When stroke survivors’ self-ratings are inconsistent with the ratings of others: a cohort study examining biopsychosocial factors associated with impaired self-awareness of functional abilities
Kate V. Cameron A , Jennie L. Ponsford
A , Jennie L. Ponsford  A B , Dean P. McKenzie
A B , Dean P. McKenzie  C D and Renerus J. Stolwyk
C D and Renerus J. Stolwyk  A B *
A B *
A
B
C
D
Abstract
Stroke survivors’ self-ratings of functional abilities are often inconsistent with ratings assigned by others (e.g. clinicians), a phenomenon referred to as ‘impaired self-awareness’ (ISA). There is limited knowledge of the biopsychosocial contributors and consequences of post-stroke ISA measured across the rehabilitation journey. This multi-site cohort study explored biopsychosocial correlates of ISA during subacute rehabilitation (inpatient) and at 4 months post-discharge (community-dwelling).
Forty-five subacute stroke survivors participated (Age M (s.d.) = 71.5 (15.6), 56% female), and 38 were successfully followed-up. Self-assessments were compared to those of an independent rater (occupational therapist, close other) to calculate ISA at both time points. Survivors and raters completed additional cognitive, psychological and functional measures.
Multivariate regression (multiple outcomes) identified associations between ISA during inpatient admission and poorer outcomes at follow-up, including poorer functional cognition, participation restriction, caregiver burden, and close other depression and anxiety. Regression models applied cross-sectionally, including one intended for correlated predictors, indicated associations between ISA during inpatient admission and younger age, male sex, poorer functional cognition, poorer rehabilitation engagement and less frequent use of non-productive coping (adjusted R2 = 0.60). ISA at community follow-up was associated with poorer functional cognition and close other anxiety (adjusted R2 = 0.66).
Associations between ISA and poorer outcomes across the rehabilitation journey highlight the clinical importance of ISA and the value of assessment and management approaches that consider the potential influence of numerous biological and psychosocial factors on ISA. Future studies should use larger sample sizes to confirm these results and determine the causal mechanisms of these relationships.
Keywords: acquired brain injury, appraisal discrepancy, biopsychosocial, cognition, insight, rehabilitation, self-awareness, stroke.
Introduction
Impaired self-awareness (ISA) arises when ‘a patient, affected by a brain dysfunction, does not recognise the presence or appreciate the severity of deficits in sensory, perceptual, motor, affective or cognitive functioning’ (Orfei et al. 2007, pp. 3075–3076). This condition often appears in the early stages of post-stroke recovery and affects one-third to three-quarters of stroke survivors (Starkstein et al. 1992; Hartman-Maeir et al. 2003). Assessment commonly involves comparing a survivor’s self-assessment with their treating clinician’s assessment, and any resulting discrepancies indicate ISA.
In stroke, studies have focussed on ISA for physical impairments (e.g. awareness of hemiplegia) (Nurmi and Jehkonen 2014). Knowledge of ISA for functional abilities (e.g. independent living tasks and the skills required to perform them) is limited, despite these abilities being the focus of rehabilitation. Accordingly, this study sought to identify factors associated with post-stroke ISA for functional abilities. Improved knowledge of associated factors may support clinicians in identifying those at risk of post-stroke ISA and may also lead to improved clinical management for those stroke survivors evidencing ISA.
Research gaps also exist regarding the outcomes of ISA in stroke survivors. Most research in stroke has focused on ISA during inpatient admissions, perhaps because many cases improve within 3–6 months (Hier et al. 1983; Vocat et al. 2010), but the absence of cohort studies means we know little about the long-term outcomes of those who evidence post-stroke ISA during their recovery (Jehkonen et al. 2006). This knowledge is critical in determining the clinical significance of post-stroke ISA and in selecting appropriate community supports.
In this paper, the term ‘impaired self-awareness’ has been used to align with existing nomenclature and refers to discrepancies between stroke survivor self-assessment and the assessment of a proxy rater (e.g. clinician or close other). However, it is important to avoid conflating discrepancies with the underreporting of impairments by the stroke survivor. Discrepancies may arise from reporting errors on the part of the proxy rater, the stroke survivor, or both. Furthermore, in some instances it may be particularly difficult to determine whose assessment is most accurate, especially when evaluating higher-order functions such as cognition, emotional functions and participatory abilities, which tend to be more challenging to assess objectively and to quantify deviations from premorbid functioning (Avlund 1997; Cameron et al. 2020). It is arguably more important to focus on the extent of rating inconsistency between survivors and clinicians/close others than to determine whose assessment most closely approximates truth. Hence while the term ‘impaired self-awareness’ is used in this research paper, the methodological and interpretative framework employed by the authors focusses on the extent of rating consistency between parties in their assessments of stroke survivor functioning.
Early explanatory frameworks of ISA were unidimensional and had a strong focus on biomedical and neurocognitive drivers (Weinstein and Kahn 1955; Crosson et al. 1989; McGlynn and Schacter 1989; Heilman et al. 1998; Toglia and Kirk 2000). Modern conceptual advances have, however, shaped ISA as a multidimensional construct that is the product of multiple contributing factors (Marcel et al. 2004; Orfei et al. 2007; Orfei et al. 2009; Jenkinson et al. 2011). In line with these advances, Clare, Ownsworth and Morris applied the biopsychosocial model of health (Engel 1980) to develop an explanatory framework of ISA (Clare 2004; Ownsworth et al. 2006), proposing that ISA arises from the combined influences of biological, psychological and socio-environmental factors. These factors interact with each other to influence presentations of ISA and hence it is useful to examine these collectively. The biopsychosocial framework’s flexibility and multidimensionality offers considerable explanatory power, demonstrating effectiveness in other populations affected by ISA, such as dementia (Ownsworth et al. 2006; Clare et al. 2012; Lacerda et al. 2020) and traumatic brain injury (TBI) (Niemeier et al. 2014; Belchev et al. 2017); however, there are limited similar applications in stroke.
To progress this, variables of clinical significance can be identified from research in other affected clinical populations (e.g. TBI) and tested in a biopsychosocial framework with stroke survivors to identify those associated with post-stroke ISA. Research examining ISA in dementia and TBI populations is comparatively more advanced and has identified a number of factors associated with ISA, though there is considerable inter-study variation in the direction and strength of these associations and thus the findings cannot be regarded as definitive. Nonetheless, research has identified associations between ISA and cognitive impairment (Amanzio et al. 2013; Zimmermann et al. 2017), neuroanatomical lesion sites (Pia et al. 2004; Zamboni et al. 2013; Terneusen et al. 2022), greater injury severity (Sherer et al. 2005; Zimmermann et al. 2017), coping mechanisms (Tagai et al. 2020), personality traits such as neuroticism and hypersensitivity to deficits (Belchev et al. 2017; Martyr et al. 2022), emotional distress (Martyr et al. 2022; Wheeler et al. 2022), younger age (Sherer et al. 2003b; Hertzog and Dunlosky 2011; Zimmermann et al. 2017) increased caregiver burden (Kelleher et al. 2016), social constructions of disability (Yeates et al. 2006), compromised rehabilitation engagement (Fleming and Strong 1995), limited functional gains from treatment (Gialanella et al. 2005; Ownsworth and Clare 2006; Smeets et al. 2017), and poorer community reintegration (Jehkonen et al. 2001; Robertson and Schmitter-Edgecombe 2015). Thus research conducted in dementia and TBI populations has helpfully identified factors that may be relevant when seeking to determine factors that represent predictors and outcomes of ISA in stroke survivors. To test their relevance in stroke, these factors must be collectively examined in a stroke-only sample because stroke differs from dementia and TBI on variables that may influence associations, such as illness trajectory, post-injury impairment profile and other clinical and demographic characteristics (World Health Organization 2019). To our knowledge, there is no research in stroke-only populations that collectively examines biopsychosocial factors associated with ISA for functional abilities.
