Changes in healthcare-associated infections after the introduction of a national hand hygiene initiative
Adrian G. Barnett A D , Katie Page A , Megan Campbell A , David Brain A , Elizabeth Martin A , Shirley Winters A , Lisa Hall A B , David Paterson B C and Nicholas Graves A BA Institute of Health and Biomedical Innovation, Queensland University of Technology, Qld 4059, Australia.
B Centre for Healthcare Related Infection Surveillance and Prevention, Queensland Health, Qld 4006, Australia.
C The University of Queensland Centre for Clinical Research, Qld 4029, Australia.
D Corresponding author. Email: a.barnett@qut.edu.au
Healthcare Infection 19(4) 128-134 https://doi.org/10.1071/HI14033
Submitted: 18 September 2014 Accepted: 7 October 2014 Published: 10 November 2014
Journal Compilation © Australasian College for Infection Prevention and Control 2014
Abstract
Introduction: Interventions that prevent healthcare-associated infections should lead to fewer deaths and shorter hospital stays. Cleaning hands with soap and water or alcohol rub is an effective way to prevent the transmission of organisms, but compliance is sometimes low. The National Hand Hygiene Initiative in Australia aimed to improve hand hygiene compliance among healthcare workers, with the goal of reducing rates of healthcare-associated infections.
Methods: We examined if the introduction of the National Hand Hygiene Initiative was associated with a change in infection rates. Monthly infection rates for six types of healthcare-associated infections were examined in 38 Australian hospitals across six states. Infection categories were: bloodstream infections, central-line associated bloodstream infections, methicillin-resistant and methicillin-sensitive Staphylococcus aureus, Staphylococcus aureus bacteraemia and surgical site infections.
Results: The National Hand Hygiene Initiative was associated with a statistically significant reduction in infection rates in 11 out of 23 state and infection combinations studied. There was no change in infection rates for nine combinations, and there was an increase in three infection rates in South Australia.
Conclusions: The intervention was associated with reduced infection rates in many cases. The lack of improvement in nine cases may have been because they already had effective initiatives before the national initiative’s introduction.
Additional keywords: intervention, nosocomial.
Implications
-
The National Hand Hygiene Initiative was broadly successful as it was associated with reduced infection rates in many states and infection types.
-
The initiative may have been counter-productive in South Australia because of a potential shift in resources away from existing infection-control strategies.
Introduction
Healthcare-associated infections increase the risk of death and cause longer stays in hospital.1 Colonisations and infections can occur when microorganisms are transferred from the hands of healthcare workers to the environment and to patients. Hand hygiene is a key strategy for breaking the transmission cycle from healthcare workers, patients and the environment. The 2014 Society for Healthcare Epidemiology of America (SHEA) guidelines called hand hygiene a ‘fundamental strategy for the prevention of pathogen transmission in healthcare facilities’.2
The success of hand hygiene programs depends on high rates of compliance among hospital staff. Studies of compliance have shown highly variable rates from below 50%3,4 to close to 90%.5 The Australian National Hand Hygiene Initiative (NHHI) aimed to improve hand hygiene compliance and monitor its effectiveness in reducing infections (www.hha.org.au). The initiative was based on the World Health Organization’s ‘Clean care is safer care’ campaign.6,7 The NHHI aimed to achieve sustained improvements in hand hygiene compliance by using: ongoing education, regular hand hygiene compliance auditing using the ‘5 moments’ program,6 and standardised assessment of Staphylococcus aureus bloodstream (SAB) infection rates.8 The aim was for every hospital in Australia to adopt the initiative, and it is now mandatory as part of the National Safety and Quality Health Service Standards.
