Attitudes towards antimicrobial stewardship: results from a large private hospital in Australia
Menino O. Cotta A B D , Megan S. Robertson C , Mark Tacey B , Caroline Marshall A B , Karin A. Thursky A , Danny Liew B and Kirsty L. Buising AA Victorian Infectious Diseases Service, Melbourne Health, The Peter Doherty Institute, Parkville, Vic. 3010, Australia.
B Department of Medicine, Royal Melbourne Hospital Campus, University of Melbourne, Parkville, Vic. 3010, Australia.
C Clinical Trials and Research Centre, Epworth HealthCare, Richmond, Vic. 3121, Australia.
D Corresponding author. Email: menino.cotta@unimelb.edu.au
Healthcare Infection 19(3) 89-94 https://doi.org/10.1071/HI14008
Submitted: 4 March 2014 Accepted: 11 April 2014 Published: 28 May 2014
Journal Compilation © Australasian College for Infection Prevention and Control 2014
Abstract
Introduction: An effective hospital-wide antimicrobial stewardship (AMS) program requires engagement with all healthcare professionals involved in antimicrobial use. It is therefore useful to consider attitudes and perceptions among clinical stakeholders in Australian private hospitals before introducing AMS in these facilities. The aim of this study was to describe perceptions and attitudes towards antimicrobial resistance, antimicrobial use, AMS interventions, and willingness to participate.
Methods: A 26-item attitudinal survey was distributed to visiting specialists, nurses and pharmacists at a large (500 bed) private hospital in Australia. Survey questions utilised ‘Yes/No’ responses and a 7-point Likert scale ranging from ‘strongly agree’ to ‘strongly disagree’. Descriptive analyses were performed and Chi-squared tests conducted.
Results: There were a total of 331 respondents (80 physicians, 58 surgeons, 78 anaesthetists, 105 nurses and 10 pharmacists). The response rate was 42% among clinicians, 100% among pharmacists and 13% among nurses. Only half of the respondents were willing to participate in proposed AMS interventions. A larger proportion of respondents believed that antimicrobial resistance was more of a serious problem in other Australian hospitals compared with the surveyed hospital (62% v. 45%, P < 0.001). Fifty-eight percent agreed that improving prescribing at the hospital would reduce antimicrobial resistance. Twenty-nine percent of respondents had previous exposure to AMS, with pharmacists and physicians more likely to have heard of AMS compared with surgeons, anaesthetists and nurses (P = 0.016 and P < 0.001 respectively).
Conclusions: This study highlights the challenge of making antimicrobial resistance a relevant local issue in private hospitals and engaging key health professionals before implementing change.
Implications
-
Successful implementation of antimicrobial stewardship (AMS) programs requires buy-in from relevant clinical stakeholders.
-
A survey of attitudes towards, and perceptions about antimicrobial use and antimicrobial resistance may be useful to determine the level of engagement among these clinical stakeholders, and to help identify promoters of AMS and the professions that may require more awareness and education.
Introduction
Antibiotics are prescribed to between 19 and 59% of patients in acute hospitals,1 yet up to half of these antibiotic prescriptions may be judged to be inappropriate. Inappropriate and excessive use of antimicrobials can accelerate the development of antimicrobial resistance amongst local pathogens.2,3 Antimicrobial stewardship (AMS) initiatives have previously been effective in changing prescribing patterns and curtailing inappropriate use of antimicrobials in hospitals.4–6
In order to develop these effective AMS interventions and change antimicrobial prescribing habits, it has been suggested that there is a need to better understand clinicians’ underlying perceptions about antimicrobial use and the issue of antimicrobial resistance.7–9 There is increasing evidence that an effective hospital-wide AMS program needs to have multidisciplinary input from and engagement with all health professions involved in the use of antimicrobials, not just prescribers.10 Previous studies have assessed clinicians’ knowledge and beliefs about antimicrobial use and resistance,11–19 however, only one of these included healthcare professionals outside of medical prescribers, and this study was conducted at a hospital with a pre-existing AMS program.18
In Australia, AMS programs have now been incorporated into new National Safety and Quality Health Service (NSQHS) standards and are part of a new accreditation scheme mandatory for all hospitals;20 thus there is an imperative to comply with these standards by having an AMS program in place. Although the Australian private hospital sector contributes approximately one-third of all hospital beds and treats 40% of all patients,21 there is currently a lack of AMS activities occurring in this sector.22 Importantly, there are no data within these facilities on what attitudes currently exist towards antimicrobial resistance and antimicrobial use as well as what perceptions clinical stakeholders may have about the benefit of AMS.
