Understanding the role of the fructose-1,6-bisphosphatase gene for enhancing the photosynthetic rate in Arabidopsis thaliana
Fatima Gulzar A , Raza Ahmad B , Suk-Yoon Kwan C , Zulqurnain Khan A , Sulaiman Ali Alharbi D , Mohmmad Maroof Shah B , Shoaib ur Rehman A , Maria Siddique E , Mohammad Javed Ansari F G , Irum Shahzadi B , Muhammad Abu Bakar Saddique A , Muhmmad Zahid Ishaq H and Ummara Waheed
A , Muhmmad Zahid Ishaq H and Ummara Waheed  A *
A *
A
B
C
D
E
F
G
H
Abstract
Transgenic Arabidopsis thaliana (ecotype Columbia) was successfully transformed with the gene fructose-1,6-bisphosphatase (FBPase) and named as AtFBPase plants. Transgenic plants exhibited stable transformation, integration and significantly higher expressions for the transformed gene. Morphological evaluation of transgenic plants showed increased plant height (35 cm), number of leaves (25), chlorophyll contents (28%), water use efficiency (increased from 1.5 to 2.6 μmol CO2 μmol−1 H2O) and stomatal conductance (20%), which all resulted in an enhanced photosynthetic rate (2.7 μmol m−2 s−1) compared to wild type plants. This study suggests the vital role of FBPase gene in the modification of regulatory pathways to enhance the photosynthetic rate, which can also be utilised for economic crops in future.
Keywords: Arabidopsis thaliana, C3, C4, CO2, FBPase, genetic transformation, photorespiration, photosynthesis.
Introduction
Plant photosynthesis is a critical process underpinning the survival of most organisms on Earth, by capturing solar energy and making it available within ecosystems. Photosynthesis comprises of two processes: light and dark reactions. During dark reactions the major enzyme RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) performs carboxylation, i.e. fixation of carbon dioxide, producing two molecules of 3-phosphoglycerate (3PGA) (Song et al. 2023). In addition to carboxylation, RuBisCO also performs oxygenation (i.e. fixing oxygen) (Bowes et al. 1971) and produces one 3PGA molecule with another two molecules of phosphoglycolate (2PG). The production of phosphoglycolate molecules is toxic for plants and blocks the functions of RuBisCO and triosephosphate isomerase. To circumvent this toxic effect, the glycolate catabolic reaction takes place during photorespiration, producing one molecule of PGA, ammonia and carbon dioxide (Heyduk 2022). In this process, C3 type plants waste up to 25% of fixed carbon dioxide and 6.1% of energy (Mizokami et al. 2019).
In contrast, C4 photosynthetic plants are provided with carbon concentrating mechanisms (CCM) that involve minimal photorespiration, resulting in maximum energy conservation (Long et al. 2019). In comparison to plants with the C3 mechanism, C4 plants exhibit 6% higher solar energy conversion (Ferrari et al. 2022). However, it is also established that photorespiration is vital for plants’ existence and its mechanism cannot be completely avoided (Ainsworth and Rogers 2007). Therefore, CO2 and energy losses should be reduced through setting up the shorter and more efficient 2PG metabolic pathway in C3 plants (Tan and Chen 2023). Numerous efforts have been made for the modification of C3 plants to minimise the effect of photorespiration, but RuBisCO modification is a challenging task (Tan and Chen 2023). However, advanced biotechnological methods can serve the purpose, like genetic engineering of potential genes that can modify the photosynthetic pathway to minimise the energy loss and enhance the photosynthesis rate (Dalal et al. 2015). Such genes are found to be present in cyanobacteria that contain different 2PG metabolising pathways and can minimise the losses created by photorespiration (Arrivault et al. 2019).
One such gene is fructose-1,6 B-bisphosphatase (FBPase). It is present in cyanobacteria and has the potential to elevate the levels of photosynthesis in C3, as it plays a vital role between the regenerative phase of the Calvin cycle and starch biosynthesis and also activates irreversible reactions (Mizokami et al. 2019). Cyanobacterial genes have previously been used to transform the photosynthetic mechanism, with promising results (Mitchell et al. 2020).