Thus, there is the need for a cohort study that examines biopsychosocial factors associated with post-stroke ISA over the course of recovery to identify potential predictors and outcomes of ISA. Our study therefore investigated associations between biopsychosocial variables and post-stroke ISA at inpatient rehabilitation (‘inpatient’ stage) and again at 4 months post-discharge (‘community-dwelling’ stage) in a representative stroke sample. Given the extensive number of possible biopsychosocial variables, those with the most empirical support were selected for inclusion in this study. Despite some variables demonstrating more convincing associations with ISA in other populations, considerable variation still exists between study findings and thus we refrained from posing directional hypotheses, electing to conduct an exploratory investigation instead. To maximise the number of associations identified in the data, this study examined concurrent associations (i.e. variables measured at the same time point) and associations across time points. This facilitated exploration of predictors and outcomes of ISA. Specifically, the study examined the relationship between ISA at inpatient admission and concurrently occurring factors; between ISA at inpatient admission and factors occurring at community follow-up; and between ISA at community follow-up and concurrently occurring factors.
Materials and methods
Ethics approval for this multi-site prospective observational cohort study was granted by the participating hospitals and university (HREC Ref. 12380B). The study is reported according to STROBE guidelines (Vandenbroucke et al. 2007; von Elm et al. 2007).
Participants
Participants were consecutively recruited from two inpatient stroke rehabilitation wards in Melbourne, Australia between April 2013 and April 2014. Inclusion criteria were diagnosis of recent stroke (confirmed by medical team and imaging); minimum 18 years old; and English fluency. Survivors were excluded if they had comorbid neurological pathology, severe cognitive or language impairment preventing valid completion of assessment measures, or a significant current psychiatric illness.
Measures
The Patient Competency Rating Scale (PCRS) (Prigatano et al. 1986) is a measure of ISA developed for TBI and since used satisfactorily in stroke (Fischer et al. 2004; Noé et al. 2005; Barskova and Wilz 2006; Smeets et al. 2012). The PCRS enjoys widespread popularity, and in a comprehensive review, the PCRS was one of only three ISA measures deemed to have acceptable psychometric and conceptual properties (Smeets et al. 2012). The measure produces a discrepancy score of ±120 and positive values indicate patient ‘overestimation’ compared to the independent rater. There are several different methods for calculating PCRS discrepancy scores (Fleming et al. 1996). Consistent with the study’s focus on the presence of discrepancies rather than overall determinations of accuracy, we converted the discrepancy scores on each item to absolute values and summed for a total absolute discrepancy score. Further details of the measure are provided in Table 1.
| Construct | Assessment tool | Description and administration | Scale | |
|---|---|---|---|---|
| Impaired self-awareness | Patient Competency Rating Scale (PCRS) (Prigatano et al. 1986) | Stroke survivors rated their abilities on a broad range of functions, including activities of daily living (ADLs), applied cognitive skills, and emotional and interpersonal skills. |
| |
| Stroke survivors’ ratings were compared with an independent rater (clinician or close other) who used an equivalent form to rate the survivor on the same items. | ||||
| Discrepancy scores were calculated by subtracting clinician/close other ratings from survivor ratings on each item, converting to an absolute value, and summing. | ||||
| Stroke severity | The National Institutes of Health Stroke Scale (NIHSS) (Brott et al. 1989) | Medical staff rated the stroke survivor during acute hospital admission. Ratings were transcribed from the stroke survivor’s medical record. | ||
| Cognitive impairment | Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005) | Stroke survivors completed the cognitive screening tool. | ||
| Motor functioning | Functional Independence Measure (FIM) – motor subscale (Granger et al. 1986) | Clinicians and close others rated how much assistance was required for the stroke survivor to carry out basic ADLs such as self-care, toileting and transfers. | ||
| Functional cognition | Functional Independence Measure (FIM) – cognition subscale (Granger et al. 1986) | Clinicians and close others rated how much assistance was required by the stroke survivor to carry out daily activities that draw upon social cognition and communication skills. | ||
| Participation restriction | Mayo Portland Adaptability Inventory-4 Participation Index (MPAI-4-PI) (Malec and Lezak 2003) | Clinicians and close others estimated the degree to which the stroke survivor was restricted from fully participating in life activities such as employment, financial management, and socialising. | ||
| Rehabilitation engagement | Hopkins Rehabilitation Engagement Scale (HRES) (Kortte et al. 2007) | Clinicians rated stroke survivor engagement in rehabilitation. | ||
| Depression | Hospital Anxiety and Depression Scale (HADS) – depression subscale (Zigmond and Snaith 1983) | Stroke survivors and close others self-reported symptoms of depression. This measure has been validated for use in both clinical and non-clinical samples (Bjelland et al. 2002). | ||
| Anxiety | Hospital Anxiety and Depression Scale (HADS) – anxiety subscale (Zigmond and Snaith 1983) | Stroke survivors and close others self-reported symptoms of anxiety. This measure has been validated for use in both clinical and non-clinical samples (Bjelland et al. 2002). | ||
| Coping style | Coping Scale for Adults – Short Form (CSA-SF) (Frydenberg and Lewis 1996). Subscales used: | Stroke survivors reported how often they used each strategy to cope with overall concerns. | ||
| Quality of life | Assessment of Quality of Life (AQoL-4D) (Hawthorne and Osborne 2005) | AQoL-4D is a multi-attribute utility health-related quality of life instrument that calculates an overall utility score. Stroke survivors reported on four dimensions of life quality. | ||
| Caregiver burden | Zarit Burden Interview (ZBI) (Zarit et al. 1980) | Close others responded to items assessing aspects of carer burden. |
Details of demographic, stroke, functional, psychological and caregiver measures are summarised in Table 1. The study used patient-reported measures to assess a number of subjective constructs, including psychological distress, quality of life and coping style. Self-report was deemed a valid approach given its routine use in research examining correlates of ISA (e.g. Orfei et al. 2007; Smeets et al. 2017; Dromer et al. 2021; Martyr et al. 2022), and literature supporting reliable self-report of these symptoms in persons with mild to moderate cognitive impairment (Trigg et al. 2007; Frank et al. 2011; Ismail et al. 2017).
Procedure
Stroke survivors were approached on the rehabilitation ward. After providing informed consent, the stroke survivor completed researcher-administered measures assessing ISA (PCRS), mood, cognition, and coping style. Their occupational therapist subsequently completed the PCRS and questionnaires assessing the survivor’s motor functioning, functional cognition, and rehabilitation engagement. Parties were blinded to each other’s responses to reduce potential bias. During the inpatient stage, stroke survivors also nominated a close other (most often a family member or friend) to participate in the community stage of the study.
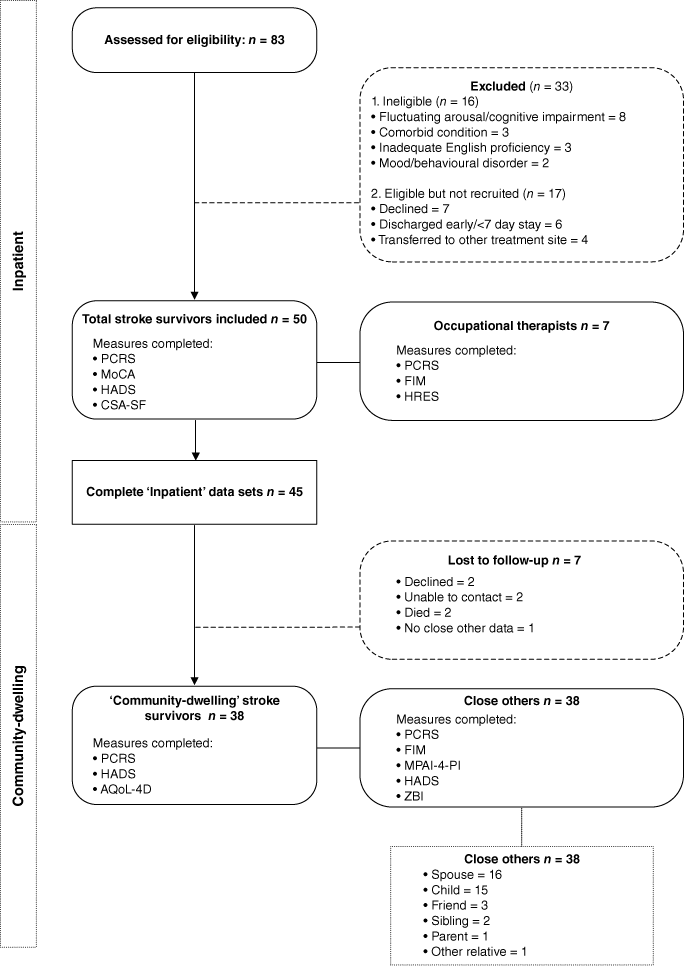
Approximately 4 months post-discharge, stroke survivors again completed the PCRS and measures assessing quality of life and emotional distress. Separately to the survivor and with the aid of a research assistant, the close other provided informed consent prior to completing the PCRS and assessments of the survivor’s motor, cognitive, and participation abilities. Close others also reported on their own emotional distress and carer burden. The decision to have close others (rather than clinicians) complete the ISA measure at community follow-up was informed by recognition that close others would have the most frequent opportunities to observe stroke survivor functioning in the community, and also practical constraints (inpatient clinicians did not have continued contact with the stroke survivor). Fig. 1 provides an overview of assessments administered at each time point.