In a previous paper we examined the change in SAB rates after the introduction of the NHHI.9 The results were mostly positive, with a reduction in four out of six states and no change in two states. However, only examining SAB may be too narrow a view as the NHHI may have reduced other infections as well, and multiple outcomes should be used to evaluate infection-prevention initiatives.10 Detrimental effects also need to be considered as it is possible that the focus of the NHHI on SAB may have reduced attention on the prevention of other infections or caused resources to be redirected from other programs. The latest SHEA practice recommendations include hand hygiene as a strategy for: methicillin-resistant Staphylococcus aureus (MRSA),2 central line-associated bloodstream infections (CLABSI),11 surgical site infections (SSI),12 and Clostridium difficile.13 Including all the potential benefits is key for considering the overall economic costs and benefits of the NHHI.14,15
We tested the effectiveness of the Australian National Hand Hygiene Initiative by examining whether it was associated with a reduction in six types of infection rates. We used an observational quasi-experimental design based on monthly infection rates. We obtained data from six of the eight states and territories, and present separate results for each state and territory due to differences between the states in pre-existing hand hygiene practices.
Methods
Our hypothesis was that the intervention changed the monthly rates of infections. We did not specify a direction for this change, so all hypotheses tests were two-sided. The analysis plan was developed a priori and no post-hoc tests were made.
Data
Data on healthcare-associated infections (HAIs) are routinely collected by Australian hospitals and reported both to their state or territory health authority, and nationally for performance monitoring. The hospitals chosen were: the five largest public hospitals (by number of acute beds) in New South Wales, Victoria, Queensland, Western Australia and South Australia; the three largest public hospitals in Tasmania; and the single main public hospital in the Northern Territory and Australian Capital Territory. This gave 30 hospitals. We then selected the next largest 20 public hospitals Australia-wide to give 50 hospitals in total. We requested all the available monthly data for the 50 hospitals.
Infections were defined according to each state and territory.16 Although there are differences between states in how infections are defined this is not of concern for our analyses that focus on changes within a state.
We analysed data by jurisdiction, as we knew there were slight differences in data collection and definitions used. Data was collected for surveillance purposes by infection-control practitioners.
We checked to ensure that the data had been collected in line with the respective jurisdictional definitions for healthcare-associated infections. As such, colonisations and screening specimens, and community-associated infections were excluded.
The data used here were provided to us by individual hospitals or via the state units who support healthcare-associated infection surveillance including validating infection numbers. We further verified the data quality and checked the infection definitions used. Sufficient data for all time periods were not available for the Northern Territory or Victoria.
The roll-out of the NHHI included education and auditor training. The roll-out was implemented at different times across the country. As collection of auditing data formed the basis of the intervention, we used the first report of auditing data for each hospital to be the start of the intervention.
The study was approved by the appropriate Human Research Ethics Committees in each state and territory, and the release of data was additionally approved through the research governance processes appropriate to each hospital. The study was also approved by the Queensland University of Technology Human Research Ethics Committee.
Study design
We used a before-and-after quasi-experimental design17 by comparing the infection rates after the intervention with those before. The complete details of the methods are in our previous paper which only examined SAB infection.9
We ran the analyses separately for each infection type in each state and territory as the intervention was implemented on a state basis, with overall co-ordination at both a state and national level. There were also important differences between states in terms of average infection rates and pre-existing hand hygiene campaigns and infection-prevention policies. Hence it was thought likely that the effect of the intervention would vary by state and territory.
