Genotypic variation in soil water use and root distribution and their implications for drought tolerance in chickpea
Ramamoorthy Purushothaman A B , Lakshmanan Krishnamurthy A F , Hari D. Upadhyaya A C D , Vincent Vadez A and Rajeev K. Varshney A EA International Crops Research Institute for the Semiarid Tropics (ICRISAT), Patancheru 502 324, Telangana, India.
B Jawaharlal Nehru Technological University Hyderabad (JNTUH), Hyderabad 500 085, Telangana, India.
C Department of Agronomy, Kansas State University, 2004 Throckmorton PSC, 1712 Claflin Road, Manhattan, KS 66506, USA.
D UWA Institute of Agriculture, University of Western Australia, 35 Stirling Highway, Crawley, Perth, WA 6009, Australia.
E School of Plant Biology and Institute of Agriculture, The University of Western Australia, 35 Stirling Highway, Crawley, Perth, WA 6009, Australia.
F Corresponding author. Email: lkm1949@gmail.com
Functional Plant Biology 44(2) 235-252 https://doi.org/10.1071/FP16154
Submitted: 24 April 2016 Accepted: 16 September 2016 Published: 28 November 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Chickpeas are often grown under receding soil moisture and suffer ~50% yield losses due to drought stress. The timing of soil water use is considered critical for the efficient use of water under drought and to reduce yield losses. Therefore the root growth and the soil water uptake of 12 chickpea genotypes known for contrasts in drought and rooting response were monitored throughout the growth period both under drought and optimal irrigation. Root distribution reduced in the surface and increased in the deep soil layers below 30 cm in response to drought. Soil water uptake was the maximum at 45–60 cm soil depth under drought whereas it was the maximum at shallower (15–30 and 30–45 cm) soil depths when irrigated. The total water uptake under drought was 1-fold less than optimal irrigation. The amount of water left unused remained the same across watering regimes. All the drought sensitive chickpea genotypes were inferior in root distribution and soil water uptake but the timing of water uptake varied among drought tolerant genotypes. Superiority in water uptake in most stages and the total water use determined the best adaptation. The water use at 15–30 cm soil depth ensured greater uptake from lower depths and the soil water use from 90–120 cm soil was critical for best drought adaptation. Root length density and the soil water uptake across soil depths were closely associated except at the surface or the ultimate soil depths of root presence.
Additional keywords: drought tolerance, field phenotyping, root length density, soil water utilisation.
Introduction
Chickpea (Cicer arietinum L.) is the second most widely grown pulse after dry (common) bean (Phaseolus vulgaris L.) globally, with a total production of 14.2 million tons from an area of 14.8 million ha and a productivity of 0.96 t ha–1 (FAOSTAT 2014). About 90% of world’s chickpea is grown under rainfed conditions (Kumar and Abbo 2001), where the crop grows and matures on a progressively depleting soil moisture profile (Kashiwagi et al. 2013) and generally experiences terminal drought stress (DS). Drought causes substantial annual yield losses up to 50% in chickpea, which equals to a loss of US $ 900 million, and the productivity remained constant for the past six decades (Ryan 1997; Ahmad et al. 2005; Bantilan et al. 2014). With the changing climate scenarios and continuous population explosion, there is a great need to develop high-yielding chickpea varieties with improved drought tolerance (Evans 1998; Gaur et al. 2014). Improvements of chickpea yields under DS have been achieved, mostly by breeding short duration cultivar that mature before the water deficit becomes too severe (Kumar et al. 1985; Kumar and Rao 2001) with an often observed penalty in grain yield due to underutilisation of the available growing season. To overcome this self-imposed penalty, relatively longer (medium) duration chickpea cultivars have to be improved either though drought avoidance or tolerance mechanisms (Sabaghpour et al. 2003).
Given the progressively receding moisture and increasing temperature at the terminal growth phase of typical chickpea growing environments, increased rooting depth and root distribution have been found to provide a significant advantage in yield increase, making the root system an essential part of drought avoidance (Jordan et al. 1983; Jones and Zur 1984; Ludlow and Muchow 1990; Silim and Saxena 1993; Kashiwagi et al. 2005). Root growth simulation studies have not only confirmed the importance of deeper root systems and root proliferation on grain yield across several years and environments in USA (Sinclair 1994) but also on chickpea under Iranian conditions (Soltani et al. 1999). The chickpea simulation studies have also showed that early maturity, increasing drought avoidance through deep and profuse root system and higher transpiration efficiency were the traits most likely to result in higher yield under terminal DS (Soltani et al. 2000). At the same time, as experienced in wheat, excessive root growth early in the growing season can also be counterproductive for increased yield production by exhausting soil water reserves before the plant is able to complete its life cycle (Richards and Passioura 1989). Additionally, the metabolic costs (production and maintenance of tissues, measured in units of carbon) of soil exploration by root systems are substantial, and can exceed 50% of daily photosynthesis (Lambers et al. 2002). The timing of enhanced root growth was also shown to be critical for drought tolerance. Greater soil water extraction at the reproductive stages, but not the overall root biomass (determined by minirhizotron evaluations), was shown to be important for yield formation under DS (Zaman-Allah et al. 2011). Therefore, these controversial views on the decisive association of large and deep root system with the grain yield under DS needs to be settled by generating more details on the root distribution, soil water use and the plant biomass productivity under DS. The debate on this association is strongly influenced by the wide fluctuations in water availability and root turnover under DS (Cutforth et al. 2013).