Data analysis
Although no formal power analysis was undertaken, a target sample size of 50 was selected for this study as it was deemed achievable given the stroke population in the study sites and the time and resource constraints of the study (Lakens 2022). We calculated estimates of effect size (eta-squared and adjusted R2) and present 95% confidence intervals.
To investigate relationships between concurrent variables and ISA at inpatient and community-dwelling stages, we firstly used simple linear regression to examine the relationship between each individual predictor and the outcome variable (PCRS discrepancy). Subsequently, all predictors were entered simultaneously into multiple linear regression to examine how the variables performed together (Austin and Steyerberg 2015). Variance inflation factors (VIF) ranged from 1.35 to 5.54, suggesting mild to moderate levels of multicollinearity (Vittinghoff et al. 2005; Hair et al. 2010). This raised potential concerns about the multiple linear model’s capacity to identify associations and thus cautioned against relying solely on these results.
In addition to traditional multiple linear regression, we therefore used a contemporary variable selection method, known as LASSO regression (Least Absolute Shrinkage and Selection Operator), to identify the independent variables most strongly associated with ISA. Importantly, LASSO is less affected by multicollinearity than conventional multiple linear regression (Tibshirani 1996; Harrell 2015). Although guidelines such as 20 observations plus five observations per independent or predictor variable have long been proposed (Khamis and Kepler 2010), a recent simulation study (Austin and Steyerberg 2015) suggested that a ratio of at least two observations per variable be employed. LASSO is more flexible than multiple linear regression in regard to minimum sample sizes, with the limitation being that the number of independent variables is less than or equal to the number of observations in the data set (Kirpich et al. 2018), making it uniquely suited to the present analysis, keeping in mind the sample size. Finally, LASSO down-weights less important variables and gradually shrinks their coefficients towards zero, and so is less affected by overfitting and multicollinearity than earlier approaches such as stepwise regression in identifying useful predictors from the available set.
Coefficients that remained in the LASSO model were descriptively compared to coefficients in the multiple linear regression model, and predictors whose coefficients were of similar size and direction were regarded as having explanatory importance. LASSO attempts to maximise the overall fit of the model and does not readily incorporate P values or 95% confidence intervals of the individual predictors (Harrell 2015). LASSO was performed using the GLMSELECT procedure in SAS 9.4 (SAS Institute Incorporated, Cary, North Carolina, 2014) and reported as per Cnossen et al. (2017). Final models were selected using the Schwarz or Bayesian Information Criterion (BIC) (Schwarz 1978; Harrell 2015), a measure of model performance weighed against model complexity/number of variables, taking number of observations into account. All other analyses were conducted using IBM SPSS 25 (IBM Corporation, New York, 2017).
While the above-mentioned analyses sought to identify variables that were associated with ISA as an outcome and at a single time point, a separate set of analyses focussed on identifying associations between variables measured at community follow-up and ISA detected during inpatient admission. To this end, a multivariate (multiple outcomes) regression (Dattalo 2013) examined the effect of ISA at inpatient admission on multiple community-dwelling outcome variables, controlling for the potential confounders of age, sex and stroke severity. Partial eta squared values provided a measure of variance explained (Dattalo 2013; Harrell 2015).
Results
Participants
Fig. 1 illustrates participant flow through the study. Stroke survivor characteristics are summarised in Table 2.
| Inpatient phase (n = 45) | Community-dwelling phase (n = 38) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Range | Frequency | Percentage | Mean | s.d. | Range | Frequency | Percentage | |||
| Age (years) | 71.5 | 15.6 | 33 to 92 | – | – | 69.6 | 15.9 | 33 to 91 | – | – | ||
| Sex | Male | – | – | – | 21 | 47 | – | – | – | 18 | 47 | |
| Female | – | – | – | 24 | 53 | – | – | – | 20 | 53 | ||
| Education (years) | 11.2 | 2.2 | 7 to 18 | – | – | 11.6 | 2.0 | 8 to 18 | – | – | ||
| Days post stroke | 55.7 | 33.4 | 12 to 154 | – | – | 189.8 | 45.2 | 125 to 333 | – | – | ||
| NIHSS | 7.7 | 5.2 | 1 to 21 | – | – | 8.2 | 5.4 | 1 to 21 | – | – | ||
| Stroke mechanism | Ischaemic | – | – | – | 27 | 60 | – | – | – | 21 | 55 | |
| Haemorrhagic | – | – | – | 18 | 40 | – | – | – | 17 | 45 | ||
| Lesion hemisphere | Left | – | – | – | 16 | 36 | – | – | – | 15 | 39 | |
| Right | – | – | – | 29 | 64 | – | – | – | 23 | 61 | ||
| Previous strokes | Yes | – | – | – | 11 | 24 | – | – | – | 9 | 24 | |
| No | – | – | – | 34 | 76 | – | – | – | 29 | 76 | ||
| Discharge destination | Home | – | – | – | 34 | 76 | – | – | – | 32 | 84 | |
| Care facility | – | – | – | 11 | 24 | – | – | – | 6 | 16 | ||
| FIM – motor | 64.0 | 19.7 | 13 to 89 | – | – | 75.7 | 16.1 | 19 to 91 | – | – | ||
| FIM – cognition | 28.3 | 5.6 | 9 to 35 | – | – | 31.0 | 4.5 | 17 to 35 | – | – | ||
| PCRS total discrepancy | 35.2 | 17.2 | 4 to 80 | – | – | 15.2 | 15.5 | 0 to 66 | – | – | ||
| MoCA | 21.3 | 5.5 | 7 to 30 | – | – | – | – | – | – | – | ||
| HRES | 23.1 | 5.1 | 13 to 30 | – | – | – | – | – | – | – | ||
| HADS – depression (survivor) | 4.2 | 3.6 | 0 to 15 | – | – | 4.8 | 4.5 | 0 to 19 | – | – | ||
| HADS – anxiety (survivor) | 4.9 | 3.9 | 0 to 20 | – | – | 3.6 | 3.0 | 0 to 10 | – | – | ||
| CSA–SF – dealing with the problem | 55.5 | 15.3 | 24 to 87 | – | – | – | – | – | – | – | ||
| CSA-SF – non-productive coping | 44.7 | 13.5 | 21 to 90 | – | – | – | – | – | – | – | ||
| MPAI–4–PI | – | – | – | – | – | 13.9 | 7.9 | 1 to 28 | – | – | ||
| AQoL–4D | – | – | – | – | – | 0.48 | 0.29 | –0.02 to 1.00 | – | – | ||
| HADS – depression (close other) | – | – | – | – | – | 2.5 | 3.1 | 0 to 10 | – | – | ||
| HADS – anxiety (close other) | – | – | – | – | – | 4.6 | 3.5 | 0 to 15 | – | – | ||
| ZBI | – | – | – | – | – | 19.2 | 17.0 | 0 to 53 | – | – | ||
Eighty-three stroke survivors were approached. Fifty met eligibility and consent requirements to participate, and 45 of these had complete data. Of the 50 stroke survivors who participated in the inpatient component of the research and the 33 who were approached but declined or were ineligible, there were no statistically significant differences in age (t (81) = −1.18, P = 0.24), sex (X2 (1) = 0.173, P = 0.68), stroke mechanism (X2 (2) = 1.14, P = 0.57) or affected hemisphere (X2 (2) = 0.129, P = 0.94). The sample was broadly representative of the stroke population on key demographic variables including age (M (s.d.) 71.49 (15.60)) and sex (53% female) (Australian Institute of Health and Welfare 2018).