Statistical methods
The regression model for the counts of infections in hospital i in month t was:
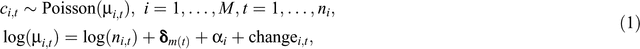
where M is the total number of hospitals and ni is the number of months observed in hospital i. A Poisson distribution is ideal for modelling counts.18
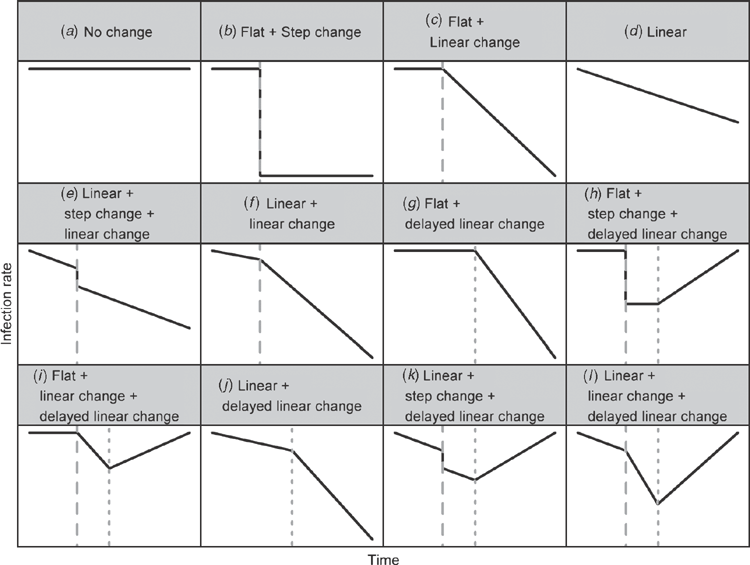
We examined the change in infection rates after the intervention by examining 12 possible changes over time, and example changes are shown in Fig. 1. Models A and D adhere to the null hypothesis that the intervention had no impact on rates. Models K and L allow a potential delayed increase in rates once the intervention effect has worn off. We selected the best model for each state and infection type as that with the best fit to the data according to the Akaike Information Criteria.19
The offset in the equation, log(ni,t), divides the mean counts, μi,t, by the denominator. The denominator was 100 procedures for SSIs, 1000 line days for CLABSIs, and 10 000 bed days or in-patient days for all other infections. Including a denominator helped control for changes over time, such as long-term trends in increasing hospital use and seasonal changes in hospital admissions.
We controlled for any seasonal patterns in infection rates using a categorical variable for month (δ). We used a random intercept in each hospital (αi) to control for differences in the average infection rates between hospitals. We were not interested in differences in infection rates between hospitals, but were instead interested in the within-hospital change due to the intervention, and the average within-hospital change per state.
For the best model in each state we estimated the percentage change in infection rates after the intervention, together with 95% confidence intervals.
All analyses were conducted in R (www.r-project.org) version 3.0.1
Results
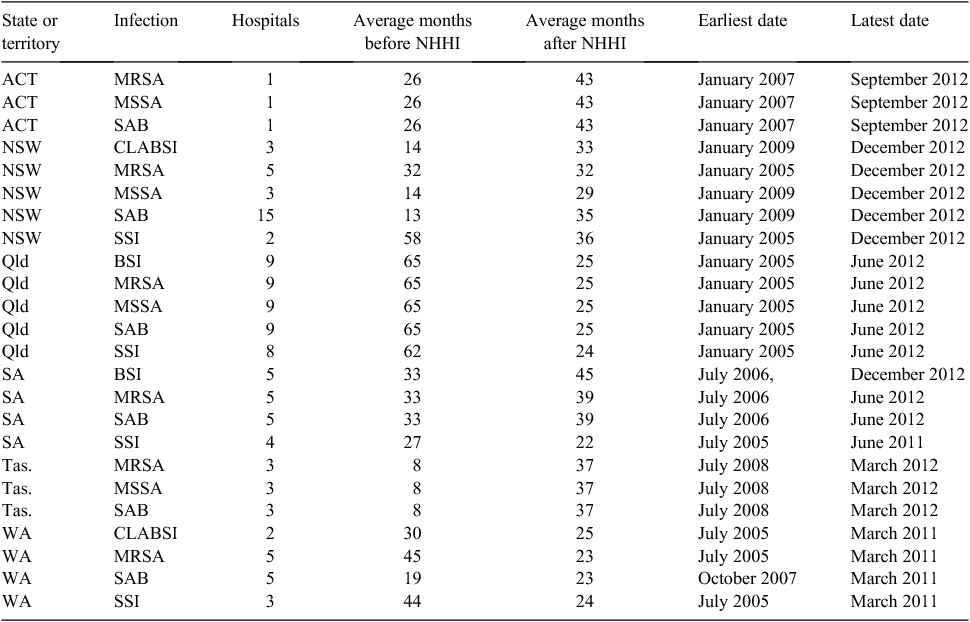
We obtained sufficient usable data for 38 of the 50 largest public hospitals in Australia in six states. For these hospitals we had 684 years of monthly infection rates across six infection types. The average number of months before the NNHI’s introduction was 39 per hospital, with an average post-intervention time of 30 months. Summary statistics on the available months of data and dates are in Table 1.
