What does cognitive screening reveal about early cognitive performance following endovascular clot retrieval and intravenous thrombolysis in acute ischaemic stroke?
Sam Humphrey A B , Kerryn E. Pike
A B , Kerryn E. Pike  A C D , Brian Long B E , Henry Ma F G , Robert Bourke B , Danielle Byrne H , Bradley Wright A and Dana Wong
A C D , Brian Long B E , Henry Ma F G , Robert Bourke B , Danielle Byrne H , Bradley Wright A and Dana Wong  A *
A *
A
B
C
D
E
F
G
H
Abstract
Little is known regarding cognitive outcomes following treatment with endovascular clot retrieval (ECR) and intravenous tissue plasminogen activator (t-PA). We aimed to determine if there were any differences on a measure of cognitive screening between patients treated with ECR, t-PA, and those who were managed conservatively.
The medical records of ischaemic stroke patients admitted to Monash Medical Centre between January 2019 and December 2019 were retrospectively reviewed. Information extracted from medical records included age, sex, National Institutes of Health Stroke Scale at presentation, location of occlusion, treatment type, medical history, and cognitive screening performance measured by the Montreal Cognitive Assessment (MoCA).
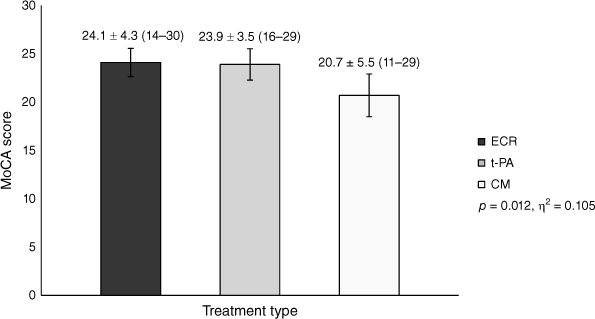
Eighty-two patients met the inclusion criteria (mean age = 66.5 ± 13.9; 49 male, 33 female). Patients treated with ECR performed significantly better on the MoCA (n = 36, 24.1 ± 4.3) compared to those who were managed conservatively (n = 26, 20.7 ± 5.5). Performance for patients treated with t-PA (n = 20, 23.9 ± 3.5) fell between the ECR and conservative management groups, but they did not significantly differ from either.
Our retrospective chart review found that ischaemic stroke patients treated with ECR appear to perform better on cognitive screening compared to patients who are managed conservatively. We also found that patients treated with ECR and t-PA appear to have similar cognitive screening performances in the acute stages following ischaemic stroke, although this finding is likely to have been impacted by group differences in stroke characteristics and may reflect the possibility that the ECR group performed better than expected based on their stroke severity.
Keywords: cognition, cognitive screening, endovascular clot retrieval, intravenous thrombolysis, ischaemic stroke, stroke rehabilitation, thrombectomy, tissue plasminogen activator.
Introduction
Endovascular clot retrieval (ECR) is a highly effective treatment for ischaemic stroke, reducing both death and disability in treated patients (Goyal et al. 2016). Although previous studies have largely focused on functional outcomes, little is known regarding cognitive outcomes following ECR, despite cognition being identified as the number one research priority relating to life after stroke (Pollock et al. 2014). Although existing evidence is limited, early research exploring cognition following ECR has been positive, with treated patients demonstrating better cognitive test performance compared to standard medical care (López-Cancio et al. 2017; Xu et al. 2017; Lattanzi et al. 2020). Despite these promising results, further studies are required to determine if these findings are reproducible, as is more research investigating cognitive outcomes following treatment with intravenous tissue plasminogen activator (t-PA). A recent systematic review highlighted the need for more robust studies in this area, as the effect of intravenous thrombolysis on post-stroke cognition remains unclear (Broome et al. 2016).