Efficient water uptake ability was found to be essential for yield improvement (Fischer et al. 1998; Blum 2009; Wasson et al. 2012) and this efficiency is recognised to rely on the size and activity of the root system (Gregory 1994; Gowda et al. 2012). Timely and enhanced soil water uptake by equally large root systems seems to be one of the most promising approaches for enhancing drought tolerance in legumes and this association was documented in many studies (Kamoshita et al. 2000; Okada et al. 2002; Kashiwagi et al. 2006, 2015; Bernier et al. 2009; Bandyopadhyay 2014). However, there are other studies that contradict this view on the basis of root system sufficiency, soil water environments and on the timings of soil water uptake. Moreover, the association between root proliferation and soil water uptake can be fundamental to prove that yield losses mainly rely on soil water availability. Thus to provide a comprehensive understanding of the contribution of roots to grain yield, it is critical to link this association through the soil water utilisation. Therefore, the objectives of this study were to (i) assess the extent of root distribution and soil water uptake across soil depths and crop growth stages under water deficit and field capacity conditions; and (ii) relate this information with the known drought reactions of diverse chickpea genotypes.
Materials and methods
Plant material and crop management
Twelve chickpea genotypes viz., ICC 4958, ICC 8261, ICC 867, ICC 3325, ICC 14778, ICC 14799, ICC 1882, ICC 283, ICC 3776, ICC 7184, Annigeri, and ICCV 10 with close phenology but good contrasts for root development, drought response and canopy temperature depressions were chosen for this study and were field-evaluated on a Vertisol (fine montmorillonitic isohyperthermic typic pallustert) during the post-rainy season, in 2009–10 and 2010–11, at ICRISAT, Patancheru (17°30ʹ N, 78°16ʹE; altitude 549 m) in peninsular India. The water holding capacity of this field in lower limit: upper limit was 0.26 : 0.40 cm3 cm–3 for the 0–15 cm soil layer, and 0.30 : 0.47 cm3 cm–3 for the 105–120 cm soil layer. The available soil water up to 120 cm depth observed in this study was 216 mm in 2009–10 and 207 mm in 2010–11. The bulk density was 1.35 g cm–3 for the 0–15 cm soil layer and 1.42 g cm–3 for the 105–120 cm soil layer (El-Swaify et al. 1985). The field used was solarised using polythene mulch during the preceding summer primarily to protect the crop from wilt causing fungi Fusarium oxysporum f. sp, among other benefits and damages (Chauhan et al. 1988).
The fields were prepared in to broad bed and furrows with 1.2 m wide beds flanked by 0.3 m furrows. Surface application and incorporation of 18 kg N ha–1 and 20 kg P ha–1 as di-ammonium phosphate were carried out. The experiment was conducted in a randomised complete block design (RCBD) with three replications. Seeds were treated with 0.5% Benlate (EI DuPont India Ltd) + Thiram (Sudhama Chemicals Pvt. Ltd) mixture for both 2009–10 and 2010–11 seasons. The seeds were hand-sown manually at a depth of 2–3 cm maintaining a row to row distance of 30 cm and a plant to plant distance of 10 cm with in rows with a row length of 4 m on 31 October 2009 and 20 November 2010. About 82 seeds were used for each 4 m row and at 10 days after sowing (DAS) the plants were thinned maintaining a plant-to-plant spacing of 10 cm. A 20 mm irrigation through sprinklers was applied immediately after sowing to ensure uniform seedling emergence. Subsequently, plants were grown under rainfed condition to impose terminal DS and irrigated once in 15–20 days as an optimally irrigated (OI) treatment. The plots were kept weed free by hand weeding and intensive protection were taken against pod borer (Helicoverpa armigera).
Root sample extraction and processing
Steel soil core tubes (50 mm in diameter) were used to collect soil + root sample up to 120 cm at a time interval of every 10 days from mid-vegetative period of crop growth. Each samples comprised of two or three cores and all these cores were pooled depth-wise to increase the sample size. The extracted soil core was separated in to subcores of 15 cm each having eight subcores out of 120 cm. The soil sample containing roots were soaked in water overnight, soil was mixed with tap water to form a suspension, and the roots were recovered by passing the soil-water suspension through a 2 mm wire mesh sieve. Chickpea roots were then separated from the organic debris and weed roots manually by floating the sample material on water in trays. Recovered roots were suspended in a transparent tray with 2–3 mm film of water for easy dispersion of roots and scanned using a scanner. Total root length of each sample was measured using the image analysis system (WinRhizo, Regent Instruments Inc.). The roots were kept for oven drying at 70°C for 72 h (to constant weight). Root dry weight (RDW in g m–3) was estimated for each depth or for total depth separately. A total of ultimate two soil depths (15 + 15 cm) RDWs were considered as deep RDW. Root length density (RLD) was estimated as cm cm–3 of soil from the root length (RL) of the subcore as root length (cm) per volume of soil core (cm3).
Soil moisture measurement
The TRIME-soil moisture probe was used to measure the available soil moisture content in the field. TRIME access tubes, with a depth of 150 cm and an inner diameter of 4.2 cm (0.1 cm wall-thickness), were installed in each plot. TRIME-FM (IMKO) instrument connected with a cylindrical 18 cm long probe that can access the entire depth of access tube measures and directly converts measured transit-times in terms of soil water-contents displayed on its front panel. These measurements were taken in both the OI and DS. The amount of soil moisture (in volumetric terms) at each 15 cm depth interval was recorded up to 120 cm. There were 36 access tubes each under DS and OI conditions in which both TRIME TDR observations and the manual gravimetic soil moistures were measured separately for establishing the soil depth-wise calibration curves. The TDR soil moisture observations were corrected using the correction factor specific to soil depth and season. Moisture content of the surface soil (0–15 cm) was measured only through gravimetry. When required the soil water held in each soil horizon of 15 cm depth was summed up to 1.2 m.