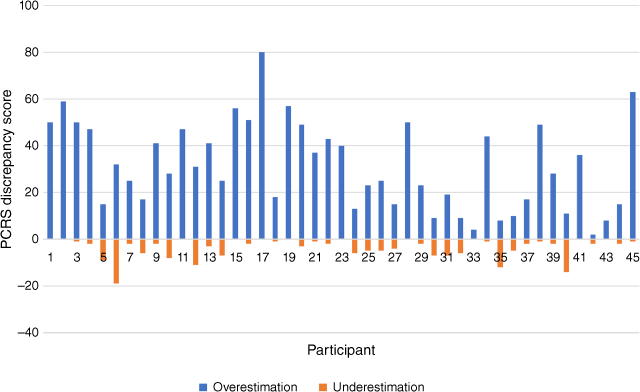
As mentioned earlier, this study made use of absolute scores, consistent with a conceptual focus on the presence of discrepancies, rather than a focus on the direction of the discrepancy or seeking to determine the ‘accuracy’ of each individual’s rating. It should be noted, however, that there were relatively few instances of stroke survivors underestimating their abilities relative to the clinician or close other (i.e. a negative PCRS score). Fig. 2 depicts the distribution of PCRS discrepancy scores for each survivor-clinician dyad. The figure illustrates the extent to which each survivor has overestimated and underestimated their functional abilities relative to the clinician’s assessment. It can be seen that in only two cases, the number of underestimations exceed overestimations and thus most individuals overestimated their functional abilities relative to clinicians.
In total, 38 stroke survivors and their close other provided usable data for the community-dwelling assessment and participated on average 124.7 (s.d. 34.3) days after inpatient assessment (range 82–246). There were no statistically significant differences in age (t (43) = −1.916, P = 0.06), sex (X2 (1) = 1.091, P = 0.30), stroke mechanism (X2 (1) = 0.451, P = 0.50), stroke severity (t (43) = 1.525, P = 0.14), stroke hemisphere (X2 (1) = 0.176, P = 0.67), discharge destination (X2 (1) = 1.522, P = 0.22), or inpatient ISA score (t (43) = 1.382, P = 0.17) between the 38 stroke survivors who participated in the community follow-up component, and the seven who did not.
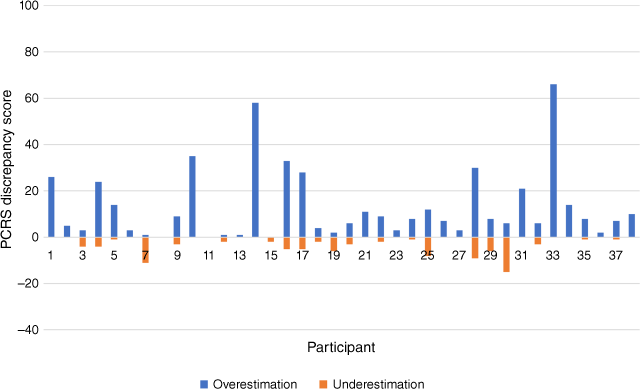
Mean discrepancy scores on the ISA measure at community follow-up (M (s.d.) 15.2 (15.5)) were smaller than those measured during inpatient admission (M (s.d.) 35.2 (17.2)), suggesting that ISA was less pronounced at community follow-up. Similar to the trend observed during inpatient admission, the majority of participants overestimated their functioning relative to the other rater – in this instance, the nominated close other. In total, six participants were more likely to underestimate, than overestimate, their functional abilities compared to their nominated close other. Fig. 3 depicts the distribution of PCRS discrepancy scores for each survivor-close other dyad.
Outcomes
Table 2 provides descriptive data for the measured variables at both time points.
All simple, multiple linear and LASSO results are presented in Table 3. Simple linear regressions (each predictor entered singly, into a separate regression) showed greater ISA (i.e. larger PCRS scores) was significantly associated with increased time since stroke and with lower scores on measures of functional cognition, cognitive impairment, rehabilitation engagement, and non-productive coping style. For example, each increase of one unit in FIM cognition score was associated with a decrease of 2.17 in mean PCRS score, regression coefficient = −2.17, 95% CI = −2.85 to −1.49, P < 0.001. Multiple linear regression modelling with all predictors entered simultaneously (F(16,28) = 4.27, P < 0.001, R2 = 0.71, adjusted R2 = 0.54) showed only functional cognition had a statistically significant association with ISA (regression coefficient = −2.72, 95% CI = −3.75 to −1.69, P < 0.001). Age narrowly failed to reach statistical significance (P = 0.057).
| Simple linear regression | Multiple linear regression | LASSO | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Regression coefficient | P | 95% CIA | Regression coefficient | P | 95% CIA | Shrunken coefficient | |
| Age | −0.13 | 0.458 | −0.46 to 0.21 | −0.38 | 0.057 | −0.76 to 0.01 | −0.33 | |
| Sex – female | −4.13 | 0.428 | −14.55 to 6.29 | −6.06 | 0.213 | −15.81 to 3.68 | −6.24 | |
| Sex – male | Ref | |||||||
| Education | −0.27 | 0.827 | −2.70 to 2.17 | 0.15 | 0.878 | −1.82 to 2.12 | – | |
| Days post stroke | 0.16 | 0.038* | 0.10 to 0.31 | −0.04 | 0.676 | −0.22 to 0.14 | – | |
| Stroke mechanism – haemorrhagic | 0.63 | 0.906 | −10.05 to 11.31 | 2.56 | 0.538 | −5.85 to 10.97 | – | |
| Stroke mechanism – ischaemic | Ref | |||||||
| Lesion hemisphere – right | −2.54 | 0.641 | −13.44 to 8.37 | −0.54 | 0.909 | −10.11 to 9.02 | – | |
| Lesion hemisphere – left | Ref | |||||||
| NIHSS | 0.26 | 0.616 | −0.77 to 1.28 | −0.18 | 0.766 | −1.41 to 1.05 | – | |
| Previous strokes – no | −5.42 | 0.370 | −17.49 to 6.65 | −9.02 | 0.109 | −20.19 to 2.15 | – | |
| Previous strokes – yes | Ref | |||||||
| FIM – motor | −0.12 | 0.350 | −0.39 to 0.14 | 0.13 | 0.534 | −0.29 to 0.55 | – | |
| FIM – cognition | −2.17 | <0.001* | −2.85 to −1.49 | −2.72 | <0.001* | −3.75 to −1.69 | −2.19 | |
| MoCA | −1.08 | 0.020* | −1.98 to −0.17 | 0.18 | 0.737 | −0.88 to 1.23 | – | |
| HRES | −1.52 | 0.002* | −2.44 to −0.60 | −0.07 | 0.887 | −1.12 to 0.97 | −0.17 | |
| HADS – depression | 0.13 | 0.859 | −1.36 to 1.62 | 0.45 | 0.570 | −1.16 to 2.07 | – | |
| HADS – anxiety | −0.62 | 0.362 | −1.97 to 0.74 | −0.20 | 0.760 | −1.51 to 1.12 | – | |
| CSA–SF dealing with the problem | −0.42 | 0.413 | −1.45 to 0.61 | −0.16 | 0.772 | −1.25 to 0.94 | – | |
| CSA–SF non-productive coping | −1.14 | 0.048* | −2.26 to −0.01 | −0.57 | 0.252 | −1.56 to 0.53 | −0.93 | |
| R 2/Adjusted R 2 | 0.71/0.54 | 0.65/0.60 | ||||||
LASSO shrinkage was then applied to the 16 predictors to obtain the final set of independent predictors and their shrunken regression coefficients. The LASSO procedure selected 5 of the original 16 predictors. The LASSO coefficients of these predictors were of the same direction and of similar size to the corresponding multiple linear regression coefficients, and hence were regarded as having explanatory importance. Specifically, greater ISA was associated with younger age, male sex, and lower scores on measures of functional cognition, rehabilitation engagement, and non-productive coping. LASSO analyses indicated that the five predictors together accounted for 65% (R2 = 0.65) of the possible variation in ISA (adjusted R2 = 0.60) (Table 3).
