Adaptation and feasibility of START online, a multicomponent intervention for Australian carers of people with dementia: a pilot randomised controlled trial
Michelle Kelly A * , Kaylene Kilham A , Alison Walter A , Karen Bell-Weinberg A , Gill Livingston B and Briony Dow C D
A * , Kaylene Kilham A , Alison Walter A , Karen Bell-Weinberg A , Gill Livingston B and Briony Dow C D
A
B
C
D
Abstract
There are more than 400 000 Australians living with dementia and an estimated 200 000 carers who provide unpaid or informal care for a person with dementia. Around a third of those live in regional and rural Australia. The objective of this study was to test the feasibility and acceptability of the adapted telehealth STrAtegies for RelaTives (START) program within the Australian healthcare context.
A two-armed, randomised controlled pilot trial was conducted. Twenty-eight family carers were assigned to the START 8-week manualised coping program or cognitive behaviour therapy (CBT) delivered in a university psychology clinic. Carers completed pre- and post-intervention questionnaires to determine the acceptability of the program. Standardised measures of mood and perceived carer burden were also administered to explore evidence for limited efficacy using Reliable Change Indices.
Eighteen carers were allocated to the START program and 10 to the CBT program. At completion, 10 of 13 commencers in START completed, whereas four of eight eligible CBT carers completed. START carers reported higher overall satisfaction and acceptance of the intervention compared to carers who underwent CBT. Furthermore, the telehealth modality was shown to be a practical and acceptable method of intervention delivery, and satisfaction was rated highly (8.5/10). Referral to and demand for the program proved the largest challenge. Improvement in mood was indicated for some carers who completed START.
These findings indicate preliminary evidence for aspects of feasibility of the START intervention for supporting carers in their caring roles via telehealth delivery. Further investigation is needed to determine intervention efficacy for the treatment of mental health symptomology via telehealth. Any future trial examining dementia carers should first establish strong referral pathways and linkages with primary healthcare and diagnostic services.
Australian and New Zealand Clinical Trials Registry (ACTRN12617000413325).
Keywords: carers, carer support program, cognitive behaviour therapy, dementia, e-health, feasibility, START, telehealth.
Introduction
Dementia symptoms, including cognitive impairment and loss of inhibition, affect behaviour and are typically progressive and irreversible (Haro et al. 2014). These symptoms not only have major health and social consequences for those with a diagnosis of dementia, but also affect their carers. Care for people with dementia is provided within the home by families (informal care), in the home by formal paid carers, or in residential aged care. The natural progression of the disease requires increasing amounts of care, with the majority of care provided at home by family, either a partner or spouse (around 35% of carers), an adult child (41%), or other family members (15%) (NATSEM 2017). There are approximately 200 000 informal ‘carers’, roughly 0.8% of the Australian population, caring for a person with dementia (NATSEM 2017). Of the more than 70% of people living in the community with dementia, approximately 46% rely solely on their support (NATSEM 2017).
Although this caring role can provide positive outcomes of increased family togetherness and companionship (Netto et al. 2009), carers take on many complex and often physically and emotionally challenging tasks (Mahoney et al. 2005) with approximately 40% having clinically significant levels of anxiety and depression (Pinquart and Sörensen 2003; Cuijpers 2005). These adverse mental health impacts are in part related to the presence of behavioural and psychological symptoms of dementia (Haro et al. 2014). Dementia also leads to financial burden for families and the wider community. In 2018, it was estimated that dementia cost Australia over $15 billion, with the cost of hospitalisation and formal nursing care accounting for 88% of this expenditure (NATSEM 2017). Furthermore, family carers provide an average of between 4 and 55 hours of unpaid care per week (in addition to other commitments), saving the Australian economy $5.5 billion per year (Access Economics 2010) and close to £11.6 billion in the UK (Prince et al. 2014). These ongoing psychological, physical, and financial burdens have long been associated with poorer mental health (Etters et al. 2008; Temple and Dow 2018). Consequently, research that advances the development of effective interventions to maintain carer capacity and quality of life and accommodate their responsibilities is vital to the ongoing wellbeing of the carer and the person with dementia, as well as the wider community (Cooper et al. 2010; Temple and Dow 2018).
Several systematic reviews have examined interventions aimed at improving the overall wellbeing of carers of people with dementia (e.g. Cooke et al. 2001; Selwood et al. 2007; Gilhooly et al. 2016; Scott et al. 2016; Dickinson et al. 2017). There is good evidence that individual behaviour management training and individual or group-focused coping-strategy programs are effective but education or supportive therapy alone is not. In short, the evidence suggests that individualised, multicomponent interventions are the most effective for improving wellbeing and enabling carers to provide at-home care for longer (Hoe et al. 2007; Selwood et al. 2007; Parker et al. 2008; Dickinson et al. 2017). Specifically, programs should cover psychoeducation, individualised strategies for managing behavioural change, and psychological strategies for managing stress and other challenges that come with caring for people with dementia. Further, it has been suggested that only multicomponent interventions are effective in also reducing carer burden due to the broad nature of burden as a construct for measurement (Acton and Kang 2001). Accordingly, Livingston et al. (2014) adapted a US group-based caregiver skills program (Coon et al. 2003) into a coping program for UK carers to be delivered individually. The program, STrAtegies for RelaTives (START), involves eight (1 hour) face-to-face sessions focused on dementia education and coping skills plus relaxation strategies, and it was found in randomised controlled trials to be clinically and cost effective at 4 and 8 months, as well as 2 and 6 years after delivery, in reducing symptoms of anxiety and depression and improving quality of life (Li et al. 2014; Livingston et al. 2014). Those who received treatment as usual were found to be four and five times more likely to display clinical depression at the 8-month and 2- and 6-year follow-ups (Livingston et al. 2020). Although the majority of carers were provided START face-to-face, occasionally sessions occurred via telephone; however, this was not further evaluated. Overall, START was shown to be practical and acceptable to most carers (Sommerlad et al. 2014).
Telehealth services are particularly important within the Australian context (Bradford et al. 2016), as healthcare services are less accessible for those living in regional, rural, and remote areas where there are fewer healthcare professionals (Schirmer 2017). Limited and unreliable public transportation (van Gaans and Dent 2018) and psychological and health/mobility limitations (Somenahalli 2015) cause additional barriers for older health service seekers in both rural/remote and urban areas. Further, carers experience a range of barriers when accessing appropriate respite care, restricting capacity to attend face-to-face appointments (Neville et al. 2015), all of which add to the burden of caring for a person with dementia. Globally, COVID-19 has also highlighted the difficulty and importance of providing consistent service provision via a face-to-face modality. Telehealth is a flexible modality, with a growing body of research highlighting the benefits of this approach in meeting carer needs (Czaja and Rubert 2002; Torp et al. 2008; Wilz and Soellner 2015). Given START has not yet been evaluated in the Australian context, nor utilising technology, this was an appropriate next step in demonstrating the clinical utility of this program.
The aim of the current research was to adapt the START intervention to suit Australian carers and determine the feasibility and acceptability of this adapted program within the Australian healthcare context using Bowen’s framework (Bowen et al. 2009). We examined START in comparison to cognitive behaviour therapy (CBT), an evidence-based intervention for the treatment of anxiety and depression (Hollon et al. 2006). Given there are few supports for carers of people with dementia available, the growing number of people with dementia living in the community, and carer reports of distress, it was expected there would be adequate demand for the adapted program to meet study objectives. We aimed to investigate the practicality of implementing the START program within a university training clinic. It was predicted that carers would rate the START program as more relevant to their difficulties than the CBT program. It was further predicted that carers would report acceptability of the telehealth application of the START program and that this would prove a feasible delivery method. Finally, we expected to demonstrate some limited evidence for efficacy for participants in both programs for mood and carer burden measures.