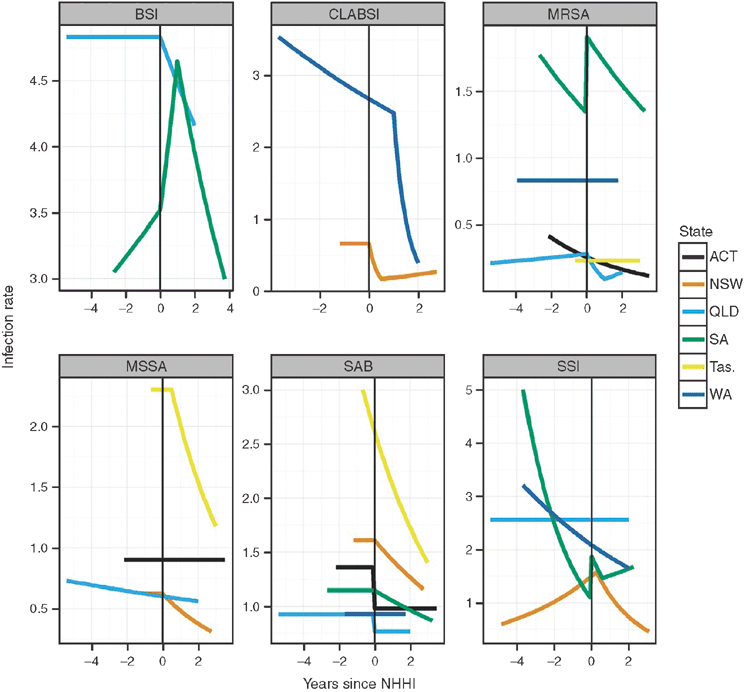
The estimated infection rates before and after the NHHI are in Fig. 2 (the estimates with the monthly observed rates are shown in Fig. S1, available as supplementary material for this paper). Some large reductions in infection rates are clear, such as the reduction in BSI in Queensland, CLABSI in Western Australia, and MSSA in Tasmania. A lack of change is also evident for some combinations, such as MRSA in Western Australia, MSSA in the ACT, and SAB in Tasmania. In two cases (CLABSI in NSW and MRSA in Queensland) an initial reduction in rates was followed by a later increase.
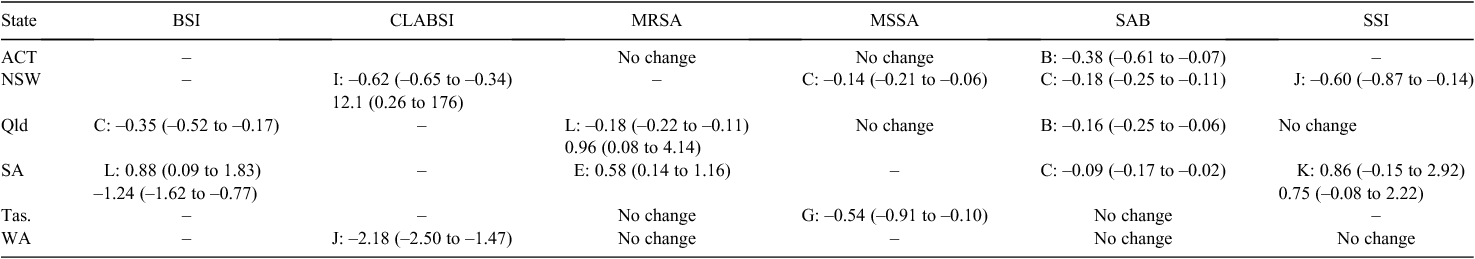
The estimated changes in infection rates are in Table 2. There was no change in infections for 9 out of the 23 comparisons made. Thirteen of the changes were statistically significant. The one not statistically significant change was SSI in South Australia. Most of the changes were an immediate or delayed reduction in rates. A noticeable exception to this pattern was South Australia where rates increased for three of the four infection types studied.
|
The change in rates needs to be interpreted in combination with the best fitting model. For example the reduction in SAB rates in the ACT of 0.38 is an immediate one-off decrease (Fig. 2), whereas the reduction in SAB rates in NSW of 0.18 was a linear decrease per year, meaning that after 2 years the estimated reduction in rates was 0.36.
