and there was no longer a significant baseline difference between the two groups according to SF-36 social functioning and physical functioning.
Discussion
The primary aim of the current study was to determine whether a new multicomponent intervention for minor stroke survivors (SUN) reduced unmet needs compared with usual care at 1 and 3 months post-hospital discharge. The results suggested that a suite of mixed format, evidence-based education and support tools did not fully meet all the unmet needs of minor stroke survivors during their transition from hospital back into everyday life. Participation in the new intervention led to significant improvements in emotional wellbeing but no significant changes in unmet need, social participation, health-related quality of life, return to work and social group membership. The control group reported a significant need for existing support to continue that was not seen in the intervention group.
Growing evidence suggests that people with minor stroke can experience persisting stroke-related impairments and restrictions spanning a number of domains, including cognition, fatigue, physical function and mental health (Marsh et al. 2018; McHutchison et al. 2019; Finch et al. 2020; Suda et al. 2020; Vlachos et al. 2021). In many cases, these needs can remain unmet by current services (Finch et al. 2017) due to subtle impairments not easily detected in the supported hospital environment. In the present study, although participants in the intervention received more support than the control group, this did not lead to a significant improvement across all areas of unmet need. Participation in the new intervention led to significant improvements in emotional wellbeing, physical function and social function but not in unmet need, social participation, health-related quality of life, return to work and social group membership. The control group reported a significant need for existing support to continue that was not seen in the intervention group. A number of factors may be at play, including that the intervention did not fully meet the support needs of all participants or that the underpowered sample may have obscured potentially meaningful findings.
Emotional disturbances in the form of depression and anxiety are reported by approximately 43% of people with minor stroke (Vlachos et al. 2021), with effects persisting 3 years post-stroke (McHutchison et al. 2019). The new intervention led to significant improvements in self-reported emotional wellbeing according to the SF-36 that were not seen in the control group. This may have at least partially reflected the wellbeing focus of the intervention through the emotional wellbeing education session, information in the minor stroke information sheet and related questions on the GP checklist.
Stroke has been linked to social isolation and reduced social participation (Hinojosa et al. 2011; Woodman et al. 2014). However, participants in the present study, regardless of whether they were in the intervention or control group, reported no significant changes in their social group membership over time according to the EXITS. It is possible that this lack of change may have also been impacted by COVID-19 lockdowns and social distancing requirements that occurred during the study. Although participants in both groups reported significant improvements over time on the MPAI-4 (which measures ability, adjustment and participation), there was no significant difference between the groups. This suggests that participants overall became more able to participate socially as time progressed, regardless of whether they received the intervention or not.
By chance, the intervention group started the study with poorer scores according to the SF-36 physical functioning and social functioning; however, for both subscales, participants in the intervention group improved to the point that there was no significant statistical difference between the groups. This suggests a more positive trajectory in the intervention group; however, more evidence is needed, particularly because we found evidence that patients with poorer physical functioning were less likely to engage with the intervention. This is important because better physical and social functioning post-stroke have been associated with improved quality of life after stroke (Northcott et al. 2016; Pucciarelli et al. 2017).
Previous research has found that individuals who were working before their stroke reported better outcomes than patients who are not working (Marsh et al. 2018). In the present study, there was no significant difference between the groups in terms of work status at T1 or T3. Although more participants had returned to work by T3, with more participants working in the intervention group than the control group, the difference between the groups was not significant. It is possible that the lack of difference between the two groups may have been due to the large number of participants who were not working (and were retired) at the time of their stroke. It is possible that different results may be observed in a study with a larger number of working participants on study entry. An additional contributing factor to the lack of difference between the two groups in the present study may have been that the return-to-work measure may not have been sufficiently sensitive to change to work hours, duties, responsibilities and performance. Future research could explore this in more detail.