The current study reports the successful transformation of the FBPase cyanobacterial gene in model plant Arabidopsis thaliana through floral dip Agrobacterium mediated transformation. The transgenic plants showed significantly higher gene expression, with enhanced plant growth, increased stomatal conductance, improved chlorophyll contents and higher water retaining capacity; resulting in improved photosynthesis when compared to the wild plants. This study will open new avenues to genetically transform the economically important crops that are driven by C3 photosynthesis mechanism.
Materials and methods
Vector construction
The gene sequence of Synechocystis sp. strain PCC 680 was retrieved from https://www.kazusa.or.jp/database/ and specific primers were generated for constructing an expression vector of FBPase (see Supplementary material S1). The cloned gene was sequenced and transferred into a shuttle vector provided with 35 cauliflower mosaic virus (CaMV) 35S dual promoter, tobacco etch virus 5′-untranslated region (5′-UTR) translation enhancer (TEV), chloroplast transit peptide sequence from tomato smaller subunit of RuBisCO (chlTP) and 35S terminator. The cassette of gene expression was then loaded onto pCAMBIA2300 and was named pSFB (Fig. 1) (Sowjanya et al. 2019). Finally, the expression vector was transformed to GV3101strain of Agrobacterium tumefaciens using the freeze–thaw method.
Representation of vector pSFB utilised for transformation of Arabidopsis thaliana plants expressing FBPase. 35SPro, cauliflower mosaic virus promoter; TEV, transcriptional enhancer; chlTP, transit peptide from the sequence of the small subunit of RuBisCO (tomato); FBPase, fructose-1,6-bisphosphatase; 35S Ter, the terminating sequence from the cauliflower mosaic virus.

Growth of A. tumefaciens strain AGL1
Agrobacterium culture was streaked on a Luria Broth (LB) medium plate having 50 mg L−1 kanamycin then allowed to grow at 28°C. After 48 h a single colony was picked, re-suspended in 5% sucrose solution and kept in incubation under 200 rpm shaking overnight until the optical density (OD) reached 0.8.
Plant material and growth conditions
Sterilised seeds of A. thaliana (ecotype Columbia) were grown on half-strength Murashige and Skoog (MS) media in vitro at MNS University of Agriculture, Multan, Pakistan. Growth conditions were kept under a 16 h light and 8 h dark photoperiod, 60% relative humidity and 120 μmol m−2 s−1 light intensity. Two-week-old seedlings were then transferred to pots provided with peat moss (Abbasi et al. 2021). The bolts that initially appeared were cut off to gain maximum growth for enhanced transformation proficiency.
Genetic transformation of Arabidopsis
The floral dip method was used to transform Arabidopsis. Young flowers were dipped into the Agrobacterium and sucrose solution for 3–5 min. Inoculated seeds were surface sterilised using 70% ethanol for 3 min. MS growth media supplemented with 50 mg L−1 of the antibiotic kanamycin was used to culture the inoculated seeds. After the second week, plants grown with healthy roots systems were carefully chosen to screen the foreign gene integration (Clough and Bent 1998).
Analysis of transgenic plants by PCR
Genomic DNA of all transgenic plant lines (transgenics) was extracted through the CTAB method (cetyl trimethylammonium bromide; Ling et al. 2011). As a negative control, DNA of non-transformed Arabidopsis plants (wild) was used. Polymerase chain reaction (PCR) was used to detect the putative transformed plants.
The amplification process was performed as follows:
Initial denaturation: 95°C for 5 min
Denaturation: 94°C for 30 s
Primer annealing: 52.5°C for 45 s
Extension: 72°C for 30 s
Final extension: 72°C for 10 min
PCR products were loaded on a 1% agarose gel stained with ethidium bromide.
Analysis of foreign gene expression in transgenic plants
In order to evaluate the transcription and stability of transgene, both transgenic and wild plants were grown in the greenhouse. Total RNA (DNA free) was extracted, using an RNA prep Pure Plant Kit (TIANGEN). Complementary DNA (cDNA) was synthesised using PrimeScript1RT reagent kit (Takara, China). For quantitative real-time PCR (qPCR), Premix Ex TaqTM II (Takara) was used and PCR amplification was performed using a CFX connect Real-Time PCR detection system (Rehman et al. 2021). ACTIN 7 (ACT7) (Ferreira et al. 2023), a housekeeping gene, was used as an internal control.
