Saltbush seedlings (Atriplex spp.) shed border-like cells from closed-type root apical meristems
Alison R. Gill A and Rachel A. Burton
A and Rachel A. Burton  A *
A *
A
Abstract
Australian saltbush (Atriplex spp.) survive in exceptionally saline environments and are often used for pasture in semi-arid areas. To investigate the impact of salinity on saltbush root morphology and root exudates, three Australian native saltbush species (Atriplex nummularia, Atriplex amnicola, and Atriplex vesicaria) were grown in vitro in optimised sterile, semi-hydroponic systems in media supplemented with different concentrations of salt (NaCl). Histological stains and chromatographic techniques were used to characterise the root apical meristem (RAM) type and root exudate composition of the saltbush seedlings. We report that saltbush species have closed-type RAMs, which release border-like cells (BLCs). Monosaccharide content, including glucose and fructose, in the root mucilage of saltbush was found to be uniquely low, suggesting that saltbush may minimise carbon release in polysaccharides of root exudates. Root mucilage also contained notable levels of salt, plus increasing levels of unidentified compounds at peak salinity. Un-esterified homogalacturonan, xyloglucan, and arabinogalactan proteins between and on the surface of BLCs may aid intercellular adhesion. At the highest salinity levels, root cap morphology was altered but root:shoot ratio remained consistent. While questions remain about the identity of some components in saltbush root mucilage other than the key monosaccharides, this new information about root cap morphology and cell surface polysaccharides provides avenues for future research.
Keywords: Atriplex spp., border-like cells, homogalacturonan, monosaccharides, root apical meristem, root exudates, root morphology, root mucilage, salinity, Saltbush.
Introduction
The root cap plays a key role in root protection and growth (Kumpf and Nowack 2015; Ropitaux et al. 2019), wherein cells undergo changes as they move from their origin in the root apical meristem (RAM) to the cap periphery. When the cells reach the periphery, they separate from each other and from the root cap, and are exuviated into the rhizosphere as border or border-like cells (BLCs) (Driouich et al. 2007; Mravec 2017). Border cells, such as those found in pea (Pisum sativum) and maize (Zea mays), are immediately released into solution as single cells when the root tip is immersed in water, while BLCs are shed as organised layers that remain attached together after immersion (Driouich et al. 2010; Ropitaux et al. 2019; Kumar and Iyer-Pascuzzi 2020). The RAM, which is the zone of dividing cells that produces all cells in the root, can have different patterns of cell organisation (Kumar and Iyer-Pascuzzi 2020). The closed-type RAM is characterised by clonally distinct tiers of cells that can be traced back to their origin, while open-type RAMs are less organised, with cells originating from a large zone without clonally distinct tiers (Groot et al. 2004; Hamamoto et al. 2006). While border cells and BLCs both originate from the RAM, once released from the root tip into the rhizosphere border cells are single, non-attached cells while BLCs remain attached to each other (Ropitaux et al. 2019).
Both border cells and BLCs secrete mucilage, which is an extruded water-soluble gel that aids in root lubrication, pathogen defence, nutrient acquisition, attraction of beneficial microbes and abiotic stress tolerance, including against drought, heat and salinity stresses (Knee et al. 2001; Badri and Vivanco 2009; Baetz and Martinoia 2014; Kawasaki et al. 2018; Sasse et al. 2018; Driouich et al. 2021). Mucilage is thought to play a role in plant tolerance to salinity stress by regulating water ascent and storage, exchanging cations, and sodium fixation (Ghanem et al. 2010; Nazari 2021; Ninmanont et al. 2021).
Mucilage is comprised of both high molecular weight components, such as polysaccharides, proteins, phenolic acids, lipids, and extracellular DNA, and low molecular weight components including organic acids, amino acids, and nucleic acids (Oburger and Jones 2018; Driouich et al. 2021). While the composition of root mucilage differs between plant species and phenological stage, common monosaccharides include arabinose, fucose, galactose, glucose, mannose, rhamnose, and xylose (Knee et al. 2001). These sugars have been found in the root mucilage of pea (P. sativum), cowpea (Vigna unguiculate), wheat (Triticum aestivum), maize (Z. mays), and rice (Oryza sativa) (Bacic et al. 1986; Moody et al. 1988; Knee et al. 2001), and can be measured using chromatographic techniques. In addition, pectins present in root mucilage are believed to play a role in BLC adhesion and may contribute to salinity stress tolerance, thus warranting investigation in species that can tolerate high levels of salt (Durand et al. 2009; Hayashi and Kaida 2011; Tenhaken 2015; Lutts et al. 2016). Microscopy, including immunofluorescence techniques, can be used to visualise the location of polysaccharides present in and around the root cap and its component cells.
As plants release up to 40% of their net fixed carbon into the rhizosphere as sloughed off cells and mucilage, root exudates comprise a significant energy sink (Hawes et al. 2016). For this reason, there is increasing interest in the impact root exudates have on the rhizosphere. However, research on the function and composition of root exudates has been limited due to the methodological difficulties in obtaining sufficient amounts for analysis. While exudates can be collected directly from the rhizosphere, these samples are likely to be altered due to microbial decomposition and growth, soil sorption, and contamination (Kawasaki et al. 2018; Oburger and Jones 2018). To limit microbial contamination, seeds may be germinated on filter paper or agar, but this method does not simulate real soil-like mechanical forces (Knox et al. 2020). Sterile, semi-hydroponic growth systems that use a relevant matrix like glass beads, although still not perfectly mimicking conditions in real soil environments, are therefore the preferred option for studying root exudates in vitro as they provide mechanical impedance while avoiding complications with microbial interactions. Entirely hydroponic systems are ineffective since mucilage is soluble, making it extremely difficult to collect or study in situ. To collect root exudates, root tips are commonly suspended in sterile water, followed by processing of the liquid that contains the solubilised exudates (Moody et al. 1988; Knee et al. 2001; Kawasaki et al. 2016). Novel methods, including spinning trays of germinated seeds at low centrifugal forces, have also been developed but are not applicable for all seed sizes and types (Zickenrott et al. 2016). In the past, the methodological difficulties involved in producing and collecting root exudates have prevented major progress in root exudation research.
Saltbush (Atriplex spp.) are a group of native Australian halophytic shrubs that release root mucilage into the rhizosphere and are well-suited to survival in highly saline, semi-arid regions of Australia. Saltbush species have been successfully established as valuable and nutritious sources of pasture for sheep and cattle (Descheemaeker et al. 2014; Norman et al. 2017). Old man saltbush (Atriplex nummularia) is the most common forage species due to its very high salinity and drought tolerance while river saltbush (Atriplex amnicola) has a high tolerance to waterlogging, and bladder saltbush (Atriplex vesicaria) is the most naturally ubiquitous species. Currently, there is little known about saltbush root morphology and RAM organisation, as well as root function in salinity tolerance. As root exudates increase plant tolerance to abiotic stresses, it is proposed that they may assist saltbush survival in highly saline environments, up to 350 mM NaCl (Liddicoat and McFarlane 2007), by regulating water ascent and ion transport.
The overall objective of this work was to characterise the root morphology and root exudates of seedlings of three Australian saltbush species (A. nummularia, A. amnicola, and A. vesicaria) under different salinity levels using an optimised semi-hydroponic system. The specific aims were to firstly characterise the RAM type of saltbush, including defining the type of border cells. The second aim was to determine the monosaccharides and polysaccharides present in saltbush root exudates and finally to understand how salinity impacts root growth, root morphology, and root exudation in saltbush seedlings.
Materials and methods
Liquid growth media
A half strength Hoagland Modified Basal Salt solution (815 mg L−1, pH 5.4, Phyto Technology Laboratories, USA) buffered with 2 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.0, Glentham Life Sciences, UK) was prepared. Salinity treatments were formulated by the addition of sodium chloride (NaCl; Chem-Supply, Australia) to final salt concentrations of 12, 70, 120, 230 and 310 mM NaCl, which encompasses the range where saltbush has been found to survive (Liddicoat and McFarlane 2007; Nedjimi 2014).