Crop utilised soil water, from the root inhabited soil layers, was calculated as follows:
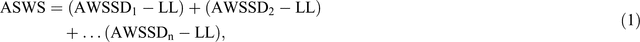
where ASWS is available soil water at sowing, ASWS D1 is available soil water at sowing in soil depth 1 (0–15 cm), ASWS D2 is available soil water at sowing in soil depth 2 (15–30 cm), ASWS Dn is available soil water at sowing in soil depth n and LL is the lower limit for plant uptake:
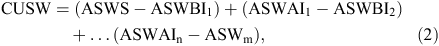
where CUSW is crop utilised soil water (mm), ASWS is available soil water at sowing (mm), ASWBI1 is available soil water before the first irrigation or rain, ASWAI1 is available soil water immediately after the first irrigation or rain, ASWBI2 is available soil water before the second irrigation or rain, ASWAIn is available soil water before the nth irrigation or rain and ASWm is available soil water at crop maturity.
Statistical analysis
The replication-wise data on phenotypic traits observed at different crop growth stages in 2009–10 and 2010–11 were subjected to statistical analysis using one way ANOVA. Significance of means was estimated through F value for each trait. The means derived from the ANOVA were used for correlations, regressions using Genstat software (12th edn) and path coefficient analysis using MINITAB Release 14.1 software. Variance components due to genotypes (σ2 g) and error (σ2e) and their standard errors were determined. Here, the replication was treated as a fixed effect and the genotype (G) × DAS (D) × treatment (T) × year (Y) interaction as random. The variance due to (G) (σ2 g) and G × D × T × Y interaction (σ2gdty) and their s.e. were determined. Broad sense heritability (h2b) was estimated as h2b = σ2 g/(σ2g + (σ2e/r)) where r was the number of replications (Lush 1940; Searle 1961).
Results
Weather pattern
In both the years, the rain received before the cropping season was >850 mm, well distributed and more than enough to ensure complete charging of the soil profile. Rains during cropping summed to 44 mm during 9 to 19 DAS in 2009–10 and 12.6 mm during 19 to 22 DAS in 2010–11 that delayed the onset of drought but the terminal DS did built up (data not shown). There was another rain (39 mm) at 75 DAS during 2009–10, but at this stage under DS the early or medium maturing accessions had already crossed the stage of responsiveness. Overall, the minimum temperatures were higher, particularly during the critical third and fourth week of December, and maximum temperatures were lower during 2009–10 (Fig. 1). Relatively cooler minimum temperatures and maximum temperatures at vegetative period were observed in 2010–11. The cumulative evaporation was relatively higher during 2009–10 cropping season than the subsequent year, except during the reproductive period in 2010–11, influenced by the higher vapour pressure deficit (VPD).
Genetic variation in root length density and crop utilised soil moisture
There was a large range of genotypic variation in RLD and crop utilised soil moisture (CUSM), measured at different growth stages, in both drought treatments and years. In comparison to DS in 2009–10, the RLD (trial mean) was high under OI only in the surface soil depths. However, the RLD of mid- and deeper soil layers were higher under DS except at 90 DAS in 2010–11 compared with OI. Trial mean of CUSM was higher under OI across growth stages and years with very few exceptions (Tables 1, 2 and Tables S1, S2, available as Supplementary Material to this paper). The genotype × DAS, genotype × DAS × treatment (drought), genotype × DAS × treatment (drought) × year (environment) interactions were statistically significant for RLD and CUSM across different stages of crop growth with very few exceptions (Table S3).
The severity of DS was relatively high in 2010–11 compared with 2009–10 due to the rainfall occurrence during the experiment (Fig. 1). Across growth stages, drought treatment and years, highest range of genetic variation for RLD (difference between the ranges) was observed at the surface soil layers followed by penultimate soil depths except at the 90 DAS measurement in 2010–11 (Tables 1, 2). The trial mean of RLD was found to be the highest at the surface soil layer across growth stages in both drought treatments and years (Tables 1, 2, S1, S2). Among the soil depths, the extensive increase (from one crop stage to other stage) in RLD was found to be occurring at the distal roots under DS whereas it occurred at the surface and mid-soil layer under OI (data not shown). Genotypes varied significantly (P = >0.001), for RLD measured across growth stages and drought treatments in both the years. The heritability of RLD was high across growth stages, drought treatments and years. Under DS, the range of heritability for RLD in 2009–10 was 0.225 to 0.917, 0.498 to 0.913 and 0.628 to 0.972 for 35, 50 and 80 DAS, respectively. Similarly, in 2010–11 it was 0.901 to 0.977, 0.621 to 0.848, 0.607 to 0.859, 0.618 to 0.910, 0.563 to 0.876 and 0.632 to 0.913 for 35, 45, 55, 65, 75 and 90 DAS respectively (Tables 1, 2).