The gene specific primers that were used:
| AtAct7-F:ATCAATCCTTGCATCCCTCAGC | R:GGACCTGACTCATCGTACTCAC | |
| FBPase: F-CTAATCGGTGAAAGTCGGGA | R-3GCGGCAAATAATACGGTTT |
Each experiment was conducted in triplicate.
Physiological characterisation
After molecular characterisation, the seeds were collected from seven independent transgenic lines, grown on the MS medium and then transferred to peat moss in the same conditions mentioned in ‘Plant material and growth conditions’ to monitor the following physiological characterisation.
Measurement of plant height and number of leaves
Both transgenic and wild plants were monitored at different growth stages till maturity. In order to evaluate the plant growth and height, plant length of transgenics was measured manually with the help of a scale. Plant height was measured after 40 days of sowing, at the stage of full maturity when the flowers at the top of the plant were fully opened. A minimum of seven plants were taken to obtain the estimate of plant height. Leaf numbers were counted at 32 days after sowing.
Measurement of photosynthesis related parameters
Rate of photosynthesis, water use efficiency and stomatal conductance of transgenic and wild plants of the same age were measured using Ciras-3, and chlorophyll content was measured using SPAD-502 Plus (Ling et al. 2011). The measurements were taken from the plants 40 days after sowing, and excluding the midrib of the leaf.
Statistical analysis
All experiments were performed in a completely randomised design (CRD) in triplicate. Descriptive statistics and estimates of variance were calculated using Minitab version (ver. 17). Data normality was checked using Barlett’s test of homogeneity and the Anderson–Darling Normality Test. Sample variance was calculated using one-way ANOVA. The least significant difference (l.s.d.) post hoc test was used to further check the variance among the studied plants. A significance level of P ≤ 0.05 was used for data verification.
Results
Molecular characterisation of putative transformants
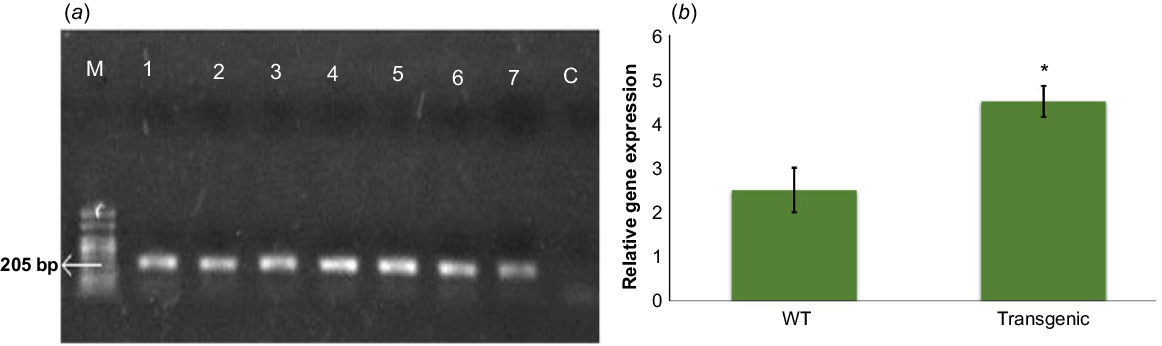
Plants that showed resistance to the selection media provided with antibiotic resistance gene were considered as putative transformants. Out of twenty plants, ten were selected for PCR analysis, amongst which seven plants were found positive for the presence of FBPase gene (Fig. 2a). Expression analysis revealed that overexpression of the FBPase gene was significantly higher in transgenic as compared to the wild type plants (Fig. 2b).
(a) PCR based amplified bands of the FBPase gene in Arabidopsis plants. Molecular weight markers (M): Invitrogen 1 kb ladder, FBPase PCR product (205 kb). Lanes 1–7: FBPase positive plants (transgenics) showing bands of expected size. Lane C: Negative control (wild type plant DNA of Arabidopsis. (b) Relative expression pattern analyses of wild type (WT) and transgenic Arabidopsis plants estimated and normalised using Actin 7 as the internal control by qPCR. Expression analysis revealed that overexpression of the FBPase gene was significantly higher in transgenics, as compared to the wild type plants. * indicates P ≤ 0.05. Error bar denotes standard error.