The aim of this study was to describe perceptions and attitudes towards antimicrobial resistance, antimicrobial use and AMS among all key healthcare professionals at a large Australian private hospital.
Methods
This survey formed the first part of a larger project examining attitudes and perceptions to AMS among private hospital stakeholders. Subsequent components of the project involved focus group discussions, semi-structured interviews and on-site observations.
A 26-item survey (available as Supplementary material for this paper) was conceptualised and constructed by a multidisciplinary research group (the expert panel) including infectious diseases physicians, clinical microbiologists, intensive care physicians, AMS pharmacists and nurse practitioners. The survey collected information on respondents’ beliefs about the significance of antimicrobial resistance as a problem, perceptions about factors contributing to antimicrobial resistance, experience with antimicrobial resistance, awareness of AMS, perceptions of antimicrobial prescribing at the hospital and attitudes towards potential AMS interventions.
The sections on significance of antimicrobial resistance as a problem, contributing factors, antimicrobial prescribing at the hospital, potential AMS interventions, and willingness to participate were scored using a seven-point Likert scale. Likert responses ranged from ‘strongly agree’ to ‘strongly disagree’ or ‘not a problem’ to ‘a very serious problem’. Survey validity, usability and generalisability were established through trials of several iterations of the survey after gaining feedback from the expert panel as well as conducting field tests using subjects from different professional healthcare backgrounds who were not included in the study sample.
The final version of the survey was distributed by email to all visiting specialists, registrars, nurses and pharmacists in a 490-bed private hospital. Approximately 800 employed nurses and 10 contracted pharmacists worked at the hospital. There were 512 clinicians who were considered ‘active visiting specialists’ as they had admitted patients during the previous 3-year period. To encourage the response rate among clinicians and nurses, hard copies of the survey were distributed at clinical meetings, in hospital departments such as operating theatres and the intensive care unit, as well as most wards in the hospital. These hard copies were collected periodically by the investigators. The survey was actively promoted by members of hospital executive at regular business meetings to encourage participation.
Although a formal AMS program had yet to be introduced hospital-wide at the study site, formation of an AMS committee and endorsement of AMS by hospital executive had recently been undertaken.
The survey collected data on each respondent’s role, speciality (if applicable) and the number of years of experience post-primary qualification, but the identity of respondents was otherwise kept unknown. Respondents were given 6 weeks to complete the survey and reminders were regularly sent by email from hospital executive. The current study formed part of a range of AMS activities that had previously been granted ethics approval by the institutional review board.
Statistical methods
Categorical data were presented as proportions that were ‘in agreement’ or viewed antimicrobial resistance as a ‘serious problem’ (i.e. with a ‘6’ and ‘7’ Likert scale response). Differences amongst professions were tested using Pearson’s Chi-squared test, or when sample size was smaller than 10 for any category, Fisher’s exact test was used. A two-tailed p-value of 0.05 was considered statistically significant. STATA statistical analysis software (version 12) was used (StataCorp, College Station, TX, USA).
Results
There were a total of 331 respondents, of whom 80 (24%) were physicians, 58 (18%) surgeons, 78 (24%) anaesthetists, 105 (32%) nurses and 10 (3%) pharmacists. The response rate was 42% among clinicians, 100% among pharmacists and 13% among nurses.
Antimicrobial resistance
Antimicrobial resistance was viewed as a serious problem in Australian hospitals by 62% of respondents, whilst only 45% believed it was a serious problem in the surveyed hospital (P < 0.001). There were similar proportions of respondents that viewed antimicrobial use in the Australian community and Australian hospitals as contributing to antimicrobial resistance at the surveyed hospital (51% and 56%, respectively). Fewer respondents (34%), however, believed that antimicrobial use in the Australian animal and/or agricultural sectors contributed to this resistance (P < 0.001).