Usage of the new intervention was lower than expected, suggesting that the intervention did not fully meet the support needs of people with minor stroke. However, those participants who attended the sessions reported that they felt the sessions were beneficial. It is possible that the lower than anticipated intervention participation may have contributed to the lack of significant findings. Participants in the intervention group were less likely to attend if they had poorer physical functioning, according to the SF-36. It is possible that physical difficulties may have prevented these participants from attending. Surprisingly, however, when COVID-19 restrictions started and telehealth sessions were offered instead (removing the need to physically travel to the session location) participant uptake of the education sessions did not increase. For some participants, technology limitations prevented uptake of the telehealth sessions. Future research could explore the lower usage in more detail.
It is also worth noting that relatively lower feedback ratings were received for ‘hearing about others experience’ and ‘appropriate venue’. In the case of the first item, it is possible that this might have been impacted by the lower than expected attendance at the sessions (one–six participants per session). For the second item, it is possible that the physical location of the face-to-face sessions was not favoured by participants. It is possible that the shift to virtual sessions due to COVID-19 restrictions may have impacted the ratings for both items. Further research should explore these elements in more detail to improve the participant experience of the intervention. Additional attention could also be given to circumnavigating the reasons provided for non-attendance in the current study, including age and information provision method. As participants’ comments about non-attendance indicated that some participants did not view the sessions as relevant and that they may have either not received or dismissed messages/reminders about the sessions, it would be beneficial for future research to explore what session content would have been more relevant, how the nature and potential benefits of attendance may have been better communicated, and the most engaging and attention-grabbing format for upcoming session reminders.
Clinical and research implications
The results of the current study suggest that a new multicomponent intervention did not fully meet all the unmet needs of minor stroke survivors during their transition from hospital back into everyday life. The greatest potential benefit of the intervention may be in improving emotional wellbeing and physical functioning after minor stroke rather than unmet need, social participation, health-related quality of life, return to work and social group membership. The results highlight the importance of health professionals providing people with minor stroke access to information about minor stroke and services after hospital discharge, and that the most beneficial format and content of this information is unknown. As usage of the components was less than expected, the study also highlights that there is not a one-size-fits-all approach to this post-hospital discharge information. Future research needs to explore the components in detail, ideally within a co-design framework, to modify the intervention. Consideration should also be given to a fully telehealth model for the education sessions. Given the key positive outcome of the intervention was in wellbeing, future iterations of the intervention should explore this in more detail. Specifically, there is a need for future research to explore the mechanisms underpinning improvements in wellbeing (such as through the use of more comprehensive qualitative interviews) so that those components of the intervention that are relevant for enhancing wellbeing could then be amplified in subsequent iterations of the intervention. It would also be beneficial to explore the predictors of intervention uptake and response, so that the intervention can be designed, evaluated and implemented accordingly. Future research will also need to explore the power issues of the current study, potentially through multisite recruitment. The cost-effectiveness of the intervention could also be explored.
Limitations
The results of the present study were limited by the COVID-19 pandemic. As outlined earlier, due to the associated lockdowns and research restrictions, recruitment was lower than intended. Similarly, the education sessions were planned to be delivered in face-to-face mode but were required to move to online format. Further research is required, therefore, to refine and evaluate the program with delivery of sessions via telehealth. The study findings may also be limited by the restriction of data collection to a single site. As treatment fidelity was not included in the evaluation, future research could explore this element. Additionally, as the definition of minor stroke we used was predominantly neurologically based, including the use of the NIHSS, it is possible that participants with undetected memory, attention and executive difficulties may have been included in the study, potentially impacting intervention engagement and response. Furthermore, as our exclusion criteria were other neurological conditions or previous stroke, it is possible that participants with other medical and psychiatric disorders were eligible and/or participated, which may have further influenced engagement and/or response to treatment. Finally, self-report measures were a key element of the study. It is possible that self-reports of function and limitations may not reflect actual ability and function.
Conclusion
The current study explored the efficacy of an evidence-based, multicomponent service pathway for people with minor stroke (SUN) compared to usual care. Despite limitations due to COVID-19, the new intervention led to improvements in self-reported emotional wellbeing and non-significant improvements in other domains. Further research is required to refine the intervention and trial the intervention in a large, multisite study. Regardless, the new intervention has the potential to provide a service pathway for people with minor stroke that could be utilised within other health services throughout Australia and internationally.