Plant height
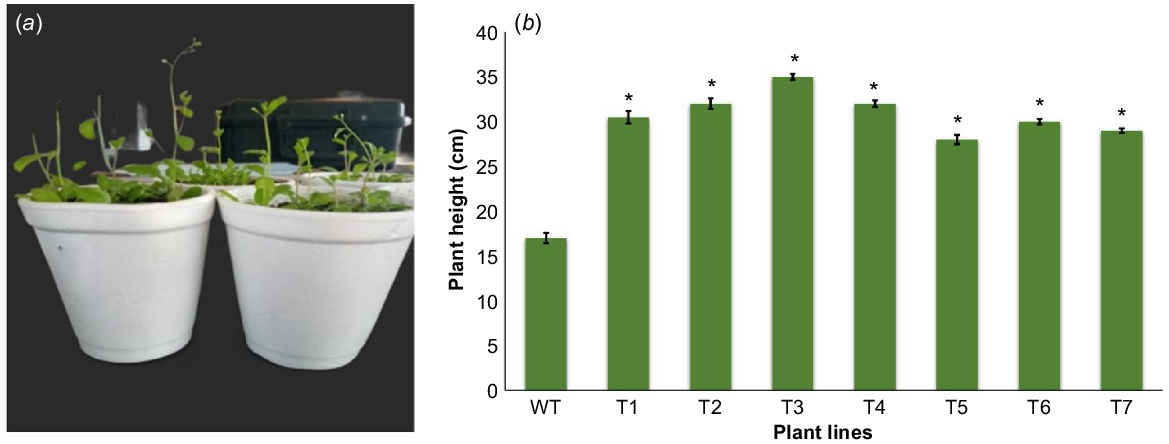
Transgenic plants showed early bolting as compared to the wild plants (Fig. 3a). After 35 days of sowing transgenic plants showed significantly greater heights, with a maximum of 34.5 cm in the T3 line; the minimum was recorded as 24.4 cm in the T6 line. In comparison, amongst wild type plants the maximum recorded height was only 17.5 cm (Fig. 3b).
Comparison of plant height between transgenic and wild plants at maturity. (a) Representative photograph of transgenic and wild Arabidopsis showing early bolting and increased height. (b) Comparison of plant heights between transgenic (T1–T7) and wild (WT) plants. Data are shown as the mean ± s.e. of seven plants. Vertical bars represent the plant height and the asterisk sign indicates significant difference (P = 0.05) according to Student’s t-test.
Number of leaves and chlorophyll contents
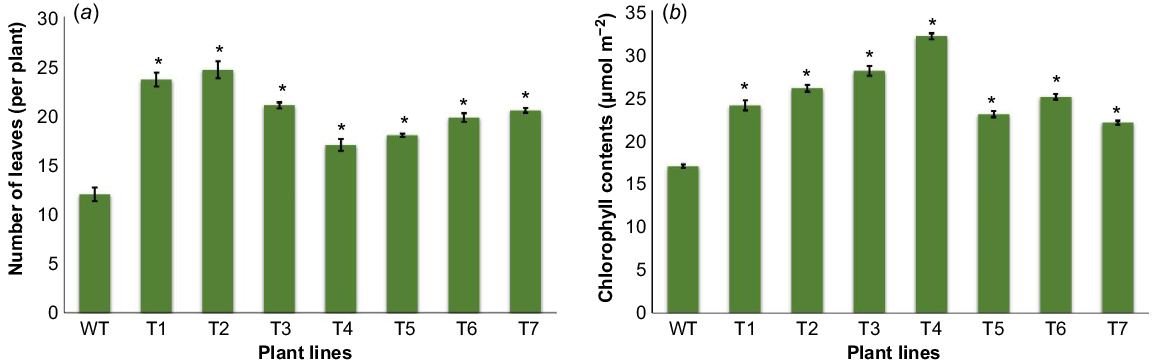
After 32 days of growth, the number of leaves were counted for both transgenic and wild plants. It was observed that transgenic plants gained maximum of 25 more leaves than wild plants that grew with a maximum of 12 leaves (Fig. 4a). Data indicated that transgenic plants accumulated more chlorophyll content as compared to wild plants. The highest record of chlorophyll content was 30.5 μmol m−2 in as compared to the highest recorded in wild plants, 19 μmol m−2 (Fig. 4b).