Patient care and antimicrobial prescribing
Thirty-six per cent of respondents believed that antimicrobial resistance affected patients under their care, while less than a third (31%) believed that there was antimicrobial prescribing at the hospital that did not comply with current national antimicrobial prescribing guidelines (Table 1).
A significantly higher proportion of physicians and nurses indicated agreement with the statement that antimicrobial resistance affected patients under their care (both 45%), compared with the other professional groups of surgeons, anaesthetists and pharmacists with proportions of 22%, 26% and 30%, respectively. (P < 0.001). All of the pharmacists surveyed believed that improving antimicrobial prescribing would help decrease antimicrobial resistance, with this proportion being significantly higher than with the other health professions (P = 0.006).
Proposed AMS initiatives and willingness to participate
Willingness to participate in AMS interventions was equivocal (Table 2). In comparison to the other professional groups, there was a significantly higher proportion of pharmacists in agreement to a formal antimicrobial usage policy (P = 0.007), introduction of local antimicrobial prescribing guidelines (P = 0.006), and the introduction of a specialist team giving antimicrobial prescribing advice (P = 0.002). Surveyed pharmacists were also more willing to participate in any AMS interventions introduced at the hospital (P = 0.002), and were also in support for an introduction of a decision support computer application, although this was not statistically significant (P = 0.053).

|
Non-compliance with Therapeutic Guidelines: Antibiotic23
A 50% or greater non-compliance with Therapeutic Guidelines: Antibiotic23 was estimated by 38% of respondents for all antimicrobial prescriptions and by 41% of respondents for surgical prophylaxis prescriptions (Fig. 1). The proportion of surveyed pharmacists who believed this (i.e. 80% for each set of prescriptions) was significantly higher compared with the other professions (P = 0.007 and P = 0.019, respectively). Surveyed anaesthetists were more likely to estimate 50% or greater non-compliance for surgical prophylaxis prescriptions when compared with surveyed surgeons (P = 0.012).
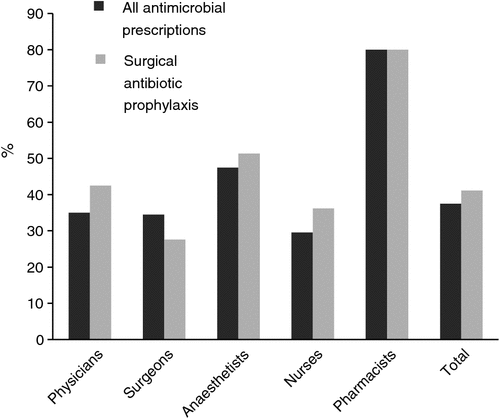
|
Experience with antimicrobial resistance and AMS
The proportion of surgeons who responded as having been involved in the care of a patient with a resistant infection and stated noticing an increasing number of antimicrobial resistant infections over the past 10 years was significantly less compared with other respondents (P < 0.001 for both) (Table 3). A significantly higher proportion of physicians (48%) reported having worked in healthcare facilities with AMS programs (P < 0.001). Pharmacists and physicians were significantly more likely to have heard of AMS compared with the other professions (P = 0.016 and P < 0.001 respectively).

|
Discussion
This attitudinal survey represents the first multi-disciplinary study involving all key health professionals involved in antimicrobial use in the Australian private hospital sector. Survey responses indicate that a great deal of work needs to be undertaken to address these issues before implementation of any hospital-wide AMS program.
As with previously reported perception surveys,13,14,16,19 there was a prevailing view among survey respondents that antimicrobial resistance was more of a serious problem in other Australian hospitals compared with the surveyed hospital. In addition, only around one third of respondents believed that antimicrobial resistance directly affected patients under their care. These findings highlight the challenge of making antimicrobial resistance a relevant local issue among health professionals.
Of note, surgeons and anaesthetists were least likely to agree that antimicrobial resistance affect patients under their care. A potential reason for this perception could be that surgical and anaesthetic staff often do not get involved in the management of antimicrobial resistance as they are more likely to seek advice from and transfer care to physicians in circumstances of infection.15,17 Although only a minority of respondents believed there was non-guideline compliant antimicrobial prescribing (i.e. 31%), the proportion nearly doubled (58%) in the belief that improving prescribing would help decrease antimicrobial resistance. This proportion, however, is somewhat modest when compared with results from a 2004 survey which yielded 97% agreement that better antimicrobial use would help reduce resistance,15 and suggests that education linking antimicrobial prescribing and antimicrobial resistance will need to be a priority at the hospital.
