Advances in CRISPR/Cas9 technology: shaping the future of photosynthetic microorganisms for biofuel production
Samreen Arshad A , Muhammad Luqman Qadir A , Nazim Hussain A * , Qurban Ali B * , Shiming Han C * and Daoud Ali D
B * , Shiming Han C * and Daoud Ali D
A
B
C
D
Handling Editor: Suleyman Allakhverdiev
Abstract
Use of fossil fuels causes environmental issues due to its inefficiency and and imminent depletion. This has led to interest in identifying alternative and renewable energy sources such as biofuel generation from photosynthetic organisms. A wide variety of prokaryotic and eukaryotic microorganisms, known as microalgae, have the potential to be economical and ecologically sustainable in the manufacture of biofuels such as bio-hydrogen, biodiesel, bio-oils, and bio-syngas. By using contemporary bioengineering techniques, the innate potential of algae to produce biomass of superior quality may be enhanced. In algal biotechnology, directed genome modification via RNA-guided endonucleases is a new approach. CRISPR/Cas systems have recently been frequently used to modify the genetic makeup of several aquatic and freshwater microalgae. The majority of research has used the Cas9-driven Type II system, one of two classes and six unique kinds of CRISPR systems, to specifically target desired genes in algae, and knock them out and down, or both. Using CRISPR technology to modify its genetic makeup, microalgae has produced more biomass and increased in lipid content. This review highlights the attempts made so far to target microalgae genome modification, discusses the prospects for developing the CRISPR platform for large-scale genome modification of microalgae, and identifies the opportunities and challenges in the development and distribution of CRISPR/Cas9 components.
Keywords: biodiesel, biofuel, bio-oil, CRISPR–Cas9, cyanobacteria, genome-editing, microalgae, photosynthetic microorganisms.
Introduction
Biofuels are energy-dense compounds that originate from the by-products of living things, including bacteria, microalgae, and plants, or that are produced by biological procedures. The world’s growing population demands greater energy resources to maintain and improve its living standards. Global energy needs may be met in part by biofuels. Fossil fuels have long been the main form of energy, but its consumption is inefficient and their burning results in ecological problems (Razzak et al. 2013; Voloshin et al. 2015). Concerns about the effects of global warming, the worldwide energy recession, the depletion of fossil resources, and surging crude oil costs have all increased interest in energy produced from renewable sources (Behera et al. 2015). This provides an opportunity for the replacement of fossil fuels with ecologically beneficial and more sustainable sources of energy like biofuels. Following recent developments in the manufacture of microbial biofuels, direct energy transformation from microorganisms to produce biofuels has been recognised as one alternative to fossil fuels. A viable and innovative method for producing biomass pathways that might be explored for a biofuel production process is the cyanobacteria or microalgae biofilm culture, which can be used to cultivate microalgae or cyanobacteria to produce biofuel (Demirbas 2009; Heimann 2016; Junaid and Gokce 2024). For the past couple of decades, the most renowned means of producing biofuels has been plant biomass. A growing body of evidence indicates that algal biomass is a viable source for the production of biofuels. Both plants and algae have the ability to photosynthesise, a process where sugars are produced from the assimilation of atmospheric carbon dioxide using solar energy. Photosynthesis is the primary method used by plant and algae to grow; and the production of biomass is the first step needed to make biofuel. This is shown by the way in which green algae and plants fix carbon in the natural world (Voloshin et al. 2015). Depending on how they are produced, biofuels are classified in four generations: (1) first generation biofuels are produced from edible sources such like starch; (2) second generation biofuels are produced from lignocellulose compounds derived from non-edible plant material; (3) third generation biofuels are produced from microalgae and cyanobacteria; and (4) fourth generation biofuels are produced from genetically engineered microorganisms like including yeast, fungi, cyanobacteria, and microalgae (Alalwan et al. 2019).
Between 2016 and 2040, the output of bioenergy is predicted to rise from 9.7 × 106 GJ per day to 4.6 × 107 GJ per day (IEA 2022), and the ecological consequences of biofuels will depend on how they are generated. First generation biofuels has several negative economic, social, and ecological effects that could be lessened with the adoption of environmentally-friendly biofuel manufacturing methods, which include native perennial plants, microalgae cultivation methods produced on low-biodiversity or degraded landscapes, and recyclable wastes. These effects would primarily be mitigated by less competition in agricultural output and ecological diversity. Before becoming widely used, these innovative biofuel generation options must be thoroughly evaluated for economic sustainability as well as their ability to meet ecological objectives (such as lowering poverty, reducing the effects of global warming, preserving biodiversity, providing clean water, and reducing eutrophication). To guide decisions and put renewable production of biofuel strategies into practice, thorough assessments that consider the ecological and social implications of biofuel development are required. This could entail developing and standardising a range of climate change metrics, the creation of standardised evaluations of the environmental effects of biofuel manufacturing processes, the emergence of thorough case investigations that include several economic and sustainability goals, enhanced estimates of the practicality and revenue for prospective biofuel manufacturing, and the creation of comprehensive evaluations to comprehend the ecological and socio-economic consequences of biofuel manufacturing alternates. With time, technological advancements could increase the revenue potential of more environmentally friendly options for producing biofuels; at present, estimates place this profitability at USD19–62 for lignocellulosic feedstocks and USD13–8949 GJ-1 for microalgae manufacturing mechanisms (Carriquiry et al. 2014). In the interim, many policies (such as laws, levies, subsidies, economic and market-oriented methods, and collaborations in applied research) can encourage the creation and use of more environmentally-friendly methods for producing biofuels. These regulations have to be stated at the international, national, and local levels. A crucial piece of technology for addressing the long-term energy needs of transportation while minimising changes to land usage is third generation biofuels. For example, less than 2% of every nation’s agricultural land would need to be dedicated to microalgal agriculture to meet their present local transportation energy needs. These countries include Australia, Mexico, Iran, Egypt, India, Brazil, South Africa, Canada, Indonesia, and Saudi Arabia (Correa et al. 2019).
Other potential uses of photosynthetic microorganisms is the production of valuable compounds, such as pigments, polyunsaturated fats, and vitamins, the immediate utilisation of biomass (such as aquaculture feed and dietary supplements), and ecological purposes (such as treatment of waste water, manufacturing of biofuel, and elimination of CO2) (Fernandes et al. 2015; Kayani et al. 2025). Prokaryotes (cyanobacteria) and microalgae are unicellular photosynthetic microorganisms. The main disadvantages of producing biofuel on an industrial scale from these sources arepoor yield, lack of feedstock supply, feedback inhibition, the existence of inhibitory mechanisms in different species, and the organisms’ resistance to biofuel formation. For genome modification, a variety of strategies are used, which includes clustered regularly interspaced short palindromic repeats/associated protein (CRISPR), zinc finger nuclease (ZFNs), and Transcription Activator-like Effector Nuclease (TALEN). CRISPR/Cas has become of the most remarkable and quickly expanding of these (Tanveer et al. 2024). The effective generation of biofuels from photosynthetic microorganisms like algae and cyanobacteria can be achieved by modifying their genomes by using the CRISPR/Cas9-mediated gene editing technology. The CRISPR/Cas9 system enables higher biomass and lipid production leading to the efficient production of biofuels (Lakhawat et al. 2022; Fatima et al. 2023).
This review provides an overview of photosynthetic organisms, identifies promising biofuel-genrating microbials, discusses the use of CRISPR/Cas9 technology in microalgae and cyanobacteria, and describes future perspectives of creating a CRISPR framework for highly efficient genome modification of photosynthetic microorganisms.
Revolutionising biofuel production: integration of photosynthetic microorganisms and CRISPR/Cas9
Overview of photosynthetic cyanobacteria and algae
The most significant types or groups of microalgae are (1) diatoms (Bacillariophyceae), which are the most abundant living form of phytoplankton and make up the largest group biomass on Earth; (2) green algae (Chlorophyceae); (3) blue-green algae (Cyanophyceae), which have characteristics of both bacteria and algae, with rapid photosynthetic rates (Vassilev and Vassileva 2016); (4) golden algae (Chrysophyceae); and (5) red algae (Rhodophyceae). Numerous important biomolecules, including carbohydrates and lipids were present in microalgal biomass and might be transformed into third generation biofuels. The rapid growth of microalgae allows for more efficient use of scarce land resources to meet the enormous demand for biofuels without the risk of a biomass shortfall. Other advantages include lower water use compared to land crops, highly efficient carbon dioxide reduction, lower nitrogen oxide emission, and increased profitability. Commercial microalgal farming facilities is also hampered by the greater capital expenditures and greater intensive resource input compared with conventional agriculture (Li et al. 2008). Cars have been fuelled using biodiesel, which is made commercially or in private facilities via a mono-alcoholic trans-esterification process (Demirbas 2007). The manufacturing of biodiesel is difficult because feedstock is expensive and there is competition for available land that is needed for food production. A viable substitute source of lipids for the generation of biodiesel is the microalgal species that would collect lipids to a considerable extent in their biomass. The necessity to lower the cost of producing microalgae directly competing with traditional energy sources is highlighted (Chisti 2007). Commercial biodiesel production facilities using algal oils have been constructed by private companies. The processing of biomass at elevated temperatures without oxygen results in the production of bio-oil and bio-syngas. It is shown that bio-oils may be used to generate electricity by cofiring with fossil fuels like natural gas or diesel, or by using external combustion (such as in Stirling engines and steam cycles) or internal combustion (such as in diesel and gas turbine systems) (Li et al. 2008). Conventional biomass derived from agriculture and forestry sources have been the subject of the majority of research. Microalgae bio-oils are generally of greater quality than bio-oils derived from wood, according to a few recent studies (Demirbaş 2006).
The vital biological method of photosynthesis uses light and dark reactions to convert inorganic carbon into organic material. Light exposure too great for microalgae can result in photoinhibition, which lowers the production effectiveness of adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide phosphate (NADPH). To address this, non-photochemical quenching is used. Through the Calvin-Benson cycle, CO2 is transformed into carbohydrates in the dark processes. To attain high rates of photosynthetic activity even in low CO2 settings, microalgae have developed a carbon dioxide concentrating mechanism (CCM). To maintain internal and external electrolyte, and pH balance, CCM controls the ratio of light-to-dark reactions. There are differences in the structural characteristics of CCM between eukaryotic microalgae and cyanobacteria. Cyanobacteria have a specific characteristic called carboxysomes, vital to the consequent rise in the concentration of CO2 locally. Many pigments, including carotenoids, phycobiliproteins, and chlorophylls, participate in the light-capturing and photoprotective activities in microalgae during photosynthesis (Abbas et al. 2021; Hu et al. 2023; Naeem et al. 2024).
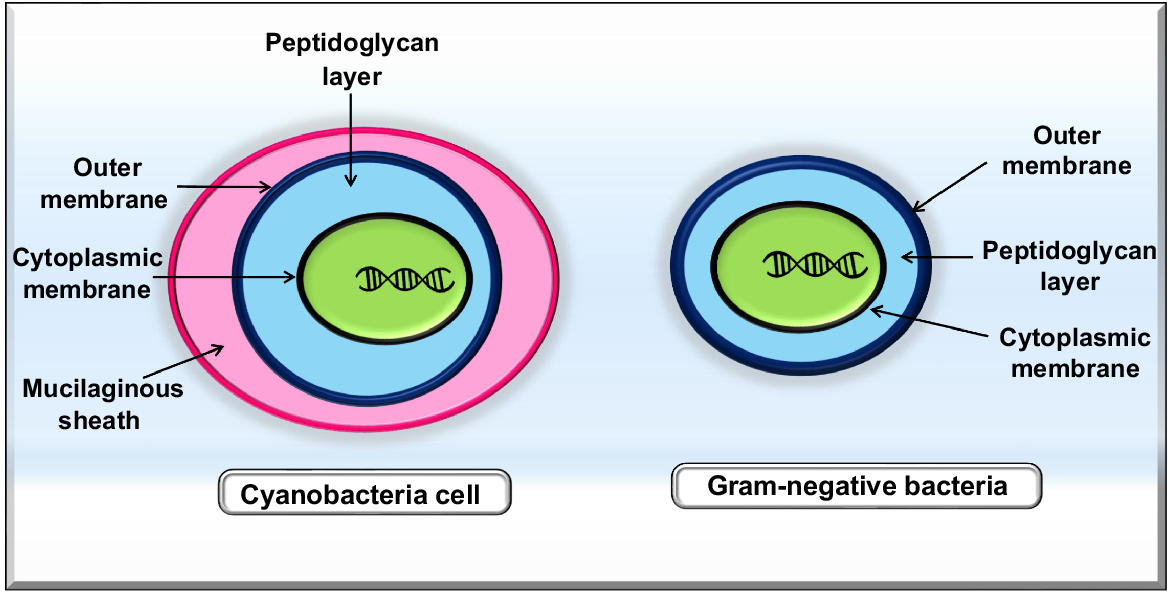
Ranging from marine and freshwater environments to terrestrial environments, cyanobacteria survive in the most extreme settings such as hypersaline ecosystems, frozen systems, and geothermal environments. The cells of cyanobacteria are surrounded by a complex, multilevel framework that protects them from stresses in their environment (Mehdizadeh Allaf and Peerhossaini 2022). A thick coating of peptidoglycan surrounds Gram-positive bacteria, while Gram-negative bacteria have a thin peptidoglycan cell wall with lipopolysaccharide in their external membrane (Silhavy et al. 2010). Cyanobacteria are unique in that they are Gram-negative bacteria but posssess a thick peptidoglycan coating (10–700 nm) like Gram-positive bacteria (Mehdizadeh Allaf and Peerhossaini 2022). Certain cyanobacteria also possess external non-flagellar attachments, pili or fimbriae, which play roles in movement, attachment, biofilm development, and DNA uptake (Schuergers and Wilde 2015; Javed et al. 2024). Fig. 1 compares cyanobacteria cells and Gram-negative bacteria cells.