Plant growth
Seeds of three saltbush species (Atriplex nummularia L., Atriplex amnicola W., and Atriplex vesicaria Heward ex. Benth) (Succession Ecology, South Australia; Nindenthana Seeds, Western Australia) were surface sterilised in 4% (v/v) bleach, 95.9% (v/v) ethanol and 0.1% (v/v) Triton for 10 min with gentle shaking. Sterilised seeds were germinated under aseptic conditions in a sterile, semi-hydroponic system, which consisted of a plastic tissue culture container (46 × 92 mm) (ThermoFisher, Australia) filled with 1 mm glass beads (BioSpec Products, USA) up to 25 mm depth. Aluminium foil was placed around the bottom of the container (also to a depth of 25 mm), blocking most light to the glass beads to mimic soil conditions. The glass beads were saturated with 4 mL of liquid growth media. Seeds were placed on the bed of glass beads (15 seeds per container) and four containers for each species were incubated in a growth cabinet for 7 days (day/night cycle of 25°C/15°C, 16 h/8 h; light intensity of 410 μmol m−2 s−1). As seeds were placed on top of the glass beads for germination, shoots were exposed to light while roots grew into the dark, glass bead substrate (see Supplementary Fig. S1). To ensure that root elongation was not negatively affected by the growth method chosen (i.e. the saturated glass bead system), root length in glass beads was compared to root length on filter paper in Petri dishes, both with 4 mL liquid growth media. There were no significant differences in root length of the same species between the growth systems (data not shown).
Root morphology
At 7 days after sowing, seedlings were scanned in 2D using an EPSON Expression 10000 XL scanner with WinRhizo programming. Root length and total seedling length were estimated using ImageJ. Root:shoot ratio was calculated.
Seeds for whole mount microscopy were prepared as described above but were sown on 10 cm round Petri dishes with one filter paper and 4 mL liquid growth media. Roots were mounted on glass slides in a 0.001% solution of Calcofluor White 2MR (CFW) (1 g L−1) with Evan’s Blue (0.5 g L−1) stain (Fluka Analytical, USA) or a solution with a 50:50 mixture of 0.01% ruthenium red and 0.005% crystal violet stains (Sigma-Aldrich, USA). Images were captured on a Zeiss Axio Imager M2 (Germany) with attached Zeiss AxioCam MR R3 camera at 5× magnification.
Roots grown on glass beads for 7 days were fixed in 4% formaldehyde, 0.25% glutaraldehyde and 4% sucrose in 1× phosphate-buffered saline (PBS) for 2 h, washed in 1× PBS and cleared in ClearSee for 3 weeks (Ursache et al. 2018). Roots were stained for 18 h with 0.1% Direct Red 23 (Sigma-Aldrich, USA) in ClearSee according to the protocol by Ursache et al. (2018) and imaged using a Nikon A1 confocal laser-scanning microscope (USA) with the 561 nm (Direct Red) laser line.
Immunofluorescence labelling was conducted as described by Durand et al. (2009), with roots fixed as above. Anti-pectin monoclonal antibodies (mAbs) used were LM19 (Plant Probes, UK), which recognises un-esterified homogalacturonan and LM20 (Plant Probes, UK), which recognises esterified homogalacturonan (Verhertbruggen et al. 2009). LM15 (Plant Probes, UK) and JIM8 (Plant Probes, UK) were also used to recognise xyloglucan and arabinogalactan proteins (AGPs) respectively. Roots were examined using a Nikon A1 confocal laser-scanning microscope (USA) using the 488 nm (immunofluorescence labelling) and the 561 nm (Direct Red) laser lines. Control experiments were performed by omission of the primary antibody. An average of 3–5 root apices were examined for each treatment.
Root exudation
After 7 days, growth liquid was removed from the glass beads in the four containers using a pipette and combined into a bulk sample for each treatment. Glass beads were washed twice with 25 mL Milli-Q water (mqH2O) to further extract residual root exudates. Both washes were combined with the bulk sample for each treatment. The extracts were filtered through a Grenier 40 μm EASYstrainer, stored at −20°C, and freeze-dried to a constant weight following the method described by Phan et al. (2016). Extracts were rehydrated in mqH2O to 5 mg mL−1 and heated at 60°C for 2 h. The soluble and insoluble fractions were separated, frozen at −20°C, and freeze-dried to obtain weights for both fractions.
The insoluble fractions were weighed and then rehydrated in mqH2O water on glass slides, stained with 0.1% Direct Red 23 (Sigma-Aldrich, USA) and imaged using a Zeiss Axio Imager M2 (Germany) with attached Zeiss AxioCam MR R3 camera. After visualisation and identification of cells, this fraction was not analysed further.
The soluble fractions were rehydrated to 5 mg mL−1, aliquoted into technical replicates, and frozen at −20°C for compositional analyses as follows. Na+ concentrations in the water-soluble fractions were measured using a flame photometer (Sherwood, model 420, UK).
Samples were diluted in water (1/100) and separated using high performance anion exchange chromatography with a pulsed amperometric detector (HPAEC–PAD) on a Dionex ICS-5000 as per Lim (2018). Standards used included maltose in the concentrations described in Lim (2018). Three technical replicates were analysed for each treatment.
Monosaccharide profiles were also determined using reverse phase high performance liquid chromatography (RP-HPLC) as described by Burton et al. (2011), with modifications. Aliquots of 800 μL 5 mg mL−1 root exudates dissolved in water were hydrolysed with 200 μL 5 M sulphuric acid at 100°C for 3 h and 1/5 dilutions prepared. The internal standard was 0.1 mM 2-deoxy glucose. External standards were included as follows: glucose at 8, 40, and 200 μM; mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, galactose, and fucose all at 1.6, 8, and 40 μM. Chromatographic analysis of these PMP-derivatives was carried out on an Agilent 1200 LC using Phenomenex Kinetex (with a C18 2.6 μm 3 × 100 mm 100A column) with UV detection at 250 nm. Modifications to the run gradient were eluents: (A) 10% acetonitrile in 40 mM ammonium acetate; (B) 70% acetonitrile; and (C) 40 mM acetic acid. The gradient ran at 92% A and 8% B for 9.5 min, 83% A and 17% B for 0.5 min, 90% B and 10% C for 1.5 min, 92% A and 8% B for 3 min, with a total run time of 14.5 min at a constant flow rate of 0.8 mL min−1. Three technical replicates were analysed for each treatment.
Statistical analysis
We tested the effect of salinity concentration (12, 70, 120, 230 and 310 mM NaCl), saltbush species (A. nummularia, A. amnicola, and A. vesicaria), and their interaction on root morphology and root exudate traits using two-way ANOVAs with Tukey’s multiple comparisons test using GraphPad Prism (ver. 9). Graphs were plotted in GraphPad Prism (ver. 9).