The secondary increase in CLABSI rates in New South Wales is very large with a mean increase of 12.1. This is because the CLABSI rates were very low before the increase (Fig. 2) and as we used a multiplicative Poisson model the change in rates appears large relative to this low baseline.
Discussion
The estimated impact of the NHHI was generally positive with a statistically significant reduction in infection rates for 11 out of 23 comparisons. In ongoing work we will examine whether these reductions are large enough to translate into a conclusion that the National Hand Hygiene Initiative was cost-effective.
In four instances infection rates were already decreasing before the NHHI was introduced and the intervention failed to decrease rates further – for example, MRSA rates in ACT (Fig. 2). These results indicate that existing programs were already working well and it may have been wiser to implement the NHHI when these rates became flatter. We recommend plotting average infection rates over time before introducing any intervention aimed at reducing infection rates in order to avoid introducing potentially unnecessary interventions. However, it is possible that without the introduction of the National Hand Hygiene Initiative the change in rates may have worsened. The program may have been successful in maintaining a declining trajectory. We could have examined this if we had contemporary wards or hospitals that did not receive the intervention.
The results from South Australia were quite different to the other states, as rates increased for three of the four infection types studied although there was a decrease in SAB rates. It is possible that the shift of infection-control resources to hand hygiene and SAB auditing disrupted existing infection-control practices in South Australia. The rise in BSI infections (Fig. 2) may be coincidental and a rise in BSI infections at this time was predominantly from non-device sources which are not directly associated with hand hygiene.20 However, it is possible infection-control resources at a hospital level were shifted away from other programs (such as surveillance, management of intravascular devices and environmental cleaning) to allow for increased hand hygiene auditing, resulting in the NHHI having unintended consequences.
For two cases (CLABSI in NSW and MRSA in Queensland) an initial reduction in rates was followed by a later increase. This could be because the initial benefit of the training and attention of the NHHI had worn off, or because the very low rates for these two infections were followed by some regression to the mean.
We stress that any negative associations shown here do not imply that hand hygiene is not worthwhile. Rather the negative associations imply that a national program to increase hand hygiene compliance was not always effective, and is likely dependent on a range of local and contextual factors.
Limitations
Our results show an association between the introduction of the NHHI and subsequent changes in infection rates. We should be cautious about ascribing causation from our estimates, especially because the timing of the intervention was not randomised and there were no control hospitals that did not receive the NHHI, which increases the risk of confounding due to other changes over time.
Other changes to infection-control practice and policy occurred in most of the hospitals during the study period. The timing of these changes varied between hospitals and through interviews with infection-control staff we found no evidence of them occurring concurrently with the introduction of the hand hygiene intervention. Overall, we believe that such potential changes are unlikely to confound the observed associations between the NHHI and monthly infection rates, at least systematically so.
The question of whether the intervention improved hand hygiene compliance rates is not part of this paper.21 This is because the next stage of our research is to estimate both the running costs and hence the cost-effectiveness of the intervention,15 and as the major costs are due to infections we need to know whether and by how much the intervention reduced infections.
We examined a range of possible changes to infection rates due to the intervention (Fig. 1) and in some cases there were multiple changes (e.g. MRSA in Queensland) which means the results cannot be summarised using a single number. Also reductions associated with the NHHI were sometimes immediate (e.g. SAB in ACT) and sometimes gradual (e.g. SAB in NSW). This makes it difficult to numerically compare the impact between states. Such comparisons would be possible using simple statistical models that assume that rates were flat before the intervention with a step or linear change post-intervention.22 However, these models ignore the possibility that rates were already declining before the intervention began. For example, the MRSA data from Grayson et al.22 show a statistically significant reduction in MRSA clinical isolates per 100 patient days of –0.018 per month (95% CI –0.024 to –0.011, P-value <0.001) associated with a hand hygiene culture-change program when using Model C (Fig. 1) which assumes rates were flat before the intervention. However, re-analysing the data using Model F, which allows a linear decrease in rates before the intervention, the mean reduction associated with the program is reduced to –0.007 and is not statistically significant (95% CI –0.019 to 0.006, P-value = 0.31). Model F also gives a better fit to the data.