Cyanobacteria are the biggest group of photosynthetic prokaryotes and can capture solar energy and carry out photosynthesis via chlorophyll a absorbing CO2 and producing O2, similar to higher plants (Angermayr et al. 2009). The phycobilin pigments, phycocyanin and phycoerythrin, are blue and red auxiliary photosynthetic pigments that cyanobacteria make along with chlorophyll a (green pigment). These pigments allow the bacteria to flourish in low light. A proven defence against photooxidative damage, carotenoids are another substance produced by cyanobacteria (Barsanti and Gualtieri 2006; Zakar et al. 2016). ATP and NADPH of cyanobacteria are similar to those of eukaryotic cells as they both process light energy. Cyanobacteria may engage in carbon fixation and the synthesis of organic compounds by transforming CO2 into biomass and generating alcohols, fatty acids, and carbohydrates that can be used as sustainable sources of biofuels, which serve as an energy source (Quintana et al. 2011). Their rapid growth rates and high biomass output are other properties of cyanobacteria that make them suitable for the manufacture of biofuels. During cell stress, cyanobacteria can create large quantities of lipids. Unlike eukaryotic microalgae, cyanobacteria are easily genetically manipulated to produce biomass or other desirable end products from atmospheric carbon (Sarsekeyeva et al. 2015). Photobioreactors (PBRs) and open ponds are frequently utilised in the bulk generation of microalgae on a commercial level (Codd et al. 2016), which means less competition for land needed for food production. Among the various configurations, open ponds are the earliest and least costly; however, the culture suspension in open ponds is easily contaminated by other microorganisms, and it is challenging to monitor variations in temperature and transpiration losses. Nevertheless, this system offers better control over heat and food circulation, exposure to sunlight, pH, and pollution prevention methods. However, the generation of biomass in PBRs is expensive. Suspended microorganisms can be exposed to more light if the PBR volume is mixed efficiently. Not all bacterial species can withstand the extreme strain brought on by mixing mechanically (Mehdizadeh Allaf and Peerhossaini 2022). Encouraging green buildings with power-efficient bio-solar facades, and utilising double-walled rectangular PBR screens, is one of the most creative ways to cultivate cyanobacteria for atmospheric carbon fixation and renewable energy sources (Mazard et al. 2016). The biomass generated in the bioreactors may be collected and transferred to a biorefinery to extract valuable chemical compounds.
Use of CRISPR/Cas9 technology in genetic engineering
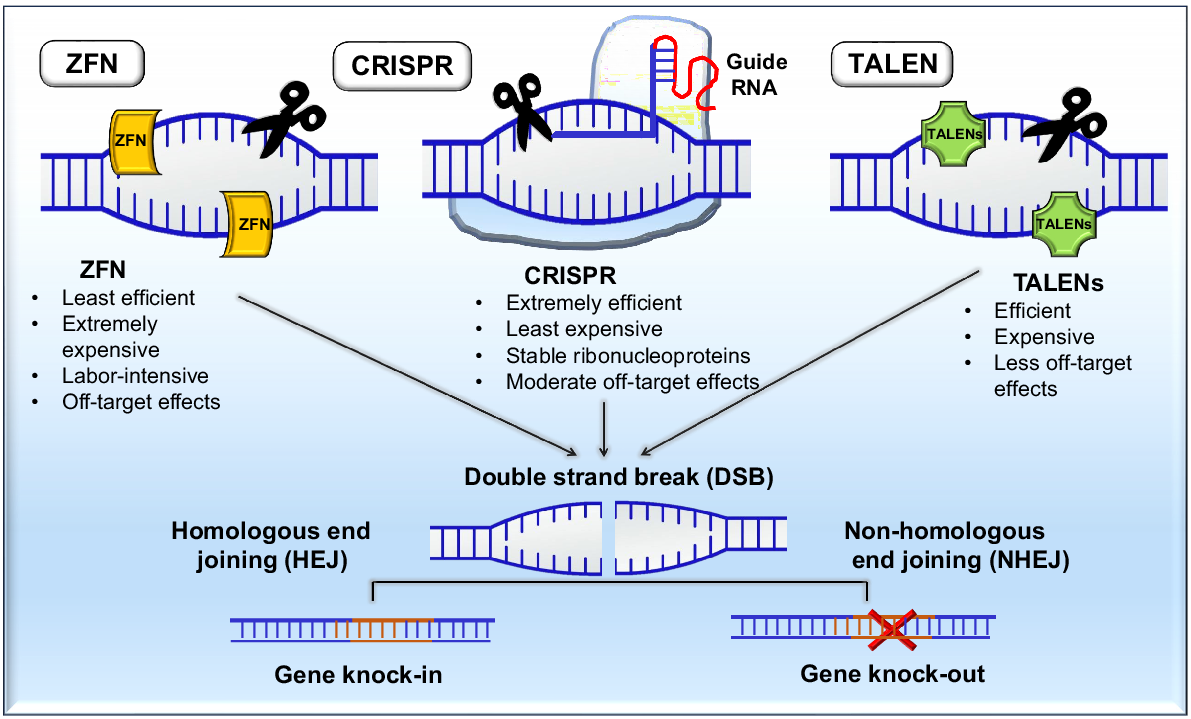
Targeted genome editing methods have gained popularity because they allow for exact genome-level in situ alterations. The traditional in vivo gene targeting technique uses arduous, lengthy, and inefficient normal homologous recombination. The ability to carry the catalytic domains of the restriction endonuclease FokI, which produces a double-strand break (DSB) with cohesive overhangs allowed ZFN and TALEN to overcome it. These tools were crucial in the extraction of gene function information through targeted genome modification. However, as research has progressed, the CRISPR/Cas approach has emerged, which was borrowed from the adaptive immune response of bacteria (Doudna and Charpentier 2014). This development has improved the reliability of functional genomics by facilitating the easy and precise modification or suppression of gene activity (Hsu et al. 2014). ZFNs are synthetic DNA-binding proteins with two domains linked by a linker sequence. They provide sequence specificity through a zinc-finger DNA binding domain and a restriction endonuclease FokI-mediated DNA cleaving domain. Relying on repetitive regions, TALENs have been beneficial for targeted gene editing. However, their intricacy, challenges, and cost have limited their use, prompting researchers to develop a simpler, effective, and cost-effective method for precise genome alteration. As a consequence, the CRISPR/Cas9 system was created to provided the highest level of genome editing techniques. CRISPR development began in 1987 when non-repetitive short sequences (spacers) and a 29-nucleotide repeat were observed in Escherichia coli by Ishino et al. (1987). In 2012, researchers reported that CRISPR technology may be applied to genome modification and they carried out an in vitro study to characterise DNA targeting by Cas9 (Gupta et al. 2019). In eukaryotic cells, Cas9-based genome modification was initially developed in 2013 (Barrangou and van der Oost 2013). Two separate groups carried out the first genome-wide Cas9 screening in 2014 (Shalem et al. 2014; Wang et al. 2014). Since then, CRISPR/Cas9 has been successfully used for multiple tasks, including high-throughput gene screening, chromosomal locus live-cell categorisation, single-stranded RNA editing, and gene knockout. Fig. 2 summarises the concepts of ZFN, TALENs, and CRISPR.
Potential of CRISPR/Cas9 to improve biofuel production from photosynthetic microorganisms
Certain marine and freshwater microalgae have had their genomes altered through the widespread usage of CRISPR/Cas systems. The majority of research has utilised the Cas9-driven system to specifically knock down desired genes in algae, knock them out, or both. Microalgae, such as diatoms, have shown promise in using CRISPR technology to express certain genes and produce industrial features like increased biomass production and lipid content. The utilisation of genetically modified microorganisms (GMOs) such as microalgae to produce fourth generation biofuel has the potential to address the problems associated with the depletion of fossil resources. Green algae and diatoms that have undergone genetic manipulation are being assessed as biological models for their potential industrial biodiesel production by adjusting their gene expression patterns in different experimental setups (Patel et al. 2019). The sustainable and promising feedstock for the manufacturing of biodiesel is triacylglycerols, which are a type of storage lipid that many microalgae can create in 20–50% of cases when exposed to photo-oxidative stress or other unfavourable environmental circumstances (Bhujade et al. 2017). Industrial-scale biofuel generation requires the large-scale growth of high-biomass algae. To integrate into the techno-economical paradigm, regulated development of efficient variants is very necessary throughout the year to extract lipid-rich biomass from genetically modified microalgae (Kumar 2015). It is important to clarify the expression patterns of native algae under diverse conditions by examining RNA-seq data to find the relevant transcription factors (TF), promoters, and genes by investigating genes with differential expression (Wang et al. 2018). Using high-throughput RNA-Seq data analysis, 20 putatively bnuiregulated TFs of the mircoalgae Nannochloropsis gaditana were found in the nitrogen-deprived cells. These TFs were then removed from the oleaginous genome of the alga to determine which TFs are essential for the distribution of total carbon among biomass and lipids. Zn(II)2Cys6 TF knock-out strains had 45–55% more lipids than wild-type algae (Ajjawi et al. 2017). Thus, one potential method to discover the target genes or TFs to be edited by a CRISPR system is to further increase the biomass and lipid yield of different industrial algae by functional annotation of large-scale RNA-seq data. To create algal cell factories as a resilient living bioreactor system, high-efficiency techniques for the transformation of ribonucleoproteins and CRISPR/Cas9 plasmids and the selection of transformants have been devised (Wang et al. 2016; Sharma et al. 2018). The FTSY gene, which codes for the antenna chlorophylls in the industrial alga Coccomyxa sp. strain KJ, results in pale-yellow mutant colonies when it loses its function. Using CRISPR/Cas9 RNP-based methods, this gene has been altered to produce a more resilient strain of Coccomyxa that can serve as a factory for biofuel cells (Yoshimitsu et al. 2018). Using type-II CRISPR techniques, N. gaditana has been altered to produce mutants with higher lipid outputs by deleting the TF Zn(II)2Cys6, which controls the expression of genes associated with the transition of total carbon to lipid (Ajjawi et al. 2017).
Due to their photosynthetic capacity, cyanobacteria have a lower requirements of carbon dioxide than eurkaryotes, making them suitable as a source of biofuel (Naduthodi et al. 2018). Genome engineering using CRISPR/Cas9 has been effectively used in cyanobacteria, leading to the suppression of genes encoding for aldehyde dehydrogenases/reductases, polyhydroxyalkanoate synthase, Green Fluorescent Protein, and ADP-glucose phosphorylase (Yao et al. 2016). The expression of the exogenous (eYFP) and endogenous (glgC) genes was repressed in Synechococcus elongatus strain PCC 7942 (Huang et al. 2016), whereas the native genes that encode the phycobilisome and carboxysome subunits were downregulated (Gordon et al. 2016). Additionally, CRISPRi suppressed the glutamine synthetase I gene, which resulted in twice as much lactate (Gordon et al. 2016) and ammonium build-up (Higo et al. 2018). S elongatus strains UTEX 2973 and PCC 7942 were susceptible to the cytotoxic impacts of the Cas9 protein. To counteract the harmful impact, the Cas9 protein was produced from a vector that prevents replication at the ideal temperature of Synechococcus. It was demonstrated that Cas9 may induce double-strand breaks (DSB) in S. elongatus strain PCC 7942, which leads to cell death (Li et al. 2016; Wendt et al. 2016). To increase carbon flow into the oxidative pathway of the TCA cycle, phosphenolpyruvate carboxylase and citrate synthase genes were knocked in using Cas9-assisted homology-directed repair (Li et al. 2016).
The development of the CRISPR/Cas system allows for highly efficient targeted genome editing of cyanobacteria and microalgae to produce industrial features like biomass and lipid production, which lead to the manufacture of biofuels.
Challenges in biofuel production
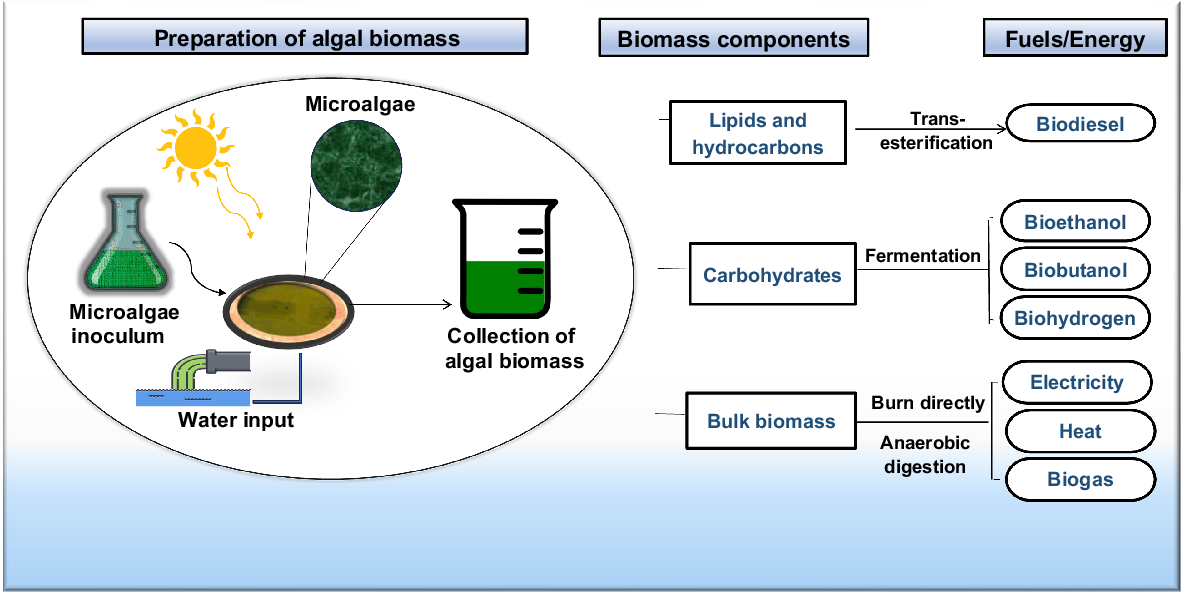
Due to the emission of greenhouse gas (GHG) associated with fossil fuel use, there is increased research and development efforts in identifying alternative and sustainable fuels. The imminent depletion of various fossil fuel sources in the next 50 years as well as the significant ecological damage caused by urban smog, acid rain, and climate change have led to a focus on reducing the amount of carbon released into Earth’s atmosphere. Transition towards renewable energy sources, such as wind, solar, and biofuels is beginning (Callegari et al. 2020). Biofuel includes a variety of products made from biomasses or their by-products such as bioethanol, vegetable oils, biodiesel, biohydrogen, bioethers, biomethanol, and synthetic biofuels (Lin et al. 2014). Fig. 3 shows how microalgal biomass can be converted to biofuel.