Results
Root apical meristem organisation
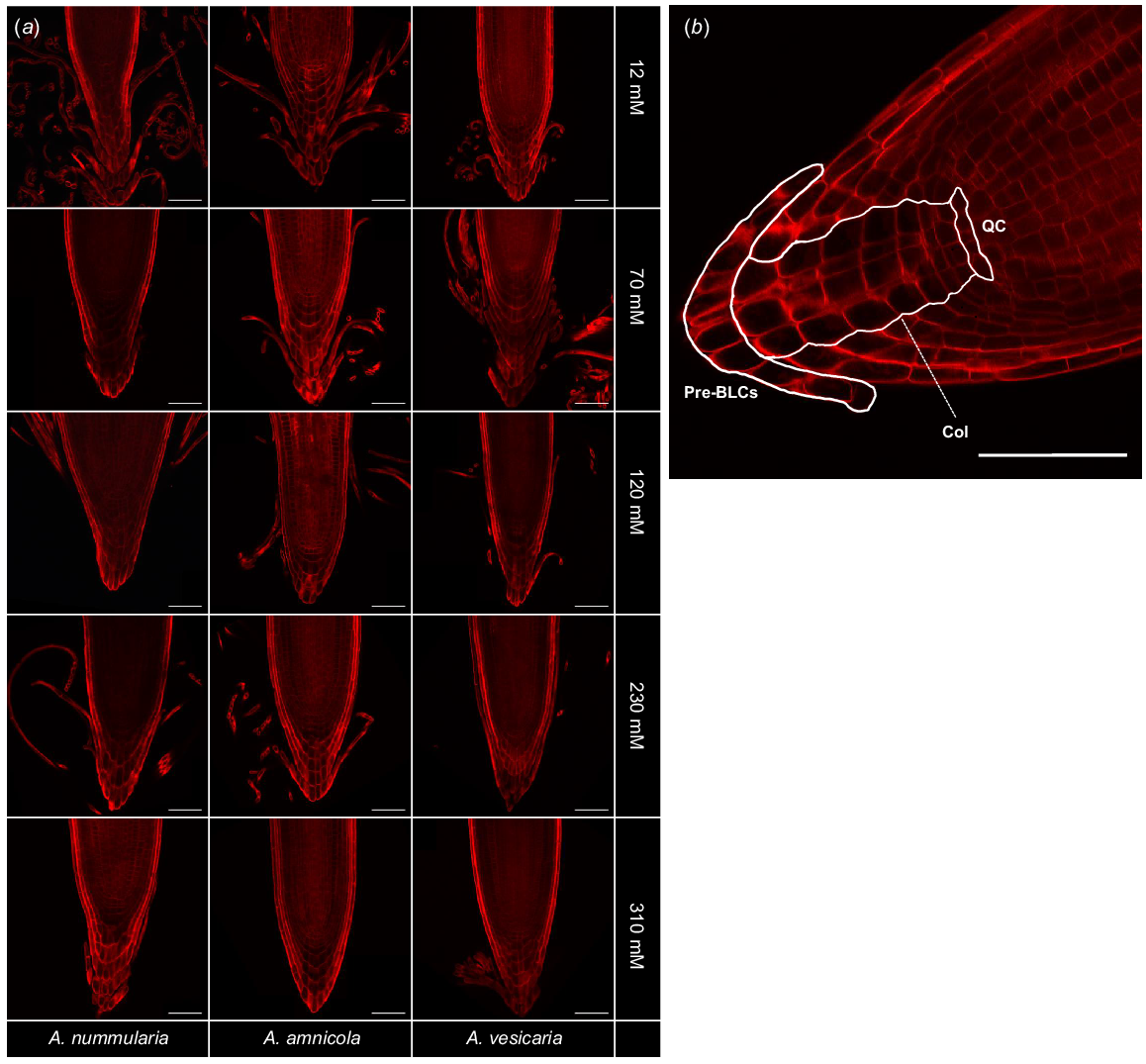
To characterise the RAM type of the saltbush species, root caps were probed with Direct Red 23, a fluorescent dye that is highly specific for crystalline cellulose microfibrils (Ropitaux et al. 2019). Direct Red 23 strongly stained saltbush cell walls but did not stain the surrounding mucilage. Cell lineages in the RAM were distinct and organised, indicating that saltbush have closed-type RAMs (Fig. 1). Saltbush released long, thin strands of cells that did not separate from each other after being released from the root surface and formed sheets at the end of the root tip, indicative of BLCs.
(a) Fluorescence microscopy of root caps of three species of saltbush (Atriplex nummularia, A. amnicola, A. vesicaria) grown in vitro at five salinity concentrations (12, 70, 120, 230, and 310 mM NaCl). Staining with Direct Red 23 reveals saltbush have border-like cells (BLCs). Images are representative of n = 3–5 replicates. (b) Representative image of A. amnicola grown at 12 mM NaCl shows that saltbush have closed-type root apical meristems with distinct cell lineages. Pre-BLCs, cells that will become border-like cells once detached from the root cap; Col, columella cells; QC, position of presumptive quiescent centre. Scale bars = 100 μm.
Root exudate quantification
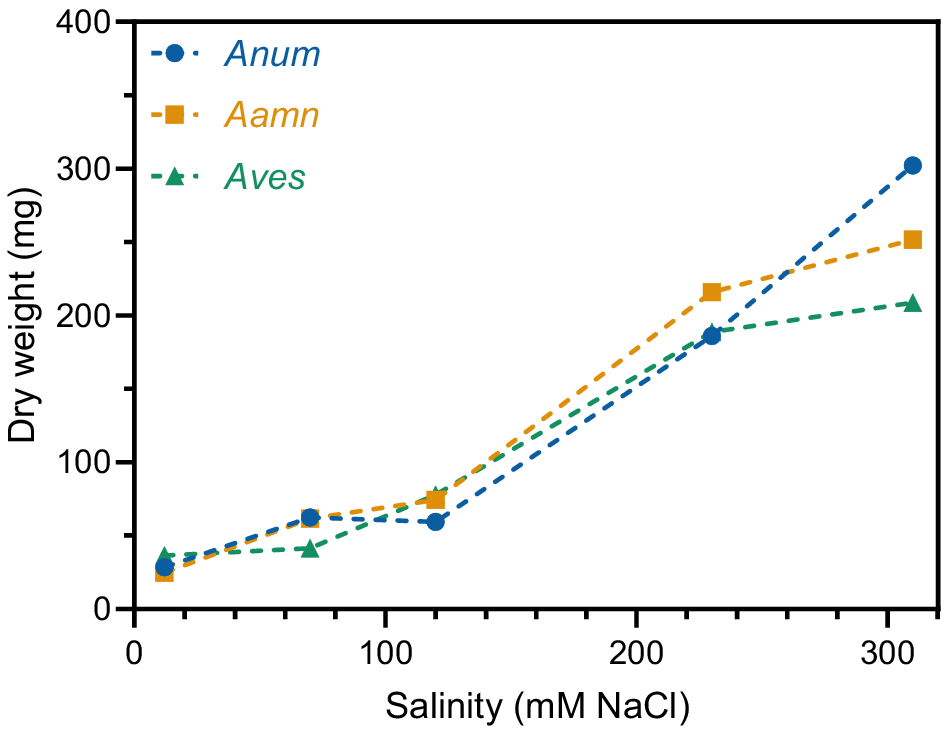
Material from the four tissue culture containers for each treatment were combined into a bulk sample. The total dry weight of root exudates increased as salinity concentration increased in all saltbush species. At 12 mM NaCl, A. nummularia, A. amnicola, and A. vesicaria had very low total root exudate yields of 28.5 mg, 24.8 mg, and 36.4 mg, respectively (Fig. 2). This increased up to 59.5 mg, 74.4 mg, and 77.8 mg for A. nummularia, A. amnicola, and A. vesicaria, respectively, at 120 mM NaCl. The dry weight of root exudates was numerically greatest at 310 mM NaCl for all three species of saltbush, with A. nummularia showing the greatest increase to 302.3 mg (Fig. 2).
Total dry weight of root exudates in three saltbush species at five salinity concentrations (12, 70, 120, 230, and 310 mM NaCl). Root exudates from each treatment (four tissue culture containers) were pooled into a bulk sample, so statistical analysis is not presented. Anum, Atriplex nummularia; Aamn, Atriplex amnicola; Aves, Atriplex vesicaria.
Root exudate analysis for monosaccharide identification
To define the different components of root exudates produced by saltbush, water-soluble and water-insoluble fractions were separated, freeze-dried, and weighed. The water-soluble fraction of root exudates, referred to hereafter as root mucilage, increased from the lowest salinity concentrations up to a maximum at 230 mM NaCl (Fig. 3a). The water-soluble fraction comprised 51 to 60% of the total weight for the saltbush species at 12 mM NaCl. This increased with salinity levels such that the water-soluble fraction was 95 to 99% at 230 mM NaCl and 90 to 93% at 310 mM NaCl (Fig. 3a).
Comparison of the (a) water-insoluble to water-soluble (root mucilage) fractions of root exudates and (b) the relative quantities of salt, monosaccharides and unknown compounds in the root mucilage of saltbush at five salinity concentrations. Anum, Atriplex nummularia; Aamn, Atriplex amnicola; Aves, Atriplex vesicaria.