We examined all infection types independently rather than looking for a common overall effect on infections. It is likely that hand hygiene will impact on some infections more than others, as transmission pathways and dynamics vary. Each of the infections listed is in some way potentially prevented through hand hygiene. However other infection-control programs will also have an effect. For example transmission of Clostridium difficile is prevented by good environmental cleaning, and many surgical site infections can be prevented through appropriate antibiotic prophylaxis. In addition, it is likely that focusing heavily on one infection-control intervention, such as hand hygiene auditing, may come at an opportunity cost, when other activities end up with less resources.
Overall, we have shown that the NHHI was successful in reducing infection rates in roughly half of the cases studied. The rates remained unchanged in nine cases and increased in three cases. These results are fundamental for informing the overall cost-effectiveness of the initiative. We should be cautious about ascribing causation to the associations found here given the quasi-experimental design and retrospective analysis plan. Ideally, large initiatives such as this should prospectively plan studies based on the existing data and interventions, the current need, and the contextual factors likely to influence the success in each location.
Conflict of Interest
None of the authors have any conflicts of interest.
Funding
Financial support: This work was supported by a National Health and Medical Research Council partnership (grant number 553081) with financial and in kind support from: Australian Commission on Safety and Quality in Health Care, Hand Hygiene Australia, and jurisdictional health departments.
References
[1] Barnett AG, Page K, Campbell M, Martin E, Rashleigh-Rolls R, Halton K, Paterson D, Hall L, Jimmieson N, White K, Graves N. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: a case-control study. BMJ Open 2013; 3| The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: a case-control study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpvVars74%3D&md5=8a8c2f4c77f642c889b47f8209560b32CAS | 24176795PubMed |
[2] Calfee D, Salgado C, Milstone AM, Harris A, Kuhar D, Moody J, Aureden K, Huang S, Maragakis L, Yokoe D. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35 772–96.
| Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update.Crossref | GoogleScholarGoogle Scholar | 24915205PubMed |
[3] Cantrell D, Shamriz O, Cohen MJ, Stern Z, Block C, Brezis M. Hand hygiene compliance by physicians: marked heterogeneity due to local culture? Am J Infect Control 2009; 37 301–5.
| Hand hygiene compliance by physicians: marked heterogeneity due to local culture?Crossref | GoogleScholarGoogle Scholar | 18834749PubMed |
[4] Pittet D. Compliance with hand disinfection and its impact on hospital-acquired infections. Journal of Hospital Infection 2001; 48 S40–S46.
| Compliance with hand disinfection and its impact on hospital-acquired infections.Crossref | GoogleScholarGoogle Scholar | 11759025PubMed |
[5] Hui S, Ng J, Santiano N, Schmidt H-M, Caldwell J, Ryan E, Maley M. Improving hand hygiene compliance: harnessing the effect of advertised auditing. Healthc Infect 2014; 19 108–13.
| Improving hand hygiene compliance: harnessing the effect of advertised auditing.Crossref | GoogleScholarGoogle Scholar |
[6] Pittet D, Allegranzi B, Boyce J. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect Control Hosp Epidemiol 2009; 30 611–22.
| The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations.Crossref | GoogleScholarGoogle Scholar | 19508124PubMed |
[7] World Health Organization. WHO guidelines on hand hygiene in health care: first global patient safety challenge. Clean care is safer care. 2009.