Data availability
The data that support the findings in this study are available on request from the corresponding author, EF. The data are not publicly available due to containing information that could compromise the privacy of participants.
Acknowledgements
The authors would like to thank the participants and their families for their involvement in the study. The authors would also like to thank the Princess Alexandra Hospital Hyper-Acute Stroke Unit and the Princess Alexandra Hospital allied health and pharmacy directors and their departments for support of the project and education sessions. Thanks are also extended to Judith Coombes from the Pharmacy Department at the Princess Alexandra Hospital for assistance with the education sessions and Bronte Whitlock for assistance with feedback scoring.
References
Allen SM, Mor V (1997) The prevalence and consequences of unmet need: contrasts between older and younger adults with disability. Medical Care 35, 1132-1148.
| Crossref | Google Scholar | PubMed |
Bushnell CD, Kucharska-Newton AM, Jones SB, Psioda MA, Johnson AM, Daras LC, Halladay JR, Prvu Bettger J, Freburger JK, Gesell SB, Coleman SW, Sissine ME, Wen F, Hunt GP, Rosamond WD, Duncan PW (2021) Hospital Readmissions and Mortality Among Fee-for-Service Medicare Patients With Minor Stroke or Transient Ischemic Attack: Findings From the COMPASS Cluster-Randomized Pragmatic Trial. Journal of the American Heart Association 10(23), e023394.
| Crossref | Google Scholar | PubMed |
Cruwys T, Steffens NK, Haslam SA, Haslam C, Jetten J, Dingle GA (2016) Social Identity Mapping: A procedure for visual representation and assessment of subjective multiple group memberships. British Journal of Social Psychology 55, 613-642.
| Crossref | Google Scholar | PubMed |
Eames S, Hoffmann T, Worrall L, Read S, Wong A (2013) Randomised controlled trial of an education and support package for stroke patients and their carers. BMJ Open 3(5), e002538.
| Crossref | Google Scholar | PubMed |
Edwards DF, Hahn M, Baum C, Dromerick AW (2006) The impact of mild stroke on meaningful activity and life satisfaction. Journal of Stroke and Cerebrovascular Diseases 15(4), 151-157.
| Crossref | Google Scholar | PubMed |
Finch EC, Foster MM, Fleming J, Aitken PD, Williams I, Cruwys T, Worrall L (2017) Undetected and underserved: the untold story of patients who had a minor stroke. The Medical Journal of Australia 206(8), 337-338.
| Crossref | Google Scholar | PubMed |
Finch E, Foster M, Cruwys T, Fleming J, Aitken P, Jaques K, Williams I, Shah D (2019) Meeting unmet needs following minor stroke: the SUN randomised controlled trial protocol. BMC Health Services Research 19(1), 1-7.
| Crossref | Google Scholar | PubMed |
Finch E, Foster M, Fleming J, Cruwys T, Williams I, Shah D, Jaques K, Aitken P, Worrall L (2020) Exploring changing needs following minor stroke. Health & Social Care in the Community 28, 347-356.
| Crossref | Google Scholar | PubMed |
Finch E, Foster M, Fleming J (2021) Disrupted biographies: making sense of minor stroke after hospital discharge. Disability and Rehabilitation 43(18), 2632-2639.
| Crossref | Google Scholar | PubMed |
Fryer CE, Luker JA, McDonnell MN, Hillier SL (2016) Self management programmes for quality of life in people with stroke. Cochrane Database of Systematic Reviews [8] CD010442.
| Crossref | Google Scholar | PubMed |
Green TL, King KM (2007) The trajectory of minor stroke recovery for men and their female spousal caregivers: literature review. Journal of Advanced Nursing 58(6), 517-531.