Distinction have now been made to distinguish the origins of feedstock in an attempt to classifiy biofuels. Biofuels initially originated from animal or vegetable fats and oils, cellulose, sugar, and starch supplied from different animal or agricultural raw materials. First generation feedstocks for biofuel were derived from vegetable oils, and feedstock from soy bean (Glyine max), corn (Zea mays), sugar cane (Saccharum officinarum), and wheat (Triticum aestivum) (Jin et al. 2011; Azad et al. 2015). In addition to being expensive and demanding strict growth guidelines, these feedstocks also posed ethical and ecological development challenges, such as the continuing rise in prices for staple foods in developing nations (Renzaho et al. 2017) and the unsustainable conversion of forests to agricultural land (Khanna et al. 2017; Irfan and Mirara 2024). Moreover, their use resulted in major ecological problems, including contaminated soil and water, increased GHG emissions, a decline in biodiversity, the transmission of pests and crop diseases, and decreased energy and cultivation yields for crops like soy bean, corn, and sugar cane (Agarwal et al. 2017). Other food crops such as peanuts (Arachis hypogaea), rapeseed (Bassica napus), and sugar beet (Beta vulgaris) have also been used as substrates for first generation biofuels. Except for certain mechanical and sporadic thermal preliminary processing, fermentation was the primary method used to produce first generation biofuels.
Unlike first generation biofuels that used food crops as a feedstock, second generation biofuels use wood-based cellulosic or lignocellulosic biomasses that are unsuitable as food sources (Bhatia et al. 2017). Depending on its origin, a specific feedstock may also be classified as both first and second generation. For example, vegetable oil is a first generation biofuel; however, it is regarded as a second generation biofuel when that oil is considerd waste and is no longer unsuitable as a food source (Saravanan et al. 2018). Ideally, second generation feedstocks are cultivated on marginal land (Gelfand et al. 2013) because a second generation fuel should have no value as food source, and also require minimal water or fertiliser inputs. There are several processes for extracting energy from second generation feedstocks, including thermochemical processes like torrefaction, gasification, and pyrolysis. For example, lignocellulosic feedstock undergoes such multi-step processing before ethanol is produced. The most widely used second generation feedstocks are grasses (e.g. Indian grass, Sorghastrum nutans; Miscanthus spp., switchgrass, Panicum virgatum), seed crops (e.g. oil palm, Elaeis guineensis; rapeseed; species from the genera Jatropha and Camelina), waste vegetable oil, and municipal waste (e.g. sludge from wastewater purification, yard clippings, landfill gas) (Ho et al. 2014). The carbon footprint of these feedstocks is significantly lower than conventional fossil fuels (Naik et al. 2010). Grass is an ideal second generation feedstock because it grow quickly on marginal land with minimal fertiliser need, and can be harvested regularly (Cheng and Timilsina 2011).
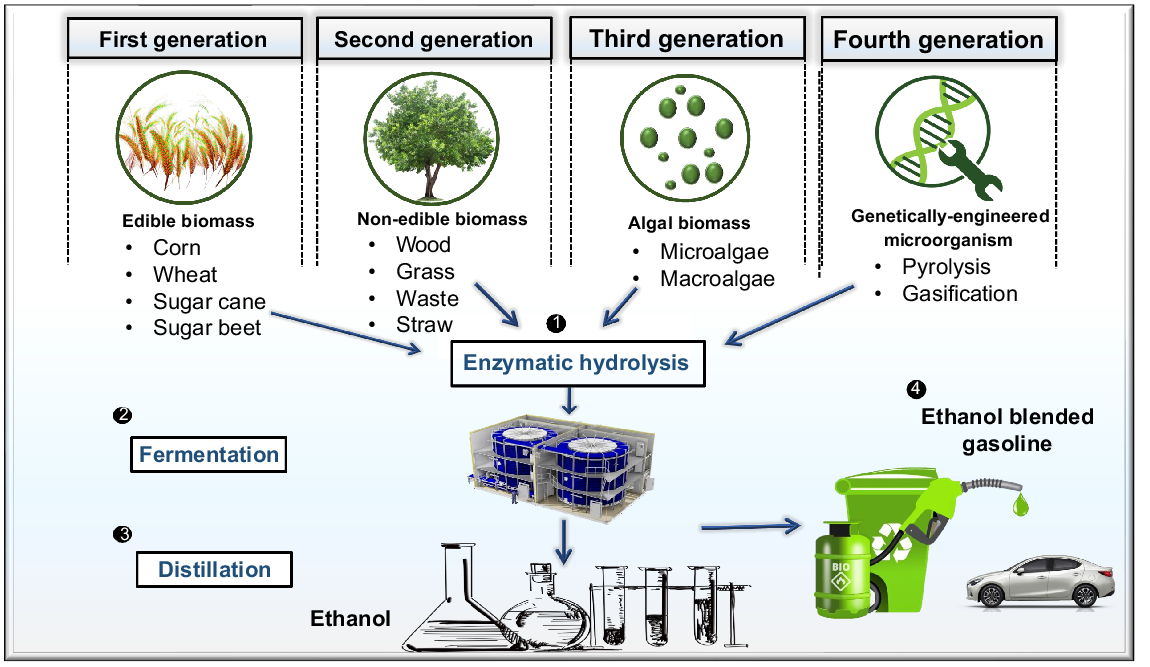
Third generation biofuels are derived from cyanobacteria and microalgae, and undergo processes such as lipid extraction and fermentation to generate energy. Fourth generation biofuels include cyanobacteria, yeast, fungi, and microalgae that have undergone genetic engineering (Vassilev and Vassileva 2016). Fig. 4 summarises the four generations of biofuels.
Enhancing lipid metabolism in cyanobacteria and microalgae for the production of biofuel is a challenge for researchers. Strain selection (Mallick et al. 2016), genetic intricacy (Zhu et al. 2022), growth-lipid accumulation trade-offs (Zhu et al. 2022), cultivation difficulties (Li-Beisson and Peltier 2013), economic feasibility (Zhu et al. 2022), environmental stress reactions (Wang et al. 2024), technological constraints, and interdisciplinary methods (Falfushynska 2024) are a few of these. The effectiveness and economic feasibility of employing these living things as long-lasting biofuel resources are hampered by these challenges. For biofuel production to be successful, cost-effective techniques must be developed and technological constraints must be addressed.
Types of CRISPR/Cas systems
Based on the structure of effector complexes and endonucleases that each class, type, and subtype of the CRISPR system exhibits, comparative microbial genetics has played a major role in finding and categorising these systems. Two main categories, Class 1 and Class 2, have been determined. Type I, Type III, and an uncommonly basic Type IV CRISPR/Cas system are part of Class 1. In Archaea, Type I and Type III networks dominate; however, in Eubacteria, they are less common. The RNA recognition motif fold, extra small and large components, and repeat-associated mysterious proteins (RAMPs) provide the framework of the intricate effector complex structures of Types I and III systems. One Cas5 and many Cas7 molecules are present in these effector complexes. The ubiquitous proteins Cas1 and Cas2 identify foreign DNA (spacer DNA) regions surrounded by a Protospacer Adjacent Motif (PAM) during the first phase of CRISPR/Cas processing (Patel et al. 2019). The Cas3 gene, which produces a large protein with helicase and DNase action, is the primary component of Type I CRISPR (Plagens et al. 2015). In addition, complexes of the RAMP superfamily have been seen to be formed by the proteins Cas4, Cas5, Cas6, and Cas7. All Type III systems have the characteristic Cas10 gene, which codes for a protein with several domains including a Palm domain (a variation of the RNA recognition motif) regarded as a major component of Type III crRNA–effector complexes and identical to the primary domain of many cyclases and nucleic acid polymerases (Makarova et al. 2015). The two main proteins of Type IV, Cas5 and Cas7, make it an extremely basic type. Other proteins that are the components of the Type IV CRISPR system include dinG, Cas6-like proteins, Cas8-like proteins, and Cas11. The core of the other five distinct forms of CRISPR, except Type IV, is known to be the Cas1 and Cas2 genes (Koonin et al. 2017).
Class 2 effector modules are composed of just one multi-domain unit that is organised more simply than Class 1. There are three forms of CRISPR/Cas systems in Class 2: (1) Type II; (2) Type V; and (3) Type VI. Three primary effector endonucleases (Cas1, Cas2, and Cas9) as well as a little trans-activating crRNA that is complementary to the CRISPR repeat DNA sequence are characteristics of the Type II system. Csn2 and Cas4 are needed for spacer recruitment in Subtype II-A and II-B networks, respectively. Howvever, Subtype II-C needs Cas1, Cas2, and Cas9 (Bernheim et al. 2017). Effector endonuclease Cpf1, which is employed in certain species instead of Cas9 (Type II CRISPR), has been anticipated to be Type V. It has been shown that Cpf1 is an effective RNA-guided endonuclease that can cleave targets without the need for tracrRNA, in contrast to Cas9. In comparison, CRISPR/Cpf1 is smaller, does not include tracrRNA, and can identify the T-rich PAM sequence TTTN on the guide RNA’s 5′ side. It differs from Cas9 in that it employs an NGG PAM (5′-NGG-3′ sequence that is recognised by the Cas9 enzyme in CRISPR–Cas9 gene editing) on its 3′ end. With just one RNA molecule (crRNA), Cpf1 produces random cuts and double-stranded breaks that result in restriction staggered overhangs. The Cpf1 produced from Acidaminococcus, Francisella novicida strain U112, and bacteria from the Gram-negative family Lachnospiraceae caused breaks at 19 bp after the PAM on the targeted strand and 23 bp on the opposite strand, as demonstrated by documented research findings on mammalian cells (Zetsche et al. 2015). Although Cpf1 lacks the HNH endonuclease region of Cas9, it still has a RuvC-like endonuclease site, suggesting that Cpf1 acts differently from Cas9. Some of the drawbacks of Cas9, like its G-rich PAM demand and blunt dual-stranded cuts, may be addressed by using an alternative endonuclease. In terms of magnitude, reduced toxic effects, and modification, the Cpf1-based Type V system is becoming more and more popular in the scientific community since it is relatively easier to grasp (Patel et al. 2019). The three subtypes of Type VI feature two HEPN domains that are expected to exhibit RNase activity. Experimental validation of VI-A (Cas13a) and VI-B (Cas13b) effectors’ interference activity has been completed (Koonin et al. 2017). Table 1 outlines the classes, subtypes, and common effector endonucleases among each class.
Structure and mechanism of CRISPR/Cas9 system
The CRISPR/Cas9 system is comprised of two parts: (1) Cas9, which is synthesised an endonuclease protein that produces double-strand breaks (DSB) in DNA; and (2) a guide RNA (gRNA), which is a short piece of RNA made up of a structure required to attach to Cas9 (Gupta et al. 2019). With the help of two distinct nuclease active domains named HNH and RuvC, which participate in the splitting of the sense and antisense strands of DNA, respectively, Cas9 snips double-stranded DNA at a location of three base pairs upstream of PAM. Few Cas9 proteins that have been designed to contain deactivated RuvC or HNH regions have been synthesised lately. These are referred to as dCas9 (dead Cas9). As the Cas9 protein may bind to almost any complementary region found in any genome, attempts are being undertaken to create a functionally inactive dCas9 by modifying the original codon and introducing point mutations that would block the transcription of a variety of genomic loci. The names ‘StCas9’ and ‘SpCas9’ refer to the Cas9 from Streptococcus thermophilus and Streptococcus pyogens, respectively, while ‘StdCas9’ and ‘SpdCas9’ refer to their corresponding dead Cas9 proteins. By integrating with transcriptional repressor proteins or gene transcription factors, dCas9 is extremely beneficial in adjusting the expression patterns of certain genes (Sternberg et al. 2014). An approximately 20-nucleotide spacer known as a protospacer, identifies the locus in the genome to be modified. The nuclease will only cause a double-strand break if the PAM has NGG at the 3′ terminus of the spacer (for Cas9).