Comparison of leaf numbers and chlorophyll contents between transgenic and wild plants. (a) Comparison of leaf numbers between transgenic (T1–T7) and wild (WT) plants. (b) Chlorophyll content analysis in both transgenic (T1–T7) and wild (WT) plants. Data presented as the mean ± s.e. of seven plants. Vertical bars represent the leaf numbers and chlorophyll contents. The asterisk sign indicates significant difference (P = 0.05) according to Student’s t-test.
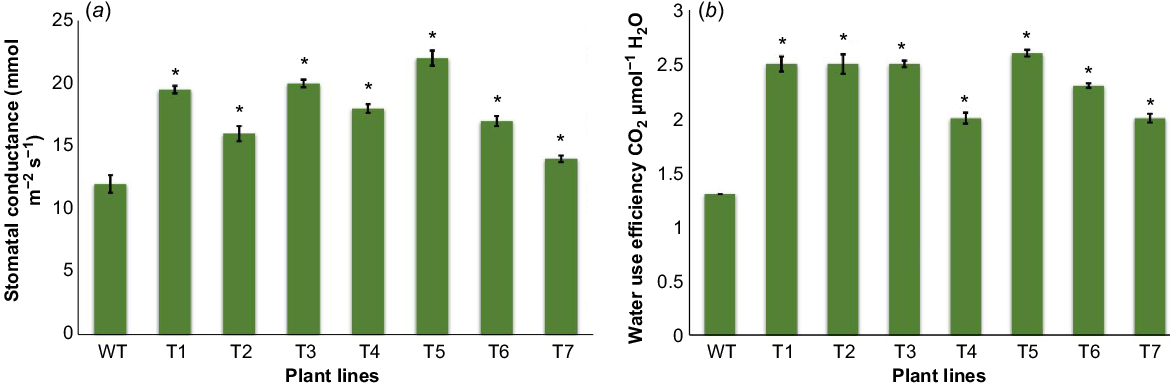
Stomatal conductance and water use efficiency
The maximum level of stomatal conductance in the transgenic plants was 22 mmol m−2 s−1 as compared to 12 mmol m−2 s−1 in wild plants (Fig. 5a). Water use efficiency was increased to a maximum of 2.6 μmol CO2 μmol−1 H2O in comparison to 1.5 μmol CO2 μmol−1 H2O in wild plants (Fig. 5b).
Stomatal conductance and water use efficiency analysis between transgenic and wild plants. (a) Graphical representation for stomatal conductance rate in both (T1–T7) and wild (WT) plants. (b) Water use efficiency analysis in both transgenic (T1–T7) and wild (WT) plants. Data presented as the mean ± s.e. of seven plants. Vertical bars represent chlorophyll content, the asterisk sign indicates significant difference (P = 0.05) according to Student’s t-test.