Methods
Study design
This is a two-armed, randomised controlled pilot study with blinded (completed independently by participants online) measures completed pre-, 1-week post-, and 3-months post-intervention. The study has been reported according to the CONSORT checklist for pilot trials (Eldridge et al. 2016).
Participants
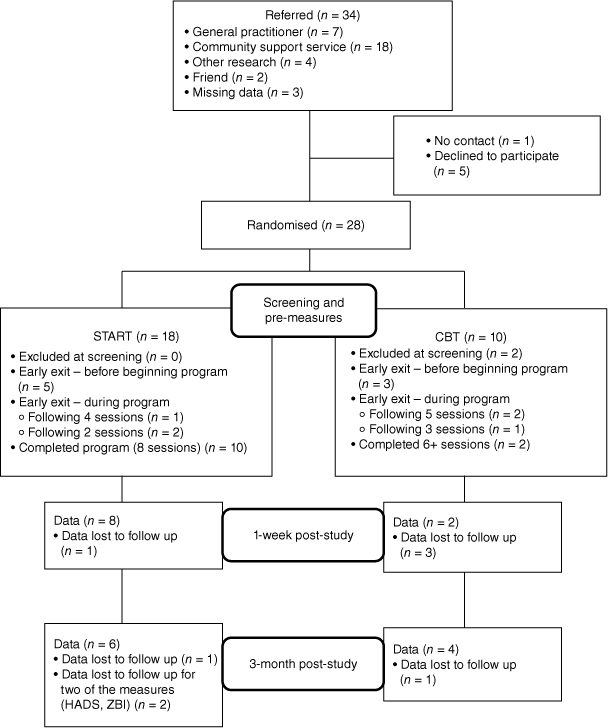
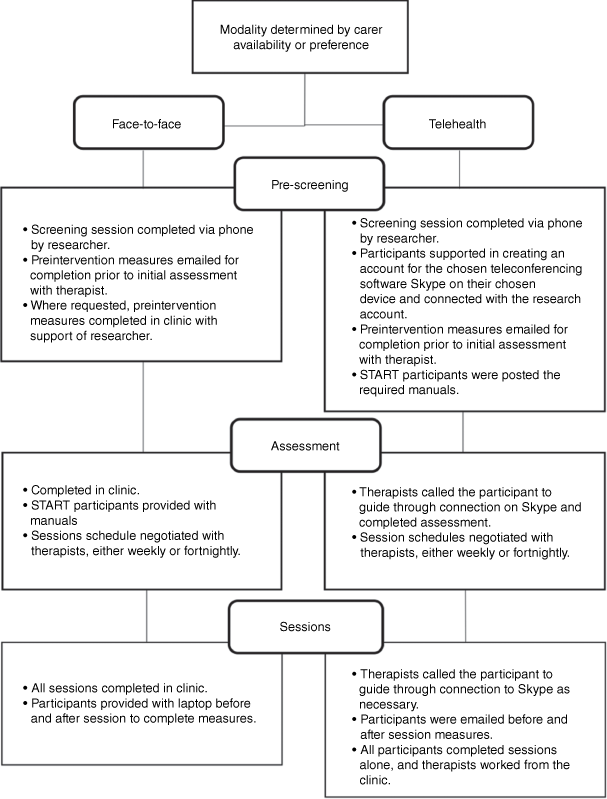
Community dwelling carers were recruited through Geriatricians, mental health services, General Practitioners (GPs), carer support, and community organisations. Referrers either provided researchers with contact details with participant consent or encouraged eligible individuals to self-refer. The majority of carers were randomly assigned to the START intervention group or the CBT group via blocked randomisation at a ratio of 2:1 respectively. Given recruitment difficulties, the final three participants were allocated to the START program. They were then screened for suitability and completed pre-intervention measures. The recruitment and engagement details are provided in Fig. 1. Carer availability and preferences determined the method of program delivery (face-to-face or telehealth (videoconferencing and/or phone conferencing); see Fig. 2).
Carers were eligible for inclusion if they were over the age of 18 years of age, provided current practical or emotional support on at least a daily basis for a person with dementia, and had adequate English language proficiency. Initially, only eligible carers with clinically significant affective symptoms and or self-reported carer burden were to be included; however, these criteria were relaxed due to (1) requests by carers who self-reported that they were experiencing distress as a result of their caring role and otherwise had no formal support available to them, and (2) difficulty recruiting enough carers that met the strict inclusion criteria. This was considered an acceptable variation to protocol given the original START randomised control trial had no inclusion requirement related to current symptomology (Livingston et al. 2014). Exclusion criteria were current major psychiatric illness (e.g. psychosis) on the part of the carer, high suicidality, or significant cognitive difficulties. Carers were aware that they would be offered an intervention but were blinded to randomisation.
Therapists were provisionally registered postgraduate students enrolled in the Master of Clinical Psychology program, with the exception of one therapist, who was a Clinical Psychologist, and an academic staff member at the institution. Participation was voluntary, and therapy hours as part of this study contributed to clinical placement requirements. All therapists received weekly supervision by a Clinical Psychologist as well as frequent interaction with a Clinical Psychologist trained in the delivery of START.
Measures/instruments
The primary outcome for this study was the feasibility of a novel intervention for use within the Australian context. We examined six of the eight areas outlined by Bowen and colleagues; the extent to which there was demand for the START program in a regional location; the implementation, practicality, adaptation, and acceptability; and limited efficacy testing (Bowen et al. 2009). We further assessed the practicality and acceptability of telehealth via comparison of participant self-report across modalities, in addition to participant-rated satisfaction and confidence in accessing the program using telehealth.
Outcomes were assessed via a questionnaire designed specifically for this study. The Carer Experience Survey was conducted 1-week post- and 3-months post-program with up to 12 questions in each. This survey measured acceptability and adaptation regarding telehealth delivery of the program (e.g. ‘Were your expectations met?’) rated on an 11-point Likert Scale (0–10) ranging from ‘not met’ to ‘exceeded’ and perceived relevance of the program (e.g. ‘How relevant was the program?’) rated on an 11-point Likert Scale (0–10) ranging from ‘not relevant’ to ‘extremely relevant’, as well as some open responses (optional). Carers were also asked to rate their experience according to mode of program delivery (face-to-face or telehealth, e.g. ‘Attending the clinic is an acceptable way to receive this program’ or ‘Online was an acceptable way to receive this program’) on an 11-point Likert Scale (0–10) ranging from ‘strongly disagree’ to ‘strongly agree’ (measuring practicality and acceptability).