| Crossref | Google Scholar | PubMed |
Grimley RS, Rosbergen IC, Gustafsson L, Horton E, Green T, Cadigan G, Kuys S, Andrew NE, Cadilhac DA (2020) Dose and setting of rehabilitation received after stroke in Queensland, Australia: a prospective cohort study. Clinical Rehabilitation 34(6), 812-823.
| Crossref | Google Scholar | PubMed |
Hamre C, Fure B, Helbostad JL, Wyller TB, Ihle-Hansen H, Vlachos G, Ursin M, Tangen GG (2020) Balance and gait after first minor ischemic stroke in people 70 years of age or younger: a prospective observational cohort study. Physical Therapy 100(5), 798-806.
| Crossref | Google Scholar | PubMed |
Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C (2004) Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke 35(3), 731-735.
| Crossref | Google Scholar | PubMed |
Haslam C, Holme A, Haslam SA, Iyer A, Jetten J, Williams WH (2008) Maintaining group memberships: social identity continuity predicts well-being after stroke. Neuropsychological Rehabilitation 18(5–6), 671-691.
| Crossref | Google Scholar | PubMed |
Heinemann AW, Sokol K, Garvin L, Bode RK (2002) Measuring unmet needs and services among persons with traumatic brain injury. Archives of Physical Medicine and Rehabilitation 83(8), 1052-1059.
| Crossref | Google Scholar | PubMed |
Hinojosa R, Haun J, Sberna Hinojosa M, Rittman M (2011) Social isolation poststroke: relationship between race/ethnicity, depression, and functional independence. Topics in Stroke Rehabilitation 18(1), 79-86.
| Crossref | Google Scholar | PubMed |
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348, 1687.
| Crossref | Google Scholar | PubMed |
Intercollegiate Stroke Working Party (2023) National Clinical Guideline for Stroke for the UK and Ireland. Available at www.strokeguideline.org.
Johnson W, Onuma O, Owolabi M, Sachdev S (2016) Stroke: a global response is needed. Bulletin of the World Health Organization 94(9), 634-364A.
| Crossref | Google Scholar | PubMed |
Krishnamurthi RV, Ikeda T, Feigin VL (2020) Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology 54(2), 171-179.
| Crossref | Google Scholar | PubMed |
Lennon S, McKenna S, Jones F (2013) Self-management programmes for people post stroke: a systematic review. Clinical Rehabilitation 27(10), 867-878.
| Crossref | Google Scholar | PubMed |
Lorig KR, Sobel DS, Stewart AL, Brown J.r BW, Bandura A, Ritter P, Gonzalez VM, Laurent DD, Holman HR (1999) Evidence suggesting that a chronic disease selfmanagement program can improve health status while reducing hospitalization: a randomized trial. Medical Care 5-14.
| Crossref | Google Scholar | PubMed |
Malec JF, Lezak MD (2008) Manual for the Mayo-Portland Adaptability Inventory (MPAI-4) for adults, children and adolescents. Centre for Outcome Measurement in Brain Injury (COMBI). Available at www.tbims.org/combi/mpai
Marsh EB, Lawrence E, Hillis AE, Chen K, Gottesman RF, Llinas RH (2018) Pre-stroke employment results in better patient-reported outcomes after minor stroke: Short title: Functional outcomes after minor stroke. Clinical Neurology and Neurosurgery 165, 38-42.
| Crossref | Google Scholar | PubMed |
McHutchison CA, Cvoro V, Makin S, Chappell FM, Shuler K, Wardlaw JM (2019) Functional, cognitive and physical outcomes 3 years after minor lacunar or cortical ischaemic stroke. Journal of Neurology, Neurosurgery & Psychiatry 90(4), 436-443.
| Crossref | Google Scholar | PubMed |
Molenberghs G, Thijs H, Jansen I, Beunckens C, Kenward MG, Mallinckrodt C, Carroll RJ (2004) Analyzing incomplete longitudinal clinical trial data. Biostatistics 5(3), 445-464.