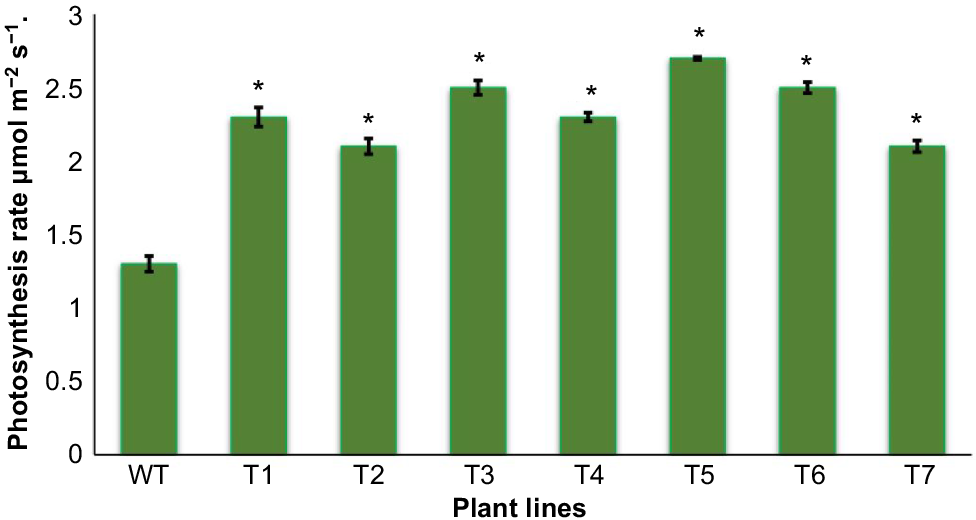
Photosynthesis rate
Transgenic plants exhibited enhanced photosynthetic rate. Among all the seven tested lines, 2.7 μmol m−2 s−1 was recorded as the highest rate; whereas the highest photosynthetic rate in wild plants was 1.3 μmol m−2 s−1 in wild plants (Fig. 6).
Discussion
In the current scenario of environmental pollution, temperature and CO2 levels are elevated to a greater level, which negatively affects crop growth and productivity (Song et al. 2023). Environmental stress has become exacerbated in C3 type plants, as they must perform photorespiration, which ultimately causes lower photosynthetic activity (Ferrari et al. 2022). There is an immense need to adopt methods that can modify C3 type plants to minimise the energy loss created by photorespiration. Various methods have been formulated and adopted for mechanising the C3 photosynthetic pathway, like RuBisCO modification and engineering a C4 mechanism of photosynthesis, but the success rate is low as it is quite difficult to bypass photorespiration (Tan and Chen 2023). Cyanobacteria are known to have independent routes for the catabolism of toxic molecules of glycolate into products that are non-toxic. Therefore, cyanobacterial genes have become an interesting and valuable tool as they are in wide use in genetic transformation of plants with a C3 mechanism, for enhancement of biomass and photosynthesis (Satta et al. 2023). Previously, overexpression of the cyanobacterial gene SBPase in tobacco resulted in an elevated photosynthetic rate (Mitchell et al. 2020). In this report, we were successful in achieving an enhanced photosynthesis rate and change in related parameters like chlorophyll contents, stomatal conductance and water use efficiency. In transgenic plants, 2.7 µmol m−2 s−1 was recorded as maximum rate of photosynthesis as compared to the 1.3 µmol m−2 s−1 in wild plants (Fig. 6). In addition, a maximum of 30.5% chlorophyll contents, 22% stomatal conductance and 2.6 μmol CO2 μmol−1 H2O water use efficiency was recorded as compared to 1.5 μmol CO2 μmol−1 H2O in the wild plant. These results give a clear indication that the transformed gene showed its maximum expression (Fig. 2a). Previously, along with photosynthetic rate, increased leaf surface and chlorophyll contents have been enhanced by using antisense cyanobacterial genes for modifying the photosynthetic mechanism in C3 plants (Sahrawy et al. 2004).
Plant height in the transgenic plants was higher than the wild type plants (Fig. 3). In this respect, our results are in line with previous experimental studies in potato and Cannabis sativa when transformed with glycolate dehydrogenase genes of cyanobacteria (Nölke et al. 2014). The increased vegetative growth is attributed either to activation of metabolic activities within non-photosynthetic tissues by cyanobacterial enzymes like dehydrogenases and fructose diphosphatase, or alternatively to plants having saved energy by minimising photorespiration, with that energy subsequently redirected to carbohydrate synthesis (Long et al. 2015). It is also suggested that FBPase genes control the carbon metabolism and enhance the sugar contents, which ultimately results in the enhanced phenotypic characters like increased plant height, leaf number and multiple branching (Otori et al. 2017).