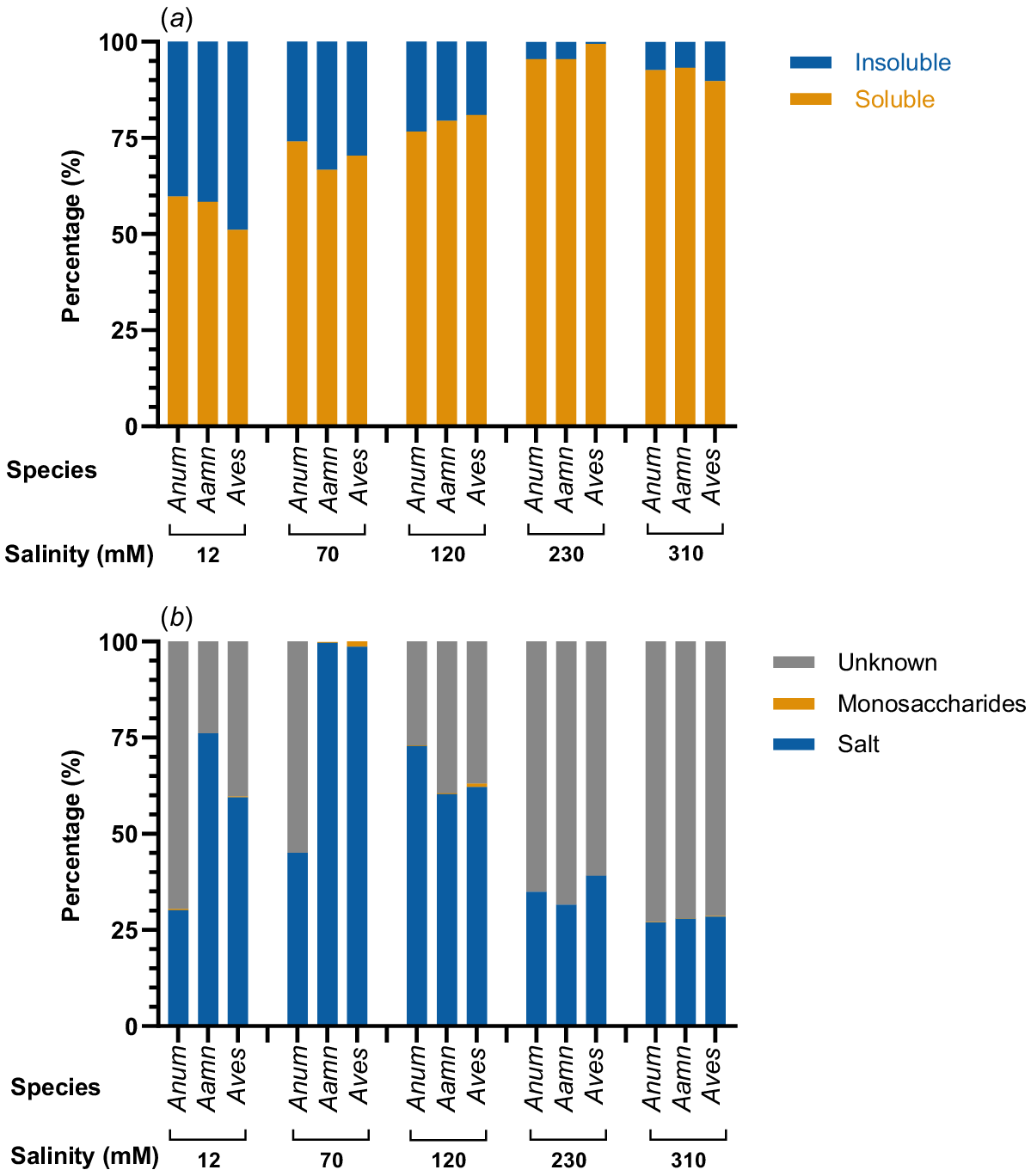
The greatest percentage of known compounds in the root mucilage of saltbush was attributed to salt (Fig. 3b). Salt concentration was greatest at medium external salinity concentrations of 70 mM NaCl for A. amnicola and A. vesicaria, and 120 mM NaCl for A. nummularia. At 70 mM NaCl, the mucilage of A. amnicola and A. vesicaria was comprised almost completely of salt with very low amounts of monosaccharides. However, at the higher external salt concentrations of 230 mM and 310 mM NaCl, the percentage of salt in the mucilage dropped and the percentage of unknown compounds was greatest. A. nummularia root mucilage had the greatest percentage of unknowns compared to the other species, but the lowest percentage of salt.
Monosaccharide analysis of saltbush root mucilage showed the most prominent peaks in the low-molecular weight range between 0 and 6 min, with only very minor peaks observed in the high molecular weight range (Fig. S2). In the low molecular weight range, all three saltbush species showed peaks at 3.5 min and 3.8 min, indicative of glucose and fructose, respectively (Fig. S2). Trace amounts of mannose, arabinose and galactose, were also present at some, but not all, salinity levels (Table 1). Mannose only appeared in A. vesicaria at 12 mM NaCl. Overall, A. vesicaria contained the greatest range and quantity of monosaccharides, predominantly at low to moderate salinity concentrations (Table 1).
| Salinity concentration (mM) | Average (% w/w) ± s.d. | |||||
|---|---|---|---|---|---|---|
| Mannose | Glucose | Arabinose | Galactose | |||
| Blank | 12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 70 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 120 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 230 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 310 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.00 | ||
| Atriplex nummularia | 12 | 0.00 ± 0.00 | 0.23 ± 0.02 | 0.04 ± 0.00 | 0.07 ± 0.01 | |
| 70 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 120 | 0.00 ± 0.00 | 0.18 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 230 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 310 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.05 ± 0.00 | ||
| Atriplex amnicola | 12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 70 | 0.00 ± 0.00 | 0.30 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 120 | 0.00 ± 0.00 | 0.25 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 230 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 310 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.05 ± 0.02 | ||
| Atriplex vesicaria | 12 | 0.03 ± 0.01 | 0.15 ± 0.02 | 0.03 ± 0.00 | 0.04 ± 0.01 | |
| 70 | 0.00 ± 0.00 | 1.28 ± 0.07 | 0.02 ± 0.00 | 0.04 ± 0.01 | ||
| 120 | 0.00 ± 0.00 | 0.87 ± 0.12 | 0.03 ± 0.01 | 0.00 ± 0.00 | ||
| 230 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 310 | 0.00 ± 0.00 | 0.15 ± 0.02 | 0.00 ± 0.00 | 0.04 ± 0.01 | ||
Monosaccharide profiles were generated by RP-HPLC of PMP derivatives. n = 3. Grey shading indicate results greater than 0.00% w/w.
The water-insoluble fraction of the exudates was determined to mainly comprise damaged and broken BLCs that had become enmeshed in mucilage (Fig. S5). This fraction was not analysed further.
Root exudate microscopy for polysaccharide identification
Initially, general polysaccharide stains were applied to the extruded root mucilage. Calcofluor white (CFW) staining indicated the presence of β-glucans, likely callose, cellulose and xyloglucan (Harrington and Hageage 2003). CFW staining was also present in the cell walls of saltbush roots, suggesting that they contain β-glucans (Fig. S3a). Staining with ruthenium red and crystal violet indicated the presence of acidic polysaccharides, most likely pectins, in the root mucilage and BLCs (Fig. S3b).