Developing a better understanding of cognitive outcomes following different acute treatments for ischaemic stroke will help provide clinicians with more accurate prognostic information regarding cognition, with the prevalence of post-stroke cognitive impairment thought to range from 20 to 80% (Sun et al. 2014). We also know there is a strong relationship between cognition and longer-term functional and participation outcomes, with a recent meta-analysis indicating that post-stroke cognitive impairment is associated with enduring activity limitations and participation restrictions (Stolwyk et al. 2021). Therefore, further investigation of how acute treatments impact cognition can help clinicians identify patients who are at increased risk of developing post-stroke cognitive impairment and facilitate early, tailored cognitive rehabilitation interventions that have the potential to lead to better longer-term functional outcomes for stroke survivors.
We aimed to determine if there were any differences on a measure of cognitive screening between patients treated with ECR, t-PA, and those who were managed conservatively (received neither ECR nor t-PA) in the acute stages following ischaemic stroke.
Methods
Study setting
This retrospective study was conducted at Monash Medical Centre in Melbourne, Australia. Monash Medical Centre is a 640-bed teaching and research hospital in south-east Melbourne with a catchment population of over 1.2 million people. The hospital is one of two statewide ECR centres that provide a 24/7 service for patients across the state of Victoria in accordance with the statewide service protocol for ECR delivery (State of Victoria, Department of Health and Human Services 2018). We received approval from the Monash Health Human Research Ethics Committee. As the study setting was retrospective with no patient contact, informed consent was waived.
Participants
The medical records of ischaemic stroke patients admitted to Monash Medical Centre between January 2019 and December 2019 were retrospectively reviewed. Demographic and clinical information extracted from the records for each patient included age, sex, National Institutes of Health Stroke Scale (NIHSS) at presentation, location of occlusion, treatment type, and medical history. We also attempted to extract a measure of functional outcome on discharge; however, modified Rankin Scale scores were not consistently recorded in patient files. Patients who had an ischaemic stroke that was not due to large vessel occlusion were excluded. For the purpose of this study, large vessel occlusions refer to occlusions within the internal carotid artery; first, second, and third segments of the middle cerebral artery; first and second segments of the anterior cerebral artery; first and second segments of the posterior cerebral artery; vertebral artery; and basilar artery (Rennert et al. 2019). Patients with a history of previous stroke, brain injury, existing neurological or neurodegenerative condition, and those with a non-English speaking background were also excluded from the study.
Screening tool
Cognitive screening performance was measured by the Montreal Cognitive Assessment (MoCA) in the first 10 days following an ischaemic stroke. The MoCA is a quick and easy‐to‐administer 30-point cognitive screening tool covering several cognitive domains, including visuospatial and executive skills, memory, attention, language, abstract reasoning, and orientation (Nasreddine et al. 2005). The MoCA was originally developed to detect mild cognitive impairment and dementia; however, it has since been widely validated in the stroke population and is a feasible screening tool for the detection of cognitive impairment in the acute stages after stroke (Chiti and Pantoni 2014; Potocnik et al. 2020; Munthe-Kaas et al. 2021; Wei et al. 2023). There is, however, some debate regarding the optimal cut-off score for the detection of post-stroke cognitive impairment, with a recent meta-analysis finding the optimal cut-off score is 21/22 and not the original recommended cut-off score of 26 (Wei et al. 2023). The MoCA was adopted for this retrospective study, as it was commonly administered to stroke patients at Monash Medical Centre as part of standard care during the data collection period. It was, however, not given to every patient for reasons such as visual or communication difficulties or in cases where it is not clinically indicated. Some cognitive assessments may also be deferred until patients are transferred to the sub-acute setting for inpatient rehabilitation. Patients who were not administered the MoCA during their acute hospital admission were excluded from the study.
Statistical analysis
Descriptive statistics were computed for all study variables. Continuous variables were compared using t-tests and analysis of variance (ANOVA) or the Mann–Whitney U test and Kruskal–Wallis test, based on normality. Continuous variables were described with mean and standard deviation. Categorical variables were compared with chi-square or likelihood-ratio tests and described as frequencies and percentages. Demographic information, clinical information, and the screening measure were compared between the three treatment conditions. Statistical significance was set at a P-value of <0.05 and effect size was measured by partial eta squared and Cohen’s d. Statistical analysis was performed using IBM SPSS Statistics 28.