In addition, such genes also take control to regulate the contents of the carbohydrates in the transgenic plants (Sahrawy et al. 2004). It was later confirmed that mutant lines of cyanobacterial gene produced the plants with two-fold lower photosynthesis (Rojas-González et al. 2015). On the other hand, with the use of mutant FBPase genes, an increased amount of H2O2 was noted and decreased amount of organic acid was also reported. This supports the use of the FBPase gene, not only for controlling photosynthetic machinery enhancement, but also for improving resistance against oxidative stress in transgenic plants.
The success rate of transformation is always understood to be dependent upon many factors like the choice of plant, transformation methods and also the candidate gene (Waheed et al. 2016). Arabidopsis has been a great choice for transformation experiments as it is acquiescent to genetic modification due to its simpler genome and shorter life cycle. The method of genetic transformation was robust, economic and gave stable integration. It also raised the chance of successful transformation chances and, last but not least, the candidate gene does not interfere with the regulatory and growth mechanism of the host plants (Fernie and Yan 2019). However, the results of photosynthesis related tests are required to better understand the mechanism at the transcription level.
Conclusions
The current study reports a robust and successful genetic transformation of A. thaliana to express the cyanobacterial gene FBPase, leading to an enhanced photosynthetic rate (up to 2.7 μmol m−2 s−1) with higher chlorophyll contents, stomatal conductance and water use efficiency. However, a few areas still need to be explored, like differential transcriptomic analysis of transgenic crops, so that this gene can be utilised to enhance photosynthetic rate in economic crops as well.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Conceptualisation: Ummara Waheed, Raza Ahmed; Methodology: Ummara Waheed, Raza Ahmed, Suk-Yoon Kwan; Formal analysis and investigation: Fatima Gulzar, Zulqurnain Khan; Writing – original draft preparation: Fatima Gulzar, Ummara Waheed, Mohmmad Maroof Shah; Writing – review and editing: Shoaib ur Rehman, Muhammad Abu Bakar Saddique, Muhmmad Zahid Ishaq, Maria Siddique, Irum Shahzadi, Sulaiman Ali Alharbi, Mohammad Javed Ansari; Supervision: Ummara Waheed. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This project was supported by Researchers Supporting Project Number (RSP2024R5) King Saud University, Riyadh, Saudi Arabia.
References
Abbasi AZ, Bilal M, Khurshid G, Yiotis C, Zeb I, Hussain J, Baig A, Shah MM, Chaudhary SU, Osborne B, Ahmad R (2021) Expression of cyanobacterial genes enhanced CO2 assimilation and biomass production in transgenic Arabidopsis thaliana. PeerJ 9, e11860.
| Crossref | Google Scholar | PubMed |
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258-270.
| Crossref | Google Scholar | PubMed |
Arrivault S, Alexandre Moraes T, Obata T, Medeiros DB, Fernie AR, Boulouis A, Ludwig M, Lunn JE, Borghi GL, Schlereth A, Guenther M, Stitt M (2019) Metabolite profiles reveal interspecific variation in operation of the Calvin–Benson cycle in both C4 and C3 plants. Journal of Experimental Botany 70, 1843-1858.
| Crossref | Google Scholar | PubMed |
Bowes G, Ogren WL, Hageman RH (1971) Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochemical and Biophysical Research Communications 45, 716-722.
| Crossref | Google Scholar | PubMed |
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735-743.
| Crossref | Google Scholar | PubMed |
Dalal J, Lopez H, Vasani NB, Hu Z, Swift JE, Yalamanchili R, Dvora M, Lin X, Xie D, Qu R, Sederoff HW (2015) A photorespiratory bypass increases plant growth and seed yield in biofuel crop Camelina sativa. Biotechnology for Biofuels 8, 175.
| Crossref | Google Scholar |
Fernie AR, Yan J (2019) De novo domestication: an alternative route toward new crops for the future. Molecular Plant 12, 615-631.
| Crossref | Google Scholar | PubMed |
Ferrari RC, Kawabata AB, Ferreira SS, Hartwell J, Freschi L (2022) A matter of time: regulatory events behind the synchronization of C4 and crassulacean acid metabolism in Portulaca oleracea. Journal of Experimental Botany 73, 4867-4885.