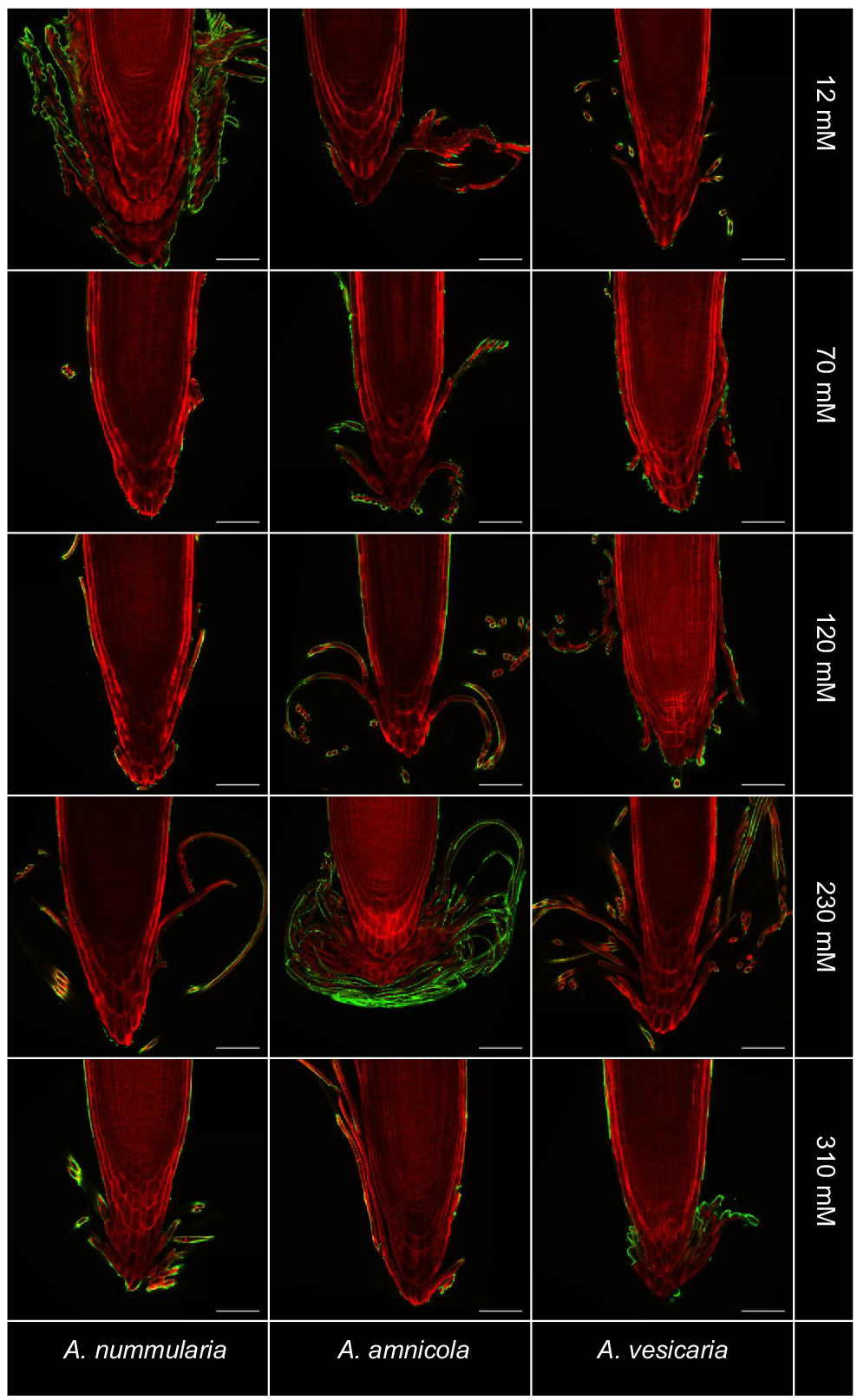
Immersion immunofluorescence labelling was used in combination with tissue clearing to investigate polysaccharide distribution within root exudates of saltbush. Staining with mAb LM19, which binds to un-esterified and partially esterified homogalacturonan, was detectable on the surface of BLCs produced by all three saltbush species at all salinity levels, but was not present inside cells (Fig. 4) (Verhertbruggen et al. 2009). Secondary antibody-only controls did not show any labelling with LM19 (Fig. S4). LM20, which labels esterified homogalacturonan, did not bind to cell surfaces (data not shown).
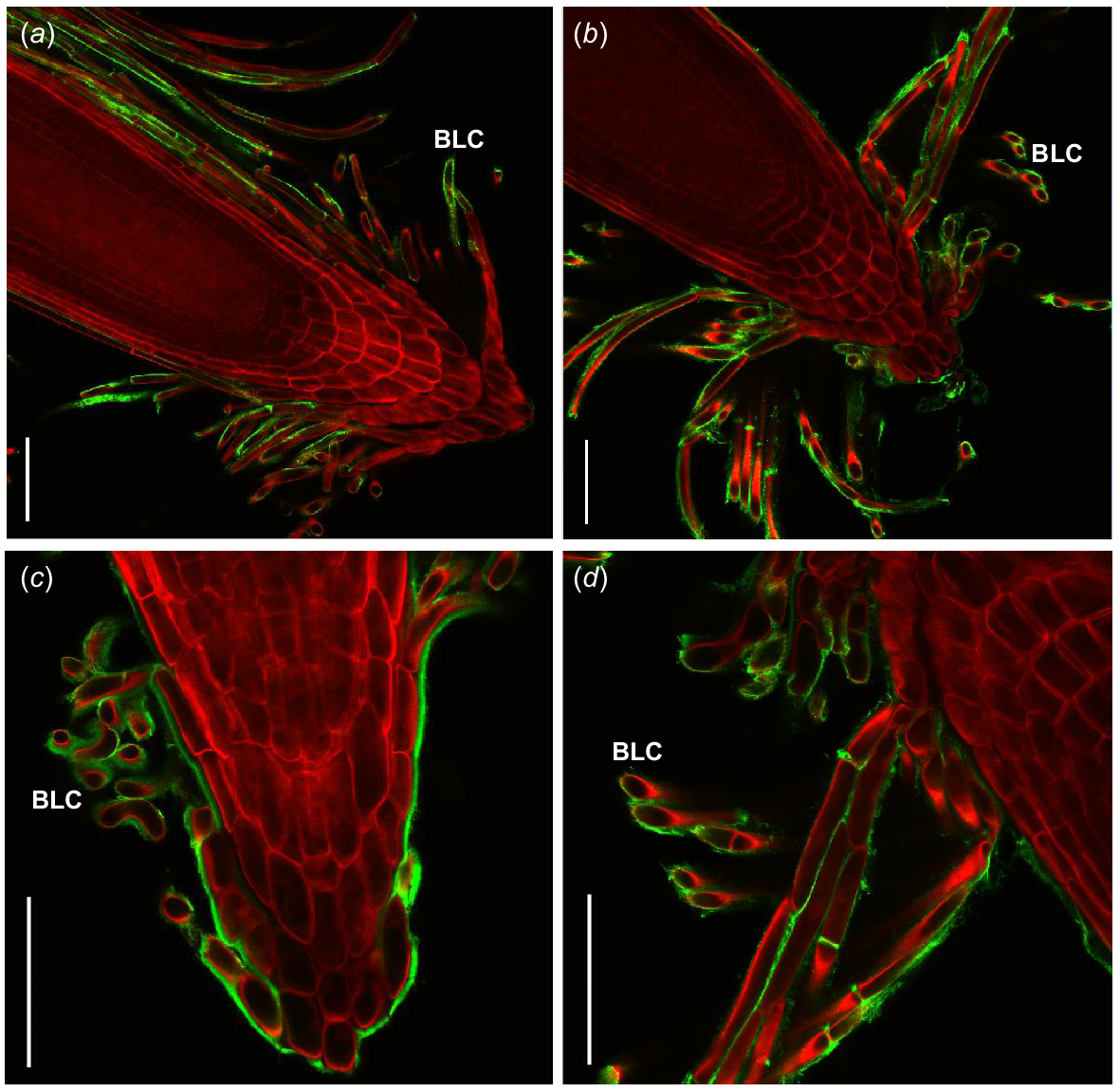
Immunofluorescent labelling of saltbush root caps with LM19 (green) indicates the presence of un-esterified homogalacturonan on the surface of border-like cells. Roots are stained with Direct Red 23 (red). Images are representative of n = 3–5 replicates. Scale bars = 100 μm.
Immersion immunofluorescence labelling was also used to investigate the occurrence of xyloglucan and AGPs in the root tissue using mAbs LM15 and JIM8 respectively. LM15 and JIM8 both bound strongly to the surfaces of BLCs (Fig. 5), as seen for LM19.
Immunofluorescent labelling of saltbush root caps (green) indicates the presence of xyloglucan and AGPs on the surface of border-like cells (BLCs). Roots are stained with Direct Red 23 (red). Images are representative. JIM8 recognised AGPs in (a) Atriplex vesicaria at 120 mM NaCl and (c) Atriplex nummularia at 70 mM NaCl. LM15 recognised xyloglucan in (b) Atriplex amnicola at 120 mM NaCl and (d) A. amnicola at 120 mM NaCl. Scale bars = 100 μm.
Root growth
Roots grown on glass beads were measured 7 days after sowing to determine whether the root length of young saltbush seedlings is affected by salinity. The only significant differences between root length at different salinity concentrations occurred in A. vesicaria (Fig. 6). A. vesicaria showed the greatest average root length at all salinity concentrations. At 230 mM NaCl, A. vesicaria had an average root length of 32.4 mm while A. amnicola and A. nummularia had average root lengths of 9.6 mm and 15.6 mm, respectively (P < 0.0001, Fig. 6a). A. amnicola and A. nummularia had consistent average root lengths across the range of salinity concentrations, while A. vesicaria peaked at 230 mM NaCl (Fig. 6a). Time to germination was not significantly different between species, with germination on glass beads within 7 days varying between 67% and 100% (data not shown).