In the naturally-developed CRISPR system, Cas9 forms the final splicing complex by binding with two separately generated RNA molecules named crRNA and tracrRNA. The tracrRNA serves as a link between the crRNA and the Cas9, whereas the crRNA targets a particular genomic sequence to initiate dsDNA breaks. Eventually, the molecules crRNA and tracrRNA are united to create a single gRNA, a shorter RNA molecule with the same effectiveness as crRNA-tracrRNA (Sander and Joung 2014). The 5′ end of the gRNA contains 17–20 nucleotides that are complementary to the target DNA (Ran et al. 2013). When the gRNA binds to Cas9, it closely resembles the stem and loop framework (scaffold) of the crRNA–tracrRNA complex, which allows for precise genome modification (Fu et al. 2014). Consequently, a particular nucleotide sequence corresponding to the target DNA was needed for the construction of a unique gRNA for the target regions, after which was a PAM motif without changing the scaffold. Because of this characteristic, the CRISPR–Cas9 system outperforms ZFNs and TALENs, which depend on the protein’s DNA binding domains to identify the target regions (Nowak et al. 2016).
The most used CRISPR system for modifying the genomes of algae is Type II. The endonucleases of Staphylococcus aureus (SaCas9) and S. pyogenes Cas9 (SpCas9) have been extensively explored for modifying the genomes of algae. For targeted genome modification in algae, the integrative plasmid containing codon-optimised SpCas9 or SaCas9 and the unique single gRNA have been used (Jiang and Weeks 2017). Gene silencing, which ultimately results in InDel (insertion/deletion) mutations in the algal genome, occurs after the Cas9–gRNA complex binds to the target DNA region (Hsu et al. 2013; Shin et al. 2016).
The helicase activity of Cas9 disassembles the target DNA sequence as soon as the Cas9/gRNA complex recognises the target region, allowing the 5′ end of gRNA to pair up with the corresponding DNA strand to generate an RNA–DNA hybrid region. The parallel and anti-parallel strands of RNA-DNA are cut by the Cas9 RuvC and HNH domains. Therefore, three bases upwards from PAM, a blunt-ended DSB is produced by Type II CRISPR activity (Sander and Joung 2014; Jeon et al. 2017; Irfan et al. 2023; Abbas et al. 2024). Either homology-dependent recombination (HDR) or non-homologous end joining (NHEJ) is used to repair these damages. The open reading frame (ORF) shifts when the NHEJ repair pathway produces InDel mutations in target regions. The Csn2 protein inhibits the NHEJ repair pathway (Bernheim et al. 2017). In contrast, homologous recombination of donor DNA or expression cassette close to the protospacer is the foundation of HDR. To insert new genetic material into the intended genome of algae by specialised editing, such as the addition of a distinctive mutation or a whole functioning gene cassette, scientists often use the HDR process. For both the right and left sides of the editing site, an external DNA template (like a gene cassette or fluorescent label) with 400–1000 bp homology is needed (Zaboikin et al. 2017). The minimum distance between a potential cutting point and the nucleotide to be modified in a Type II CRISPR/Cas9 system is often estimated to be about 50 bp. The efficacy of HDR considerably reduces by 10% when this distance drops to roughly 30 bp. As a result, algal genome developers in the Type II CRISPR system often choose 100–1000 bp for HDR-based modification. In general, the expression cassette’s enhanced integration into the algal genome is provided by homology regions that are around 1000 bp on both sides of the genome (Jiang and Weeks 2017). However, no correlation has been shown to date within the measurement of the distance between the site of the nucleotide change and the cutting region and HDR efficacy with the Type V CRISPR/Cpf1 framework. The discovery of this connection may open up the path to very effective targeted genetic modification in algae (Paquet et al. 2016).
When the intended expression cassette is inserted into the target places, the original gene loses its intrinsic function and the donor DNA is knocked in; however, the target gene is knocked out and new characteristics are introduced into the genome (Shin et al. 2016). To address these problems, attempts have been centred around neutral sites; i.e. those that do not encode any metabolic functions. The brilliance of CRISPR/Cas9 technology resides in its ability to edit many genes concurrently in a cell by delivering numerous created gRNAs targeting distinct target sequences. Cas9 produces blunt ends at the appropriate target regions as an outcome. This is called multiplex editing (Ajjawi et al. 2017). In recent years, multiplex editing has been used to create desirable commercial characteristics and coproducts in green algae.
Genomic modification of cyanobacteria and algae using CRISPR/Cas9
The plasmid-dependent method: integrative and replicative mechanisms
Ideal promoters, 3′UTR (trailer sequence; aids in terminating translation and post-transcriptional genetic expression), and 5′UTR (leader sequence; aids in translation initiation) regions, ribosomal binding sites (RBS), and transcriptional terminator sequences are necessary for the expression of a specific foreign gene in eukaryotes (Rasala et al. 2010). Algal CRISPR/Cas9 editing is still in its early stages. Target gene implantation into cells may occur via either the integrative process or the replicative process. For the addition of a foreign gene into the genome of an algae and its expression, these two approaches need distinctive elements. Integrative DNA cassettes or plasmids containing selection indicators in algae are often employed to produce Cas9 and gRNA in algal cells (Wang et al. 2012; Urtubia et al. 2016). However, the cloning of Cas9 and gRNA cassettes uses replicative plasmids with an algae-derived replication origin and indicator, or reporter genes (Poliner et al. 2018). These methods take a lot of time to complete since they need to assemble many parts.
Advanced DNA polymerases exhibiting excellent processivity and robust proofreading capacity are used in overlapping PCR techniques to amplify and fuse smaller DNA fragments, resulting in the production of massive molecular weight assembly products such as unique expression cassettes (Bryksin and Matsumura 2010). Compared to the traditional restriction-ligation method, it has brought a fresh perspective to the DNA synthesis process. To create an appropriate expression cassette that can be assembled with additional essential DNA fragments for either replication within different cells of algae or incorporation in their specific genomes, it is mainly useful in the assembly of promoters, ORF, RBS, and terminators. Larger DNA pieces or DNA having very substantial GC contents may sometimes cause problems during assembly, making it extremely difficult to successfully build replicative or integrative cassettes (Li et al. 2018). Many post-assembly quality control assessments are needed to ensure the effective production of DNA. These assays include amplifying and sequencing multiple assembled intersections to verify the exact assemblage of different constituent fragments of DNA (such as promoters, ORFs, RBS, and terminators) and the detection of undesired mutations in assembled genes, antibiotic cassettes, terminators, and promoters that result in any frame-shift that causes the target expression of proteins to fail. Integrative DNA cassettes typically include 3′UTR and 5′ UTR sections, which are often limited to introns and are necessary for efficient gene expression. Only the 5′UTR is present in a large number of algal vectors, though. A multiple cloning site (MCS) for cloning both cas9 and gRNA, weaker inducible promoters for the production of both Cas9 and/or gRNA, a possible transcriptional terminator to end the transcription of cloned genes, and a minimum of one antimicrobial-resistant cassette to select transformants are the remaining components (Naduthodi et al. 2018). The Cas9/gRNA complex must have the nuclear localisation signal (NLS) added at either the C or N terminal end to target the nucleus for modification. The way the amino acids in the NLS region affect the Cas9 nuclease’s activity is yet unknown. To obtain high effectiveness of precise modification in the genome, it is thus important to determine the optimal NLS for Cas9 that is particular to each type of cell. However, for a small number of green algae, replicative plasmids that regulate the algal system have hardly ever been created. These replicative plasmids are propagated both in bacterial cells as extra-chromosomal DNA and inside the algal cytoplasm due to their distinct origin of replications (ori). In addition, they have a promoter, MCS, a putative transcriptional terminator, and reporter gene cassette or a selectable marker for the efficient identification of transformants (Ajjawi et al. 2017). Steady expression is necessary for industrial strains to prevent complications. The inserted genes of genetically altered algae may sometimes escape out of open ponds for a variety of unexplained causes (Henley et al. 2013). Therefore, for massive cultivation, strains with fully integrated gene cassettes inside their genomes are recommended. As a result, integrative plasmids are preferred by most biotechnologists for expressing Cas9 and distinct gRNAs in the nuclear genomes of different industrial algae.
Even though it may be challenging to prove, temporary expression of gRNA and Cas9 cassettes has the benefit of not altering the genome via planned or spontaneous integration of foreign DNA cassettes. Jiang et al. (2014) showed that constitutively-produced Cas9 may be harmful, but that gRNA and Cas9 can be expressed transiently for rapamycin-sensitive mutants of Chlamydomonas reinhardtii.
Cas9 ribonucleoprotein (RNP)-dependent approach
There are many instances of algal genetic modification using RNP. Refined Cas9 protein and in vitro-synthesised gRNA are used in this approach. Algal cells are directly exposed to the pre-assembled Cas9 protein and gRNA using electroporation or a biolistic technique (Greiner et al. 2017). Cas9 RNPs were electroporated directly into C. reinhardtii cells. These RNPs were designed to target mutations in three loci: (1) ChlM that encodes Mg-protoporphyrin IX S-adenosyl methionine O-methyl transferase; (2) MAA7 that encodes the beta subunit of tryptophan synthase (TSB); and (3) CpSRP43 that encodes chloroplast SRP43 (Baek et al. 2016). Compared to plasmid-based techniques, RNP-based procedures are relatively easier and provide special benefits. However, the Cas9-gRNA complex generated through this method necessitates strict quality control procedures; in particular, it is critical to show that PCR-amplified DNA exhibits in vitro DNA cleavage ability at the intended target region (Shin et al. 2016). For this strategy, it is preferable to optimise Cas9 and gRNA quantity for the synthesis of functional RNP and a reliable transformation/RNP way of distribution in cells. Furthermore, Cas9 protein load may be decreased by titrating the level of Cas9 protein for every kind of cell. When PCR amplification of a particular gRNA is performed using a lengthy oligo surrounded by a target DNA recognition domain at the 5′ end, a plasmid construct containing a gRNA scaffold may be used as a template. For the purpose of gRNA molecule transcription in vitro, these PCR amplicons normally include a terminator, a particular gRNA gene, and a T7/Sp6 promoter. For the intended RNA production, this procedure demands extensive in vitro transcription optimisation of the process and gRNA purification technology. To attain significant transformation efficacy, the in vitro constructed RNP complex must undergo several optimizations throughout its transformation into algal cells. Growth phase and culture density are important factors to consider while preparing algae-competent cells. About 0.3–0.5 OD overnight developed cultures (dark phase) are quite suitable for achieving better efficiency of transformation in Chlorella and Chlamydomonas. The optimal application of electroporation techniques varies according to the strain. Electroporation is the most common method for achieving effective transformation in C. reinhardtii (Jiang et al. 2014).
Editor cell lines of microalgae
Gene sequences expressing Cas9 endonuclease and gRNA have been integrated into integrative vectors and converted into algal cells by biolistic or electroporation techniques in the majority of findings. After integrating into the algal genome, these DNA sequences begin to replicate (Wang et al. 2016). Lately, there have been efforts to create cytosolic replicating episomal plasmids (Poliner et al. 2018). In the majority of the algae, this technique was proven effective. This technique involves inserting a Cas9 expression cassette into the genome of the chassis algae to create Cas9 protein-expressing editor cell lines. These algae cells either include a promoter shift for the regulated expression of Cas9 endonuclease or can manufacture Cas9 endonuclease continuously without Cas9 adverse effects. Classified as ‘editor cells’, these Cas9-producing cells undergo direct modification via unique gRNA cassettes. Cas9 generates double-stranded direct breaks upstream to PAM, and the Cas9/gRNA group generated gRNA-DNA complex regions inside the algal genomes at different loci. To obtain deletion variants for 20 TFs, Ajjawi et al. (2017) created an editor cell line of N. gaditana that was able to constantly manufacture Cas9. These editor cell lines were subsequently modified with 20 different gRNAs. But using N. gaditana’s Cas9-expressing editor cell line, just 18 TFs could be produced as knockout variants.
Off-target actions of Cas9
A lack of knowledge on the specificity of gRNA-guided Cas9 endonuclease increases the likelihood of off-target mutagenesis consequences, in which the Cas9–gRNA complex mutates the genome in a way that is not planned or targeted and causes unwanted changes. If the guide RNA sequence exhibits over 12 nucleotide matches in the PAM-proximal domain (seed sequence), the assembly is predicted to adhere and cause a dsDNA break. Research conducted on mammalian and bacterial cells revealed that mismatches in the seed region’s 10–12 bp often result in a reduction or even elimination of the intended cleavage action. Although the Cas9–gRNA complex may attach to several locations throughout the genome, dsDNA breakage is contingent upon the precise positioning of the PAM sequence. The PAM for the commonly utilised Cas9 from S. pyogenes (SpCas9) is normally NGG, and it must be right next to the protospacer’s 3′ end (18–20 bp DNA–gRNA duplex domain). NAG (binding signal for Cas9, which is a protein used for gene editing) may also be tolerated to some amount when the SpCas9/gRNA hybrid exists more than target DNA, as shown by both in vitro and in vivo cleavage evidence (Hsu et al. 2013; Greiner et al. 2017). A significant factor influencing the accuracy of dsDNA breakage is the intracellular Cas9 quantity. For instance, misalignment of gRNA to distinct target regions may be accepted in the presence of large quantities of the Cas9–gRNA complex. It is reported that optimal or low Cas9 expression would increase precision and decrease unwanted impacts (Chen et al. 2017). Remarkably, the majority of research has focused on finding the trans-acting elements and promoters for Cas9 and gRNAs that have limited expression.