| Crossref | Google Scholar | PubMed |
Northcott S, Moss B, Harrison K, Hilari K (2016) A systematic review of the impact of stroke on social support and social networks: associated factors and patterns of change. Clinical Rehabilitation 30(8), 811-831.
| Crossref | Google Scholar | PubMed |
Op Reimer WJ, Scholte de Haan RJ, Rijnders PT, Limburg M, Van den Bos GA (1999) Unmet care demands as perceived by stroke patients: deficits in health care? Quality in Health Care 8(1), 30-35.
| Crossref | Google Scholar | PubMed |
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50(12), e344-e418.
| Crossref | Google Scholar | PubMed |
Pucciarelli G, Vellone E, Savini S, Simeone S, Ausili D, Alvaro R, Lee CS, Lyons KS (2017) Roles of changing physical function and caregiver burden on quality of life in stroke: A longitudinal dyadic analysis. Stroke 48(3), 733-739.
| Crossref | Google Scholar | PubMed |
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 11(1), 32.
| Crossref | Google Scholar | PubMed |
Stroke Foundation (2019) ‘My Stroke Journey.’ (Stroke Foundation) Available at https://strokefoundation.org.au/What-we-do/For-survivors-and-carers/My-Stroke-Journey
Stroke Foundation (2020) Clinical Guidelines for Stroke Management. Retrieved from https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management [19 September]
Suda S, Nishimura T, Ishiwata A, Muraga K, Aoki J, Kanamaru T, Suzuki K, Sakamoto Y, Katano T, Nishiyama Y, Mishina M, Kimura K (2020) Early cognitive impairment after minor stroke: associated factors and functional outcome. Journal of Stroke and Cerebrovascular Diseases 29(5), 104749.
| Crossref | Google Scholar | PubMed |
Tellier M, Rochette A (2009) Falling through the cracks: a literature review to understand the reality of mild stroke survivors. Topics in Stroke Rehabilitation 16(6), 454-462.
| Crossref | Google Scholar | PubMed |
Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, Thabane M, Giangregorio L, Dennis B, Kosa D, Borg Debono V, Dillenburg R, Fruci V, Bawor M, Lee J, Wells G, Goldsmith CH (2013) A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Medical Research Methodology 13(1), 92.
| Crossref | Google Scholar | PubMed |
Turner GM, Backman R, McMullan C, Mathers J, Marshall T, Calvert M (2018) Establishing research priorities relating to the long-term impact of TIA and minor stroke through stakeholder-centred consensus. Research Involvement and Engagement 4(1), 2.
| Crossref | Google Scholar | PubMed |
Turner GM, Aquino MRJV, Atkins L, Foy R, Mant J, Calvert M (2022) Factors influencing follow-up care post-TIA and minor stroke: a qualitative study using the theoretical domains framework. BMC Health Services Research 22(1), 235.
| Crossref | Google Scholar | PubMed |
Vlachos G, Ihle-Hansen H, Wyller TB, Brækhus A, Mangset M, Hamre SC, Fure B (2021) Cognitive and emotional symptoms in patients with first-ever mild stroke: The syndrome of hidden impairments. Journal of Rehabilitation Medicine 53(1), 1-8.
| Crossref | Google Scholar |
von Weitzel-Mudersbach P, Andersen G, Hundborg HH, Johnsen SP (2013) Transient ischemic attack and minor stroke are the most common manifestations of acute cerebrovascular disease: a prospective, population-based study–the Aarhus TIA study. Neuroepidemiology 40(1), 50-55.
| Crossref | Google Scholar | PubMed |
Woodman P, Riazi A, Pereira C, Jones F (2014) Social participation post stroke: a metaethnographic review of the experiences and views of community-dwelling stroke survivors. Disability and Rehabilitation 36(24), 2031-2043.
| Crossref | Google Scholar | PubMed |
Zerna C, Thomalla G, Campbell BCV, Rha JH, Hill MD (2018) Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. The Lancet 392(10154), 1247-1256.
| Crossref | Google Scholar | PubMed |