| Crossref | Google Scholar | PubMed |
Ferreira MJ, Silva J, Pinto SC, Coimbra S (2023) I choose you: selecting accurate reference genes for qPCR expression analysis in reproductive tissues in Arabidopsis thaliana. Biomolecules 13(3), 463.
| Crossref | Google Scholar | PubMed |
Heyduk K (2022) Evolution of Crassulacean acid metabolism in response to the environment: past, present, and future. Plant Physiology 190, 19-30.
| Crossref | Google Scholar | PubMed |
Ling Q, Huang W, Jarvis P (2011) Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynthesis Research 107, 209-214.
| Crossref | Google Scholar | PubMed |
Long SP, Marshall-Colon A, Zhu X-G (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56-66.
| Crossref | Google Scholar | PubMed |
Mitchell MC, Pritchard J, Okada S, Zhang J, Venables I, Vanhercke T, Ral J-P (2020) Increasing growth and yield by altering carbon metabolism in a transgenic leaf oil crop. Plant Biotechnology Journal 18, 2042-2052.
| Crossref | Google Scholar | PubMed |
Mizokami Y, Sugiura D, Watanabe CKA, Betsuyaku E, Inada N, Terashima I (2019) Elevated CO2-induced changes in mesophyll conductance and anatomical traits in wild type and carbohydrate-metabolism mutants of Arabidopsis. Journal of Experimental Botany 70, 4807-4818.
| Crossref | Google Scholar | PubMed |
Nölke G, Houdelet M, Kreuzaler F, Peterhänsel C, Schillberg S (2014) The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnology Journal 12, 734-742.
| Crossref | Google Scholar | PubMed |
Otori K, Tamoi M, Tanabe N, Shigeoka S (2017) Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Bioscience, Biotechnology, and Biochemistry 8, 1470-1477.
| Crossref | Google Scholar |
Rehman SU, Qanmber G, Tahir MHN, Irshad A, Fiaz S, Ahmad F, Ali Z, Sajjad M, Shees M, Usman M, Geng Z (2021) Characterization of vascular plant one-zinc finger (VOZ) in soybean (Glycine max and Glycine soja) and their expression analyses under drought condition. PLoS ONE 16, e0253836.
| Crossref | Google Scholar | PubMed |
Rojas-González JA, Soto-Súarez M, García-Díaz Á, Romero-Puertas MC, Sandalio LM, Mérida Á, Thormählen I, Geigenberger P, Serrato AJ, Sahrawy M (2015) Disruption of both chloroplastic and cytosolic FBPase genes results in a dwarf phenotype and important starch and metabolite changes in Arabidopsis thaliana. Journal of Experimental Botany 66(9), 2673-2689.
| Crossref | Google Scholar | PubMed |
Sahrawy M, Ávila C, Chueca A, Cánovas FM, López-Gorgé J (2004) Increased sucrose level and altered nitrogen metabolism in Arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose-1,6-bisphosphatase. Journal of Experimental Botany 55(408), 2495-2503.
| Crossref | Google Scholar | PubMed |
Satta A, Esquirol L, Ebert BE (2023) Current metabolic engineering strategies for photosynthetic bioproduction in cyanobacteria. Microorganisms 11, 455.
| Crossref | Google Scholar |
Song Z, Wang L, Lee M, Yue GH (2023) The evolution and expression of stomatal regulators in C3 and C4 crops: implications on the divergent drought tolerance. Frontiers in Plant Science 14, 1100838.
| Crossref | Google Scholar | PubMed |
Sowjanya BA, Narayana BD, Shreyas S (2019) Enhancement of photosynthetic efficiency of C3 plants. International Journal of Current Microbiology and Applied Sciences 8, 775-786.
| Crossref | Google Scholar |
Tan B, Chen S (2023) Defining mechanisms of C3 to CAM photosynthesis transition toward enhancing crop stress resilience. International Journal of Molecular Sciences 24, 13072.
| Crossref | Google Scholar |
Waheed U, Harwood W, Smedley M, Kwak S-S, Ahmad R, Shah MM (2016) Comparison of agrobacterium mediated wheat and barley transformation with nucleoside Diphosphate Kinase 2 (NDPK2) gene. Pakistan Journal of Botany 48, 2467-2475.
| Google Scholar |