Optimising PAM sequences to boost Cas9 productivity
Specificity may be significantly impacted by the quantity of seed matches to target regions in the genome, which are then followed by NGG. For instance, compared to fewer than 10,000 CGTCG + NGG sites, there are over 1 million AAGGA + NGG sites in the mouse genome (Wu et al. 2014). Consequently, it is anticipated that gRNA sequences containing AAGGA3′ + NGG would decrease Cas9 selectivity and perhaps increase off-target reactions. The length of the gRNA’s target region is another crucial factor in increasing specificity. A shortened gRNA target region (17–18 nucleotides) was used in research that significantly enhanced specificity, probably as a result of inadequate gRNA loading in Cas9 (Fu et al. 2014). Other important strategies include gRNA scaffold engineering in silico, appending two additional guanine nucleotides to the 5′ end (just before the complementarity site), regulating Cas9–gRNA stability in cells, seed match genomic site accessibility, and epigenetic variables (Patel et al. 2019). A novel method for enhancing specificity included altering a single nuclease domain of Cas9, which has the ability to cleave a single strand of target DNA. Recognised as ‘nickase’, this mutant Cas9 causes a break in single-stranded DNA that is effectively repaired by the cells. When nickase is directed toward two adjacent areas of opposing DNA strands, a double-stranded break is formed, which then causes ‘Indels’ in the target site. Nickase can at times additionally cleave off-target sites via unknown processes (Shao et al. 2018). Double-stranded sequential cuts are only produced by the functioning RuvC-like endonuclease domain of the Cpf1 endonuclease, which has HNH site removed. The main drawback of this approach is that Cas9-induced nicks may, by unidentified processes, result in alterations in the off-target regions. Because of its numerous distinct qualities, Type V CRISPR endonuclease Cpf1 may be used as a substitute for Cas9 endonuclease. But Cpf1 has particular with PAM sequence specificity. According to reports, Cpf1 (LbCpf1) interacts with C-containing PAM sequences that are not at optimal levels, including TCCA, CCCA, or TCTA causing structural alterations that allow for a broad range of specificity that extends across TTTN (Yamano et al. 2017).
Applications of CRISPR/Cas9 in biofuel production
CRISPR/Cas9 editing in microalgae increases lipid content. It is a preferred tool for modifying genomes for biofuel production. Lignin pathways in plants hinder biofuel production by affecting sugar release. Key resources to boost microalgae bioengineering were discussed. Microalgal bioengineering includes enhancing photosynthesis, lipid production, and biomolecule production.
Optimising photosynthetic efficiency and biomass production
Enhanced photosynthetic efficiency is crucial for increasing microalgae biomass production, which is essential for utilising microalgae as a future feedstock. The process of carbon fixation relies on various factors, with the selectivity and speed of the Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo enzyme) being particularly important. RuBisCo can convert CO2 and O2 into 3-phosphoglycerate and 2-phosphoglycolate, respectively, with the latter being harmful to the cells. Occurring in the peroxisome and mitochondrion, photorespiration utilises 2-phosphoglycolate to release CO2, thereby hindering photosynthesis (Spreitzer et al. 2005). Though not entirely successful, attempts have been made to use genetic engineering to increase RuBisCo specificity and the rate of catalysis. Alternatively, adjusting the cultivation system design to enhance CO2 supply can help mitigate RuBisCo selectivity issues. However, genetically modifying RuBisCo is a more favourable approach than selecting efficient variants from nature to improve its catalytic rate. For instance, replacing the small subunit of Chlamydomonas RuBisCo with that of spinach (Spinacia oleracea), sunflower (Helianthus annuus), or Arabidopsis thaliana has been shown to enhance carboxylation efficiency and CO2/O2 specificity. Although this hybrid enzyme displayed a 3–11% rise in specificity, its velocity remained unchanged. The conserved region of the small submit of RuBiCo has many amino acid residues that have been identified as potential targets for enhancing its catalytic efficiency (Genkov et al. 2010). Another strategy involves regulating RuBisCo activity, as demonstrated by significantly increased photosynthetic biomass production in Nannochloropsis oceanica upon RuBisCo increased overexpression. In addition to RuBisCo, enzymes like sedoheptulose 1,7-bisphosphatase (SBPase), fructose−1,6-bisphosphatase (FBPase), and fructose 1,6-bisphosphate aldolase (FBA) in the Calvin cycle regeneration phase are crucial for manipulating photosynthetic activity (Wei et al. 2017). Recent studies have shown that overexpressing cyanobacterial FBA can boost the photosynthetic capacity of Chlorella vulgaris (Yang et al. 2017), while overexpression of Chlamydomonas SBPase enhances photosynthetic activity in Dunaliella bardawil (Fang et al. 2012). Although FBPase overexpression enhances photosynthetic efficiency in higher plants, it can have adverse effects on growth and photosynthesis in Chlamydomonas under increased CO2 conditions. This is primarily because of the increased conversion of fructose−1,6-bisphosphate to fructose−6-phosphate, resulting in reduced glyceraldehyde−3-phosphate levels (Dejtisakdi and Miller 2016). These findings suggest that targeting the FBPase-catalyzed reaction may not be the most effective method to improve the accumulation of biomass and photosynthetic efficacy of microalgae.
In contrast, light blocking often causes photo-limitation, which hinders the development of microalgae with elevated cell densities. The photosynthetic process is saturated by the high light at the cell surface layers, resulting in photoinhibition. Excess energy is released by non-photochemical quenching. The lower levels of light in the deeper cell layers compel them to engage in photorespiration rather than photosynthesis at the same time. In the end, less biomass is produced because to the inferior photosynthetic efficiency caused by this unequal dispersion of light. Reducing the antenna or light-harvesting complex size is one way to improve the transmission of light and capacity for absorption. For instance, in comparison to a chlorophyll b mutant, enhanced photosynthetic activity as well as growth rate were obtained by reducing chlorophyll b levels in Chlamydomonas by RNAi-mediated suppression of chlorophyllide a oxygenase (Perrine et al. 2012). Similarly, a C. vulgaris mutant produced by random mutagenesis of the chloroplast signal recognition particle (CpSRP43) showed improved photosynthetic efficiency with lower non-photochemical quenching and greater biomass revenue (Shin et al. 2017). The mutant also had reduced antenna size and lower levels of chlorophyll a and b. An alternative strategy included engineering the diatom Phaeodactylum tricornutum to perform intracellular spectrum recompositing in order to improve light absorption and increase the generation of biomass (Fu et al. 2017). In this instance, surplus blue light energy is absorbed by the excessively expressed fluorescent green protein and released as green light, which accessory pigments may pick up. As a result, by greater penetration of emitted green light, spectral recompositing increases light absorption decreases non-photochemical quenching, and may relieve photoinhibition in extremely dense cell cultures. Coral−algae symbionts have been shown to use a similar process to distribute available light energy equally to adapt to the light conditions in the deep sea (Smith et al. 2017). While genetic engineering offers valuable insights into photosynthetic efficiency, most findings are based on model algal systems and have yet to be applied on a large scale. Furthermore, the design of cultivation systems significantly impacts photosynthetic efficiency and productivity. Hence, a more comprehensive approach, such as adjusting the flux balance of the Calvin cycle for enhanced CO2 fixation or targeting multiple factors simultaneously for a synergistic effect is necessary.
Optimising and enhancing lipid production
Lipids derived from microalgae have garnered significant attention because of their high yield and noteworthy nutraceutical value. The quality, quantity, and specific types of lipids produced by microalgae contribute to diversifying their potential applications and also impact the characteristics of biodiesel if intended for fuel purposes (Shekh et al. 2016). For researchers engaged in this field, lipid productivity stands out as a crucial factor in selecting suitable strains. Indeed, the composition of lipids accumulated by microalgae significantly influences its commercial viability for applications in food, feed, or fuel. Throughout the years, efforts have been made to strike a balance between enhancing the lipid content of microalgae through multiple methods while maintaining lipid production levels high. Nevertheless, the genetic modification of robust strains to boost lipid production remains a promising avenue for enhancing the economic efficiency of the process. In recent times, research has focused on manipulating genes involved in lipid biosynthesis by either knocking them out or overexpressing them to assess their impact on lipid accumulation. An example of this is the overexpression of acetyl-CoA carboxylase (ACCase), the enzyme responsible for the production of fatty acid, which was initially attempted in 1996 (Dunahay et al. 1996). Despite observing a 2- to 3-fold rise in ACCase activity following overexpression, this did not translate into increased lipid accumulation. However, the simultaneous upregulation of ACCase and malic enzyme, which facilitates the conversion of malate to pyruvate, proved effective in enhancing lipid accumulation in Dunaliella salina (Talebi et al. 2014). Conversely, it is well-documented that a system’s metabolic flow may be impacted by transcriptional control, as transcription factors can target many regulatory sites within the metabolic pathway. Manipulating transcription factors to upregulate genes involved in lipid biosynthesis has shown promise in boosting lipid accumulation. For instance, the downregulation of a transcription regulator zinc and cysteine complexes (ZnCys) in N. gaditana caused the lipid content to rise twice (Ajjawi et al. 2017). Various approaches have been explored to enhance lipid yields by halting the breakdown of produced lipids. For example, a knock-out mutant of the phospholipase A2 gene in C. reinhardtii resulted in a rise in total lipid concentration of 64.25% (Shin et al. 2019). In a separate study, silencing the cht7 gene, which encodes a TAG lipase, resulted in a remarkable tenfold rise in TAG levels (Tsai et al. 2014). Most recently, the utilisation of CRISPR/Cas9-based technology for genetic editing in C. vulgaris involved the construction of a Cas9 fragment with sgRNA targeting the omega-3 fatty acid desaturase (fad3) gene, resulting in a substantial 46% (w/w) rise in lipid accumulation (Lin and Ng 2020).
Recent advances in CRISPR/Cas9 technology for optimising biofuel production pathways
CRISPR/Cas emerges as a key genome modification tool for various organisms. In 1987, the CRISPR locus was found in E. coli (Ishino et al. 2018), providing adaptive immunity against foreign DNA. It consists of repeated sequences with spacers transcribed into crRNAs that bind to Cas proteins, directing cleavage of target DNA. Cas nuclease creates DSB in genomic DNA for genome editing in eukaryotic cells, leading to knockout mutants. Zinc finger and TALEN endonucleases have also been used for genome editing, but CRISPR/Cas is popular due to guide RNAs providing accuracy and flexibility (Zhang et al. 2013). New Cas proteins with variable endonuclease activity are constantly being found, allowing for enhanced targeted genome editing. The most widely utilised nuclease for CRISPR/Cas-mediated genome modification is Cas9 from S. pyogenes. CRISPR/Cas tool is utilised for various applications like creating knockout mutants, gene integrations, and transcriptional regulations (Ishino et al. 2018). The technique is still in its early stages in microalgae contrary to other organisms. CRISPR-based multiplexed gene knockout and knock-in, as well as CRISPR interference or activation, have been explored for manipulating target genes and metabolic pathways in algal species to produce industrially significant biomolecules (Dhokane et al. 2023).
Chlamydomonas reinhardtii
C. reinhardtii represents an optimal ideal organism for genetic engineering because of its extensively characterised genome, simple cultivation methods, and the availability of a wide range of genetic tools for manipulation. The pioneering work in 2014 marked the initial successful utilisation of CRISPR/Cas9 in C. reinhardtii, showcasing the potential for precise gene editing in this particular organism (Jiang et al. 2014). Since then, a multitude of investigations have been carried out to enhance and refine the CRISPR/Cas9-mediated modification in C. reinhardtii. DNA-free RNA-guided synthetic nucleases (RNPs) were effectively utilised by producing efficient 0.56% and 0.46% knockouts of the CpFTSY and zeaxanthin epoxidase (ZEP) genes, respectively (Baek et al. 2016). They next eliminated the ZEP gene in C. reinhardtii strain CC-4349 that had a high carotenoid output, advancing this study. This led to a significant 56-fold rise in zeaxanthin levels without lowering lutein levels. Feeding this modified strain to chickens led to the production of fortified eggs with elevated lutein levels (2-fold) and zeaxanthin levels (2.2-fold) (Baek et al. 2018). The possibility of using vector-derived CRISPRi to suppress the CrPEPC1 gene and effectively reroute the carbon stream to increase lipid synthesis, which demonstrated how CRISPRi may be used to modify target gene expression in C. reinhardtii to improve desired phenotypes (Kao and Ng 2017). Furthermore, by disrupting the α-branch of the lycopene epsilon cyclase (LCYE) gene and blocking lutein synthesis through ZEP gene knockout, another study used RNP-mediated CRISPR/Cas9 technology to generate exceptionally pure zeaxanthin. This led to a 60% increase in zeaxanthin yield when compared to the parental strain. In a subsequent investigation, ZEP and the ADP-glucose pyrophosphorylase (AGP) genes were targeted to create a double mutant. This resulted in the accumulation of lutein (2.93 mg g−1), zeaxanthin (3.12 mg g−1), and lipids (450.09 mg g−1) in an N-deprived environment, and an 81% increase in oil output with enhanced macular pigment production (Song et al. 2020).
Researchers also disrupted key enzymes in the polyamine biosynthesis pathway to investigate putrescine accumulation, revealing the pivotal role of ornithine decarboxylase 1 (ODC1) as the limiting factor in putrescine build-up. Their findings indicated that augmenting ornithine decarboxylases and functionally knocking out amine oxidase 2 (AMX2) to prevent putrescine degradation resulted in a tenfold rise in cellular putrescine levels, yielding 200 mg L−1 (Freudenberg et al. 2022).
In conclusion, the ability to accurately and effectively modify the Chlamydomonas genome has greatly increased with the use of CRISPR/Cas genome editing technologies. These developments have enabled scientists to explore the functions of individual genes and create strains with specific traits for a wide range of uses.
Picochlorum spp.