The highest range of variation among genotypes for CUSM was observed at the penultimate soil depths across growth stages under DS and at the mid soil depths under OI particularly at 30–45 cm in both 2009–10 and 2010–11 (data not shown). The trial mean of CUSM was the highest at the surface soil layers at 35 DAS. And with the increase in crop growth stage, it was found to be the highest at the soil depth 45–60 cm during the reproductive stage across drought treatment and years (Tables 1, 2, S1, S2). Among the soil depths, the extensive soil moisture uptake had occurred at the penultimate to distal rooting zone soil depths under DS whereas at the mid-soil layer (30–60 cm) under OI (data not shown). The variation among the genotypes (σ2 g (α level)) for CUSM was poor for all the soil depths at 35 DAS and such variation became highly significant from the flowering stage of crop growth onwards except for the surface soil layer in both the drought treatments and years (Tables 1, 2, S1, S2). The heritability of RLD was largely low to moderate across growth stages under both drought treatments and years. Under DS, the range of heritability for CUSM in 2009–10 was 0.174–0.380, 0.129–0.699 and 0.281–0.822 for 35, 50 and 80 DAS respectively. Similarly, in 2010–11 it was 0.012–0.289, 0.093–0.299, 0.126–0.437, 0.137–0.627, 0.092–0.629 and 0.035–0.696 for 35, 45, 55, 65, 75 and 90 DAS respectively.
Association of RLD with CUSM
Compared with OI, all the genotypes produced higher RLD at the penultimate soil layers as a response of DS except at 35 DAS and also the RLD varied from one genotype to other extensively under DS (data not shown). The mean CUSM at the final stage of crop growth was found to be about 1-fold less under DS (Table 3).

|
In both the years under both drought treatments, the linear regression between the roots (RLD) present in any soil zone and the amount of soil water utilised (CUSM) from that zone was found to be significantly positive explaining a major part of the CUSM variation in all the samplings and across crop growth stages except at the surface soil layers or the freshly roots descended soil zones with a few exceptions in the year 2009–10 (Figs 2, 3 and Figs S1 and S2, available as Supplementary Material to this paper). These associations between RLD and CUSM were the closest in most active soil water absorption zones such as 0–15 cm at 35 DAS, 75–90 cm at 50 DAS and 60–75 at 80 DAS under DS in 2009–10 and, 30–45 cm at 35 DAS, 45–60 cm at 45 and 55 DAS, 75–90 cm at 65 DAS, 60–75 cm at 75 DAS in 2010–11 (Figs 2, 3). None of the soil depths have shown any significant relationship between RLD and CUSM at 90 DAS in 2010–11, as most of the genotypes had attained maturity and the root activity ceased.
Under OI, the closest association between RLD and CUSM was found to occur in 0–15 cm soil depth at 35 DAS, 30–45 cm at 50 DAS and 90–105 at 80 DAS in 2009–10 and 15–30 cm at 35 DAS, 60–75 cm at 45 DAS, 30–45 cm at 55 DAS, 45–60 cm at 65 DAS, 105–120 cm at 75 DAS and 75–90 cm at 90 DAS in 2010–11 (Figs S1, S2).
Growth stage, soil depths and genotypes interactions in soil water uptake
The soil water uptake is a continuous process and the rate of uptake would differ from one soil depth to the other, depending on various parameters such as root distribution, soil water availability and plant age. In 2009–10 under DS, the average CUSM between 36 and 50 DAS was comparatively high from 15 to 60 cm soil depths and was found to be the maximum at 15–30 cm (5.0 mm) and 45–60 cm soil depths (4.9 mm) (Fig. 4a). At this stage, the most active or maximum water mining soil zones of the genotypes varied (data not shown). From the 15–30 cm soil depth, the drought tolerant genotype ICC 14799 (5.92 mm), the highly drought tolerant genotype ICC 867 (5.81 mm) and the widely adapted variety ICCV 10 (5.81 mm) extracted the maximum soil water (Table 4), whereas, the large rooting drought tolerant genotype ICC 4958 extracted 5.97 mm from 60–75 cm soil depth and 3.49 mm from the 75–90 cm soil depths (Table 4). Similarly, the highly drought tolerant genotype ICC 14778 extracted 4.90 mm from 60–75 cm and 3.44 mm from 75–90 cm soil depths. The water uptake of highly drought sensitive genotype ICC 3776 was consistently lower in all soil depths. The small rooting genotypes ICC 283 extracted 5.40 mm at 30–45 cm soil depth, the locally adapted variety Annigeri extracted 6.06 mm and the small rooting genotype ICC 1882 extracted 5.99 mm from 45–60 cm soil water.

|

|
The average CUSM during the reproductive crop growth period, 51 to 80 DAS, was comparatively high in soil depths 45 – 90 cm and was found to be maximum at 60–75 cm (11.5 mm) followed by the immediately adjacent soil depths (Fig. 4b; Table 4). At this stage, CUSM from the 0–15 cm soil layer was negative due the rainfall occurred during that period (Fig. 1). Excepting the surface soil layer, the average CUSM from the soil layer 15–30 cm was the lowest (1.89 mm) as soil water from this depth was already utilised in the vegetative phase of crop growth (Fig. 4b; Table 4). The CUSM of the small rooting genotype ICC 1882 was maximum (12.2 mm) from the 45–60 cm soil depth with the maintenance of a moderate level of soil water uptake from deeper soil layers (Table 4). When the soil water uptake from the penultimate soil layers alone (90–120 cm) was considered, the drought tolerant ICC 867 and ICC 14799 and the best adapted Annigeri and ICCV 10 had used above average quantities and, the drought sensitive genotype ICC 7184 and the large rooting drought tolerant genotype ICC 4958 had used below average quantities of soil water extraction.