(a) Root length and (b) root:shoot ratio in three saltbush species at five salinity concentrations (12, 70, 120, 230, and 310 mM NaCl) measured seven days after sowing. Lines are guide to sight only. Anum, Atriplex nummularia; Aamn, Atriplex amnicola; Aves, Atriplex vesicaria. Shown are observed means ± s.e. (n = 2–10). Significant differences in root length occur only in A. vesicaria at different salinity concentrations (in green). *P < 0.05; **P < 0.01.
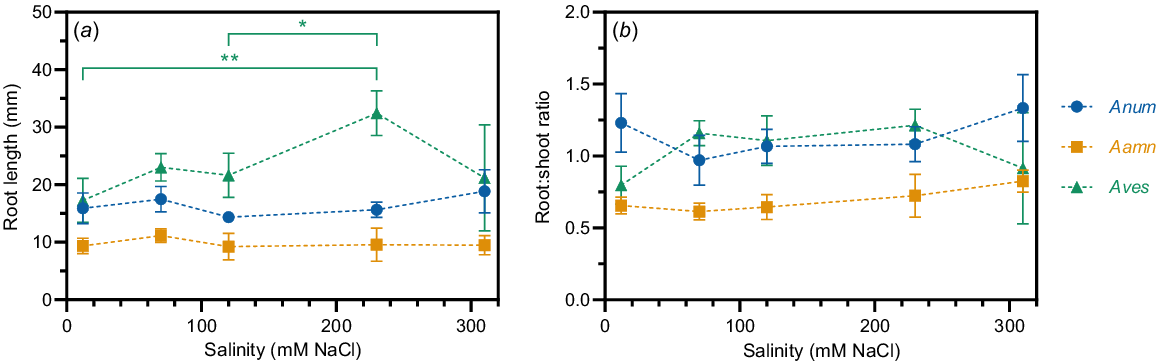
Root:shoot ratio was not significantly different between salinity concentrations within each species (Fig. 6b). A. amnicola root:shoot ratio was significantly lower than the other two species (P < 0.0001, Fig. 6b). A. nummularia and A. vesicaria showed identical root:shoot ratios of 0.56 at 310 mM NaCl (Fig. 6b).
Discussion
Saltbush species have closed-type root apical meristems that shed border-like cells
Here, we report that saltbush species have closed-type RAMs, as shown by cell organisation in multiple, well-defined layers (Fig. 1). Closed-type RAMs are characterised by clonally-distinct tiers of initials where cells have a defined lineage and can be traced back to their origin (Hamamoto et al. 2006). Kumpf and Nowack (2015) report two distinct different tissue types: (1) the central columella root cap; and (2) the peripheral lateral root cap. Columella cells are distal to the quiescent centre while lateral root cap cells are long, thin cells located on the periphery of the columella cells. As the columella and lateral root caps differentiate, they are displaced towards the root periphery. When they reach the root periphery, the oldest cells are exuviated into the rhizosphere as border cells or BLCs (del Campillo et al. 2004).
Closed-type RAMs have been observed in many families, including Brassicaceae with Arabidopsis thaliana (Hamamoto et al. 2006), Linaceae, Asteraceae, Lamiaceae and Poaceae (Groot et al. 2004; Rost 2011; Saito et al. 2019). Here, we extend the occurrence of closed-type RAMs to saltbush in the Amaranthaceae family and to the first halophyte. Notably, we report that Atriplex species release BLCs into the rhizosphere rather than border cells. Cells sloughed off from saltbush roots into the rhizosphere are arranged in organised layers that remain attached to each other when immersed in water and are therefore defined as border-like. These cells do not become dispersed individually, and thus cannot be classified as border cells, which separate once in contact with water (Driouich et al. 2010).
Saltbush roots are surrounded both by long, thin BLCs that detach from the epidermal layer in the maturation region of the root and short, fat BLCs derived from columella cells that form clumps around the root cap (Fig. 1). The distribution of BLCs around the root cap may play a role in stress tolerance by offering physical protection to the growing root region for the greatest period of time. The distribution pattern is different to Arabidopsis, where BLCs exist only as clumps of fat cells around the root cap (Durand et al. 2009).
Saltbush root mucilage contains minimal monosaccharides
In this experiment, we used two chromatography techniques to ascertain the monosaccharides present in saltbush root mucilage, as well as microscopy techniques to visualise polysaccharides in and around the root cap. Monosaccharide analysis using RP-HPLC and HPAEC-PAD both indicated that low molecular weight species are present in the saltbush root mucilage. Glucose is present in all three species in the greatest concentrations at low to medium salinity, as well as low concentrations of arabinose and galactose (Table 1). Fructose was also determined to be present in saltbush root mucilage (Fig. S2).
There are limited reports of root mucilage in other halophytic species. In one study with seedlings of Kosteletzkya virginica, rhamnose, galactose, and glucose were found in the greatest quantities compared to fucose, arabinose, xylose, and mannose (Ghanem et al. 2010). In this study, 100 mM NaCl salinity slightly decreased the glucose content in root mucilage. In our study, A. nummularia showed a similar result for glucose, with a decrease in glucose at 120 mM NaCl compared to 12 mM NaCl.
However, only around 1.5% of the total mucilage extract was identified as monosaccharides (Fig. 3), which is exceptionally low compared to cereal crops such as maize and wheat, where root mucilage is comprised of 80–100% monosaccharides (Ray et al. 1988; Knee et al. 2001; Amicucci et al. 2019). This unexpected difference may represent a tactic for the conservation of carbon output via limiting polysaccharides within root mucilage, assisting plants in surviving in harsh environments (Zickenrott et al. 2016). Salinity concentrations between 70 and 230 mM NaCl, when monosaccharides were present in the root mucilage in the greatest quantities, might potentially represent the most optimal range for the growth of saltbush, allowing carbon release into the soil for advantageous but not essential processes such as attracting beneficial soil microorganisms, repelling pathogenic microorganisms, or root lubrication (McCully 1999; Minz et al. 2013; Baetz and Martinoia 2014; Sasse et al. 2018). Conversely, stress responses may be triggered in saltbush grown at very high external salt concentrations, resulting in the allocation of carbon elsewhere in the plant, such as for the production of osmoprotectants or salt bladders (Jamil et al. 2011).
As monosaccharides are present in minimal quantities in the root mucilage, there must be a substantial quantity of other compounds present that are not detectable using RP-HPLC and HPAEC-PAD methods (Fig. 3b). These could include salt as well as a range of proteins, organic acids, phenolics, and AGPs (Bais et al. 2006; Jones et al. 2009; Kawasaki et al. 2018). The nature and roles of this collection of compounds produced by plant roots as a measure to mitigate a number of stresses has recently been reviewed (Chai and Schachtman 2022). This review highlights the complexity of root exudates and indicates that, as discriminatory analytical methods become better, more compounds are likely to be identified. We measured salt in the root mucilage using flame photometry and found that large quantities are present, although the increased root mucilage quantities produced under high salinity are not attributed to greater amounts of salt (Figs 2, 3). The greatest quantity of salt in the root mucilage occurs at 12–120 mM NaCl, and is reduced at higher salinity levels. Importantly, this shows that the increased exudate weights at higher salinity (Fig. 2) cannot be attributed only to higher salt contents, but must also include other compounds. At increased external NaCl concentrations, salt may be transported to epidermal bladder cells on leaves for storage, which reduces the salinity effects on the plant and decreases NaCl in root mucilage (Kiani-Pouya et al. 2017). This supports findings from Aslam et al. (1986) that suggest an accumulation of ions in the tissues of A. amnicola at high external NaCl concentrations. Additional studies that include a de-salting step prior to monosaccharide analysis would provide further clarity on the levels of salt and quantities of other as yet unidentified compounds present in the root mucilage, which are likely to belong to the list identified by previous researchers (Bais et al. 2006; Chai and Schachtman 2022).