The multivariate regression model, representing the association between a single predictor (ISA at inpatient stage) and 10 community-dwelling outcome variables simultaneously, while controlling for age, sex, and stroke severity, was statistically significant (F(10,24) = 3.78, P = 0.004). Greater levels of ISA during inpatient admission were associated with lower scores on measures of functional cognition and participation restriction, and with higher scores on measures of caregiver burden, close other depression and close other anxiety (Table 4). Functional cognition explained the most variance (ηp2 = 0.33).
| Variable | B | P | 95% CIA | Partial eta-square | |
|---|---|---|---|---|---|
| Discharge destination – care facility | 0.00 | 0.512 | −0.01 to 0.01 | 0.01 | |
| Discharge destination – home | Ref | ||||
| FIM – motor | −0.16 | 0.312 | −0.49 to 0.16 | 0.03 | |
| FIM – cognition | −0.16 | <0.001* | −0.24 to −0.08 | 0.33 | |
| MPAI–4–PI | 0.21 | 0.009* | 0.06 to 0.37 | 0.19 | |
| HADS – depression (stroke survivor) | 0.04 | 0.436 | −0.06 to 0.14 | 0.02 | |
| HADS – anxiety (stroke survivor) | −0.01 | 0.747 | −0.08 to 0.06 | 0.00 | |
| AQoL-4D | −0.00 | 0.202 | −0.01 to 0.00 | 0.05 | |
| HADS – depression (close other) | 0.08 | 0.028* | 0.01 to 0.15 | 0.14 | |
| HADS – anxiety (close other) | 0.09 | 0.021* | 0.02 to 0.16 | 0.15 | |
| ZBI | 0.38 | 0.007* | 0.11 to 0.65 | 0.20 |
Simple linear regressions indicated larger ISA in the community setting was significantly associated with lower scores on measures of motor functioning, functional cognition, and quality of life; and with higher scores on measures of participation restriction, survivor depression, survivor anxiety, close other anxiety, and caregiver burden. In a multiple linear regression model with all 10 predictors entered simultaneously, functional cognition, survivor anxiety, and close other anxiety were statistically significant. LASSO shrinkage was then applied and selected 2 of the 10 predictors – functional cognition and close other anxiety. The two LASSO coefficients were of the same direction and of similar size to the corresponding multiple linear coefficients, and hence were considered as having explanatory importance. Specifically, higher levels of ISA in the community setting were associated with lower levels of functional cognition, and higher levels of close other anxiety. The two predictors together accounted for 68% (R2 = 0.68) of the possible variation in ISA (adjusted R2 = 0.66) (Table 5).
| Simple linear regression | Multiple linear regression | LASSO | ||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Regression coefficient | P | 95% CIA | Regression coefficient | P | 95% CIA | Shrunken coefficient | |
| Discharge destination – care facility | 12.81 | 0.062 | −0.66 to 26.28 | −0.38 | 0.947 | −11.84 to 11.09 | – | |
| Discharge destination – home | Ref | |||||||
| FIM – motor | −0.51 | <0.001* | −0.78 to −0.23 | −0.27 | 0.166 | −0.65 to 0.12 | – | |
| FIM – cognition | −2.75 | <0.001* | −3.46 to −2.04 | −3.55 | <0.001* | −4.59 to −2.51 | −2.45 | |
| MPAI-4-PI | 1.06 | <0.001* | 0.50 to 1.61 | −0.59 | 0.160 | −1.43 to 0.25 | – | |
| HADS – depression (survivor) | 1.50 | 0.007* | 0.44 to 2.56 | −1.02 | 0.107 | −2.28 to 0.24 | – | |
| HADS – anxiety (survivor) | 1.97 | 0.017* | 0.38 to 3.57 | 1.29 | 0.043* | 0.05 to 2.53 | – | |
| AQoL-4D | −25.44 | 0.003* | −41.40 to −9.48 | 1.36 | 0.891 | −18.70 to 21.41 | – | |
| HADS – depression (close other) | 1.49 | 0.069 | −0.12 to 3.10 | 0.03 | 0.965 | −1.15 to 1.20 | – | |
| HADS – anxiety (close other) | 2.23 | 0.001* | 0.92 to 3.54 | 1.67 | 0.015* | 0.35 to 3.00 | 1.06 | |
| ZBI | 0.47 | 0.001* | 0.20 to 0.73 | −0.27 | 0.061 | −0.56 to 0.01 | – | |
| R 2/Adjusted R 2 | 0.79/0.71 | 0.68/0.66 | ||||||
Discussion
This study sought to identify biopsychosocial factors associated with post-stroke ISA across the recovery period. In an important finding, greater levels of ISA during inpatient rehabilitation were associated with poorer community-dwelling outcomes for both stroke survivors and their close others. Further, in multiple linear regression analyses, ISA was associated with a number of biological and psychosocial variables, with functional cognition demonstrating a particularly robust association.
Associations between ISA during inpatient admission and community-dwelling outcomes
Greater levels of ISA during inpatient stay were associated with more participation restriction and poorer functional cognition at community follow-up. The study design precludes causal inferences; however, ISA may be a risk factor for poor community-dwelling outcomes. In a separate finding (discussed under ‘biopsychosocial factors’), greater ISA during inpatient admission was associated with poorer inpatient rehabilitation engagement. We purport that rehabilitation engagement may mediate the relationship between ISA during inpatient admission and community-dwelling outcomes. Specifically, poor rehabilitation engagement may inhibit the development of skills that facilitate independence and participation in the community. Future mediation analysis is recommended to explore these relationships further.
Greater ISA during inpatient admission was also associated with close other psychological distress and carer burden in the community. This aligns with recent research demonstrating a positive association between stroke survivor ISA and carer strain and depression in the first six months post stroke (Stein and Reynolds 2020), and with research showing similar relationships for dementia and TBI populations (Seltzer et al. 1997; Ergh et al. 2002; Moretti et al. 2006; Vogel et al. 2010). Cognitive and behavioural dysfunction are known contributors to carer burden (Greenwood et al. 2008; Byun and Evans 2015), and we suggest that discrepancies between stroke survivors and their carers regarding the presence of these difficulties likely exacerbates this distress. This finding emphasises the importance of providing appropriate education and support to carers to manage these challenges.
Associations between ISA and functional cognition
Functional cognition describes the collection of cognitive skills necessary to perform complex activities of daily living (Wesson et al. 2017). It includes aspects of metacognition, executive functioning, other cognitive functions (e.g. memory, visuospatial), performance skills (e.g. motor skills) and performance patterns (e.g. routines) (Donovan et al. 2007; Wolf et al. 2019; Giles et al. 2017, 2020). The association between greater ISA and poorer functional cognition was statistically significant and evident across all three investigative aims. There are multiple ways to interpret this association. Firstly, ISA may compromise functional cognition by limiting an individual’s capacity to benefit from rehabilitation, e.g. learning and integrating compensation techniques. Secondly, it is possible that there are similar underlying factors contributing to both functional cognition and ISA. Appraising one’s abilities plausibly requires many of the same cognitive skills involved in functional cognition, and hence deficiencies in these cognitive skills may contribute to ISA. Executive functioning, though only one component of functional cognition, may be particularly influential in light of research in other ABI populations, suggesting an association (Lezak 1993; Ownsworth and Fleming 2005; Zimmermann et al. 2017). Contributions from other cognitive components of functional cognition should not be discounted, however, and further research is necessary to better understand the association between ISA and functional cognition, including any unique contributions.
Biopsychosocial factors associated with ISA
ISA was associated with a number of biological and psychosocial variables, supporting the validity of a biopsychosocial framework of appraisal discrepancy. Specifically, greater levels of ISA during inpatient admission were associated with younger age, male sex, poorer rehabilitation engagement, and less frequent use of non-productive coping strategies.
Associations with age and sex align with previous health behaviour research, specifically research that suggests age mediates individuals’ responses to chronic illness (Pound et al. 1998) and research indicating that gender-role expectations may influence disclosure of personal problems/weaknesses and subsequent help-seeking (Wood et al. 1997; Addis and Mahalik 2003; Mackenzie et al. 2006; Alston et al. 2012; Thompson et al. 2016). The association between greater levels of ISA during inpatient admission and poorer rehabilitation engagement is clinically intuitive and supports previous findings in ABI populations (Lam et al. 1988; Malec et al. 1991). Notwithstanding, it remains an important distinct finding. As discussed earlier, we suggest rehabilitation engagement may mediate some of the other relationships observed, although further research is required.