The active soil water mining zones continued to descend with the advance in growth stages. Under DS in 2010–11, the average soil moisture uptake during various growth phases such as 35 to 45, 46 to 55, 56 to 65, 66 to 75 and 76 to 90 DAS was found to be the maximum in 15–30, 30–45, 60–75, 75–90 and 90–105 cm soil depths, respectively (Fig. 4c–g). A wide genotypic difference for CUSM was found during the growth phase of 56–65 DAS (8.56 mm) followed by 66–75 DAS (7.88 mm) as also these were the maximum water extracting soil zones across growth stages.
The soil water uptake between 35 to 45 DAS was the maximum at the soil depth 15–30 followed by 30–45 cm soil depths (Fig. 4c). At this stage the maximum rooting depth was found to be 75 cm. Except the small rooting (ICC 283 and ICC 1882) and the drought sensitive (ICC 7184 and ICC 3776) genotypes, the CUSM of the remaining (drought tolerant and adapted) genotypes were above average in soil water uptake at the soil depth 15–30 cm and the drought tolerant genotype ICC 14799 was found to be highest in CUSM (7.04 mm) (Table 5). The same type of soil water extraction behaviour was also observed at the subsequent soil depth 30–45 cm. Moreover, ICC 4958 and ICC 8261 were found to be superior in soil water extraction across soil depths at this growth stage (Table 5).
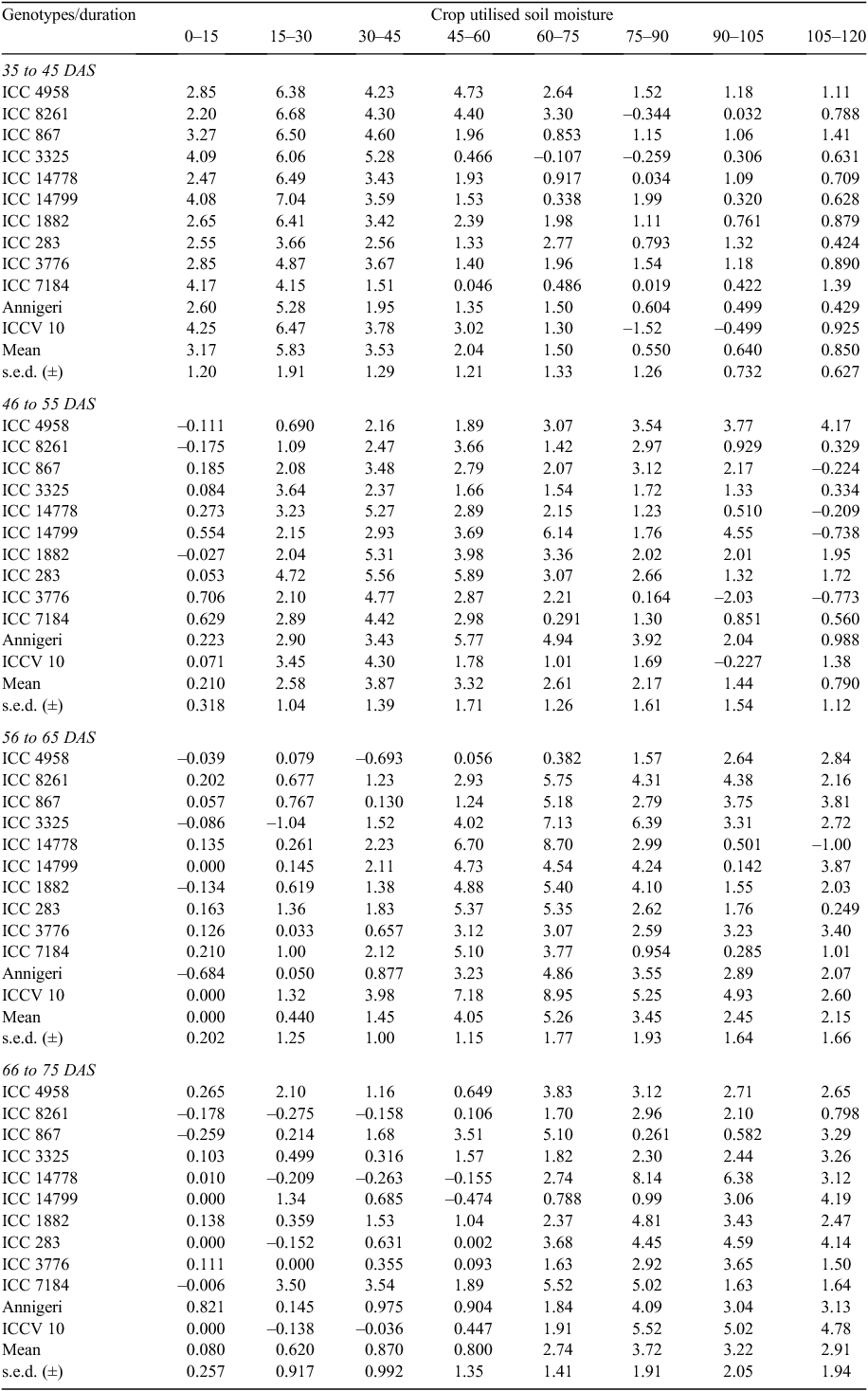
|
During 46 to 55 DAS, the maximum rooting depth was found to be 90 cm and the average soil water extraction was found maximum at 30–45 cm soil depth followed by 45–60 cm (Fig. 4d; Table 5). At this stage, the drought tolerant genotype ICC 14778 and both the small rooting genotypes (ICC 283 and ICC 1882) were found to be above average in CUSM compared with the remaining genotypes at the soil depth 30–45 (Table 5). At the same time, the genotypes which are stated to be low in CUSM at 30–45 soil depth were found to extract greater amounts of soil water at the 75–90 cm soil layer resulting in a high CUSM at this growth stage. The highly drought sensitive genotypes (ICC 7184 and ICC 3776) were found to be below average across all soil depths (Table 5).