[8] Ryan K, Russo PL, Heard K, Havers S, Bellis K, Grayson ML. Development of a standardised approach to observing hand hygiene compliance in Australia. Healthc Infect 2012; 17 115–21.
| Development of a standardised approach to observing hand hygiene compliance in Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Barnett AG, Page KP, Campbell MB, Brain D, Martin E, Rashleigh-Rolls R, Halton K, Hall L, Jimmieson N, White K, Paterson D, Graves N. Changes in healthcare-associated Staphylococcus aureus bloodstream infections after the introduction of a national hand hygiene initiative. Infect Control Hosp Epidemiol 2014; 35 1029–36.
| Changes in healthcare-associated Staphylococcus aureus bloodstream infections after the introduction of a national hand hygiene initiative.Crossref | GoogleScholarGoogle Scholar | 25026620PubMed |
[10] Mitchell BG, Collignon PJ, McCann R, Wilkinson IJ, Wells A. A major reduction in hospital-onset Staphylococcus aureus bacteremia in Australia—12 years of progress: an observational study. Clin Infect Dis 2014; 59 969–75.
| A major reduction in hospital-onset Staphylococcus aureus bacteremia in Australia—12 years of progress: an observational study.Crossref | GoogleScholarGoogle Scholar | 24973314PubMed |
[11] Marschall J, Mermel L, Fakih M, Hadaway L, Kallen A, O’Grady N, Pettis A, Rupp M, Sandora T, Maragakis L, Yokoe D. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35 753–71.
| Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update.Crossref | GoogleScholarGoogle Scholar | 24915204PubMed |
[12] Anderson D, Podgorny K, Berríos-Torres S, Bratzler D, Patchen Dellinger E, Greene L, Nyquist A-C, Saiman L, Yokoe D, Maragakis L, Kaye K. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35 605–27.
| Strategies to prevent surgical site infections in acute care hospitals: 2014 update.Crossref | GoogleScholarGoogle Scholar | 24799638PubMed |
[13] Dubberke E, Carling P, Carrico R, Donskey C, Loo V, McDonald LC, Maragakis L, Sandora T, Weber D, Yokoe D, Gerding D. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35 628–45.
| Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update.Crossref | GoogleScholarGoogle Scholar | 24799639PubMed |
[14] Graves N, Barnett A, White K, Jimmieson N, Page K, Campbell M, Stevens E, Rashleigh-Rolls R, Grayson L, Paterson D. Evaluating the economics of the Australian National Hand Hygiene Initiative. Healthc Infect 2012; 17 5–10.
| Evaluating the economics of the Australian National Hand Hygiene Initiative.Crossref | GoogleScholarGoogle Scholar |
[15] Page K, Barnett AG, Campbell M, Brain D, Martin E, Fulup N, Graves N. Costing the Australian National Hand Hygiene Initiative. Journal of Hospital Infection 2014;
| Costing the Australian National Hand Hygiene Initiative. Crossref | GoogleScholarGoogle Scholar | 25092619PubMed |
[16] Russo PL, Cheng AC, Richards M, Graves N, Hall L. Healthcare-associated infections in Australia: time for national surveillance. Aust Health Rev 2014; in press.
| 25362241PubMed |
[17] Shardell M, Harris AD, El-Kamary SS, Furuno JP, Miller RR, Perencevich EN. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis 2007; 45 901–7.
| Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies.Crossref | GoogleScholarGoogle Scholar | 17806059PubMed |
[18] Dobson AJ, Barnett AG. An Introduction to Generalized Linear Models . Boca Raton: Chapman & Hall/CRC; 2008.
[19] Burnham KP, Anderson DR. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach . Springer; 2002.
[20] Cope C, Wilkinson I. Bloodstream Infection 2012 Annual Report . SA Department for Health and Ageing; 2014.
[21] Reichardt C, Königer D, Bunte-Schönberger K, Mönch N, Schwab F, Behnke M, Gastmeier P. Three years of national hand hygiene campaign in Germany: what are the key conclusions for clinical practice? Journal of Hospital Infection 2013; 83 S11–S16.
| Three years of national hand hygiene campaign in Germany: what are the key conclusions for clinical practice?Crossref | GoogleScholarGoogle Scholar | 23453170PubMed |
[22] Grayson ML, Jarvie LJ, Martin R, Johnson PD, Jodoin ME, McMullan C, Gregory RH, Bellis K, Cunnington K, Wilson FL, Quin D, Kelly AM. Significant reductions in methicillin-resistant Staphylococcus aureus bacteraemia and clinical isolates associated with a multisite, hand hygiene culture-change program and subsequent successful statewide roll-out. Med J Aust 2008; 188 633–40.
| 18513171PubMed |