Importantly, only a half of the respondents were willing to participate in any proposed AMS intervention; these results are perhaps reflective of significant disengagement (either passive or active) to issues revolving around antimicrobial use among clinical stakeholders at the hospital. Employing subsequent qualitative methods in addition to these baseline quantitative data will likely shed more light on what factors are influential in this apparent lack of engagement.
The least favourable intervention among respondents was that of introducing a restriction-based policy that limits prescribing via an approval process (52%), while introduction of local guidelines and/or protocols together with a computer application that gives antimicrobial prescribing advice was found to be slightly more acceptable (58% and 60% respectively). These set of results are similar to previous studies and perhaps reflect the view that the most favoured interventions are those that provide information and education rather than restrict prescribing behaviour.13 It is not clear whether the overall low level of willingness to participate is reflective of either a perceived lack of time to contribute to a new intervention or an active refusal. The low level of experience with AMS suggests a degree of unfamiliarity with what it might entail.
Besides pharmacists, anaesthetists were the only other profession where the majority of respondents believed there was 50% or greater non-guideline compliance of surgical antibiotic prophylaxis prescriptions. Surgeons, on the other hand, were the only profession which believed that surgical antibiotic prophylaxis had less non-guideline compliance than all antimicrobial prescriptions combined. As surgeons and anaesthetists prescribe nearly all surgical prophylaxis prescriptions at the surveyed hospital, using surgeon-specific protocols in some cases, it was interesting to note the significant difference in perception of non-compliance in surgical prophylaxis between these two professions. Surgeons were also less likely to report being involved in the care of resistant infections or noticing an increasing number of resistant infections over the previous decade. This result, together with the perception among the majority of surgeons that antimicrobial resistance does not affect patients under their care, suggests there is a potential disconnect between the management of surgical patients and the consequences of antimicrobial resistance at the hospital.
Pharmacists seemed to be more engaged with issues around antimicrobial resistance and AMS. They were more agreeable than any other profession to the majority of proposed AMS interventions and more willing to participate in AMS interventions introduced at the hospital. A significantly higher proportion of pharmacists believed there was 50% or greater non-compliance with national prescribing guidelines for all antimicrobial prescriptions including surgical prophylaxis, and that improving antimicrobial prescribing at the hospital would help decrease resistance.
Unlike physicians, who may have heard of AMS (64%) because of having worked in healthcare facilities with AMS programs (48%), pharmacists may have become aware of AMS through other means as although 80% of pharmacists had heard of AMS, only 20% had previously worked in facilities with existing AMS programs. On the other hand, a low proportion of nursing respondents were aware of AMS. This is an important consideration for implementers of AMS at the hospital as nursing staff are beginning to play an important role in AMS interventions, such as switching from intravenous to oral antimicrobial therapy.24
Although there is formal endorsement and sponsorship of AMS by the hospital executive as well as established governance structure in the form of an AMS committee, results of the survey show that clinical stakeholders are not easily engaged in issues pertaining to antimicrobial use at the hospital. Hence, the first step of a newly introduced AMS program will be to make all five professions more aware of the significance of antimicrobial resistance in the overall care of their patients as well as the importance of judicious antimicrobial prescribing and use. Given that a large proportion of these will be visiting specialists who transiently attend at the private hospital to see their admitted patients, highlighting the importance of AMS will be all the more challenging.
Limitations to this study do exist. Selection bias may exist in the results due to the voluntary nature of the study, particularly among nursing staff where the response rate was low. Thus, it is difficult to generalise outcomes to the wider study population. No demographic information was collected to test for bias between responders and non-responders, and as such, the investigators can only speculate on whether there were any important differences between these two groups.