During 56 to 65 DAS, the maximum rooting depth was found to be 105 cm and the average soil water extraction was found maximum at 60–75 cm soil depth followed by 45–60 and 75–90 cm (Fig. 4e; Table 5). At this stage, the genotypic differences in CUSM at 60–75 cm soil depth was high compared with the other maximum soil water extracting soil depths at various DAS measurements. At this stage the soil water uptake from the surface soil depths, 0–15 and 15–30 cm, had become nearly 0 mm indicating the absence of available soil water to the crops. The widely adapted genotype ICCV 10 (8.95 mm) and the highly drought tolerant genotype ICC 14778 (8.70 mm) extracted the highest soil water at 60–75 cm soil depth (Table 5). At the same time, the genotype ICC 4958 extracted the lowest soil water (0.38 mm) followed by the drought sensitive genotype ICC 3776 (3.07 mm). Moreover, majority of the drought tolerant and adapted genotypes had exhibited above average soil water extraction at the deepest soil layer (90–105 cm) at this growth stage (Table 5).
During 66 to 75 DAS, the maximum rooting depth was 120 cm and the average soil water extraction was found to be maximum at 75–90 cm soil depth followed by 90–105 and 105–120 cm depths (Fig. 4f; Table 5). At this stage, the genotypic differences for the CUSM at 75–90 cm soil depth was maintained similar as observed in the previous measurement (56–65 DAS) (Fig. 4e; Table 5). The soil water uptake from surface soil depths 0–60 had become <1 mm. The highly drought tolerant genotype ICC 14778 extracted the highest soil water (8.14 mm) followed by the widely adapted genotype ICCV 10 (5.52 mm) at 75–90 cm soil depth (Table 5). At the same time, the highly drought tolerant genotype ICC 867 extracted the lowest soil water (0.26 mm) followed by the drought tolerant genotype ICC 14799 (0.99 mm) more due to soil water exhaustion in this soil layer. There was a clear cut discrimination of genotypes observed for soil water uptake at the deepest soil layer (105–120) as both the highly drought sensitive genotypes (ICC 3776 and ICC 7184) were below average in soil water extraction and the genotype ICC 8261 was observed as the lowest (0.80 mm). The rest of the genotypes were found to be above average in soil water extraction at this growth stage (Table 5).
During 76 to 90 DAS, the maximum rooting depth was deeper than 120 cm and the average soil water extraction was maximal at 90–105 cm soil depth followed by the two adjacent (105–120 and 75–90 cm) soil depths (Fig. 4g). The soil water uptake of surface soil depths 0–75 cm had become <1 mm. The drought tolerant genotype ICC 3325 extracted the highest soil water (4.43 mm) and the genotype ICC 14799 had extracted the lowest at 90–105 cm soil depth. At this stage, majority of the drought tolerant and adapted genotypes were below average in soil water extraction (data not shown). At the same time, the drought tolerant genotype ICC 283 extracted the highest soil water (4.68 mm) followed by the highly drought tolerant genotype ICC 14778 (4.02 mm) and the all the large rooting, early maturing and drought sensitive genotypes were found to be below average in soil water extraction at the 105–120 cm soil depth (data not shown).
Genotypes varied significantly for total CUSM, integrated across growth stages, in both drought treatments. In 2009–10 under DS, the best adapted genotypes (ICCV 10 and Annigeri), drought tolerant genotypes (ICC 867, ICC 14778, ICC 14799) and the early duration small rooting genotype ICC 283 were high in total CUSM and rest of the genotypes had utilised low amount of soil water (Table 3). Also the same genotypes, except ICC 867, exhibited high uptake of soil moisture in 2010–11 too. In addition, the large root producing genotypes (ICC 4958 and ICC 8261) were below average in total soil water utilisation across years due to a shorter duration of ICC 4958 and a likely poor soil water utilisation characteristic of a kabuli, ICC 8261 (Purushothaman et al. 2014). The total CUSM of the genotype ICC 4958 remained to be above-average up to pod filling stage (data not shown) but its early maturing nature of this genotype lead this to use low amount of soil water. The difference in water uptake between the highest and the lowest CUSM genotypes was 9.6 mm in 2009–10 and 28.5 mm in 2010–11 (Table 3). Under OI the mean total CUSM was ~45% higher than that under the DS and all the soil moisture utilisation of the drought tolerant genotypes were high to above-average and the drought sensitive genotypes (ICC 3776 and ICC 7184) were lower in total CUSM across years with a very few exceptions.