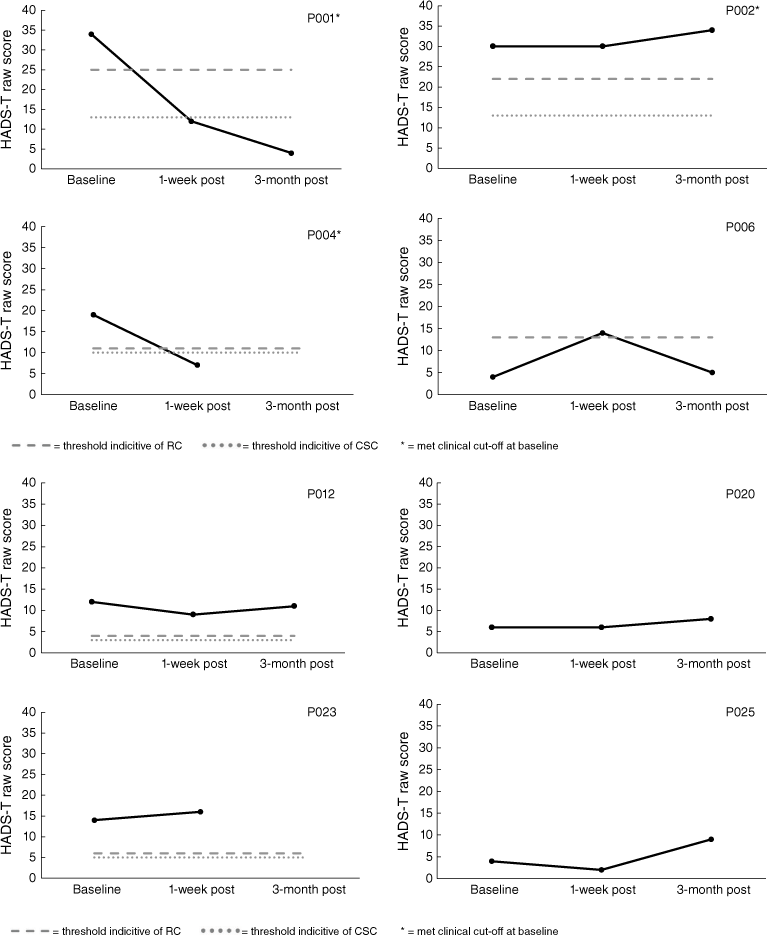
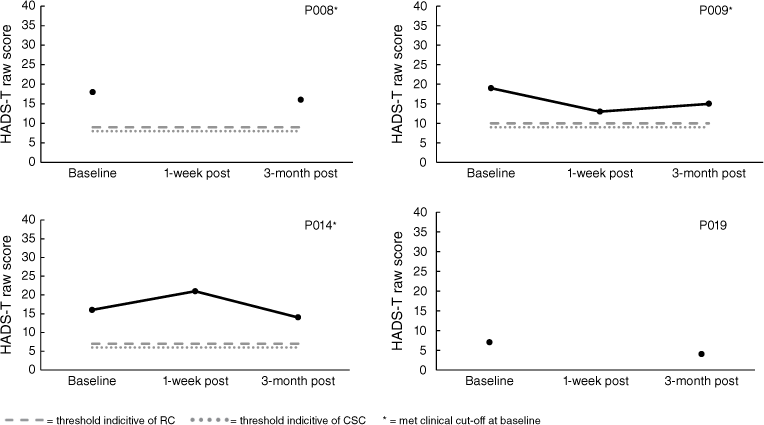
HADS (Zigmond and Snaith 1983) is a validated 14-item self-report questionnaire used to indicate anxiety and depression symptomology. The HADS has good reliability (α = 0.83), validity with correlations with the Beck Depression and Anxiety scales ranging from 0.61 to 0.83 (Bjelland et al. 2002), specificity of 0.78, sensitivity of 0.9 (HADS-A), specificity of 0.79, and sensitivity of 0.83 (HADS-D). A total score out of 42 is reported as a primary outcome measure in the UK START studies (e.g. Livingston et al. 2014). Here, the HADS total score was also utilised. For analyses, a clinical cut-off of 16+ was employed based on previous research (Bjelland et al. 2002) and was used in Li and colleagues (2014) START randomised controlled trial.
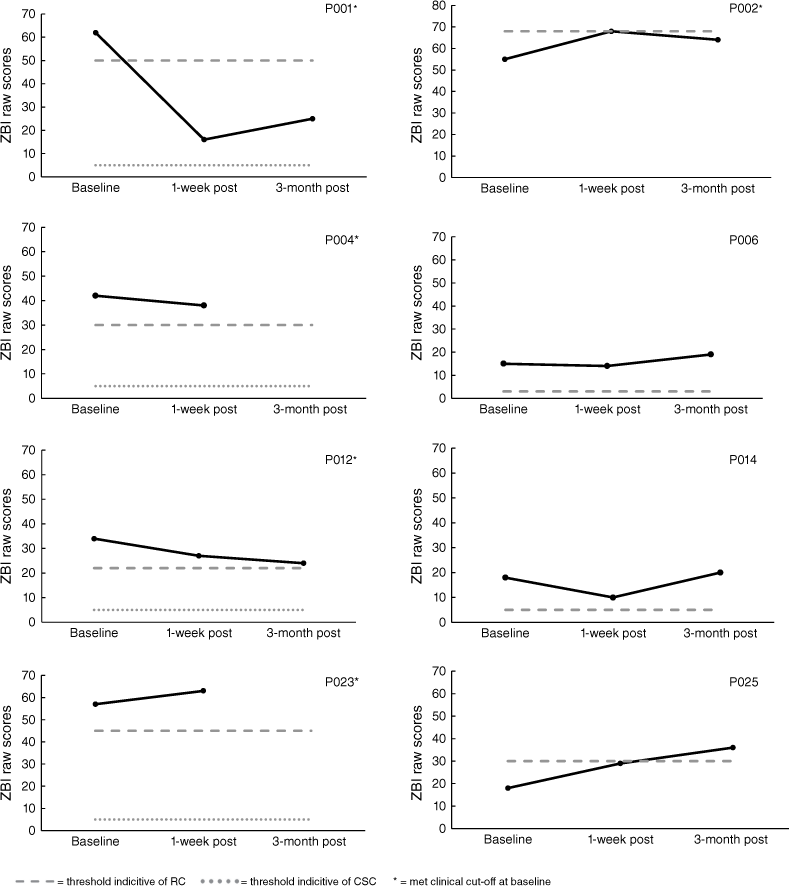
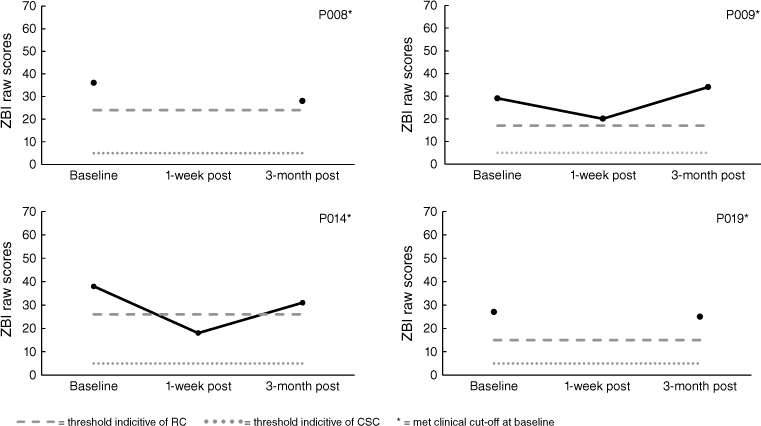
The Zarit Burden Interview (ZBI: Zarit, Reever, and Bach-Peterson 1980) is a 22-item self-report questionnaire rated on a four-point Likert Scale to determine perceived carer burden (maximum score 88). The ZBI displays excellent internal reliability (α = 0.94) (Hébert et al. 2010), test–retest reliability (ICC = 0.89), and adequate concurrent validity (r = 0.53–0.73) (Seng et al. 2010). For analysis, clinical cut-offs of 21+ indicated at least mild burden (Stagg and Larner 2015).
All data were collected using the web-based survey software and questionnaire tool Qualtrics (https://au1.qualtrics.com). Hard copy surveys were also available for carers who were not familiar with using computers. These methods ensured no bias was introduced during outcome measure collection.
All START sessions were recorded for the purposes of fidelity rating. The authors (M. K. and K. B. W.) reviewed 5% of all START sessions, rating them against a checklist, and supervisors of the therapists oversaw adherence to CBT principles, observing sessions and discussing content and plans in supervision.