Research into the genus Picochlorum has focused on the production of a variety of biomolecules such as pigments, lipids, bioactive compounds, and proteins. In 2020, a significant milestone was achieved as CRISPR/Cas9 technology was successfully employed in Picochlorum celeri to create knockout variants of two specific genes: (1) nitrate reductase; and (2) carotenoid isomerase. About 6% of the transformants aiming nitrate reductase and about 50% of the transformants aiming carotenoid isomerase had produced dysfunctional variants, as reported by Krishnan et al. (2020). This study offers valuable insights into the feasibility of CRISPR-based genome modification in Picochlorum spp., enabling the rapid generation of mutants, facilitating the elucidation of gene functions, and allowing precise manipulation of genes and metabolic pathways to develop resilient production strains.
Chlorella spp.
Chlorella spp. is abundant in lipids, rendering them a promising candidate for the synthesis of biofuels. They contain significant nutritional elements, such as high levels of essential fatty acids, minerals, vitamins, and protein. These microorganisms are extensively utilised as a nutritional supplement and functional component in food because of their promising health advantages, including support for the immune system, detoxification, and antioxidant characteristics. Nevertheless, the scarcity of genomic reservoirs for Chlorella and the limited efficacy of genetic modification pose major challenges to advancing the genetics of this genus (Dhokane et al. 2023).
The inaugural instance of the successful implementation of CRISPR-based genetic modification in Chlorella spp. was documented in 2020. In their investigation, a plasmid vector containing Cas9 and gene-specific gRNA for omega-3 fatty acid desaturase (fad3) was introduced into C. vulgaris strain FSP-E. The fad3 knockout mutants exhibited a lipid content 46% (w/w) higher than that of the wild-type. This analysis distinctly illustrated pertinent empirical data on the utilisation of CRISPR-based genetic editing in Chlorella spp. to generate resilient strains for many industrial uses (Lin and Ng 2020).
In a study by Lin et al. (2022), the CRISPRa/i system was employed to modulate gene expression in Chlorella sorokiniana strain UTEX 1602. The researchers evidenced that gene regulation through dCas9-VP64 (CRISPRa) augmented protein levels by around 60% (w/w), whereas regulation via dCas9-KRAB (CRISPRi) elevated protein content by 65%, with lipid accumulation ranging from 150 to 250 mg L−1 (wild-type, 150 mg L−1). This investigation notably introduces novel possibilities for adjusting the expression levels of target genes, thereby enhancing the yields of target molecules (Lin et al. 2022).
Other photosynthetic microorganisms modified for biofuel production
By using CRISPR/Cas9 to disrupt some genes in Parachlorella kessleri, particularly the AATPL1 gene, lipid productivity increased by more than 30% in comparison to the wild-type variant. This improvement is especially helpful for maximising lipid yields, which are necessary for the manufacturing of biodiesel (Kasai et al. 2024). Similar genetic changes have been made to Scenedesmus obliquus in an effort to increase its lipid content and developmental rates; more recently, research has concentrated on deleting genes related to competitive metabolic pathways in order to increase lipid accumulation and biomass productivity in general (Lakhawat et al. 2022). Moreover, Nannochloropsis spp., recognised for increased lipid content, has also been modified. Specifically, TAG levels have been raised, which is important for biodiesel applications (Dhokane et al. 2023). In the meantime, research is being done on the halophilic microalga D. salina (Dhokane et al. 2023), Synechocystis sp. strain PCC 6803 (Parsaeimehr et al. 2022), Isochrysis galbana, and Cylindrotheca fusiformis (Hassanien et al. 2023).
In summary, these developments show how CRISPR/Cas9 technology can be used to optimise biofuel production pathways in a variety of photosynthetic microorganisms, opening the door to more environmentally-friendly and successful biofuel sources.
Limitations, challenges, and future perspectives
There are still several challenges in genetically engineering cyanobacteria and microalgae for biofuel production. For example, the progress of genetic modification in microalgae, such as Chlorella, has been hampered by the absence of significant genomic resources (Yang et al. 2016). Tanwar et al. (2018) suggest that the poor effectiveness of transformation procedures might be attributed to the stiffness of the Chlorella cell wall. For instance, while adjusting settings during electroporation could increase cell permeability, it might also result in poor recovery after the process, which would reduce efficiency (Muñoz et al. 2018). Microalgae genetic alteration presents moral, societal, and ecological problems. To guarantee the ethical creation and distribution of GMOs, these issues needs to be addressed. This requires an understanding of the laws that govern the application of CRISPR technology (Dhokane et al. 2023). Scale-up and industrial use need a great deal of research attention from an economic perspective. Microalgal cells may be metabolically affected by high Cas9 expression levels, which would lower their rate of survival and proliferation. Because of this, Cas9 expression levels must be carefully regulated to avoid harming the host organism (Jing 2023). Continuous selection pressure is frequently necessary to keep GMOs in a stable state. Edited strains might not be able to surpass their wild-type strains without selective benefits, which could cause the desired features to gradually disappear. This is especially important in natural settings because engineered traits may be diluted or eliminated due to concurrence by unmodified variants (Feng et al. 2023). Strong DNA repair systems seen in microalgae and cyanobacteria can occasionally obstruct changes brought forth by CRISPR/Cas9. The longevity of genetic alterations over time may be impacted by these repair processes, which may unintentionally reverse edits or result in partial mutations (Patel et al. 2019).To create sustainable strains with improved features for industrial uses, these problems must be resolved.
Although microalgae and cyanobacteria could immediately transform carbon dioxide into chemicals, their concentrations were much lower than anticipated quantity in the modern industrial scenario. For example, the greatest amount of ethanol that cyanobacteria may produce is only 5.5 g L−1, but E. coli is capable of producing 10 g L−1(Lin et al. 2019). However, cell growth was reduced as a result of the interruption of the formation of glycogen, which also lowered glycogen use and increased carbon flow to the desired chemical production. In the future, it will be crucial to preserve or enhance the balance between the use of glycogen and the synthesis of more desired compounds (Gordon et al. 2016; Ni et al. 2021; Niu et al. 2021). Cyanobacteria has historically been a common host to produce chemicals and fix CO2 at the same time, through genetic modification. According to multi-omics research, any modified Cyanobacteria with exceptional characteristics increases the expression of proteins during photosynthesis. But according to some reports, (Wang et al. 2016; Kanno et al. 2017; Singh et al. 2022) native photosynthesis, in producing chemicals, is limited, relying solely on CO2 as a carbon source through the native RuBisCO pathway is not a viable option for producing chemicals and leads to decreased efficiency (Huang et al. 2016; Lin et al. 2017; Inam et al. 2024; Riaz et al. 2024). To manufacture compounds with excellent yields autotrophically, it is important to understand how to overcome the constraint of photosynthesis and raise the tolerance to CO2. A novel strategy may be to provide an alternative carbon fixation route in cyanobacteria, although this would still be difficult since the process limiting photosynthetic capacity is unclear. A lack of genetic data makes it difficult to apply the knowledge base from cyanobacterial genetic manipulation to eukaryotic microalgal gene editing. Double homologous recombination, for instance, is a popular method for cyanobacteria engineering. Based on homologous recombination, the same idea might be used for eukaryotic microalgae, such as in Chlamydomonas where the rbcS intron was successfully constructed, or in Chlorella where LB and RB were constructed. The inability of rbsc intron to introduce a gene in Chlorella revealed a significant divergence between the two species’ rbcS sequences. Therefore, research on cyanobacteria may also hasten the development of eukaryotic microalgae synthetic biology. A growing number of recently created genetic tools and techniques, such as pre-treatments such cell wall removal to increase transformation efficiency, have been published to enhance the conversion and expression mechanism (Lin et al. 2019). In a few years, it will be possible to create cyanobacteria or microalgae to make large quantities of biofuels while maintaining the same growth rate by combining multi-omics with sophisticated genome editing technologies.
Conclusion
The development of biofuels and bioenergy has attracted a lot of attention as a result of growing concerns about the finite supply of petroleum-based fuels and how they affect atmospheric CO2 levels. Researching biofuels must be commercially viable on a big scale in addition to identifying the best type of biomass to use for biofuel production. Researchers and companies are interested in cyanobacteria and microalgae because of many factors, including oxygen-dependent photosynthesis, excessive per-acre production, non-food-dependent feedstock, growth on ineffective and non-arable land, use of several kinds of sources of water (seawater, fresh, wastewater, and brackish), and the generation of high-value co-products in addition to biofuels. At the moment, bioethanol, biogas, biodiesel, and biohydrogen, are the biofuels that are most in demand globally. The review focused on the consequences of using the CRISPR/Cas9-driven gene editing technology to modify the genome of microalgae to produce biofuel more efficiently. Increased production of biomass has been obtained by CRISPR/Cas9-based gene knockdown and gene cassette insertion techniques. Microalgae and cyanobacteria modified using CRISPR/Cas9 have shown increased total lipid content, which is necessary for the synthesis of biofuel. However, an effective genetic engineering platform will be achieved through further advancements in genomes, bioinformatics, metabolomes, transcriptomes, proteomes, and multicombination of information analysis in microalgae and cyanobacteria species. This will lead to increased growth rates, improved carbon sequestration ability, more valuable substances, elevated carbon dioxide tolerance, and temperature resistance. In the future, this might aid in producing designer microalgae that are suitable for the industrial production of biofuel.
Author contributions
SA and MLQ carried out study under the supervision of NH helped in the collection of data. NH and QA helped in setting the sequence of article. NH, SH, DA and QA carried final revisions in the manuscript. All authors read and approved final version of manuscript.
Acknowledgements
This work was supported by the Key Laboratory of kiwifruit resources development and utilization of Guizhou Universities (Qian Jiaoji [2022] 054), Project of Liupanshui Normal University (No.LPSSYKYJJ201601; LPSSY2023XKTD09) and the Science and Technology project of Liupanshui City (Grant #52020-2020-0906).
References
Abbas A, Rehman A, Javed M (2021) Exploring the potential of in vitro tissue culture in breeding programs of legume and pulse crops: utilization and present condition. Bulletin of Biological and Allied Sciences Research 2021, 36.
| Crossref | Google Scholar |
Abbas A, Arshad A, Rehman A, Bukhari M, Zaman S (2024) Revolutionizing plant breeding programs with advancements in molecular marker-assisted selection. Bulletin of Biological and Allied Sciences Research 2024, 57.
| Crossref | Google Scholar |
Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, Kwok K, Peach L, Orchard E, Kalb R, Xu W, Carlson TJ, Francis K, Konigsfeld K, Bartalis J, Schultz A, Lambert W, Schwartz AS, Brown R, Moellering ER (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nature Biotechnology 35, 647-652.
| Crossref | Google Scholar | PubMed |
Alalwan HA, Alminshid AH, Aljaafari HAS (2019) Promising evolution of biofuel generations. Subject review. Renewable Energy Focus 28, 127-139.
| Crossref | Google Scholar |
Angermayr SA, Hellingwerf KJ, Lindblad P, Teixeira de Mattos MJ (2009) Energy biotechnology with cyanobacteria. Current Opinion in Biotechnology 20, 257-263.
| Crossref | Google Scholar | PubMed |
Azad AK, Rasul MG, Khan MMK, Sharma SC, Hazrat MA (2015) Prospect of biofuels as an alternative transport fuel in Australia. Renewable and Sustainable Energy Reviews 43, 331-351.
| Crossref | Google Scholar |
Baek K, Kim DH, Jeong J, Sim SJ, Melis A, Kim J-S, Jin E, Bae S (2016) DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Scientific Reports 6, 30620.
| Crossref | Google Scholar |
Baek K, Yu J, Jeong J, Sim SJ, Bae S, Jin E (2018) Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnology and Bioengineering 115, 719-728.
| Crossref | Google Scholar | PubMed |
Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S (2015) Scope of algae as third generation biofuels. Frontiers in Bioengineering and Biotechnology 2, 90.
| Crossref | Google Scholar |
Bernheim A, Calvo-Villamañán A, Basier C, Cui L, Rocha EPC, Touchon M, Bikard D (2017) Inhibition of NHEJ repair by type II-A CRISPR-Cas systems in bacteria. Nature Communications 8, 2094.
| Crossref | Google Scholar |
Bhatia SK, Kim S-H, Yoon J-J, Yang Y-H (2017) Current status and strategies for second generation biofuel production using microbial systems. Energy Conversion and Management 148, 1142-1156.
| Crossref | Google Scholar |
Bhujade R, Chidambaram M, Kumar A, Sapre A (2017) Algae to economically viable low-carbon-footprint oil. Annual Review of Chemical and Biomolecular Engineering 8, 335-357.
| Crossref | Google Scholar | PubMed |
Bryksin AV, Matsumura I (2010) Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. BioTechniques 48, 463-465.
| Crossref | Google Scholar | PubMed |
Callegari A, Bolognesi S, Cecconet D, Capodaglio AG (2020) Production technologies, current role, and future prospects of biofuels feedstocks: a state-of-the-art review. Critical Reviews in Environmental Science and Technology 50, 384-436.
| Crossref | Google Scholar |
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA (2017) Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 550, 407-410.
| Crossref | Google Scholar | PubMed |
Cheng JJ, Timilsina GR (2011) Status and barriers of advanced biofuel technologies: a review. Renewable Energy 36, 3541-3549.
| Crossref | Google Scholar |
Chisti Y (2007) Biodiesel from microalgae. Biotechnology Advances 25, 294-306.
| Crossref | Google Scholar | PubMed |
Correa DF, Beyer HL, Fargione JE, Hill JD, Possingham HP, Thomas-Hall SR, Schenk PM (2019) Towards the implementation of sustainable biofuel production systems. Renewable and Sustainable Energy Reviews 107, 250-263.
| Crossref | Google Scholar |
Dejtisakdi W, Miller SM (2016) Overexpression of Calvin cycle enzyme fructose 1,6-bisphosphatase in Chlamydomonas reinhardtii has a detrimental effect on growth. Algal Research 14, 116-126.