Results
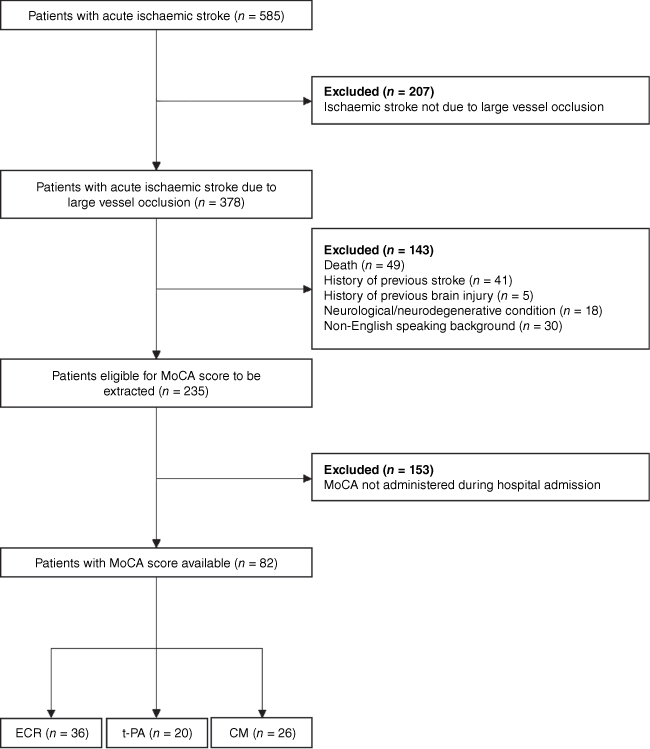
A total of 585 patients who presented to Monash Medical Centre with an ischaemic stroke were identified. Of these patients, 179 were treated with ECR, 71 were treated with t-PA, and 335 were managed conservatively. The demographic and clinical information of patients are shown in Table 1. Of the 585 patients identified, 503 did not meet the inclusion criteria and were excluded from the study (Fig. 1). Patients who met the inclusion criteria were significantly younger than the patients who were excluded from the study (mean age = 66.5 ± 13.9 vs 74.4 ± 13.2; P < 0.001) and also had a significantly lower NIHSS score (lower stroke severity) at presentation (6.2 ± 5.5 vs 9.1 ± 7.9; n = 474, P = 0.009).
| ECR (n = 179) | t-PA (n = 71) | CM (n = 335) | P-value | ||
|---|---|---|---|---|---|
| Demographic information | |||||
| Age, mean, s.d., (range) | 70.9 ± 13.8 (28–96) | 70.9 ± 13.0 (26–91) | 75.0 ± 13.4 (24–100) | 0.002A | |
| Male, n (%) | 88 (49.2) | 45 (63.4) | 182 (54.3) | 0.122B | |
| Clinical information | |||||
| NIHSS, mean, s.d., (range) | 10.6 ± 7.0 (0–27) | 5.9 ± 5.7 (0–25) | 8.1 ± 8.3 (0–34) | <0.001C | |
| Left hemisphere stroke, n (%) | 88 (52.7) | 39 (55.7) | 171 (51.7) | 0.451B | |
Of the 82 patients who met inclusion criteria, 36 were treated with ECR, 20 were treated with t-PA, and 26 were managed conservatively. The demographic and clinical information of patients are shown in Table 2. In accordance with the statewide service protocol for ECR delivery, 11 of the 36 (30.4%) patients treated with ECR were also administered t-PA whilst the decision to pursue ECR was being made. Therefore, the ECR group is better categorised as an ECR plus standard medical care group, similar to previous research in this area (López-Cancio et al. 2017; Lattanzi et al. 2020).