The START program runs for eight (1 h) one-on-one sessions with the therapist and carer. Session 1 covers what is dementia, symptoms, behaviours, and managing stress. Session 2 covers reasons for behaviour, what is the purpose of behaviour, and what might trigger and maintain behaviours. Session 3 covers making a behaviour plan, changing behaviours, and changing responses. Session 4 covers behavioural strategies and unhelpful thoughts. Session 5 covers how to better communicate with those around you to get the support needed. Session 6 covers planning for the future with links to various resources and supports that may be required. Session 7 covers pleasant event scheduling and managing mood, and Session 8 is a review session.
Revised editions of the UK START manuals (Therapist and Carer versions) were developed to acknowledge differences in language and healthcare resources between the UK and Australian healthcare systems. These changes included the provision of a supplementary booklet that provided links to national and state carer support and advocacy services. The manual was reviewed and approved by a panel of experts from the carers group from Dementia Australia as well as researchers from the University of Newcastle, the National Ageing Research Institute, and the University College London. The carer manual details session content and contains support resources and relaxation audio tracks (see University College London’s (n.d.) START website for full details on session content; https://www.ucl.ac.uk/psychiatry/research/mental-health-older-people/projects/start; and for the Australian version, see https://sites.google.com/view/sociabilitylab/current-projects). Completion of all eight sessions was deemed ‘complete’ for the purpose of acceptability; however, like for CBT below, the client could choose to cease the program early and not complete all modules.
Standard cognitive behavioural therapy was employed. We examined START in comparison to CBT, as it is the evidence-based intervention of choice for the treatment of anxiety and depression in Australia (Hollon et al. 2006). CBT, like START, has psychoeducation, distress management, and components to understand the relationship between thoughts, feelings, and behaviours. However, unlike START, those who receive CBT would spend more of the therapy focused on the self rather than the person they are caring for and those interactions. Materials deemed suitable, such as worksheets, were utilised with the discretion of the therapist and supervisor. Although 10 sessions is considered standard CBT in the Australian context due to its funding model, and is the median session number reported in reviews (Okumura and Ichikura 2014; López-López et al. 2019), the therapy was deemed complete when both therapist and client determined this to be the case, regardless of session number. All student therapists had undertaken basic training in CBT.
Procedure
All participants provided informed consent. Carers completed an initial assessment with their allocated therapist (up to 1.5 h), assessing goals, carer related concerns, and relational history with the person with dementia and risk. Intervention sessions were completed weekly or fortnightly, depending on carer availability. Carers in the START program completed eight (1 h) individualised sessions. The program included recommendations to practice the strategies and relaxation exercises between sessions. Carers in the CBT program completed unlimited (1 h) individualised sessions. Regardless of program allocation, carers were required to complete mood/burden measures across three time points: pre-intervention, 1-week post-intervention and 3-months post-intervention. Experience surveys were completed 1-week and 3-months post-intervention only. In addition, all carers completed weekly reflection and feedback questionnaires following each session.
Student therapists were required to work in the clinic 1 day a week, with each day randomly allocated to treatment type to avoid contamination. Therapists completed a pre-study survey, recording experience and expectations, in addition to weekly session feedback surveys. START therapists were required to complete a 4-h video-training module and were provided with a program manual, outlining the content for each session. All therapists were requested to complete post-study feedback surveys. The therapist experience was not a focus of this study (see Walter et al. in press).
Data analysis
Due to the study aims being primarily focused on feasibility and acceptability, power calculations were not conducted (Lancaster et al. 2004; Thabane et al. 2010). Recommendations for sample size for pilot/feasibility trials is between 24 and 50 participants (Julious 2005; Sim and Lewis 2012). As such, our recruitment target was 50 carers (allowing for attrition).
Descriptive and demographic data were examined using the IBM SPSS statistical package (V24.0, 2016). Should the study have met the maximum recruitment target, between-groups analysis would have been conducted to demonstrate limited efficacy. However, due to small sample sizes acquired, and in order to explore individual experience, data for mood and carer burden were analysed to provide description of the immediate and 3-month post-treatment data of the intervention for each carer. To examine limited efficacy, the Reliable Change Index (RCI; Jacobson and Truax 1991) was used to detect reliable change (RC) and, where appropriate, clinically significant change (CSC) for each carer for anxiety, depression, and burden over time. All calculations were completed according to the formula outlined by Jacobson and Truax (1991), using Morley and Dowzer’s (2014) Excel RCI calculator. Reference data used to determine the RC and CSC are detailed in Table 1.
| Measure | Cronbach’s alpha | Clinical norm reference | Non-clinical norm reference | CSC criterion | |
|---|---|---|---|---|---|
| HADS-T | α = 0.83 (Bjelland et al. 2002) | M = 14.80, s.d. = 7.40 (Livingston et al. 2014) | M = 9.82, s.d. = 5.98 (Crawford et al. 2001) | B | |
| ZBI | α = 0.92 (Hébert et al. 2010) | M = 38.10, s.d. = 17.00 (Livingston et al. 2014) | n/a | A |
Results
Feasibility of the START program
A total of 28 participants were referred to the research program over 2.5 years period, by GPs and other aged care and mental health services. Demographic data for all carers is presented in Table 2. Of the 18 carers in the START group, five did not begin the program. No contact could be made with three of the five carers, one carer was unable to commit due to their own health concerns, and the other carer reported a time commitment issue in addition to finding the pre-intervention questionnaires confronting. Ten (55.5%) completed START. In the CBT group, 5 of the 10 randomised carers did not begin the program. Of the eight who met the eligibility criteria, two indicated that they did not wish to participate but gave no further reason. Two withdrew, citing irrelevance to their current situation (inclusive of one who completed three sessions). Four carers (40%) were deemed to have completed their treatment following between 5 and 12 sessions, and their data were included.
| Participant | Sex | Age | Ethnicity | Marital status | Education | Occupation | Employment | Caring for | Resides with | Modality | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| START | |||||||||||
| 001A | F | 49 | White Australian | Married | University | Home duties | Nil | Mother | Yes | Clinic | |
| 002A | F | 52 | White Australian | Single | University | Fulltime carer | Nil | Mother | No | Telehealth | |
| 003A | M | 79 | White Australian | Married | TAFE | Sign writer | Retired | Spouse | Yes | d/a | |
| 004A | F | 51 | White Australian | Married | n/a | n/a | n/a | Mother | Yes | Telehealth | |
| 006A | F | 58 | Aboriginal peoples | Married | High school | n/a | Retired | Mother | No | Clinic | |
| 010B | M | 52 | White Australian | Married | n/a | Farm contractor | Fulltime | Spouse | Yes | d/a | |
| 012A | F | 77 | Irish | Married | TAFE | Small business | Retired | Spouse | Yes | Telehealth | |
| 017B | M | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 018B | F | 74 | White Australian | Married | TAFE | Small business | Retired | Spouse | Yes | Clinic | |
| 020A | F | 60 | White Australian | Married | University | Speech pathologist | Retired | Spouse | Yes | Telehealth | |
| 021B | F | 72 | White Australian | Married | University | Teacher | Retired | Spouse | Yes | Clinic | |
| 022B | F | 61 | White Australian | Married | TAFE | Director | Retired | Mother | Yes | d/a | |
| 023A | F | 73 | Dutch | Married | University | Project coordinator | Part-time | Spouse | Yes | Telehealth | |
| 024B | F | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 025A | F | 70 | White Australian | Married | High school | Administration assistant | n/a | Spouse | Yes | Telehealth | |
| 026B | F | 70 | White Australian | Married | High school | n/a | Retired | Spouse | Yes | Telehealth | |
| 027B | F | 68 | White Australian | Married | No qualification | Seamstress | n/a | Spouse | No | Telehealth | |
| 028B | F | 61 | White Australian | Single | TAFE | Aged care | Retired | Mother | Yes | Telehealth | |
| CBT | |||||||||||
| 005B | M | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 007B | M | n/a | n/a | n/a | n/a | n/a | n/a | Spouse | n/a | d/a | |
| 008A | M | 79 | Dutch | Married | High school | Panel beater | Retired | Spouse | Yes | Clinic/Telehealth | |
| 009A | M | 80 | White Australian | Married | TAFE | Supervisor | Retired | Spouse | Yes | Clinic | |
| 011B | M | 85 | Scottish | Married | TAFE | Building inspector | Retired | Spouse | Yes | Clinic | |
| 013B | F | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 014A | M | 60 | White Australian | Single | University | Software engineer | Nil | Mother | Yes | Telehealth | |
| 015B | F | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 016B | F | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | d/a | |
| 019A | F | 76 | White Australian | Married | TAFE | Secretary | Retired | Spouse | Yes | Clinic/Telehealth | |
A small amount of funds (A$7000) were obtained to run this pilot study. The funds covered minimal research assistant hours, costs of printing manuals, and a small fee-for-service payable to the student training psychology clinic, which runs in a cost-recovery model. Three student researchers as well as 15 student therapists were involved in the study. The program was successfully integrated into the student training psychology clinic, and this clinic continues to accept START referrals to date, demonstrating some evidence for implementation and practicality.