Discussion
Adaptation to terminal drought
Recent studies on the importance of size of roots to grain yields and the timing of soil water utilisation for maximising grain yields under terminal DS had drawn variable conclusions such as positive (Sponchiado et al. 1989; White and Castillo 1992; Eghball and Maranville 1993; Kramer and Boyer 1995; Lynch 1995, 2013; Pandey et al. 2000a, 2000b; Liao et al. 2004; Nord and Lynch 2009; Puangbut et al. 2009; Lopes and Reynolds 2010; Manschadi et al. 2010; Zhu et al. 2010; Franco et al. 2011; Kell 2011; Trachsel et al. 2011; Suji et al. 2012; Wasson et al. 2012, 2014; Comas et al. 2013; Jaramillo et al. 2013; Uga et al. 2013; Fenta et al. 2014; Chimungu et al. 2014a, 2014b; Lynch et al. 2014; Bishopp and Lynch 2015) and negative or null (Ritchie 1981; Dardanelli et al. 2004; CIAT 2007, 2008; Beebe et al. 2009; Itoh et al. 2009; Ma et al. 2010; Manavalan et al. 2011; Ratnakumar et al. 2009; Zaman-Allah et al. 2011; Kumar et al. 2012; Schoppach et al. 2014; Vadez et al. 2012) association between the large root system and grain yield. The roots’ direct contribution of chickpea to shoot biomass productivity and grain yield formation had been elaborately described in a previous paper of this work (Purushothaman et al. 2016). In order to develop a comprehensive understanding of the dynamic relations between the root system and soil water uptake, this work focussed on the water uptake across the whole growth period both under DS and OI environments. This work also targeted the genotypic variation in root growth and soil water utilisation of chickpea and related it to the drought tolerance ability.
The choice of genotypes for this study had been selective, with the aim to include all the genotypic variation known for drought reactions and drought related traits. These comprise four known grain yield-based drought tolerant genotypes (Krishnamurthy et al. 2010), two strong, early root growth-based drought tolerant genotypes (Kashiwagi et al. 2005), two local adaptation-based drought tolerant genotypes (Ali et al. 2002), two weak, early root growth-based drought tolerant genotypes (Kashiwagi et al. 2005) and two grain yield-based drought sensitive genotypes (Krishnamurthy et al. 2010). Purushothaman et al. (unpubl. data) presented the results from this study that had clearly demonstrated the roots to undergo large morphological and structural changes to ensure desirable distribution when adapting to terminal drought as described in wheat previously (Sharp and Davies 1985). These changes were (i) early reduction or check in root prolificacy in surface soils most likely due to the early death of ultimate branches, (ii) greater prolificacy of roots in soil depths below 30 cm, and to a much greater extent below 75 cm, thereby increasing the proportion of deeper soil roots (Lafitte et al. 2001; Mishra et al. 2001; Comas et al. 2005; Benjamin and Nielsen 2006; Guswa 2008; Henry et al. 2011), (iii) reduction in overall root biomass (Robertson et al. 1980; Sánchez-Blanco et al. 2002; dos Santos et al. 2007; Navarro et al. 2009; Álvarez et al. 2009, 2011, 2012), (iv) reduction in root thickness, thereby increase in root length within the available root biomass, and to explore more volume of soil (Bañon et al. 2003; Koike et al. 2003; Kulkarni and Deshpande 2007; Chyliński et al. 2007; Franco et al. 2008; De Sousa and Lima 2010; Álvarez et al. 2011; Wasson et al. 2012; Bandyopadhyay 2014) and (v) early senescence of root system matching the shoot system senescence with no relevance to soil water availability. Similar pattern of RLD distribution was also observed in several legumes and cereal species such as field pea, rice, canola, cowpea, sunflower and sorghum (Liu et al. 2011; Gowda et al. 2012; Cutforth et al. 2013; Moroke et al. 2005).
Largely, roots are the first organ to perceive and respond to DS, before other plant organs, and communicate this information to the shoot (Konings and Jackson 1979; Bano et al. 1993; Ritchie 1981; Sauter et al. 2001; Chaves et al. 2003; Trachsel et al. 2010; Fenta et al. 2014). Numerous other studies have shown that plant roots can sense changes in abiotic factors such as water content (Davies et al. 2002; Wilkinson and Davies 2002), oxygen content (Drew et al. 1990) and the nutrient composition (Schachtman and Shin 2007) of the soil. The soil water withdrawal patterns found to differ significantly across different soil moisture environments and variation would be highly genotype-dependent as it differs across soil depths due to its variability in root distribution (Sponchiado et al. 1980; Benjamin and Nielsen 2006; Wang et al. 2012; Cutforth et al. 2013). In this study, soil water withdrawal had been greater under OI compared with DS. The mean total soil water uptake was about one-fold higher under OI (239 mm in 2009–10; 204 mm in 2010–11) compared with the DS (126 mm in 2009–10; 112 mm in 2010–11). Despite these differences between DS and OI treatments in soil water use, the difference in soil moisture left unused at the soil profile at crop maturity between drought treatments had remained negligible (2009–10: OI = 48 mm and NI = 45.5 mm; 2010–11: OI = 90 mm and NI = 95 mm) in both the years. These results demonstrated that the plants are capable of utilising maximum amount of soil water at both DS and OI by leaving the same amount of soil moisture at the soil profile at the end of the crop cycle. Therefore, the changes in the amount of soil water uptake as seen between DS and OI would likely depend on the plant adaptive functions such as leaf area development or biomass partitioning (Hammer et al. 2009; Borrell et al. 2014), length of the growing period (Krishnamurthy et al. 2013; Purushothaman et al. 2014), energy cost for the uptake and the soil water availability. The crop itself displays homeostasis in response to DS and one such adaptive adjustment had been evident by the reduction in growing time, particularly the reproductive phase, in chickpea (Krishnamurthy et al. 2013).