| ECR (n = 36) | t-PA (n = 20) | CM (n = 26) | P-value | ||
|---|---|---|---|---|---|
| Demographic information | |||||
| Age, mean, s.d., (range) | 66.7 ± 14.5 (29–91) | 65.0 ± 15.3 (26–85) | 67.4 ± 12.5 (26–89) | 0.850A | |
| Male, n (%) | 24 (66.7) | 13 (65.0) | 12 (46.2) | 0.229B | |
| Clinical information | |||||
| NIHSS, mean, s.d., (range) | 9.1 ± 6.5 (0–28) | 3.2 ± 3.4 (0–11) | 4.6 ± 4.7 (0–20) | <0.001C | |
| Left hemisphere stroke, n (%) | 20 (58.8) | 12 (60.0) | 14 (53.8) | 0.897B | |
| Location of occlusion, n (%) | <0.001D | ||||
| Internal carotid artery | 1 (2.8) | – | – | ||
| Middle cerebral artery | |||||
| First segment (M1) | 21 (58.3) | – | 3 (11.5) | ||
| Second segment (M2) | 11 (30.6) | 4 (20.0) | 10 (38.5) | ||
| Third segment (M3) | – | 9 (45.0) | 5 (19.2) | ||
| Anterior cerebral artery | |||||
| First segment (A1) | – | – | – | ||
| Second segment (A2) | – | 4 (20.0) | – | ||
| Posterior cerebral artery | |||||
| First segment (P1) | 1 (2.8) | 1 (5.0) | 1 (3.8) | ||
| Second segment (P2) | – | 2 (10.0) | 7 (26.9) | ||
| Basilar artery | 2 (5.6) | – | – | ||
| Day of MoCA, median, (range) | 4 (1–8) | 3 (2–5) | 2 (1–10) | 0.047E | |
P‐values of <0.05 are in bold to highlight significant difference. ECR, endovascular clot retrieval; t-PA, tissue plasminogen activator; CM, conservative management; NIHSS, National Institutes of Health Stroke Scale. MoCA, Montreal Cognitive Assessment.
The overall mean age was 66.5 (±13.9) years, and 49 patients were male (59.8%). Left hemisphere strokes were slightly more common across all three treatments (57.5%). Although the exact location of occlusions differed significantly between the treatments, the majority of strokes were due to an occlusion within the middle cerebral artery (ECR = 88.9%; t-PA = 65.0%; managed conservatively = 69.2%). Although patients treated with ECR had a significantly higher NIHSS score at presentation compared to patients treated with t-PA and conservative management (CM), there was not a significant correlation between NIHSS and MoCA scores (r = −0.20, P = 0.081). The overall median time of MoCA administration was day three of the admission, and there were no statistically significant differences between administration times after interpretation of the pairwise comparisons adjusted significance values. No other statistically significant differences between treatment groups in terms of demographic or clinical information were identified.
Univariate analysis revealed a statistically significant difference in mean MoCA scores between the three different treatments (F(2,79) = 4.655, P = 0.012, η2 = 0.105). Post hoc analysis (Table 3) showed that patients treated with ECR performed significantly better on the MoCA compared to those who were managed conservatively. No significant differences were identified between patients treated with ECR and t-PA, nor patients treated with t-PA and CM; however, the latter may be due to the smaller sample size in the t-PA group given the medium-to-large effect size (Fig. 2).
| Mean difference | Std. error | 95% CI | P-value | Cohen’s d | ||
|---|---|---|---|---|---|---|
| ECR vs CM | 3.4 | 1.2 | 0.6 to 6.2 | 0.015 | 0.689 | |
| ECR vs t-PA | 0.2 | 1.3 | −2.8 to 3.2 | 0.989 | 0.051 | |
| t-PA vs CM | 3.2 | 1.4 | −0.1 to 6.4 | 0.056 | 0.694 |
ECR, endovascular clot retrieval; t-PA, tissue plasminogen activator; CM, conservative management.