Of the 13 carers who commenced the START program, 10 completed all eight sessions. Two carers completed two sessions, and a third completed four sessions. One of the three cited ‘being overwhelmed/stressed’ as reason for withdrawal, one cited hospitalisation of the person with dementia, the third cited not having enough time. The average session completion rate was 82% (6.5 sessions from 8). Accordingly, the attrition rate was calculated at 17%. When considering all who began the program, 77% of participants completed all sessions. Carers in the CBT group completed an average of seven sessions (completing 5, 5, 6 and 12 sessions).
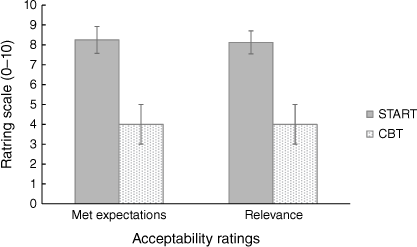
The average rating for ‘the program met expectations’ for carers who were in the START program was higher (M = 8.25, s.d. = 1.91) than for carers in the CBT program (M = 4, s.d. = 1.41). The average rating of ‘relevance’ for START program carers was higher (M = 8.13, s.d. = 1.64) compared to carers (M = 4, s.d. = 1.41) in the CBT program (Fig. 3). When asked about how helpful the START program manual was, the carers in the START group rated it on average 8/10 (s.d. = 2.5). Furthermore, all nine START carers indicated that they intended to continue using the strategies they had learnt and would refer other carers to the program, compared to two out of four carers in the CBT program.
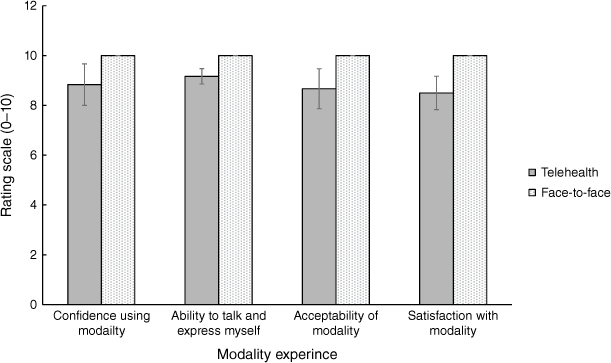
As the current research is focused on determining the adaptation to, and acceptability of, the telehealth delivery of the START program, only START carer data were examined in this regard. As can be seen in Fig. 4, all mean satisfaction scores were above 8/10 for both the telehealth (n = 7) and face-to-face (n = 2) conditions, indicating very high satisfaction with the program. Carers indicated confidence in using telehealth (M = 8.83, s.d. = 1.60) and a high ability to talk and express themselves with their therapists (M = 9.17, s.d. = 0.75). Average overall satisfaction with and reported acceptance of the telehealth mode was 8.50 (s.d. = 1.64) and 8.67 (s.d. = 1.97) respectively.
Analysis of mood (HADS-T) scores indicated that three of the nine carers in the START program met clinical cut-offs at baseline. Carers P001 and P004 indicated reliable improvement and CSC. P001 maintained these gains when followed-up at 3-months post-intervention; however, 3-month follow-up data for P004 was not obtained. Conversely, P006 displayed reliable deterioration immediately following the intervention; however, they did not meet the criteria for CSC and no longer met the criteria for reliable deterioration when followed-up at 3-months post-intervention. For the CBT program, three out of four met clinical cut-offs at baseline; nevertheless, RC was not observed for any CBT carers at 3-month follow-up. All results for the HADS-T are displayed in Figs 5 and 6. Note, there is missing 1-week post follow-up data for P008 and P019 as well as 3-month post follow-up data for P004 and P023.
Analysis of burden (ZBI) scores indicated that five of the nine carers in the START program met clinical cut-offs at baseline. Only P001 demonstrated reliable improvement, which was maintained at 3-months. P002 displayed increased burden immediately following the intervention, however, when followed-up 3-months later reliable deterioration was not demonstrated. P025 displayed reliable deterioration at 3-month post. For carers in the CBT program, all four met clinical cut-offs at baseline. P014 demonstrated reliable improvement initially but not at 3-month follow-up. All results for the ZBI are displayed in Figs 7 and 8. Note, missing 1-week post follow-up data for P008 and P019, and 3-month post follow-up data for P004 and P023.
Discussion
The primary aim of the current study was to investigate the feasibility of the adapted START program to support Australian carers to cope in their caring roles. This study further aimed to explore whether telehealth was an acceptable means of delivering the START program. Individual carer outcomes were also examined to determine whether the program demonstrated limited efficacy through a reduction in self-reported symptoms of anxiety, depression and perceived burden.
Feasibility of the intervention was examined in a number of ways, and the outcomes were variable. With regards to demand, practicality, and implementation, despite knowing that carer burden is prevalent in dementia carers in Australia, recruitment was challenging. In comparison with the original UK study (Livingston et al. 2014), it was necessary to establish new referral pathways via cold-calling GP practices, mental health, and other aged care services. This also meant relying on the practitioners in those centres to remember to offer a referral. This likely had implications for follow-through by the carer who, in some cases, may have only indicated minimal interest prior to us receiving their details, potentially explaining both the low frequency of referral and low uptake. Of the 13 carers who did begin the program, 77% completed all eight sessions, and reasons for dropout were not surprising and were consistent with other similar studies for carers of people with dementia (Gitlin et al. 2003; Livingston et al. 2014).
As expected, START carers indicated greater acceptability of their intervention, reporting higher rates of the program meeting expectations and higher levels of relevance to their situation and current needs than those in the CBT intervention. Furthermore, all carers in the START program reported that they intended to use the strategies learnt and would recommend the program to others. These benefits are similar to those identified in a previous qualitative study (Sommerlad et al. 2014). In comparison, carers in the CBT program completed an average of seven sessions, where the standard number of sessions for the treatment of depression (Linde et al. 2015) and anxiety (Kishita and Laidlaw 2017) is 10–12 (Okumura and Ichikura 2014; López-López et al. 2019). Notably, our results indicated that carers in the CBT program remained within the clinical range for reported mood symptoms. Given the low ratings of carer perceived relevance of the CBT program and average number of completed sessions, it is possible that CBT carers disengaged with the program early on due to a lack of perceived benefit and relevance. It is also possible that those being cared for experienced a deterioration of condition, therefore requiring more care, which has been identified as a mediator of carer affect and burden (Pinquart and Sörensen 2003).