An association between greater levels of ISA during admission and less frequent use of non-productive coping strategies was an unexpected finding. ‘Non-productive coping’ describes unhelpful and avoidant behaviours suggestive of poor adjustment, including wishful thinking and ignoring problems (Frydenberg and Lewis 1996); hence our finding is somewhat counterintuitive (Gracey et al. 2009). Studies in other ABI populations have reported positive associations between ISA and characteristics of non-productive coping (Finset and Andersson 2000; Krpan et al. 2007). It is unclear why our findings contradict previous research in similar populations. We suggest future research should use a mixed-methods design to further examine the relationship between coping style and post-stroke ISA.
Clinical and research implications
We have identified subacute clinical markers that may represent risk factors for post-stroke ISA, including younger age, male sex, and poor functional cognition. Assuming further independent confirmation of findings, these markers could be included in screening protocols to identify patients requiring comprehensive assessment. The association between greater levels of ISA during inpatient admission and poorer community-dwelling outcomes for stroke survivors and their close others is especially significant and, to our knowledge, this is the first cohort study to have demonstrated this. This finding emphasises the clinical significance of post-stroke ISA well beyond the period of inpatient care and perhaps even beyond the point of detectable symptoms of ISA. These findings advocate for the routine assessment of post-stroke ISA and for effective interventions and appropriate support for affected stroke survivors and their families.
Study limitations and future directions
The sample size is modest, therefore results and their generalisability should be interpreted with caution. The use of variable selection models (e.g. LASSO) is ideally suited to larger sample sizes; however, it was reassuring that multiple linear regression and LASSO analyses returned similar results, providing a degree of confidence in the results obtained. Given the modest sample size, a power calculation would have improved the study’s rigour. Furthermore, the study design precluded causal inferences, thus a longitudinal study employing a larger sample size is required to confirm findings and enable the use of more informative analyses such as time-lagged regression modelling.
We elected to have clinicians perform the inpatient ratings, and close others perform the community-dwelling ratings. This design ensured raters were the individuals with the most exposure to the stroke survivor’s functioning but meant raters were not consistent across time points. This represents a potential confound and prevented the application of a longitudinal study design. Notably though, findings on a number of key variables were similar across time points, consistent with research indicating that PCRS ratings by clinicians and close others are comparable in ABI populations (Sherer et al. 2003a). Furthermore, key variables were identified through two types of regression modelling: simultaneous multiple linear regression including all independent variables and LASSO.
The study’s modest sample size necessitated the selection of a limited number of biopsychosocial variables from the plethora that exist in the literature. The variables chosen were those that had demonstrated the most convincing associations in other ISA-affected populations; however, this meant a number of potentially promising variables were not included such as personality traits, gender, and a range of socioenvironmental variables (e.g. socio-cultural attitudes to disability, employment status). It is suggested that future studies explore potential relationships between ISA and these variables.
Conclusion
Using a representative sample of stroke survivors, we have identified a number of biopsychosocial factors associated with post-stroke ISA, and these may represent contributory factors and outcomes. Functional cognition was a particularly significant variable and presents an intriguing direction for further research. Results support biopsychosocial frameworks of ISA and emphasise the clinical significance of ISA. Findings from this study generate interesting hypotheses that future studies can address using larger sample sizes. It is clear that understanding how stroke survivors view their post-stroke functioning is of paramount importance to achieving optimal patient outcomes during inpatient care and beyond.
References
Addis ME, Mahalik JR (2003) Men, masculinity, and the contexts of help seeking. The American Psychologist 58, 5-14.
| Crossref | Google Scholar | PubMed |
Alston M, Jones J, Curtin M (2012) Women and Traumatic Brain Injury: “It’s not visible damage”. Australian Social Work 65, 39-53.
| Crossref | Google Scholar |
Amanzio M, Vase L, Leotta D, Miceli R, Palermo S, Geminiani G (2013) Impaired Awareness of Deficits in Alzheimer’s Disease: The Role of Everyday Executive Dysfunction. Journal of the International Neuropsychological Society 19, 63-72.
| Crossref | Google Scholar | PubMed |
Austin PC, Steyerberg EW (2015) The number of subjects per variable required in linear regression analyses. Journal of Clinical Epidemiology 68, 627-636.
| Crossref | Google Scholar | PubMed |
Australian Institute of Health and Welfare (2018) ‘Australia’s health 2018.’ Australia’s health series. (Canberra) Available at https://www.aihw.gov.au/reports/australias-health/australias-health-2018/contents/table-of-contents
Avlund K (1997) Methodological challenges in measurements of functional ability in gerontological research. A review. Aging Clinical and Experimental Research 9, 164-174.
| Crossref | Google Scholar | PubMed |
Barskova T, Wilz G (2006) Psychosocial functioning after stroke: psychometric properties of the patient competency rating scale. Brain Injury 20, 1431-1437.
| Crossref | Google Scholar | PubMed |
Belchev Z, Levy N, Berman I, Levinzon H, Hoofien D, Gilboa A (2017) Psychological traits predict impaired awareness of deficits independently of neuropsychological factors in chronic traumatic brain injury. British Journal of Clinical Psychology 56, 213-234.
| Crossref | Google Scholar | PubMed |
Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research 52, 69-77.
| Crossref | Google Scholar | PubMed |
Brott T, Adams HP J, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20(7), 864-70.
| Crossref | Google Scholar | PubMed |
Byun E, Evans LK (2015) Concept Analysis of Burden in Caregivers of Stroke Survivors During the Early Poststroke Period. Clinical Nursing Research 24(5), 468-86.
| Crossref | Google Scholar | PubMed |
Cameron KV, Ponsford JL, Stolwyk RJ (2020) Do stroke survivors agree with their clinicians on the extent of their post-stroke activity limitation and participation restriction? Neuropsychological Rehabilitation 30, 1430-1448.
| Crossref | Google Scholar | PubMed |
Clare L (2004) The construction of awareness in early-stage Alzheimer’s disease: a review of concepts and models. British Journal of Clinical Psychology 43, 155-175.
| Crossref | Google Scholar | PubMed |
Clare L, Nelis SM, Martyr A, Roberts J, Whitaker CJ, Markova IS, Roth I, Woods RT, Morris RG (2012) The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: testing a biopsychosocial model. International Journal of Geriatric Psychiatry 27, 167-177.
| Crossref | Google Scholar | PubMed |
Cnossen MC, Winkler EA, Yue JK, Okonkwo DO, Valadka AB, Steyerberg EW, Lingsma HF, Manley GT (2017) Development of a Prediction Model for Post-Concussive Symptoms following Mild Traumatic Brain Injury: A TRACK-TBI Pilot Study. J Neurotrauma 34(16), 2396-2409.
| Crossref | Google Scholar | PubMed |
Crosson B, Barco PP, Velozo CA, Bolesta MM, Cooper PV, Werts D, Brobeck TC (1989) Awareness and compensation in post-acute head injury rehabilitation. Journal of Head Trauma Rehabilitation 4, 46-54.
| Crossref | Google Scholar |
Donovan NJ, Kendall DL, Heaton SC, Kwon S, Velozo CA, Duncan PW (2007) Conceptualizing Functional Cognition in Stroke. Neurorehabilitation and Neural Repair 22, 122-135.
| Crossref | Google Scholar | PubMed |
Dromer E, Kheloufi L, Azouvi P (2021) Impaired self-awareness after traumatic brain injury: a systematic review. Part 2. Consequences and predictors of poor self-awareness. Annals of Physical and Rehabilitation Medicine, 64, 101542.
| Crossref | Google Scholar | PubMed |
Engel GL (1980) The clinical application of the biopsychosocial model. American Journal of Psychiatry 137, 535-544.
| Crossref | Google Scholar | PubMed |
Ergh TC, Rapport LJ, Coleman RD, Hanks RA (2002) Predictors of caregiver and family functioning following traumatic brain injury: social support moderates caregiver distress. Journal of Head Trauma Rehabilitation 17, 155-174.
| Crossref | Google Scholar | PubMed |
Fischer S, Gauggel S, Trexler LE (2004) Awareness of activity limitations, goal setting and rehabilitation outcome in patients with brain injuries. Brain Injury 18, 547-562.
| Crossref | Google Scholar | PubMed |
Fleming J, Strong J (1995) Self-Awareness of Deficits following Acquired Brain Injury: Considerations for Rehabilitation. British Journal of Occupational Therapy 58, 55-60.