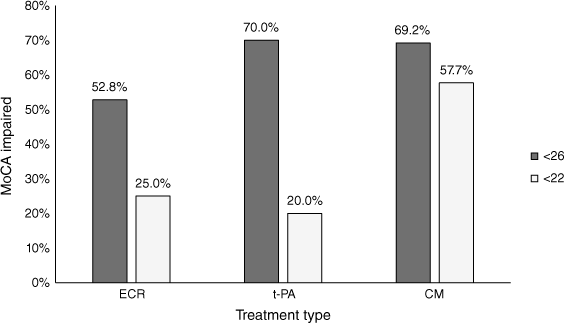
Using the recommended MoCA cut-off score of <26, there were no significant differences between the number of patients classified as cognitively impaired in each of the treatment groups (Fig. 3). Alternatively, using the stroke-specific cut-off score of <22 suggested by Wei et al. (2023), patients who were managed conservatively were significantly more likely (P = 0.009) to be classified as cognitively impaired compared to those treated with ECR and t-PA.
Discussion
Our results indicate that ischaemic stroke patients treated with ECR performed significantly better on a measure of cognitive screening compared to patients who were managed conservatively. This finding initially appears consistent with previous research in the area, which has also found that patients who receive ECR perform better on cognitive measures compared to those who receive standard medical care. It should, however, be noted that the standard medical care groups in these studies vary, with one including a combination of t-PA and CM patients whereas others include t-PA patients alone (López-Cancio et al. 2017; Xu et al. 2017; Lattanzi et al. 2020). To our knowledge, this is the first study to show that ECR patients demonstrate better cognitive performance compared to CM alone.
Interestingly, unlike previous studies, we did not find any significant cognitive differences between the ECR and t-PA groups. In the most robust study in this area to date, Lattanzi et al. (2020) found that ECR patients performed significantly better than t-PA patients on all measures in a cognitive test battery 6 months post-stroke. Similarly, Xu et al. (2017) also found that ECR patients performed significantly better on the MoCA and Mini-Mental Status Examination (MMSE) at 90-day follow-up. Given that previous research only reports findings beyond 3 months post-stroke, there may well be no acute cognitive differences between the treatments, with the earlier therapy initiation associated with t-PA potentially having a positive influence on acute cognitive performance. Indeed, Xu et al. (2017) also administered the MoCA and MMSE at baseline in their study, with their results indicating that the t-PA group actually performed slightly better than the ECR group. It is, however, important to consider differences in stroke characteristics between the ECR and t-PA groups. In our study, 58.3% of the ECR patients had an occlusion in the first (M1) segment of the middle cerebral artery, whereas 45% of the t-PA patients had an occlusion in the third (M3) segment. Patients treated with ECR also had a significantly higher NIHSS score at presentation compared to patients treated with t-PA. Stroke location and severity are well-known predictors of post-stroke cognitive impairment, with patients presenting with larger vessel occlusions and more severe strokes at increased risk of developing cognitive impairment (Pendlebury and Rothwell 2009; Jaillard et al. 2010; Levine et al. 2018; Aam et al. 2020; Rost et al. 2022). Based on the observed differences in stroke characteristics, it may well be that the ECR group are indeed entitled to a greater degree of cognitive impairment; however, this was not seen in our results, suggesting treatment with ECR may have attenuated the degree of cognitive impairment observed on screening.
Although the difference between the t-PA and CM groups was large, the smaller sample size is likely responsible for the finding not reaching statistical significance. We did, however, find that CM patients were more likely to be classified as cognitively impaired than t-PA patients when considering the stroke-specific MoCA cut-off score of <22. Previous research suggests patients treated with t-PA perform significantly better on cognitive measures in the days to weeks following ischaemic stroke when compared to conservative management (Broome et al. 2016). In a study of patients with right hemisphere stroke, Laihosalo et al. (2011) found that patients treated with t-PA demonstrated significantly better visuoconstructional abilities compared to non-treated patients 4 days post-stroke. Treated patients also demonstrated an overall reduction in the presence of visual neglect and improved attentional processing (Kettunen et al. 2012a, 2012b). Similarly, Jacquin et al. (2014) found that the severity of aphasia was significantly milder in left hemisphere stroke patients treated with t-PA 1 week post-stroke; however, these differences did not persist when measured at 3 months. Nys et al. (2006) also found no significant differences between treated and non-treated patients on a cognitive test battery 6 months post-stroke, despite observing favourable outcomes on measures of basic and instrumental activities of daily living in those who received t-PA. Our research adds to the existing knowledge that t-PA patients demonstrate better acute cognitive performance compared to CM immediately following ischaemic stroke. These benefits do, however, appear to wane after a period of gradual recovery, and further research is needed to explore longer-term cognitive outcomes in greater detail.