In summary, as hospital-wide AMS programs progressively grow into resource-intensive, multi-pronged activities involving a myriad of both clinical and non-clinical stakeholders, attitudinal surveys such as these may prove to be useful. Identifying healthcare professions that require specific strategies to improve appropriateness of antimicrobial use and highlighting proponents of AMS within the hospital are important potential outcomes. Results of this study suggest that private hospitals may have an ongoing challenge in increasing awareness and engagement among clinical stakeholders whilst also providing interventions to ensure judicious use of antimicrobials.
Conflicts of interest
None declared.
Funding
The Melbourne Health AMS Research Group is funded by a National Health and Medical Research Council (NHMRC) Project Partnership grant; partners include the Victorian Infectious Diseases Service, Victorian Department of Health, Therapeutic Guidelines Limited and Epworth HealthCare. Menino O Cotta is supported by an NHMRC postgraduate scholarship.
References
[1] Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009; 49 1496–504.| The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006.Crossref | GoogleScholarGoogle Scholar | 19842976PubMed |
[2] Hughes JM. Preserving the lifesaving power of antimicrobial agents. JAMA 2011; 305 1027–8.
| Preserving the lifesaving power of antimicrobial agents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFWlu7o%3D&md5=5686220307c8c1158408aa55d4ebc242CAS | 21343545PubMed |
[3] Fishman N. Antimicrobial stewardship. Am J Med 2006; 119 S53–61.
| Antimicrobial stewardship.Crossref | GoogleScholarGoogle Scholar | 16735152PubMed |
[4] Price J, Ekleberry A, Grover A, Melendy S, Baddam K, McMahon J, et al Evaluation of clinical practice guidelines on outcome of infection in patients in the surgical intensive care unit. Crit Care Med 1999; 27 2118–24.
| Evaluation of clinical practice guidelines on outcome of infection in patients in the surgical intensive care unit.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FhsVahug%3D%3D&md5=15c2651b33920e8cd1f6d63175386604CAS | 10548192PubMed |
[5] Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA 2000; 283 749–55.
| A controlled trial of a critical pathway for treatment of community-acquired pneumonia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ksFamug%3D%3D&md5=07a340a410bf90b6d6f53d7d0ab53211CAS | 10683053PubMed |
[6] Solomon DH, Van Houten L, Glynn RJ, Baden L, Curtis K, Schrager H, et al Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med 2001; 161 1897–902.
| Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvotFKiug%3D%3D&md5=23f2aabecfecc7d83e508912420a3573CAS | 11493132PubMed |
[7] Jarvis WR. Preventing the emergence of multidrug-resistant microorganisms through antimicrobial use controls: the complexity of the problem. Infect Control Hosp Epidemiol 1996; 17 490–5.
| Preventing the emergence of multidrug-resistant microorganisms through antimicrobial use controls: the complexity of the problem.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FivFakuw%3D%3D&md5=047c6fa0d60f17f17673aa4a23370e76CAS | 8875291PubMed |
[8] Lambert BL, Salmon JW, Stubbings J, Gilomen-Study G, Valuck RJ, Kezlarian K. Factors associated with antibiotic prescribing in a managed care setting: an exploratory investigation. Soc Sci Med 1997; 45 1767–79.
| Factors associated with antibiotic prescribing in a managed care setting: an exploratory investigation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7gvV2hug%3D%3D&md5=eeb1648255fffdde032ffd92d14e9e0cCAS | 9447627PubMed |
[9] Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282 1458–65.
| Why don’t physicians follow clinical practice guidelines? A framework for improvement.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FgsVSqtw%3D%3D&md5=8cd5731f9a7ed63ff902006d40170447CAS | 10535437PubMed |
[10] Cooke FJ, Franklin BD, Lawson W, Jacklin A, Holmes A. Multidisciplinary hospital antibiotic stewardship: a West London model. Clin Govern Int J 2004; 9 237–43.
| Multidisciplinary hospital antibiotic stewardship: a West London model.Crossref | GoogleScholarGoogle Scholar |
[11] Neu HC, Howrey SP. Testing the physician’s knowledge of antibiotic use: Self-assessment and learning via videotape. N Engl J Med 1975; 293 1291–5.
| Testing the physician’s knowledge of antibiotic use: Self-assessment and learning via videotape.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE28%2FltFagtA%3D%3D&md5=8e8a29f09ce44c5cec67291d4fc25017CAS | 1186823PubMed |
[12] Paluck E, Katzenstein D, Frankish CJ, Herbert CP, Milner R, Speert D, et al Prescribing practices and attitudes toward giving children antibiotics. Can Fam Physician 2001; 47 521–7.