Un-esterified homogalacturonan, xyloglucan, and arabinogalactan proteins are present on the surface of border-like cells
Overall, saltbush root mucilage contains only small amounts of monosaccharides, differing quantities of salt, and other unknown soluble components. To further investigate the unknown components present in root mucilage and around the root cap, we used microscopy techniques. In particular, we probed for pectic compounds, particularly homogalacturonan, because they have previously been found to increase in root mucilage under high salt treatments and play a role in cell adhesion around the root cap (Durand et al. 2009; Hayashi and Kaida 2011; Tenhaken 2015; Lutts et al. 2016). We discovered that un-esterified, or partially esterified, homogalacturonan is present on the surface of BLCs (Fig. 4) as well as xyloglucan and AGPs (Fig. 5). These compounds may glue BLCs to each other, as in Arabidopsis roots (Durand et al. 2009), since similar polysaccharides have previously been found on the surface of BLCs, including homogalacturonan and AGPs (Driouich et al. 2010; Kumar and Iyer-Pascuzzi 2020) with pectins surrounding border cells in pea (P. sativum) (Stephenson and Hawes 1994).
BLCs in saltbush remain attached to each other once released from the root surface, potentially due to the un-esterified homogalacturonan, which has been found to form gels in the presence of multivalent ions such as Ca2+. These Ca2+/pectate crosslinks hold adjacent pectate polymers together and may contribute to adhesion between cells (Willats et al. 2001; Proseus and Boyer 2012). Importantly, Arabidopsis mutants defective in homogalacturonan synthesis had altered BLC organisation (Durand et al. 2009), suggesting that homogalacturonan plays a key role in attachment between BLCs. Additionally, AGPs likely play a role in BLC adhesion, as the alteration of AGP biosynthesis in Arabidopsis results in a significant reduction in binding of BLCs to the root cap (Driouich et al. 2010).
As immersion immunofluorescence labelling only allows detection of epitopes at the surface of the tissue (Durand et al. 2009), the labelled compounds may be present either in the mucilage layer or be part of the cell walls, so further studies are needed to elucidate their location within or between cells, as well as their roles in adhesion. If they comprise an intrinsic part of the cell walls, they may not readily detach into the soluble fraction of the root mucilage that has been analysed here. The distribution of polysaccharides deserves further investigation, which may be particularly illuminating at the transmission electron microscope level. The expression patterns of the encoding genes could also be defined, particularly as roots elongate or are exposed to environmental stresses. Finally, while mucilage is secreted primarily by the root cap (Knee et al. 2001), it can also be released from root hairs and older root regions away from the root cap, so future studies should investigate polysaccharide labelling beyond the root cap alone. While the composition of polysaccharides in root mucilage is not yet well understood, different polysaccharides may play key roles in salinity tolerance, warranting further research in the future.
Increased salinity alters saltbush seedling root cap morphology but not root:shoot ratio
At 7 days after sowing, the root:shoot ratio of the three saltbush species did not differ significantly as salinity concentration increased (Fig. 6). This suggests that saltbush seedlings can survive without significant growth penalties at very high concentrations of salt, since 310 mM NaCl is 60% the salt concentration of seawater and up to seven times the amount required to seriously disrupt roots in cereal crops (Byrt et al. 2018). Root length was greater at 230 mM NaCl than 12 or 120 mM NaCl in A. vesicaria, which further supports this hypothesis. Germination time between species was not significantly different, but the number of roots measured (n = 2–10) depended on successful germination, which was variable. Future studies should investigate older saltbush seedling root lengths, ideally standardising root length measurements by time from germination, rather than time from sowing.
However, there was a noticeable effect of salinity on the saltbush root cap morphology. We found that in A. nummularia and A. vesicaria, root cap cell organisation becomes disorganised at high salinity levels of 310 mM NaCl (Figs 1 and 4). As the root cap is one of the first plant organs to come into physical contact with biotic or abiotic stresses (Kumar and Iyer-Pascuzzi 2020), these cells can undergo significant changes (Baranova et al. 2019), but research into the effect of salinity on saltbush root morphology has been limited. The reduction in cell organisation may result from a loss of control of cell division, potentially due to osmotic stress and inadequate rates of accumulation of osmotic solutes (Aslam et al. 1986). Alternatively, saltbush may have reduced root cap cell organisation at high salinity concentrations due to changes in pectin methylesterase (PME) activity (Pelloux et al. 2007). Wolf et al. (2012) suggest that a change in PME activity, which can occur due to an excess of external cations present in high salinity conditions, results in the loss of cell wall integrity and extensibility. PME may degrade homogalacturonan and other binding pectic compounds, resulting in reduced cell binding in the root cap. To test whether PME activity affects adhesion between BLCs, as well as the impact of salinity on pectin content in saltbush cells, we suggest future studies that inhibit PME in saltbush at different salinity concentrations. This has previously been investigated in Arabidopsis using catechin-derivative (-)-epi-gallocatechin gallate (EGCG) (Wolf et al. 2012).
Conclusions
We report that saltbush species have closed-type RAMs with BLCs that remain attached to each other after shedding into the rhizosphere. The monosaccharide composition of saltbush root mucilage is exceptionally low, with glucose and fructose present. In addition, homogalacturonan, xyloglucan, and AGPs were detected, which may aid in adhesion of BLCs although it is not clear if they are components of the mucilage itself or of the cell wall. While salt is present in significant quantities in the root mucilage, particularly in the range from 12 to 120 mM NaCl, there is also a large percentage of as yet unidentified components. Increased salinity up to 310 mM NaCl altered saltbush seedling root cap morphology and root mucilage production, but not root:shoot ratio. Here, we overcame the difficulties of using soil or full hydroponics for mucilage analysis by optimising a semi-hydroponic, glass bead system for saltbush seedling growth. For future experiments on older plants, a more complex, bigger glass bead system that allows for growth and mucilage collection could be developed.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of funding
Scholarship was received from the AW Howard Memorial Trust to support this research. The supporting source had no involvement in the preparation of the data or manuscript, or the decision to submit for publication.
Author contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alison Gill. The first draft of the manuscript was written by Alison Gill. Both authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy and Microanalysis Research Facility at Adelaide Microscopy, The University of Adelaide, Waite Campus, and Dr Gwenda Mayo, in particular, for their assistance in using the confocal microscope. The author is grateful to Shi Fang (Sandy) Khor and Dr Andrea Matros for their technical assistance, Tycho Neumann for their microscopy expertise, Glenn Christie and Dr Briony Horner at Succession Ecology for donating A. nummularia seeds and Dr James Cowley and Melanie Ford for their assistance in the laboratory. AG was supported by an AW Howard Memorial Trust Honours Scholarship and The Aileen & Bert Kollosche Scholarship for Study of Australian Flora. We thank the editor and two reviewers for their insightful comments on the manuscript.
References
Amicucci MJ, Galermo AG, Guerrero A, et al. (2019) A strategy for structural elucidation of polysaccharides: elucidation of a maize mucilage that harbors diazotrophic bacteria. Analytical Chemistry 91(11), 7254-7265.
| Crossref | Google Scholar |
Aslam Z, Jeschke WD, Barrett-Lennard EG, et al. (1986) Effects of external NaCl on the growth of Atriplex amnicola and the ion relations and carbohydrate status of the leaves. Plant, Cell & Environment 9, 571-580.
| Crossref | Google Scholar |
Bacic A, Moody SF, Clarke AE (1986) Structural analysis of secreted root slime from maize (Zea mays L.). Plant Physiology 80, 771-777.
| Crossref | Google Scholar |
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant, Cell and Environment 32, 666-681.
| Crossref | Google Scholar |
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends in Plant Science 19, 90-98.
| Crossref | Google Scholar |
Bais HP, Weir TL, Perry LG, et al. (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57, 233-266.
| Crossref | Google Scholar |
Baranova EN, Chaban IA, Kononenko NV, et al. (2019) Ultrastructural changes of organelles in root cap cells of tobacco under salinity. Proceedings of the Latvian Academy of Sciences, Section B: Natural, Exact, and Applied Sciences 73, 47-55.
| Crossref | Google Scholar |
Burton RA, Collins HM, Kibble NAJ, et al. (2011) Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnology Journal 9, 117-135.
| Crossref | Google Scholar |
Byrt CS, Munns R, Burton RA, et al. (2018) Root cell wall solutions for crop plants in saline soils. Plant Science 269, 47-55.
| Crossref | Google Scholar |
Chai YN, Schachtman DP (2022) Root exudates impact plant performance under abiotic stress. Trends in Plant Science 27, 80-91.
| Crossref | Google Scholar |
del Campillo E, Abdel-Aziz A, Crawford D, Patterson SE (2004) Root cap specific expression of an endo-β-1,4-d-glucanase (cellulase): a new marker to study root development in Arabidopsis. Plant Molecular Biology 56, 309-323.