Overall, our findings suggest that ECR and t-PA patients demonstrate similar cognitive screening performances initially following ischaemic stroke, with ECR patients performing significantly better than patients who are managed conservatively. There are, of course, several limitations to our study, including the relatively small sample size from a single hospital site as well as the retrospective study design. Previous studies have also suggested that although the MoCA is a useful screening tool for detecting global post-stroke cognitive impairment, it does not sufficiently identify domain-specific cognitive deficits that commonly occur after stroke (Chan et al. 2014; Demeyere et al. 2016). Indeed, the Oxford Cognitive Screen (OCS) was developed to detect these cognitive impairments and has recently been adopted as the preferred post-stroke cognitive screening measure at Monash Medical Centre (Demeyere et al. 2016; Sanctuary et al. 2023). Future research exploring cognition in the acute stages following ischaemic stroke should therefore consider using the OCS to identify domain-specific cognitive impairments. Finally, participants included in our final sample were younger and had less severe strokes than the overall sample, with this impacting the generalisability of our findings due to selection bias. Nonetheless, our study had several strengths, including being the first to compare cognitive outcomes in ECR, t-PA, and CM groups separately. Additionally, patients who met the inclusion criteria did not statistically differ in age across the three treatments, unlike the overall sample. This indicates that the observed differences in cognitive screening performance are not simply an artefact of age, with older patients seemingly less likely to be treated with ECR due to multiple medical comorbidities and disability.
Conclusion
Our retrospective chart review found that treatment with ECR is associated with better performance on a measure of cognitive screening compared to CM in ischaemic stroke due to large vessel occlusion. We also found that there were no significant differences in cognitive screening between patients treated with ECR and t-PA in the acute stages following ischaemic stroke, although this finding is likely to have been impacted by group differences in stroke characteristics and may reflect the possibility that the ECR group performed better than expected based on their stroke severity. Although promising, prospective experimental research is needed to explore these findings further, including the administration of more comprehensive cognitive assessment at longer-term follow-up. Future studies should also aim to include information regarding functional outcomes, final infarct volume, small vessel disease, and premorbid cognitive status, as these factors are also known to influence cognitive outcomes after stroke. We are currently conducting a non-randomised, three-arm parallel controlled clinical trial comparing the cognitive, emotional, and functional outcomes of ischaemic stroke patients treated with ECR and t-PA, as well as those who are managed conservatively (Australian Clinical Trials Registration Number: 12619001194156).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
References
Aam S, Einstad MS, Munthe-Kaas R, Lydersen S, Ihle-Hansen H, Knapskog A-B, Ellekjær H, Seljeseth Y, Saltvedt I (2020) Post-Stroke Cognitive Impairment—Impact of Follow-Up Time and Stroke Subtype on Severity and Cognitive Profile: The Nor-COAST Study. Frontiers in Neurology 11, 699.
| Crossref | Google Scholar | PubMed |
Broome LJ, Battle CE, Lawrence M, Evans PA, Dennis MS (2016) Cognitive Outcomes Following Thrombolysis in Acute Ischemic Stroke: A Systematic Review. Journal of Stroke and Cerebrovascular Diseases 25(12), 2868-2875.
| Crossref | Google Scholar | PubMed |
Chan E, Khan S, Oliver R, Gill SK, Werring DJ, Cipolotti L (2014) Underestimation of Cognitive Impairments by the Montreal Cognitive Assessment (MoCA) in an Acute Stroke Unit Population. Journal of the Neurological Sciences 343(1–2), 176-179.