| 1:STN:280:DC%2BD3M3ivVagtQ%3D%3D&md5=176bbfbe03d34611bb098ff74ae7188dCAS | 11281085PubMed |
[13] Wester CW, Durairaj L, Evans AT, Schwartz DN, Husain S, Martinez E. Antibiotic resistance: a survey of physician perceptions. Arch Intern Med 2002; 162 2210–6.
| Antibiotic resistance: a survey of physician perceptions.Crossref | GoogleScholarGoogle Scholar | 12390064PubMed |
[14] Giblin TB, Sinkowitz-Cochran RL, Harris PL, Jacobs S, Liberatore K, Palfreyman MA, et al Clinicians’ perceptions of the problem of antimicrobial resistance in health care facilities. Arch Intern Med 2004; 164 1662–8.
| Clinicians’ perceptions of the problem of antimicrobial resistance in health care facilities.Crossref | GoogleScholarGoogle Scholar | 15302636PubMed |
[15] Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med 2004; 164 1451–6.
| A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance.Crossref | GoogleScholarGoogle Scholar | 15249355PubMed |
[16] Brinsley KJ, Sinkowitz-Cochran RL, Cardo DM. Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the Health Belief Model as a framework. Am J Infect Control 2005; 33 175–81.
| Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the Health Belief Model as a framework.Crossref | GoogleScholarGoogle Scholar | 15798673PubMed |
[17] Guerra CM, Pereira CA, Neves Neto AR, Cardo DM, Correa L. Physicians’ perceptions, beliefs, attitudes, and knowledge concerning antimicrobial resistance in a Brazilian teaching hospital. Infect Control Hosp Epidemiol 2007; 28 1411–4.
| Physicians’ perceptions, beliefs, attitudes, and knowledge concerning antimicrobial resistance in a Brazilian teaching hospital.Crossref | GoogleScholarGoogle Scholar | 17994525PubMed |
[18] Bannan A, Buono E, McLaws ML, Gottlieb T. A survey of medical staff attitudes to an antibiotic approval and stewardship programme. Intern Med J 2009; 39 662–8.
| A survey of medical staff attitudes to an antibiotic approval and stewardship programme.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mjgsl2htA%3D%3D&md5=e494f27d770e67b6324cfdc2704f745dCAS | 19383062PubMed |
[19] Pulcini C, Williams F, Molinari N, Davey P, Nathwani D. Junior doctors’ knowledge and perceptions of antibiotic resistance and prescribing: a survey in France and Scotland. Clin Microbiol Infect 2011; 17 80–7.
| Junior doctors’ knowledge and perceptions of antibiotic resistance and prescribing: a survey in France and Scotland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FislahtQ%3D%3D&md5=d27965e2240bec9b798f28ab9a2e0d29CAS | 20132254PubMed |
[20] Australian Commission on Safety and Quality in Health Care (ACSQHC). National Safety and Quality Health Service Standards. Sydney: ACSQHC, 2011. Available from: http://www.safetyandquality.gov.au/wp-content/uploads/2011/01/NSQHS-Standards-Sept2011.pdf [verified 20 July 2013].
[21] Gee C. The contribution of the Australian Private Hospitals Sector. The Asia Pacific Journal of Health Management 2007; 2 41–6.
[22] James RS, McIntosh KA, Luu SB, Cotta MO, Marshall C, Thursky KA, et al Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps. Med J Aust 2013; 199 692–5.
| Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps.Crossref | GoogleScholarGoogle Scholar | 24237101PubMed |
[23] Antibiotic Expert Group. Therapeutic guidelines: antibiotic. Version 14. Melbourne: Therapeutic Guidelines Limited; 2010.
[24] Gillespie E, Rodrigues A, Wright L, Williams N, Stuart RL. Improving antibiotic stewardship by involving nurses. Am J Infect Control 2013; 41 365–7.
| Improving antibiotic stewardship by involving nurses.Crossref | GoogleScholarGoogle Scholar | 23069737PubMed |