| Crossref | Google Scholar |
Descheemaeker K, Smith AP, Robertson MJ, et al. (2014) Simulation of water-limited growth of the forage shrub saltbush (Atriplex nummularia Lindl.) in a low-rainfall environment of southern Australia. Crop & Pasture Science 65, 1068-1083.
| Crossref | Google Scholar |
Driouich A, Durand C, Vicré-Gibouin M (2007) Formation and separation of root border cells. Trends in Plant Science 12, 14-19.
| Crossref | Google Scholar |
Driouich A, Durand C, Cannesan MA, et al. (2010) Border cells versus border-like cells: are they alike? Journal of Experimental Botany 61, 3827-3831.
| Crossref | Google Scholar |
Driouich A, Gaudry A, Pawlak B, Moore JP (2021) Root cap-derived cells and mucilage: a protective network at the root tip. Protoplasma 258, 1179-1185.
| Crossref | Google Scholar |
Durand C, Vicre-Gibouin M, Follet-Gueye ML, et al. (2009) The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiology 150, 1411-1421.
| Crossref | Google Scholar |
Ghanem ME, Han RM, Classen B, et al. (2010) Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress. Journal of Plant Physiology 167, 382-392.
| Crossref | Google Scholar |
Groot EP, Doyle JA, Nichol SA, Rost TL (2004) Phylogenetic distribution and evolution of root apical meristem organization in dicotyledonous angiosperms. International Journal of Plant Sciences 165, 97-105.
| Crossref | Google Scholar |
Hamamoto L, Hawes MC, Rost TL (2006) The production and release of living root cap border cells is a function of root apical meristem type in dicotyledonous angiosperm plants. Annals of Botany 97, 917-923.
| Crossref | Google Scholar |
Harrington BJ, Hageage GJ (2003) Calcofluor White: a review of its uses and applications in clinical mycology and parasitology. Laboratory Medicine 34, 361-367.
| Crossref | Google Scholar |
Hawes M, Allen C, Turgeon BG, et al. (2016) Root border cells and their role in plant defense. Annual Review of Phytopathology 54, 143-161.
| Crossref | Google Scholar |
Hayashi T, Kaida R (2011) Functions of xyloglucan in plant cells. Molecular Plant 4, 17-24.
| Crossref | Google Scholar |
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Critical Reviews in Plant Sciences 30, 435-458.
| Crossref | Google Scholar |
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant and Soil 321, 5-33.
| Crossref | Google Scholar |
Kawasaki A, Donn S, Ryan PR, et al. (2016) Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS ONE 11, 1-25.
| Crossref | Google Scholar |
Kawasaki A, Okada S, Zhang C, et al. (2018) A sterile hydroponic system for characterising root exudates from specific root types and whole - root systems of large crop plants. Plant Methods 14, 114.
| Crossref | Google Scholar |
Kiani-Pouya A, Roessner U, Jayasinghe NS, et al. (2017) Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant, Cell & Environment 40, 1900-1915.
| Crossref | Google Scholar |
Knee EM, Gong F-C, Gao M, et al. (2001) Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Molecular Plant-Microbe Interactions 14, 775-784.
| Crossref | Google Scholar |
Knox OGG, Curlango-Rivera G, Huskey DA, Hawes MC (2020) Border cell counts of Bollgard3 cotton and extracellular DNA expression levels. Euphytica 216, 142.
| Crossref | Google Scholar |
Kumar N, Iyer-Pascuzzi AS (2020) Shedding the last layer: mechanisms of root cap cell release. Plants 9, 308.
| Crossref | Google Scholar |
Kumpf RP, Nowack MK (2015) The root cap: a short story of life and death. Journal of Experimental Botany 66, 5651-5662.
| Crossref | Google Scholar |
Lutts S, Qin P, Han RM (2016) Salinity influences biosorption of heavy metals by the roots of the halophyte plant species Kosteletzkya pentacarpos. Ecological Engineering 95, 682-689.
| Crossref | Google Scholar |
McCully ME (1999) Roots in soil: unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50, 695-718.
| Crossref | Google Scholar |
Moody SF, Clarke AE, Bacic A (1988) Structural analysis of secreted slime from wheat and cowpea roots. Phytochemistry 27, 2857-2861.
| Crossref | Google Scholar |
Mravec J (2017) Border cell release: cell separation without cell wall degradation? Plant Signaling & Behavior 12, e1343778.
| Crossref | Google Scholar |
Nazari M (2021) Plant mucilage components and their functions in the rhizosphere. Rhizosphere 18, 100344.
| Crossref | Google Scholar |
Nedjimi B (2014) Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochemical Systematics and Ecology 52, 4-13.
| Crossref | Google Scholar |
Ninmanont P, Wongchai C, Pfeiffer W, Chaidee A (2021) Salt stress of two rice varieties: root border cell response and multi-logistic quantification. Protoplasma 258, 1119-1131.
| Crossref | Google Scholar |
Norman H, Wilmot M, Hulm E, Young P (2017) Developing perennial shrubs to fill feed gaps on marginal soils in Australia. In: ‘Grassland resources for extensive farming systems in marginal lands: major drivers and future scenarios. Proceedings of the 19th Symposium of the European Grassland Federation’. (Eds C Porqueddu, A Franca, G Lombardi, et al.) pp. 79–81. (European Grassland Federation)
Oburger E, Jones DL (2018) Sampling root exudates – mission impossible? Rhizosphere 6, 116-133.
| Crossref | Google Scholar |
Pelloux J, Rusterucci C, Mellerowicz E (2007) New insights into pectin methylesterase structure and function. Trends in Plant Science 12, 267-277.
| Crossref | Google Scholar |
Phan JL, Tucker MR, Khor SF, et al. (2016) Differences in glycosyltransferase family 61 accompany variation in seed coat mucilage composition in Plantago spp. Journal of Experimental Botany 67, 6481-6495.
| Crossref | Google Scholar |
Proseus TE, Boyer JS (2012) Pectate chemistry links cell expansion to wall deposition in Chara corallina. Plant Signaling & Behavior 7, 1490-1492.
| Crossref | Google Scholar |
Ray TC, Callow JA, Kennedy JF (1988) Composition of root mucilage polysaccharides from Lepidium sativum. Journal of Experimental Botany 39, 1249-1261.
| Crossref | Google Scholar |
Ropitaux M, Bernard S, Follet-Gueye M-L, et al. (2019) Xyloglucan and cellulose form molecular cross-bridges connecting root border cells in pea (Pisum sativum). Plant Physiology and Biochemistry 139, 191-196.
| Crossref | Google Scholar |
Rost TL (2011) The organization of roots of dicotyledonous plants and the positions of control points. Annals of Botany 107, 1213-1222.
| Crossref | Google Scholar |
Saito S, Niki T, Gladish DK (2019) Comparison of promeristem structure and ontogeny of procambium in primary roots of Zea mays ssp. Mexicana and Z. mays “Honey Bantam” with emphasis on metaxylem vessel histogenesis. Plants 8, 162.
| Crossref | Google Scholar |
Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends in Plant Science 23, 25-41.
| Crossref | Google Scholar |
Stephenson MB, Hawes MC (1994) Correlation of pectin methylesterase activity in root caps of pea with root border cell separation. Plant Physiology 106, 739-745.
| Crossref | Google Scholar |
Tenhaken R (2015) Cell wall remodeling under abiotic stress. Frontiers in Plant Science 5, 771.
| Crossref | Google Scholar |
Ursache R, Andersen TG, Marhavý P, Geldner N (2018) A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant Journal 93, 399-412.
| Crossref | Google Scholar |
Verhertbruggen Y, Marcus SE, Haeger A, et al. (2009) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydrate Research 344, 1858-1862.
| Crossref | Google Scholar |
Willats WGT, Orfila C, Limberg G, et al. (2001) Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Journal of Biological Chemistry 276, 19404-19413.
| Crossref | Google Scholar |
Wolf S, Mravec J, Greiner S, et al. (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Current Biology 22, 1732-1737.
| Crossref | Google Scholar |
Zickenrott I-M, Woche SK, Bachmann J, et al. (2016) An efficient method for the collection of root mucilage from different plant species—a case study on the effect of mucilage on soil water repellency. Journal of Plant Nutrition and Soil Science 179, 294-302.
| Crossref | Google Scholar |