There is emerging evidence that telehealth modalities are growing in acceptance within the Australian (Dow et al. 2008; Bradford et al. 2016; Laver et al. 2020) and international contexts (Czaja and Rubert 2002; Torp et al. 2008; Wilz and Soellner 2015). The current research, albeit with a small sample, suggests that the START program delivered in Australia via telehealth offers a highly satisfactory experience, without compromising carers’ confidence or perceived ability to talk and express themselves with their therapists. This was consistent with results found by our collaborators in Melbourne, Australia (Loi et al. 2022). In addition, this study was uniquely able to demonstrate that there was little difference in reported acceptance of the START program between those who attended via telehealth and those who attended face-to-face sessions, suggesting our adaptation was successful.
Our findings are consistent with multiple other studies examining the effect of mode of delivery of various psychological therapies in therapeutic alliance and outcome (Kiropoulos et al. 2008; Andersson et al. 2012; Stiles-Shields et al. 2014; Pihlaja et al. 2018). One study (Marziali et al. 2018) specifically compared a face-to-face psychosocial support group intervention with a telehealth (videoconferencing) format for 34 carers of persons with neurodegenerative disease (1/3 Alzheimer’s Disease). They found that 95% of carers indicated that the telehealth format exceeded their expectations and felt able to communicate ‘at a deep level’. Albeit limited by sample size, our findings suggest that the use of telehealth services is comparable to face-to-face modalities for the delivery of the START program when considering the quality of the therapeutic alliance; however, further research is required to determine the influence of modality on psychological outcomes.
Although telehealth can increase access to healthcare for those living rurally and remotely, potential inequities are also introduced when technology is required for any health intervention. Specifically, both access to and knowledge of the software platforms and technology, as well as reliable internet connection, can all be barriers to clients engaging in health treatments via telehealth (Eddison et al. 2022). In the current study, we tried to ameliorate some of these barriers through negotiating space at local GPs (often the referrer) clinics, which would allow the client to attend the GP clinic and be assisted to use a computer. However, this offer was not taken up by any carers and does not always overcome the burden of travel.
In line with previous research (Hollon et al. 2006; Livingston et al. 2014) and in line with providing evidence for limited efficacy, we expected that both interventions would result in an improvement in mood for all carers. Despite there being only small numbers to examine, the Reliable Change Indices that were calculated are thought to be the most conservative estimate of CSC (Vaganian et al. 2020). We found that two out of the three START carers who met clinical cut-offs at baseline reported mood symptoms within the normal range post-intervention. Conversely, this improvement was not seen in the CBT carers. Notably, those START carers that did not reach clinical cut-offs at baseline remained in the normal range at follow-up. Li et al. (2014) reported similar results, suggesting that the START program had both prevention and intervention effects. Although we were not powered to determine efficacy, our results offer preliminary evidence for better outcomes in those who completed START compared to usual practice, again, consistent with the Melbourne study (Loi et al. 2022). Interestingly, one START carer P006 (attending sessions face-to-face) displayed reliable deterioration at 1-week post follow-up that was not maintained when followed up at 3-months. Feedback from the carer indicated that she was highly engaged throughout the intervention and valued the support of the therapist; thus, decline post-intervention may be attributed to the intervention ending and perceived loss of support (Webb et al. 2019).
We hypothesised that the carers in the START program would display a reduction in subjective burden following intervention. However, of the five carers in the START program indicating clinically significant burden at baseline, only one indicated reliable and clinically significant improvement post-intervention, whereas another met criterion for reliable deterioration immediately following the invention. In an earlier review aimed at investigating the efficacy of various interventions, authors surmised that burden may be difficult to influence via intervention (Cooke et al. 2001; Brodaty et al. 2003; Pinquart and Sörensen 2006). Although appropriate for the identification of carer burden, measures such as the ZBI may be insensitive to change, thereby offering some explanation for the null findings in the current and previous studies. Another possibility is the timing of assessment, with the skills and knowledge thought necessary to reduce carer burden taking time to implement and take effect before benefits can be observed (Cooke et al. 2001). This is consistent with the qualitative feedback provided by one carer who suggested more time between sessions might be helpful to lay down skills.
Limitations and implications for future research
Recruitment and retention of carers for this study proved difficult, presenting important implications for the feasibility of a fully powered trial. Firstly, the referral rate was quite low and very slow and required consistent contact with referrers. Those general practices that referred well were located further afar, suggesting a lack of alternative services and general social support may have played a role. Further, the diagnostic process for dementia is far from ideal with no single, direct route to assessment. This may also have played a role in referral rate with multiple different providers at different stages of the diagnostic pathway. The research also relied heavily on the recruitment support of third parties in primary healthcare and community support services. It is possible that this may have biased uptake of the program both in negative and positive ways. Overall, despite 28 carers being referred to the program, of these the researchers were not able to contact five, and five did not attend an appointment. This suggests that they may not have been on board with the referral in the first place. This is different to studies in the UK where, in many cases, a specialist clinic and medical doctor recommended the program at the point of diagnosis, potentially positively influencing uptake. In future trials, researchers should consider establishing strong partnerships with relevant health services to improve referral rates and access to carer support programs, as our results indicate that those who begin the START program are likely to remain engaged and benefit from it.
Health concerns on behalf of the carer and the person with dementia were not uncommon, with five carers citing this as a reason for not commencing the program and three carers withdrawing after commencement of the program. Furthermore, both health concerns and other commitments also contributed to carers’ perceptions that they did not have the time to commit to the program. Previous research highlights potential barriers facing carers in seeking out support. Carers can have difficulty recognising when they need support (Neville et al. 2015) or, when they do, they may feel ambivalent or guilty about seeking support (Greenwood and Smith 2015), and they can feel unworthy of self-care and engage in self-sacrificing behaviour (Furlong and Wuest 2008), especially where the intervention or support does not specifically focus on the person with dementia (Neville et al. 2015). This was evident in our study, with many carers raising concerns about the time they were spending away from the person they cared for or stating they did not have time to do the program due to medical appointments taking up a lot of time. Carers also raised some concerns in parts of the START program where the content was more focused on their wellbeing, again highlighting the importance of providing rationale for how the program can help them be a better carer, and this is reliant on them also taking care of themselves. This issue is further supported by the possible lower engagement of carers in the CBT program, which focused on the carers’ cognitions and behaviours rather than the caring dyad. This suggests that when marketing carer support programs, communication of the benefits to the person with dementia need to be prioritised.
The trial originally intended to recruit up to 50 participants, which would have enabled greater examination of between-group differences, inclusive of measures of efficacy. Due to the aforementioned challenges with recruitment, uneven group sizes, and unequal groups based on demographics, etc. (see Table 2), we were not able to meet our recruitment target and as such had to relax randomisation and adapt the analyses to suit our small numbers. We were still able to show limited evidence for the acceptability of the program delivered via telehealth, and importantly, these results were backed by a sister study conducted in Melbourne (Loi et al. 2022). Finally, it is necessary to consider self-selection of participants to modality as a potential inlet for bias in acceptability. This pilot study was conducted in a clinic where both face-to-face and telehealth appointments are available, and selection to either are generally based on client preference, often influenced by their proximity to the clinic. This process was the same for this trial, with some participants located >200 km away. Acceptability was rated high for both face-to-face and telehealth options.
Conclusion
Rates of dementia are increasing across Australia, and with the majority of persons with dementia cared for by family carers, carer-focussed interventions are warranted. Carers experience significant rates of mental and physical health concerns, and it is of clinical interest to develop appropriate support programs to limit the burden on carers and the health system. For those who commenced the program, findings indicated preliminary evidence for the acceptability of the START intervention for the support of carer needs in relation to their caring roles. Furthermore, evidence suggested that the telehealth modality is an acceptable and feasible method to carers for the delivery of the START program. Telehealth offers an innovative and flexible approach that effectively meets the needs of carers and may reduce barriers to carer access to support.