| Crossref | Google Scholar |
Demirbaş A (2006) Oily products from mosses and algae via pyrolysis. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 28, 933-940.
| Crossref | Google Scholar |
Demirbas A (2007) Recent developments in biodiesel fuels. International Journal of Green Energy 4, 15-26.
| Crossref | Google Scholar |
Demirbas A (2009) Political, economic and environmental impacts of biofuels: a review. Applied Energy 86, S108-S117.
| Crossref | Google Scholar |
Dhokane D, Shaikh A, Yadav A, Giri N, Bandyopadhyay A, Dasgupta S, Bhadra B (2023) CRISPR-based bioengineering in microalgae for production of industrially important biomolecules. Frontiers in Bioengineering and Biotechnology 11, 1267826.
| Crossref | Google Scholar |
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096.
| Crossref | Google Scholar |
Dunahay TG, Jarvis EE, Dais SS, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. Applied Biochemistry and Biotechnology 57, 223-231.
| Crossref | Google Scholar |
Falfushynska H (2024) Advancements and prospects in algal biofuel production: a comprehensive review. Phycology 4, 548-575.
| Crossref | Google Scholar |
Fang L, Lin HX, Low CS, Wu MH, Chow Y, Lee YK (2012) Expression of the Chlamydomonas reinhardtii Sedoheptulose-1,7-bisphosphatase in Dunaliella bardawil leads to enhanced photosynthesis and increased glycerol production. Plant Biotechnology Journal 10, 1129-1135.
| Crossref | Google Scholar | PubMed |
Fatima S, Cheema K, Shafiq M, Manzoor M, Ali Q, Haider M, Shahid M (2023) The genome-wide bioinformatics analysis of 1-aminocyclopropane-1-carboxylate synthase (acs), 1-aminocyclopropane-1-carboxylate oxidase (aco) and ethylene overproducer 1 (eto1) gene family of fragaria vesca (woodland strawberry). Bulletin of Biological and Allied Sciences Research 2023, 38.
| Crossref | Google Scholar |
Feng S, Xie X, Liu J, Li A, Wang Q, Guo D, Li S, Li Y, Wang Z, Guo T, Zhou J, Tang DYY, Show PL (2023) A potential paradigm in CRISPR/Cas systems delivery: at the crossroad of microalgal gene editing and algal-mediated nanoparticles. Journal of Nanobiotechnology 21, 370.
| Crossref | Google Scholar |
Fernandes BD, Mota A, Teixeira JA, Vicente AA (2015) Continuous cultivation of photosynthetic microorganisms: approaches, applications and future trends. Biotechnology Advances 33, 1228-1245.
| Crossref | Google Scholar | PubMed |
Freudenberg RA, Wittemeier L, Einhaus A, Baier T, Kruse O (2022) The spermidine synthase gene SPD1: a novel auxotrophic marker for Chlamydomonas reinhardtii designed by enhanced CRISPR/Cas9 gene editing. Cells 11, 837.
| Crossref | Google Scholar |
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnology 32, 279-284.
| Crossref | Google Scholar | PubMed |
Fu W, Chaiboonchoe A, Khraiwesh B, Sultana M, Jaiswal A, Jijakli K, Nelson DR, Al-Hrout A, Baig B, Amin A, Salehi-Ashtiani K (2017) Intracellular spectral recompositioning of light enhances algal photosynthetic efficiency. Science Advances 3, e1603096.
| Crossref | Google Scholar |
Gelfand I, Sahajpal R, Zhang X, Izaurralde RC, Gross KL, Robertson GP (2013) Sustainable bioenergy production from marginal lands in the US Midwest. Nature 493, 514-517.
| Crossref | Google Scholar | PubMed |
Genkov T, Meyer M, Griffiths H, Spreitzer RJ (2010) Functional hybrid rubisco enzymes with plant small subunits and algal large subunits: engineered rbcS cDNA for expression in chlamydomonas. Journal of Biological Chemistry 285, 19833-19841.
| Crossref | Google Scholar | PubMed |
Gordon GC, Korosh TC, Cameron JC, Markley AL, Begemann MB, Pfleger BF (2016) CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metabolic Engineering 38, 170-179.
| Crossref | Google Scholar | PubMed |
Greiner A, Kelterborn S, Evers H, Kreimer G, Sizova I, Hegemann P (2017) Targeting of photoreceptor genes in Chlamydomonas reinhardtii via zinc-finger nucleases and CRISPR/Cas9. The Plant Cell 29, 2498-2518.
| Crossref | Google Scholar | PubMed |
Gupta D, Bhattacharjee O, Mandal D, Sen MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, Chattopadhyay D, Ravichandiran V, Roy S, Ghosh D (2019) CRISPR-Cas9 system: a new-fangled dawn in gene editing. Life Sciences 232, 116636.
| Crossref | Google Scholar |
Hassanien A, Saadaoui I, Schipper K, Al-Marri S, Dalgamouni T, Aouida M, Saeed S, Al-Jabri HM (2023) Genetic engineering to enhance microalgal-based produced water treatment with emphasis on CRISPR/Cas9: a review. Frontiers in Bioengineering and Biotechnology 10, 1104914.
| Crossref | Google Scholar |
Heimann K (2016) Novel approaches to microalgal and cyanobacterial cultivation for bioenergy and biofuel production. Current Opinion in Biotechnology 38, 183-189.
| Crossref | Google Scholar | PubMed |
Henley WJ, Litaker RW, Novoveská L, Duke CS, Quemada HD, Sayre RT (2013) Initial risk assessment of genetically modified (GM) microalgae for commodity-scale biofuel cultivation. Algal Research 2, 66-77.
| Crossref | Google Scholar |
Higo A, Isu A, Fukaya Y, Ehira S, Hisabori T (2018) Application of CRISPR interference for metabolic engineering of the heterocyst-forming multicellular cyanobacterium Anabaena sp. PCC 7120. Plant and Cell Physiology 59, 119-127.
| Crossref | Google Scholar | PubMed |
Ho DP, Ngo HH, Guo W (2014) A mini review on renewable sources for biofuel. Bioresource Technology 169, 742-749.
| Crossref | Google Scholar | PubMed |
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology 31, 827-832.
| Crossref | Google Scholar | PubMed |
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262-1278.
| Crossref | Google Scholar | PubMed |
Hu J, Meng W, Su Y, Qian C, Fu W (2023) Emerging technologies for advancing microalgal photosynthesis and metabolism toward sustainable production. Frontiers in Marine Science 10, 1260709.
| Crossref | Google Scholar |
Huang C-H, Shen CR, Li H, Sung L-Y, Wu M-Y, Hu Y-C (2016) CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microbial Cell Factories 15, 196.
| Crossref | Google Scholar |
Inam S, Muhammad A, Irum S, Rehman N, Riaz A, Uzair M, Khan MR (2024) Genome editing for improvement of biotic and abiotic stress tolerance in cereals. Functional Plant Biology 51, FP24092.
| Crossref | Google Scholar |
Irfan MF, Mirara F (2024) Biochar application in improving soil health and sustainability. Bulletin of Biological and Allied Sciences Research 2024, 81.
| Crossref | Google Scholar |
Irfan U, Haider M, Shafiq M, Sami A, Ali Q (2023) Genome editing for early and late flowering in plants. Bulletin of Biological and Allied Sciences Research 2023, 45.
| Crossref | Google Scholar |
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. Journal of Bacteriology 169, 5429-5433.
| Crossref | Google Scholar | PubMed |
Ishino Y, Krupovic M, Forterre P (2018) History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. Journal of Bacteriology 200, e00580-17.
| Crossref | Google Scholar |
Javed M, Sami A, Haider M, Abbas A, Ali M, Naeem S, Amjad M, Ahmad A, Bostani R (2024) The contribution of transgenic rice to enhance grain yield. Bulletin of Biological and Allied Sciences Research 2024, 65.
| Crossref | Google Scholar |
Jeon S, Lim J-M, Lee H-G, Shin S-E, Kang NK, Park Y-I, Oh H-M, Jeong W-J, Jeong B-R, Chang YK (2017) Current status and perspectives of genome editing technology for microalgae. Biotechnology for Biofuels 10, 267.
| Crossref | Google Scholar |
Jiang WZ, Weeks DP (2017) A gene-within-a-gene Cas9/sgRNA hybrid construct enables gene editing and gene replacement strategies in Chlamydomonas reinhardtii. Algal Research 26, 474-480.
| Crossref | Google Scholar |
Jiang W, Brueggeman AJ, Horken KM, Plucinak TM, Weeks DP (2014) Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryotic Cell 13, 1465-1469.
| Crossref | Google Scholar | PubMed |
Jin C, Yao M, Liu H, Lee C-FF, Ji J (2011) Progress in the production and application of n-butanol as a biofuel. Renewable and Sustainable Energy Reviews 15, 4080-4106.
| Crossref | Google Scholar |
Junaid M, Gokce A (2024) Global agricultural losses and their causes. Bulletin of Biological and Allied Sciences Research 2024, 66.
| Crossref | Google Scholar |
Kanno M, Carroll AL, Atsumi S (2017) Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nature Communications 8, 14724.
| Crossref | Google Scholar |
Kao P-H, Ng I-S (2017) CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresource Technology 245, 1527-1537.
| Crossref | Google Scholar | PubMed |
Kasai Y, Takagi S, Ota S, Ishii K, Takeshita T, Kawano S, Harayama S (2024) Development of a CRISPR/Cas9-mediated gene-editing method to isolate a mutant of the unicellular green alga Parachlorella kessleri strain NIES-2152 with improved lipid productivity. Biotechnology for Biofuels and Bioproducts 17, 36.
| Crossref | Google Scholar |
Kayani REZ, Rustam A, Saleem U, Bilal M, Bakhtiyar M, Jamshaid S, Sohail M, Farooq K, Khan S, Rehman B (2025) Chlorophytum comosum-mediated iron nanoparticles: an eco-friendly approach for antimicrobial and dye degradation applications. Bulletin of Biological and Allied Sciences Research 2025, 94.
| Crossref | Google Scholar |
Khanna M, Wang W, Hudiburg TW, Delucia EH (2017) The social inefficiency of regulating indirect land use change due to biofuels. Nature Communications 8, 15513.
| Crossref | Google Scholar |
Koonin EV, Makarova KS, Zhang F (2017) Diversity, classification and evolution of CRISPR-Cas systems. Current Opinion in Microbiology 37, 67-78.
| Crossref | Google Scholar | PubMed |
Krishnan A, Cano M, Burch TA, Weissman JC, Posewitz MC (2020) Genome editing using Cas9-RNA ribonucleoprotein complexes in the high-productivity marine alga Picochlorum celeri. Algal Research 49, 101944.
| Crossref | Google Scholar |
Kumar S (2015) GM algae for biofuel production: biosafety and risk assessment. Collection of Biosafety Reviews 9, 52-75.
| Google Scholar |
Lakhawat SS, Malik N, Kumar V, Kumar S, Sharma PK (2022) Implications of CRISPR-Cas9 in developing next generation biofuel: a mini-review. Current Protein & Peptide Science 23, 574-584.
| Crossref | Google Scholar | PubMed |
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnology Progress 24, 815-820.
| Crossref | Google Scholar | PubMed |
Li H, Shen CR, Huang C-H, Sung L-Y, Wu M-Y, Hu Y-C (2016) CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metabolic Engineering 38, 293-302.
| Crossref | Google Scholar | PubMed |
Li-Beisson Y, Peltier G (2013) Third-generation biofuels: current and future research on microalgal lipid biotechnology. OCL 20, D606.
| Crossref | Google Scholar |
Lin W-R, Ng I-S (2020) Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzyme and Microbial Technology 133, 109458.
| Crossref | Google Scholar |
Lin T, Rodríguez LF, Shastri YN, Hansen AC, Ting KC (2014) Integrated strategic and tactical biomass–biofuel supply chain optimization. Bioresource Technology 156, 256-266.
| Crossref | Google Scholar | PubMed |
Lin P-C, Saha R, Zhang F, Pakrasi HB (2017) Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocysti s sp. PCC 6803. Scientific Reports 7, 17503.
| Crossref | Google Scholar |
Lin W-R, Tan S-I, Hsiang C-C, Sung P-K, Ng I-S (2019) Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresource Technology 291, 121932.
| Crossref | Google Scholar |
Lin J-Y, Lin W-R, Ng I-S (2022) CRISPRa/i with Adaptive Single Guide Assisted Regulation DNA (ASGARD) mediated control of Chlorella sorokiniana to enhance lipid and protein production. Biotechnology Journal 17, 2100514.
| Crossref | Google Scholar |
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJM, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV (2015) An updated evolutionary classification of CRISPR–Cas systems. Nature Reviews Microbiology 13, 722-736.
| Crossref | Google Scholar | PubMed |
Mallick N, Bagchi SK, Koley S, Singh AK (2016) Progress and challenges in microalgal biodiesel production. Frontiers in Microbiology 7, 1019.
| Crossref | Google Scholar |
Mazard S, Penesyan A, Ostrowski M, Paulsen IT, Egan S (2016) Tiny microbes with a big impact: the role of cyanobacteria and their metabolites in shaping our future. Marine Drugs 14, 97.
| Crossref | Google Scholar |
Mehdizadeh Allaf M, Peerhossaini H (2022) Cyanobacteria: model microorganisms and beyond. Microorganisms 10, 696.
| Crossref | Google Scholar |
Muñoz CF, de Jaeger L, Sturme MHJ, Lip KYF, Olijslager JWJ, Springer J, Wolbert EJH, Martens DE, Eggink G, Weusthuis RA, Wijffels RH (2018) Improved DNA/protein delivery in microalgae – a simple and reliable method for the prediction of optimal electroporation settings. Algal Research 33, 448-455.