| Crossref | Google Scholar |
Fleming JM, Strong J, Ashton R (1996) Self-awareness of deficits in adults with traumatic brain injury: how best to measure? Brain Injury 10, 1-15.
| Crossref | Google Scholar | PubMed |
Frank L, Lenderking WR, Howard K, Cantillon M (2011) Patient self-report for evaluating mild cognitive impairment and prodromal Alzheimer’s disease. Alzheimer’s Research & Therapy 3, 35.
| Crossref | Google Scholar | PubMed |
Gialanella B, Monguzzi V, Santoro R, Rocchi S (2005) Functional recovery after hemiplegia in patients with neglect: the rehabilitative role of anosognosia. Stroke 36, 2687-2690.
| Crossref | Google Scholar | PubMed |
Giles GM, Edwards DF, Morrison MT, Baum C, Wolf TJ (2017) Screening for Functional Cognition in Postacute Care and the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014. The American Journal of Occupational Therapy 71, 7105090010p1-7105090010p6.
| Crossref | Google Scholar | PubMed |
Giles GM, Edwards DF, Baum C, Furniss J, Skidmore E, Wolf T, Leland NE (2020) Making Functional Cognition a Professional Priority. The American Journal of Occupational Therapy 74, 7401090010p1-7401090010p6.
| Crossref | Google Scholar | PubMed |
Gracey F, Evans JJ, Malley D (2009) Capturing process and outcome in complex rehabilitation interventions: a “Y-shaped” model. Neuropsychological Rehabilitation 19, 867-890.
| Crossref | Google Scholar | PubMed |
Granger CV, Hamilton BB, Keith RA, Zielezny M, Sherwin FS (1986) Advances in functional assessment for medical rehabilitation. Topics in Geriatric Rehabilitation 1, 59-74.
| Crossref | Google Scholar |
Greenwood N, Mackenzie A, Cloud GC, Wilson N (2008) Informal carers of stroke survivors–factors influencing carers: a systematic review of quantitative studies. Disability & Rehabilitation 30, 1329-1349.
| Crossref | Google Scholar | PubMed |
Hartman-Maeir A, Soroker N, Oman SD, Katz N (2003) Awareness of disabilities in stroke rehabilitation – a clinical trial. Disability & Rehabilitation 25, 35-44.
| Google Scholar | PubMed |
Hawthorne G, Osborne R (2005) Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Australian and New Zealand Journal of Public Health 29, 136-142.
| Crossref | Google Scholar | PubMed |
Heilman KM, Barrett AM, Adair JC (1998) Possible mechanisms of anosognosia: a defect in self–awareness. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353, 1903-1909.
| Crossref | Google Scholar | PubMed |
Hertzog C, Dunlosky J (2011) Metacognition in Later Adulthood: Spared Monitoring Can Benefit Older Adults’ Self-Regulation. Current Directions in Psychological Science 20, 167-173.
| Crossref | Google Scholar | PubMed |
Hier DB, Mondlock J, Caplan LR (1983) Behavioral abnormalities after right hemisphere stroke. Neurology 33, 337-344.
| Crossref | Google Scholar | PubMed |
Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, Schweizer T, Mortby ME, Smith EE, Patten SB, Fiest KM (2017) Prevalence of Depression in Patients With Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Psychiatry 74, 58-67.
| Crossref | Google Scholar | PubMed |
Jehkonen M, Ahonen JP, Dastidar P, Koivisto AM, Laippala P, Vilkki J, Molnár G (2001) Predictors of discharge to home during the first year after right hemisphere stroke. Acta Neurologica Scandinavica 104, 136-141.
| Crossref | Google Scholar | PubMed |
Jehkonen M, Laihosalo M, Kettunen J (2006) Anosognosia after stroke: assessment, occurrence, subtypes and impact on functional outcome reviewed. Acta Neurologica Scandinavica 114, 293-306.
| Crossref | Google Scholar | PubMed |
Jenkinson PM, Preston C, Ellis SJ (2011) Unawareness after stroke: a review and practical guide to understanding, assessing, and managing anosognosia for hemiplegia. Journal of Clinical and Experimental Neuropsychology 33, 1079-1093.
| Crossref | Google Scholar | PubMed |
Kelleher M, Tolea MI, Galvin JE (2016) Anosognosia increases caregiver burden in mild cognitive impairment. International Journal of Geriatric Psychiatry 31, 799-808.
| Crossref | Google Scholar | PubMed |
Khamis HJ, Kepler M (2010) Sample Size in Multiple Regression: 20 + 5k. Journal of Applied Statistical Science 17, 505-517.
| Google Scholar |
Kirpich A, Ainsworth EA, Wedow JM, Newman JRB, Michailidis G, Mcintyre LM (2018) Variable selection in omics data: a practical evaluation of small sample sizes. PLoS One 13, e0197910.
| Crossref | Google Scholar | PubMed |
Kortte KB, Falk LD, Castillo RC, Johnson-Greene D, Wegener ST (2007) The Hopkins Rehabilitation Engagement Rating Scale: Development and Psychometric Properties. Archives of Physical Medicine and Rehabilitation 88, 877-884.
| Crossref | Google Scholar | PubMed |
Krpan KM, Levine B, Stuss DT, Dawson DR (2007) Executive function and coping at one-year post traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 29, 36-46.
| Crossref | Google Scholar | PubMed |
Lacerda IB, Santos RL, Belfort T, Neto JPS, Dourado MCN (2020) Patterns of discrepancies in different objects of awareness in mild and moderate Alzheimer’s disease. Aging & Mental Health 24, 789-796.
| Crossref | Google Scholar | PubMed |
Lakens D (2022) Sample Size Justification. Collabra: Psychology 8, 1-27.
| Crossref | Google Scholar |
Lam CS, Mcmahon BT, Priddy DA, Gehred-Schultz A (1988) Deficit awareness and treatment performance among traumatic head injury adults. Brain Injury 2, 235-242.
| Crossref | Google Scholar | PubMed |
Lezak MD (1993) Newer contributions to the neuropsychological assessment of executive functions. The Journal of Head Trauma Rehabilitation 8, 24-31.
| Crossref | Google Scholar |
Mackenzie CS, Gekoski WL, Knox VJ (2006) Age, gender, and the underutilization of mental health services: the influence of help-seeking attitudes. Aging & Mental Health, 10, 574-582.
| Crossref | Google Scholar | PubMed |
Malec JF, Lezak MD (2003) Manual for the Mayo-Portland Adaptability Inventory (MPAI-4). Available at http://www.timbs.org/combi/mpai [accessed 28 November 2012]
Malec JF, Smigielski JS, Depompolo RW (1991) Goal attainment scaling and outcome measurement in postacute brain injury rehabilitation. Archives of Physical Medicine and Rehabilitation 72, 138-143.
| Google Scholar | PubMed |
Marcel AJ, Tegnér R, Nimmo-Smith I (2004) Anosognosia for plegia: specificity, extension, partiality and disunity of bodily unawareness. Cortex 40, 19-40.
| Crossref | Google Scholar | PubMed |
Martyr A, Gamble LD, Nelis SM, Collins R, Alexander CM, Morris RG, Quinn C, Pentecost C, Rusted JM, Victor C, Thom JM, Matthews FE, Clare L, on behalf of the IDEAL Study Team (2022) Predictors of Awareness of Functional Ability in People with Dementia: The Contribution of Personality, Cognition, and Neuropsychiatric Symptoms – Findings from the IDEAL Program. Dementia and Geriatric Cognitive Disorders 51, 221-232.
| Crossref | Google Scholar | PubMed |
McGlynn SM, Schacter DL (1989) Unawareness of deficits in neuropsychological syndromes. Journal of Clinical and Experimental Neuropsychology 11, 143-205.
| Crossref | Google Scholar | PubMed |
Moretti R, Torre P, Antonello RM, Cazzato G (2006) Behavioral alterations and vascular dementia. Neurologist 12, 43-47.
| Crossref | Google Scholar | PubMed |
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53, 695-699.
| Crossref | Google Scholar | PubMed |
Niemeier JP, Perrin PB, Holcomb MG, Rolston CD, Artman LK, Lu J, Nersessova KS (2014) Gender differences in awareness and outcomes during acute traumatic brain injury recovery. Journal of Women’s Health 23, 573-580.