Acknowledgements
Dr Penny Rapaport (University College London), Dr Claire Nussey (University of Newcastle), Hunter Medical Research Institute.
References
Acton GJ, Kang J (2001) Interventions to reduce the burden of caregiving for an adult with dementia: A meta-analysis. Research in Nursing & Health 24(5), 349-360.
| Crossref | Google Scholar | PubMed |
Andersson G, Paxling B, Wiwe M, Vernmark K, Felix CB, Lundborg L, et al. (2012) Therapeutic alliance in guided internet-delivered cognitive behavioural treatment of depression, generalized anxiety disorder and social anxiety disorder. Behaviour Research and Therapy 50(9), 544-550.
| Crossref | Google Scholar | PubMed |
Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale: An updated literature review. Journal of Psychosomatic Research 52(2), 69-77.
| Crossref | Google Scholar | PubMed |
Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. (2009) How we design feasibility studies. American Journal of Preventive Medicine 36(5), 452-457.
| Crossref | Google Scholar | PubMed |
Bradford N, Caffery L, Smith A (2016) Telehealth services in rural and remote Australia: a systematic review of models of care and factors influencing success and sustainability. Rural and Remote Health 16(4), 4268.
| Crossref | Google Scholar |
Brodaty H, Green A, Koschera A (2003) Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society 51(5), 657-664.
| Crossref | Google Scholar | PubMed |
Cooke DD, McNally L, Mulligan KT, Harrison MJG, Newman SP (2001) Psychosocial interventions for caregivers of people with dementia: a systematic review. Aging & Mental Health 5(2), 120-135.
| Crossref | Google Scholar |
Coon DW, Thompson L, Steffen A, Sorocco K, Gallagher-Thompson D (2003) Anger and depression management: psychoeducational skill training interventions for women caregivers of a relative with dementia. Gerontologist 43(5), 678-689.
| Crossref | Google Scholar | PubMed |
Cooper C, Selwood A, Blanchard M, Walker Z, Blizard R, Livingston G (2010) The determinants of family carers’ abusive behaviour to people with dementia: results of the CARD study. Journal of Affective Disorders 121(1–2), 136-142.
| Crossref | Google Scholar | PubMed |
Crawford JR, Henry JD, Taylor EP, Crombie CM (2001) Normative data for the HADS from a large non‐clinical sample. British Journal of Clinical Psychology 40(4), 429-434.
| Crossref | Google Scholar |
Cuijpers P (2005) Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health 9(4), 325-330.
| Crossref | Google Scholar | PubMed |
Czaja SJ, Rubert MP (2002) Telecommunications technology as an aid to family caregivers of persons with dementia. Psychosomatic Medicine 64(3), 469-476.
| Crossref | Google Scholar | PubMed |
Dickinson C, Dow J, Gibson G, Hayes L, Robalino S, Robinson L (2017) Psychosocial intervention for carers of people with dementia: what components are most effective and when? A systematic review of systematic reviews. International Psychogeriatrics 29(1), 31-43.
| Crossref | Google Scholar | PubMed |
Dow B, Moore K, Scott P, Ratnayeke A, Wise K, Sims J, Hill K (2008) Rural carers online: a feasibility study. Australian Journal of Rural Health 16(4), 221-225.
| Crossref | Google Scholar | PubMed |
Eddison N, Leone E, Healy A, Royse C, Chockalingam N (2022) The potential impact of allied health professional telehealth consultations on health inequities and the burden of treatment. International Journal for Equity in Health 21(1), 91.
| Crossref | Google Scholar | PubMed |
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA (2016) CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 355, i5239.
| Crossref | Google Scholar | PubMed |
Etters L, Goodall D, Harrison BE (2008) Caregiver burden among dementia patient caregivers: a review of the literature. Journal of the American Academy of Nurse Practitioners 20(8), 423-428.
| Crossref | Google Scholar | PubMed |
Furlong KE, Wuest J (2008) Self-care behaviors of spouses caring for significant others with Alzheimer’s disease: the emergence of self-care worthiness as a salient condition. Qualitative Health Research 18(12), 1662-1672.
| Crossref | Google Scholar | PubMed |
Gilhooly KJ, Gilhooly MLM, Sullivan MP, McIntyre A, Wilson L, Harding E, et al. (2016) A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr 16(1), 106.
| Crossref | Google Scholar | PubMed |
Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D, et al. (2003) Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychology and Aging 18(3), 361-374.
| Crossref | Google Scholar | PubMed |
Greenwood N, Smith R (2015) Barriers and facilitators for male carers in accessing formal and informal support: A systematic review. Maturitas 82(2), 162-169.
| Crossref | Google Scholar | PubMed |
Haro JM, Kahle-Wrobleski K, Bruno G, Belger M, Dell’Agnello G, Dodel R, et al. (2014) Analysis of burden in caregivers of people with Alzheimer’s disease using self-report and supervision hours. Journal of Nutrician, Health and Aging 18(7), 677-684.
| Crossref | Google Scholar | PubMed |
Hébert R, Bravo G, Préville M (2010) Reliability, Validity and Reference Values of the Zarit Burden Interview for Assessing Informal Caregivers of Community-Dwelling Older Persons with Dementia. Canadian Journal on Aging 19(4), 494-507.
| Crossref | Google Scholar |
Hoe J, Katona C, Orrell M, Livingston G (2007) Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. International Journal of Geriatric Psychiatry 22(10), 1031-1036.
| Crossref | Google Scholar | PubMed |
Hollon SD, Stewart MO, Strunk D (2006) Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annual Review of Psychology 57, 285-315.
| Crossref | Google Scholar | PubMed |
Jacobson NS, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology 59(1), 12-19.
| Crossref | Google Scholar | PubMed |
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics 4(4), 287-291.
| Crossref | Google Scholar |
Kiropoulos LA, Klein B, Austin DW, Gilson K, Pier C, Mitchell J, Ciechomski L (2008) Is internet-based CBT for panic disorder and agoraphobia as effective as face-to-face CBT? Journal of Anxiety Disorders 22(8), 1273-1284.
| Crossref | Google Scholar | PubMed |
Kishita N, Laidlaw K (2017) Cognitive behaviour therapy for generalized anxiety disorder: Is CBT equally efficacious in adults of working age and older adults? Clinical Psychology Review 52, 124-136.
| Crossref | Google Scholar | PubMed |
Lancaster GA, Dodd S, Williamson PR (2004) Design and analysis of pilot studies: recommendations for good practice. Journal of Evaluation in Clinical Practice 10(2), 307-312.
| Crossref | Google Scholar | PubMed |
Laver K, Liu E, Clemson L, Davies O, Gray L, Gitlin LN, Crotty M (2020) Does Telehealth Delivery of a Dyadic Dementia Care Program Provide a Noninferior Alternative to Face-To-Face Delivery of the Same Program? A Randomized, Controlled Trial. American Journal of Geriatric Psychiatry 28(6), 673-682.
| Crossref | Google Scholar | PubMed |
Li R, Cooper C, Barber J, Rapaport P, Griffin M, Livingston G (2014) Coping strategies as mediators of the effect of the START (strategies for RelaTives) intervention on psychological morbidity for family carers of people with dementia in a randomised controlled trial. Journal of Affective Disorders 168, 298-305.
| Crossref | Google Scholar | PubMed |
Linde K, Rücker G, Sigterman K, Jamil S, Meissner K, Schneider A, Kriston L (2015) Comparative effectiveness of psychological treatments for depressive disorders in primary care: network meta-analysis. BMC Family Practice 16, 103.
| Crossref | Google Scholar | PubMed |
Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, Romeo R, et al. (2014) START (STrAtegies for RelaTives) study: a pragmatic randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of a manual-based coping strategy programme in promoting the mental health of carers of people with dementia. Health Technology Assessment 18(8), 1-242.