| Crossref | Google Scholar |
Naduthodi MIS, Barbosa MJ, van der Oost J (2018) Progress of CRISPR-Cas based genome editing in photosynthetic microbes. Biotechnology Journal 13, 1700591.
| Crossref | Google Scholar |
Naeem S, Sami A, Haider M, Ali M, Khaliq A, Akram M, Mudasar M, Ali Q, Junaid M (2024) Heat stress in citrus: a molecular functional and biochemical perception. Bulletin of Biological and Allied Sciences Research 2024, 69.
| Crossref | Google Scholar |
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renewable and Sustainable Energy Reviews 14, 578-597.
| Crossref | Google Scholar |
Ni E, Deng L, Chen H, Lin J, Ruan J, Liu Z, Zhuang C, Zhou H (2021) OsCER1 regulates humidity-sensitive genic male sterility through very-long-chain (VLC) alkane metabolism of tryphine in rice. Functional Plant Biology 48, 461-468.
| Crossref | Google Scholar | PubMed |
Niu F, Jiang Q, Sun X, Hu Z, Wang L, Zhang H, Bozhkov P (2021) Large DNA fragment deletion in lncRNA77580 regulates neighboring gene expression in soybean (Glycine max). Functional Plant Biology 48, 1139-1147.
| Crossref | Google Scholar | PubMed |
Nowak CM, Lawson S, Zerez M, Bleris L (2016) Guide RNA engineering for versatile Cas9 functionality. Nucleic Acids Research 44, 9555-9564.
| Crossref | Google Scholar | PubMed |
Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M (2016) Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125-129.
| Crossref | Google Scholar | PubMed |
Parsaeimehr A, Ebirim RI, Ozbay G (2022) CRISPR-Cas technology a new era in genomic engineering. Biotechnology Reports 34, e00731.
| Crossref | Google Scholar |
Patel VK, Soni N, Prasad V, Sapre A, Dasgupta S, Bhadra B (2019) CRISPR-Cas9 system for genome engineering of photosynthetic microalgae. Molecular Biotechnology 61, 541-561.
| Crossref | Google Scholar | PubMed |
Perrine Z, Negi S, Sayre RT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Research 1, 134-142.
| Crossref | Google Scholar |
Plagens A, Richter H, Charpentier E, Randau L (2015) DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes. FEMS Microbiology Reviews 39, 442-463.
| Crossref | Google Scholar | PubMed |
Poliner E, Pulman JA, Zienkiewicz K, Childs K, Benning C, Farré EM (2018) A toolkit for Nannochloropsis oceanica CCMP 1779 enables gene stacking and genetic engineering of the eicosapentaenoic acid pathway for enhanced long-chain polyunsaturated fatty acid production. Plant Biotechnology Journal 16, 298-309.
| Crossref | Google Scholar | PubMed |
Quintana N, van der Kooy F, van de Rhee MD, Voshol GP, Verpoorte R (2011) Renewable energy from Cyanobacteria: energy production optimization by metabolic pathway engineering. Applied Microbiology and Biotechnology 91, 471-490.
| Crossref | Google Scholar | PubMed |
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nature Protocols 8, 2281-2308.
| Crossref | Google Scholar | PubMed |
Rasala BA, Muto M, Lee PA, Jager M, Cardoso RMF, Behnke CA, Kirk P, Hokanson CA, Crea R, Mendez M, Mayfield SP (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnology Journal 8, 719-733.
| Crossref | Google Scholar | PubMed |
Razzak SA, Hossain MM, Lucky RA, Bassi AS, de Lasa H (2013) Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing – a review. Renewable and Sustainable Energy Reviews 27, 622-653.
| Crossref | Google Scholar |
Renzaho AMN, Kamara JK, Toole M (2017) Biofuel production and its impact on food security in low and middle income countries: implications for the post-2015 sustainable development goals. Renewable and Sustainable Energy Reviews 78, 503-516.
| Crossref | Google Scholar |
Riaz A, Thomas J, Ali HH, Zaheer MS, Ahmad N, Pereira A (2024) High night temperature stress on rice (Oryza sativa)–insights from phenomics to physiology. A review. Functional Plant Biology 51, FP24057.
| Crossref | Google Scholar |
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology 32, 347-355.
| Crossref | Google Scholar | PubMed |
Saravanan AP, Mathimani T, Deviram G, Rajendran K, Pugazhendhi A (2018) Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. Journal of Cleaner Production 193, 734-747.
| Crossref | Google Scholar |
Sarsekeyeva F, Zayadan BK, Usserbaeva A, Bedbenov VS, Sinetova MA, Los DA (2015) Cyanofuels: biofuels from cyanobacteria. Reality and perspectives. Photosynthesis Research 125, 329-340.
| Crossref | Google Scholar | PubMed |
Schuergers N, Wilde A (2015) Appendages of the cyanobacterial cell. Life 5, 700-715.
| Crossref | Google Scholar | PubMed |
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84-87.
| Crossref | Google Scholar | PubMed |
Shao Y, Wang L, Guo N, Wang S, Yang L, Li Y, Wang M, Yin S, Han H, Zeng L, Zhang L, Hui L, Ding Q, Zhang J, Geng H, Liu M, Li D (2018) Cas9-nickase–mediated genome editing corrects hereditary tyrosinemia in rats. Journal of Biological Chemistry 293, 6883-6892.
| Crossref | Google Scholar | PubMed |
Sharma AK, Nymark M, Sparstad T, Bones AM, Winge P (2018) Transgene-free genome editing in marine algae by bacterial conjugation–comparison with biolistic CRISPR/Cas9 transformation. Scientific Reports 8, 14401.
| Crossref | Google Scholar |
Shekh AY, Shrivastava P, Gupta A, Krishnamurthi K, Devi SS, Mudliar SN (2016) Biomass and lipid enhancement in Chlorella sp. with emphasis on biodiesel quality assessment through detailed FAME signature. Bioresource Technology 201, 276-286.
| Crossref | Google Scholar | PubMed |
Shin S-E, Lim J-M, Koh HG, Kim EK, Kang NK, Jeon S, Kwon S, Shin W-S, Lee B, Hwangbo K, Kim J, Ye SH, Yun J-Y, Seo H, Oh H-M, Kim K-J, Kim J-S, Jeong W-J, Chang YK, Jeong B-R (2016) CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Scientific Reports 6, 27810.
| Crossref | Google Scholar |
Shin W-S, Lee B, Kang NK, Kim Y-U, Jeong W-J, Kwon J-H, Jeong B-R, Chang YK (2017) Complementation of a mutation in CpSRP43 causing partial truncation of light-harvesting chlorophyll antenna in Chlorella vulgaris. Scientific Reports 7, 17929.
| Crossref | Google Scholar |
Shin YS, Jeong J, Nguyen THT, Kim JYH, Jin E, Sim SJ (2019) Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresource Technology 271, 368-374.
| Crossref | Google Scholar | PubMed |
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harbor Perspectives in Biology 2, a000414.
| Crossref | Google Scholar |
Singh G, Sharma S, Rawat S, Sharma RK (2022) Plant Specialised Glycosides (PSGs): their biosynthetic enzymatic machinery, physiological functions and commercial potential. Functional Plant Biology 49, 1009-1028.
| Crossref | Google Scholar | PubMed |
Smith EG, D’Angelo C, Sharon Y, Tchernov D, Wiedenmann J (2017) Acclimatization of symbiotic corals to mesophotic light environments through wavelength transformation by fluorescent protein pigments. Proceedings of the Royal Society B: Biological Sciences 284, 20170320.
| Crossref | Google Scholar |
Song I, Kim J, Baek K, Choi Y, Shin B, Jin E (2020) The generation of metabolic changes for the production of high-purity zeaxanthin mediated by CRISPR-Cas9 in Chlamydomonas reinhardtii. Microbial Cell Factories 19, 220.
| Crossref | Google Scholar |
Spreitzer RJ, Peddi SR, Satagopan S (2005) Phylogenetic engineering at an interface between large and small subunits imparts land-plant kinetic properties to algal Rubisco. Proceedings of the National Academy of Sciences 102, 17225-17230.
| Crossref | Google Scholar |
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA (2014) DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Biophysical Journal 106, 695A.
| Crossref | Google Scholar |
Talebi AF, Tohidfar M, Bagheri A, Lyon SR, Salehi-Ashtiani K, Tabatabaei M (2014) Manipulation of carbon flux into fatty acid biosynthesis pathway in Dunaliella salina using AccD and ME genes to enhance lipid content and to improve produced biodiesel quality. Biofuel Research Journal 1, 91-97.
| Crossref | Google Scholar |
Tanveer M, Abidin ZU, Alawadi HFN, Shahzad AN, Mahmood A, Khan BA, Qari S, Oraby HF (2024) Recent advances in genome editing strategies for balancing growth and defence in sugarcane (Saccharum officinarum). Functional Plant Biology 51, FP24036.
| Crossref | Google Scholar |
Tanwar A, Sharma S, Kumar S (2018) Targeted genome editing in algae using CRISPR/Cas9. Indian Journal of Plant Physiology 23, 653-669.
| Crossref | Google Scholar |
Tsai C-H, Warakanont J, Takeuchi T, Sears BB, Moellering ER, Benning C (2014) The protein compromised hydrolysis of triacylglycerols 7 (CHT7) acts as a repressor of cellular quiescence in chlamydomonas. Proceedings of the National Academy of Sciences 111, 15833-15838.
| Crossref | Google Scholar |
Vassilev SV, Vassileva CG (2016) Composition, properties and challenges of algae biomass for biofuel application: an overview. Fuel 181, 1-33.
| Crossref | Google Scholar |
Voloshin RA, Kreslavski VD, Zharmukhamedov SK, Bedbenov VS, Ramakrishna S, Allakhverdiev SI (2015) Photoelectrochemical cells based on photosynthetic systems: a review. Biofuel Research Journal 2, 227-235.
| Crossref | Google Scholar |
Wang B, Wang J, Zhang W, Meldrum DR (2012) Application of synthetic biology in cyanobacteria and algae. Frontiers in Microbiology 3, 344.
| Crossref | Google Scholar |
Wang T, Wei JJ, Sabatini DM, Lander ES (2014) Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80-84.
| Crossref | Google Scholar | PubMed |
Wang Q, Lu Y, Xin Y, Wei L, Huang S, Xu J (2016) Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. The Plant Journal 88, 1071-1081.
| Crossref | Google Scholar | PubMed |
Wang N, Qian Z, Luo M, Fan S, Zhang X, Zhang L (2018) Identification of salt stress responding genes using transcriptome analysis in green alga Chlamydomonas reinhardtii. International Journal of Molecular Sciences 19, 3359.
| Crossref | Google Scholar |
Wang M, Ye X, Bi H, Shen Z (2024) Microalgae biofuels: illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnology for Biofuels and Bioproducts 17, 10.
| Crossref | Google Scholar |
Wei L, Wang Q, Xin Y, Lu Y, Xu J (2017) Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Research 27, 366-375.
| Crossref | Google Scholar |
Wendt KE, Ungerer J, Cobb RE, Zhao H, Pakrasi HB (2016) CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microbial Cell Factories 15, 115.
| Crossref | Google Scholar |
Wu X, Kriz AJ, Sharp PA (2014) Target specificity of the CRISPR-Cas9 system. Quantitative Biology 2, 59-70.
| Crossref | Google Scholar | PubMed |
Yamano T, Zetsche B, Ishitani R, Zhang F, Nishimasu H, Nureki O (2017) Structural basis for the canonical and non-canonical PAM recognition by CRISPR-Cpf1. Molecular Cell 67, 633-645.e3.
| Crossref | Google Scholar | PubMed |
Yang B, Liu J, Jiang Y, Chen F (2016) Chlorella species as hosts for genetic engineering and expression of heterologous proteins: progress, challenge and perspective. Biotechnology Journal 11, 1244-1261.
| Crossref | Google Scholar | PubMed |
Yang B, Liu J, Ma X, Guo B, Liu B, Wu T, Jiang Y, Chen F (2017) Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnology for Biofuels 10, 229.
| Crossref | Google Scholar |
Yao L, Cengic I, Anfelt J, Hudson EP (2016) Multiple gene repression in cyanobacteria using CRISPRi. ACS Synthetic Biology 5, 207-212.
| Crossref | Google Scholar | PubMed |
Yoshimitsu Y, Abe J, Harayama S (2018) Cas9-guide RNA ribonucleoprotein-induced genome editing in the industrial green alga Coccomyxa sp. strain KJ. Biotechnology for Biofuels 11, 326.
| Crossref | Google Scholar |
Zaboikin M, Zaboikina T, Freter C, Srinivasakumar N (2017) Non-homologous end joining and homology directed DNA repair frequency of double-stranded breaks introduced by genome editing reagents. PLoS ONE 12, e0169931.
| Crossref | Google Scholar |
Zakar T, Laczko-Dobos H, Toth TN, Gombos Z (2016) Carotenoids assist in cyanobacterial photosystem II assembly and function. Frontiers in Plant Science 7, 173308.
| Crossref | Google Scholar |
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759-771.
| Crossref | Google Scholar | PubMed |
Zhang Y, Zhang F, Li X, Baller JA, Qi Y, Starker CG, Bogdanove AJ, Voytas DF (2013) Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiology 161, 20-27.
| Crossref | Google Scholar | PubMed |
Zhu Z, Sun J, Fa Y, Liu X, Lindblad P (2022) Enhancing microalgal lipid accumulation for biofuel production. Frontiers in Microbiology 13, 1024441.
| Crossref | Google Scholar |