| Crossref | Google Scholar | PubMed |
Chiti G, Pantoni L (2014) Use of Montreal Cognitive Assessment in Patients with Stroke. Stroke 45(10), 3135-3140.
| Crossref | Google Scholar | PubMed |
Demeyere N, Riddoch MJ, Slavkova ED, Jones K, Reckless I, Mathieson P, Humphreys GW (2016) Domain-Specific Versus Generalized Cognitive Screening in Acute Stroke. Journal of Neurology 263(2), 306-315.
| Crossref | Google Scholar | PubMed |
Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miquel MA, Donnan GA, Roos YBWEM, Bonafe A, Jahan R, Diener H-C, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, et al. (2016) Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials. The Lancet 387(10029), 1723-1731.
| Crossref | Google Scholar | PubMed |
Jacquin A, Virat-Brassaud M-E, Rouaud O, Osseby G-V, Aboa-Eboulé C, Hervieu M, Ménassa M, Ricolfi F, Giroud M, Béjot Y (2014) Vascular Aphasia Outcome after Intravenous Recombinant Tissue Plasminogen Activator Thrombolysis for Ischemic Stroke. European Neurology 71(5–6), 288-295.
| Crossref | Google Scholar | PubMed |
Jaillard A, Grand S, Le Bas JF, Hommel M (2010) Predicting Cognitive Dysfunctioning in Nondemented Patients Early after Stroke. Cerebrovascular Diseases 29(5), 415-423.
| Crossref | Google Scholar | PubMed |
Kettunen JE, Laihosalo M, Ollikainen J, Dastidar P, Nurmi L, Koivisto A-M, Jehkonen M (2012a) Rightward Bias in Right Hemisphere Infarct Patients with or without Thrombolytic Treatment and in Healthy Controls. Neurocase 18(5), 359-365.
| Crossref | Google Scholar |
Kettunen JE, Nurmi M, Koivisto A-M, Dastidar P, Jehkonen M (2012b) The Presence of Visual Neglect after Thrombolytic Treatment in Patients with Right Hemisphere Stroke. The Scientific World Journal 2012, 434120.
| Crossref | Google Scholar |
Laihosalo M, Kettunen JE, Koivisto A-M, Dastidar P, Ollikainen J, Jehkonen M (2011) Thrombolytic Therapy and Visuoperceptual Functions in Right Hemisphere Infarct Patients. Journal of Neurology 258(6), 1021-1025.
| Crossref | Google Scholar | PubMed |
Lattanzi S, Coccia M, Pulcini A, Cagnetti C, Galli FL, Villani L, Campa S, Dobran M, Polonara G, Ceravolo MG, Silvestrini M (2020) Endovascular Treatment and Cognitive Outcome after Anterior Circulation Ischemic Stroke. Scientific Reports 10(1), 18524.
| Crossref | Google Scholar | PubMed |
Levine DA, Wadley VG, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Howard G, Howard VJ, Cushman M, Judd SE, Galecki AT (2018) Risk Factors for Poststroke Cognitive Decline: The REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke 49(4), 987-994.
| Crossref | Google Scholar | PubMed |
López-Cancio E, Jovin TG, Cobo E, Cerdá N, Jiménez M, Gomis M, Hernández-Pérez M, Cáceres C, Cardona P, Lara B, Renú A, Llull L, Boned S, Muchada M, Dávalos A (2017) Endovascular Treatment Improves Cognition after Stroke: A secondary analysis of REVASCAT trial. Neurology 88(3), 245-251.
| Crossref | Google Scholar | PubMed |
Munthe-Kaas R, Aam S, Saltvedt I, Wyller TB, Pendlebury ST, Lydersen S, Ihle-Hansen H (2021) Test Accuracy of the Montreal Cognitive Assessment in Screening for Early Poststroke Neurocognitive Disorder: The Nor-COAST Study. Stroke 52(1), 317-320.
| Crossref | Google Scholar | PubMed |
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. Journal of the American Geriatrics Society 53(4), 695-699.