| Crossref | Google Scholar | PubMed |
Noé E, Ferri J, Caballero MC, Villodre R, Sanchez A, Chirivella J (2005) Self-awareness after acquired brain injury: predictors and rehabilitation. Journal of Neurology 252, 168-175.
| Crossref | Google Scholar | PubMed |
Nurmi ME, Jehkonen M (2014) Assessing anosognosias after stroke: a review of the methods used and developed over the past 35 years. Cortex 61, 43-63.
| Google Scholar |
Orfei MD, Robinson RG, Prigatano GP, Starkstein S, Rüsch N, Bria P, Caltagirone C, Spalletta G (2007) Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a systematic review of the literature. Brain 130, 3075-3090.
| Crossref | Google Scholar | PubMed |
Orfei MD, Caltagirone C, Spalletta G (2009) The evaluation of anosognosia in stroke patients. Cerebrovascular Diseases 27, 280-289.
| Crossref | Google Scholar | PubMed |
Ownsworth T, Clare L (2006) The association between awareness deficits and rehabilitation outcome following acquired brain injury. Clinical Psychology Review 26, 783-795.
| Crossref | Google Scholar | PubMed |
Ownsworth T, Fleming J (2005) The Relative Importance of Metacognitive Skills, Emotional Status, and Executive Function in Psychosocial Adjustment Following Acquired Brain Injury. The Journal of Head Trauma Rehabilitation 20, 315-332.
| Crossref | Google Scholar |
Ownsworth T, Clare L, Morris R (2006) An integrated biopsychosocial approach to understanding awareness deficits in Alzheimer’s disease and brain injury. Neuropsychological Rehabilitation 16, 415-438.
| Crossref | Google Scholar | PubMed |
Pia L, Neppi-Modona M, Ricci R, Berti A (2004) The Anatomy of Anosognosia for Hemiplegia: A Meta-Analysis. Cortex 40, 367-377.
| Crossref | Google Scholar | PubMed |
Pound P, Gompertz P, Ebrahim S (1998) Illness in the context of older age: the case of stroke. Sociology of Health and Illness 20, 489-506.
| Crossref | Google Scholar |
Robertson K, Schmitter-Edgecombe M (2015) Self-awareness and traumatic brain injury outcome. Brain Injury 29, 848-858.
| Crossref | Google Scholar | PubMed |
Schwarz G (1978) Estimating the dimension of a model. The Annals of Statistics 6, 461-464.
| Crossref | Google Scholar |
Seltzer B, Vasterling JJ, Yoder JA, Thompson KA (1997) Awareness of deficit in Alzheimer’s disease: relation to caregiver burden. Gerontologist 37, 20-24.
| Crossref | Google Scholar | PubMed |
Sherer M, Hart T, Nick TG (2003a) Measurement of impaired self-awareness after traumatic brain injury: a comparison of the Patient Competency Rating Scale and the Awareness Questionnaire. Brain Injury 17, 25-37.
| Google Scholar |
Sherer M, Hart T, Nick TG, Whyte J, Thompson RN, Yablon SA (2003b) Early impaired self-awareness after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 84, 168-176.
| Google Scholar |
Sherer M, Hart T, Whyte J, Nick TG, Yablon SA (2005) Neuroanatomic basis of impaired self-awareness after traumatic brain injury: findings from early computed tomography. Journal of Head Trauma Rehabilitation 20, 287-300.
| Crossref | Google Scholar | PubMed |
Smeets SM, Ponds RW, Verhey FR, Van Heugten CM (2012) Psychometric properties and feasibility of instruments used to assess awareness of deficits after acquired brain injury: a systematic review. The Journal of Head Trauma Rehabilitation 27, 433-442.
| Crossref | Google Scholar | PubMed |
Smeets SM, Vink M, Ponds RW, Winkens I, Van Heugten CM (2017) Changes in impaired self-awareness after acquired brain injury in patients following intensive neuropsychological rehabilitation. Neuropsychological Rehabilitation 27, 116-132.
| Crossref | Google Scholar | PubMed |
Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG (1992) Anosognosia in Patients With Cerebrovascular Lesions: A Study of Causative Factors. Stroke 23, 1446-1453.
| Crossref | Google Scholar | PubMed |
Stein MS, Reynolds FA (2020) How is carer strain related to the recovery of stroke survivors with right hemisphere dysfunction? Implications for practice. Disability and Rehabilitation 44, 693-701.
| Crossref | Google Scholar | PubMed |
Tagai K, Nagata T, Shinagawa S, Shigeta M (2020) Anosognosia in patients with Alzheimer’s disease: current perspectives. Psychogeriatrics 20, 345-352.
| Crossref | Google Scholar | PubMed |
Terneusen A, Winkens I, Van Heugten C, Stapert S, Jacobs H, Ponds R, Quaedflieg C (2022) Neural Correlates of Impaired Self-awareness of Deficits after Acquired Brain Injury: A Systematic Review. Neuropsychology Review 33, 222-237.
| Crossref | Google Scholar | PubMed |
Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K (2016) The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Family Practice 17, 38.
| Crossref | Google Scholar | PubMed |
Tibshirani R (1996) Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological) 58, 267-288.
| Crossref | Google Scholar |
Toglia J, Kirk U (2000) Understanding awareness deficits following brain injury. Neurorehabilitation 15, 57-70.
| Google Scholar | PubMed |
Trigg R, Jones RW, Skevington SM (2007) Can people with mild to moderate dementia provide reliable answers about their quality of life? Age and Ageing 36, 663-669.
| Crossref | Google Scholar | PubMed |
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Strobe I (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Medicine 4, e297.
| Crossref | Google Scholar | PubMed |
Vocat R, Staub F, Stroppini T, Vuilleumier P (2010) Anosognosia for hemiplegia: a clinical-anatomical prospective study. Brain 133, 3578-3597.
| Crossref | Google Scholar | PubMed |
Vogel A, Waldorff FB, Waldemar G (2010) Impaired awareness of deficits and neuropsychiatric symptoms in early Alzheimer’s disease: the Danish Alzheimer Intervention Study (DAISY). The Journal of Neuropsychiatry and Clinical Neurosciences 22, 93-99.
| Crossref | Google Scholar | PubMed |
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 370, 1453-1457.
| Crossref | Google Scholar | PubMed |
Wesson J, Clemson L, Crawford JD, Kochan NA, Brodaty H, Reppermund S (2017) Measurement of Functional Cognition and Complex Everyday Activities in Older Adults with Mild Cognitive Impairment and Mild Dementia: Validity of the Large Allen’s Cognitive Level Screen. The American Journal of Geriatric Psychiatry 25, 471-482.
| Crossref | Google Scholar | PubMed |
Wheeler M, Williams OA, Johns L, Chiu EG, Slavkova ED, Demeyere N (2022) Unravelling the complex interactions between self-awareness, cognitive change, and mood at 6-months post-stroke using the Y-shaped model. Neuropsychological Rehabilitation 33, 680-702.
| Crossref | Google Scholar | PubMed |
Wood W, Christensen PN, Hebl MR, Rothgerber H (1997) Conformity to sex-typed norms, affect, and the self-concept. Journal of Personality and Social Psychology 73, 523-535.
| Crossref | Google Scholar | PubMed |
World Health Organization (2019). ICD-11: international classification of diseases. Retrieved from https://icd.who.int/
Yeates G, Henwood K, Gracey F, Evans J (2006) Awareness of Disability after Acquired Brain Injury: Subjectivity within the Psychosocial Context. Neuropsychoanalysis, 8, 175-189.
| Crossref | Google Scholar |
Zamboni G, Drazich E, Mcculloch E, Filippini N, Mackay CE, Jenkinson M, Tracey I, Wilcock GK (2013) Neuroanatomy of impaired self-awareness in Alzheimer’s disease and mild cognitive impairment. Cortex 49, 668-678.
| Crossref | Google Scholar | PubMed |
Zarit SH, Reever KE, Bach-Peterson J (1980) Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 20, 649-655.
| Crossref | Google Scholar | PubMed |
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67, 361-370.
| Crossref | Google Scholar | PubMed |
Zimmermann N, Mograbi DC, Hermes-Pereira A, Fonseca RP, Prigatano GP (2017) Memory and executive functions correlates of self-awareness in traumatic brain injury. Cognitive Neuropsychiatry 22, 346-360.
| Crossref | Google Scholar | PubMed |