| Crossref | Google Scholar | PubMed |
Livingston G, Manela M, O’Keeffe A, Rapaport P, Cooper C, Knapp M, et al. (2020) Clinical effectiveness of the START (STrAtegies for RelaTives) psychological intervention for family carers and the effects on the cost of care for people with dementia: 6-year follow-up of a randomised controlled trial. The British Journal of Psychiatry 216(1), 35-42.
| Crossref | Google Scholar | PubMed |
Loi SM, Tropea J, Gaffy E, Panayiotou A, Capon H, Chiang J, et al. (2022) START-online: acceptability and feasibility of an online intervention for carers of people living with dementia. Pilot and Feasibility Studies 8(1), 41.
| Crossref | Google Scholar | PubMed |
López-López JA, Davies SR, Caldwell DM, Churchill R, Peters TJ, Tallon D, et al. (2019) The process and delivery of CBT for depression in adults: a systematic review and network meta-analysis. Psychological Medicine 49(12), 1937-1947.
| Crossref | Google Scholar | PubMed |
Mahoney R, Regan C, Katona C, Livingston G (2005) Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. American Journal of Geriatric Psychiatry 13(9), 795-801.
| Crossref | Google Scholar |
Marziali E, Donahue P, Crossin G (2018) Caring for Others: Internet Health Care Support Intervention for Family Caregivers of Persons with Alzheimer’s, Stroke, or Parkinson’s Disease. Families in Society: The Journal of Contemporary Social Services 86(3), 375-383.
| Crossref | Google Scholar |
Morley S, Dowzer C (2014) Manual for the Leeds Reliable Change Indicator: Simple Excel(tm) applications for the analysis of individual patient and group data. University of Leeds, Leeds, UK. Available at https://dclinpsych.leeds.ac.uk/wpcontent/uploads/sites/26/2018/09/Manual‐for‐Leeds‐RCI‐CSC‐calculators.pdf
Netto NR, Jenny GYN, Philip YLK (2009) Growing and gaining through caring for a loved one with dementia. Dementia 8(2), 245-261.
| Crossref | Google Scholar |
Neville C, Beattie E, Fielding E, MacAndrew M (2015) Literature review: use of respite by carers of people with dementia. Health & Social Care in the Community 23(1), 51-63.
| Crossref | Google Scholar | PubMed |
Okumura Y, Ichikura K (2014) Efficacy and acceptability of group cognitive behavioral therapy for depression: A systematic review and meta-analysis. Journal of Affective Disorders 164, 155-164.
| Crossref | Google Scholar | PubMed |
Parker D, Mills S, Abbey J (2008) Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. International Journal of Evidence-Based Healthcare 6(2), 137-172.
| Crossref | Google Scholar | PubMed |
Pihlaja S, Stenberg JH, Joutsenniemi K, Mehik H, Ritola V, Joffe G (2018) Therapeutic alliance in guided internet therapy programs for depression and anxiety disorders - A systematic review. Internet Interventions 11, 1-10.
| Crossref | Google Scholar | PubMed |
Pinquart M, Sörensen S (2003) Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging 18(2), 250-267.
| Crossref | Google Scholar | PubMed |
Pinquart M, Sörensen S (2006) Helping caregivers of persons with dementia: which interventions work and how large are their effects? International Psychogeriatrics 18(4), 577-595.
| Crossref | Google Scholar | PubMed |
Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas‐Herrera M, Wittenberg A, Adelaja R, Hu B, King B, Rehill D, Salimkumar D (2014) Dementia UK: Update. Alzheimer’s Society. Available at http://www.alzheimers.org.uk/dementiauk
Schirmer J. (2017) Carers in regional Australia: 2016 regional wellbeing survey report. Health Research Institute & Institute for Applied Ecology, University of Canberra. Available at https://www.canberra.edu.au/research/institutes/health‐research‐institute/regionalwellbeing‐survey/survey‐results/reports/2016‐reports/Carers‐report‐ONLINE‐10‐July‐2017.pdf
Scott JL, Dawkins S, Quinn MG, Sanderson K, Elliott K-EJ, Stirling C, et al. (2016) Caring for the carer: a systematic review of pure technology-based cognitive behavioral therapy (TB-CBT) interventions for dementia carers. Aging & Mental Health 20(8), 793-803.
| Crossref | Google Scholar | PubMed |
Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G (2007) Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders 101(1–3), 75-89.
| Crossref | Google Scholar | PubMed |
Seng BK, Luo N, Ng WY, Lim J, Chionh HL, Goh J, Yap P (2010) Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Annals of the Academy of Medicine, Singapore 39, 758-763.
| Crossref | Google Scholar |
Sim J, Lewis M (2012) The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. Journal of Clinical Epidemiology 65(3), 301-308.
| Crossref | Google Scholar | PubMed |
Somenahalli S (2015) Key transport and mobility issues facing seniors: evidence from Adelaide. National Seniors Productive Ageing Centre. Available at https://nationalseniors.com.au/uploads/04151097PAC_KeyTransportAndMobilityIssues_Report%20Final.pdf
Sommerlad A, Manela M, Cooper C, Rapaport P, Livingston G (2014) START (STrAtegies for RelaTives) coping strategy for family carers of adults with dementia: qualitative study of participants’ views about the intervention. BMJ Open 4(6), e005273.
| Crossref | Google Scholar | PubMed |
Stagg B, Larner AJ (2015) Zarit Burden Interview: pragmatic study in a dedicated cognitive function clinic. Progress in Neurology and Psychiatry 19(4), 23-27.
| Crossref | Google Scholar |
Stiles-Shields C, Kwasny MJ, Cai X, Mohr DC (2014) Therapeutic alliance in face-to-face and telephone-administered cognitive behavioral therapy. Journal of Consulting and Clinical Psychology 82(2), 349-354.
| Crossref | Google Scholar | PubMed |
Temple JB, Dow B (2018) The unmet support needs of carers of older Australians: prevalence and mental health. International Psychogeriatrics 30(12), 1849-1860.
| Crossref | Google Scholar | PubMed |
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. (2010) A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology 10, 1.
| Crossref | Google Scholar | PubMed |
Torp S, Hanson E, Hauge S, Ulstein I, Magnusson L (2008) A pilot study of how information and communication technology may contribute to health promotion among elderly spousal carers in Norway. Health & Social Care in the Community 16(1), 75-85.
| Crossref | Google Scholar | PubMed |
Vaganian L, Bussmann S, Gerlach AL, Kusch M, Labouvie H, Cwik JC (2020) Critical consideration of assessment methods for clinically significant changes of mental distress after psycho‐oncological interventions. International Journal of Methods in Psychiatric Research 29(2), e1821.
| Crossref | Google Scholar | PubMed |
van Gaans D, Dent E (2018) Issues of accessibility to health services by older Australians: a review. Public Health Reviews 39, 20.
| Crossref | Google Scholar | PubMed |
Walter A, Kilham K, Dow B, Rapaport P, Livingston G, Kelly M (in press) Feasibility of a Telehealth Intervention for Dementia Carers in Regional and Rural Australia.
| Google Scholar |
Webb K, Schroder TA, Gresswell DM (2019) Service users’ first accounts of experiencing endings from a psychological service or therapy: A systematic review and meta-ethnographic synthesis. Psychology and Psychotherapy: Theory, Research and Practice 92(4), 584-604.
| Crossref | Google Scholar | PubMed |
Wilz G, Soellner R (2015) Evaluation of a Short-Term Telephone-Based Cognitive Behavioral Intervention for Dementia Family Caregivers. Clinical Gerontologist 39(1), 25-47.
| Crossref | Google Scholar |
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67(6), 361-370.
| Crossref | Google Scholar | PubMed |