| Crossref | Google Scholar | PubMed |
Nys GMS, van Zandvoort MJE, Algra A, Kappelle LJ, de Haan EHF (2006) Cognitive and Functional Outcome after Intravenous Recombinant Tissue Plasminogen Activator Treatment in Patients with a First Symptomatic Brain Infarct. Journal of Neurology 253(2), 237-241.
| Crossref | Google Scholar | PubMed |
Pendlebury ST, Rothwell PM (2009) Prevalence, Incidence, and Factors Associated with Pre-Stroke and Post-Stroke Dementia: A Systematic Review and Meta-Analysis. The Lancet Neurology 8(11), 1006-1018.
| Crossref | Google Scholar | PubMed |
Pollock A, St George B, Fenton M, Firkins L (2014) Top 10 Research Priorities Relating to Life after Stroke – Consensus from Stroke Survivors, Caregivers, and Health Professionals. International Journal of Stroke 9(3), 313-320.
| Crossref | Google Scholar | PubMed |
Potocnik J, Ovcar Stante K, Rakusa M (2020) The Validity of the Montreal Cognitive Assessment (MoCA) for the Screening of Vascular Cognitive Impairment after Ischemic Stroke. Acta Neurologica Belgica 120(3), 681-685.
| Crossref | Google Scholar | PubMed |
Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS, Khalessi AA (2019) Epidemiology, Natural History, and Clinical Presentation of Large Vessel Ischemic Stroke. Neurosurgery 85(1), S4-S8.
| Crossref | Google Scholar | PubMed |
Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, Hinman JD, Dichgans M (2022) Post-Stroke Cognitive Impairment and Dementia. Circulation Research 130(8), 1252-1271.
| Crossref | Google Scholar | PubMed |
Sanctuary C, Hewitt L, Demeyere N, Kankkunen K, Oxenham DV, Simpson DB, Stolwyk RJ, Synn A, Webb SS, Marsden DL (2023) The Oxford Cognitive Screen for Use with Australian People after Stroke (OCS‐AU): The Adaptation Process and Determining Cut Scores for Cognitive Impairment Using a Cross‐Sectional Normative Study. Australian Occupational Therapy Journal 70(1), 73-85.
| Crossref | Google Scholar | PubMed |
State of Victoria, Department of Health and Human Services (2018) Endovascular clot retrieval for acute stroke; Statewide service protocol for Victoria. Retrieved from https://www.safercare.vic.gov.au/best-practice-improvement/clinical-guidance/stroke-clinical-network/endovascular-clot-retrieval-protocol
Stolwyk RJ, Mihaljcic T, Wong DK, Chapman JE, Rogers JM (2021) Poststroke Cognitive Impairment Negatively Impacts Activity and Participation Outcomes: A Systematic Review and Meta-Analysis. Stroke 52(2), 748-760.
| Crossref | Google Scholar | PubMed |
Sun J-H, Tan L, Yu J-T (2014) Post-Stroke Cognitive Impairment: Epidemiology, Mechanisms and Management. Annals of Translational Medicine 2(8), 80.
| Crossref | Google Scholar | PubMed |
Wei X, Ma Y, Wu T, Yang Y, Yuan Y, Qin J, Bu Z, Yan F, Zhang Z, Han L (2023) Which Cut-Off Value of the Montreal Cognitive Assessment Should be Used for Post-Stroke Cognitive Impairment? A Systematic Review and Meta-Analysis of Studies on Diagnostic Test Accuracy. International Journal of Stroke 18, 908-916.
| Crossref | Google Scholar |
Xu G, Dong X, Niu X, Zheng G, Wang H, Zhang F, Li L, Lv P (2017) Cognitive Function and Prognosis of Multimodal Neuroimage-Guided Thrombectomy on Mild to Moderate Anterior Circulation Infarction Patients with Broadened Therapeutic Window: A Prospective Study. European Neurology 78(5–6), 257-263.
| Crossref | Google Scholar | PubMed |