Under DS, at any specific growth stage, some specific soil depth(s) facilitated maximum water uptake and this soil zone was found to descend constantly across growing duration (Yu et al. 2007; Wang et al. 2012; Cutforth et al. 2013; Kashiwagi et al. 2015). When the integrated water uptake at the last sampling was considered, the maximum soil water uptake under DS was from 45–60 cm soil depth while under OI it was either 0–30 cm as seen in the first year or from 30 to 45 cm as in second year. In summary, when water is not limited plants prefer to utilise water more from surface soil layers (Ludlow and Muchow 1990). Plants are forced to mine deeper soil layers only when water is limited (Serraj et al. 2004; Pinheiro et al. 2005; Yu et al. 2007; Manschadi et al. 2010; Hammer et al. 2009, 2010; Wasson et al. 2012; Comas et al. 2013; Krishnamurthy et al. 2013; Lynch 2013; Steele et al. 2013). For example, in modelling exercises of soil water utilisation the root system had been considered to extract 40% of the total transpiration from the top quarter of root zone, even if the top layer is desiccated by evapotranspiration (Molz and Remson 1970) that was also confirmed to occur in chickpea (Krishnamurthy et al. 1999, 2010, 2013; Serraj et al. 2004; Kashiwagi et al. 2015).
Also the highest quantum of water any single soil depth can offer under DS, had come from 45–60 cm soil depth but from 30–45 cm soil depth or above, when irrigated. However, this should not undermine the critical nature of the water from deepest soil layers. At the last stages of crop growth (75–90 days after sowing) under DS, the four most successful genotypes (the drought tolerant ICC 867 and ICC 14799 and the best adapted Annigeri and ICCV 10) had used above average quantities from 90–120 cm soil depth and that had maximised the total water use from this soil zone. This had been the stage when the ultimate grain filling had been happening and the 90–120 cm soil depth remained as the most active water mining zone supporting the final stages of biomass partitioning to grains (Krishnamurthy et al. 2013; Wasson et al. 2012). Therefore, if the overall soil water use had to be maximised, its use has to be at a maximum in all soil layers. However, the root system finds different constraints across various soil depths such as competition from evaporation largely on the surface soil layers, soil compaction-lead higher soil resistance and poor soil aeration as the soil depths increased. The surface soil layer 15–30 cm had all the advantages such as early access by roots and better soil aeration but prone to evaporation. Therefore early use of this soil water not only enhanced the total water use but also strengthened the roots for further prolificacy in depth and density (Johansen et al. 1997; Kashiwagi et al. 2006, 2015; Purushothaman R, Krishnamurthy L, Upadhyaya HD, Vadez V, Varshney R - Unpubl. data).
Genetic variation for root system
Large genetic variation for root system prolificacy had been observed that can be visualised in to four types. The first type displayed early growth vigour as that of ICC 4958 and ICC 8261, second type displayed normal root growth in the vegetative stage and a greater growth at reproductive stage, third type poor root growth in the vegetative stage but a greater growth at reproductive stage and the fourth type poor growth across growth stages. The second and third type of root growth also had greater deep soil root proliferation or simply deep rooted. The first type of root growth was seen in ICC 4958 that escaped intense drought period (ICRISAT 1992; Saxena et al. 1993; Kashiwagi et al. 2006), with an enhanced CUSM in all the early growth stages (except at the final stage) that reflected in high levels of partitioning (Krishnamurthy et al. 2013). However, this early growth vigour did not help ICC 8261 as it grew longer and the partitioning into grains had been affected (Berger et al. 2011; Purushothaman et al. 2014). All the drought tolerant and locally adapted genotypes fell in to the categories that promoted greater root growth at least in one stage for both. However, the drought sensitive genotypes had the poor root growth across growth stages. The deeper soil root allocation (or greater rooting depth) was also another trait that was found to be associated with the drought tolerance here (R. Purushothaman, L. Krishnamurthy, H. D. Upadhyaya, V. Vadez and R. Varshney, unpubl. data).
The genotypic variation in CUSM was a close reflection of the variation observed for root length density. At the late vegetative stage (during 35–45 DAS in 2010–11), though the maximum rooting depth was up to 75 cm, the soil water uptake tend to be the maximum at the 15–30 cm soil depth. This was also generally the case in 2009–10. At this soil depth ICC 14799, ICC 867 and ICCV 10 were superior in CUSM. Clearly the CUSM of all the drought tolerant and the well adapted genotypes were above average in soil water uptake at the soil depth 15–30 cm and the drought tolerant genotype ICC 14799 was found to take up the maximum water, 7.04 mm. Roots from this zone and the efficiency soil water exploitation of this zone was found to be most critical as success in later plant growth depended on greater use of water from this zone (Kashiwagi et al. 2006, 2015). With the drought intensity increasing, the genotypic variation in drought yield depended more on water uptake from this layer. The CUSM of the small rooting (ICC 283 and ICC 1882) and the drought sensitive (ICC 7184 and ICC 3776) genotypes were poor at this stage and soil depth. Similar pattern of variation was also seen in the subsequent soil depth 30–45 cm. At this growth stage, ICC 4958 and ICC 8261 had been superior in soil water extraction across all the soil depths up to 75 cm.
At majority of early part of the reproductive growth stages (51–80 DAS in 2009–10 and 55–75 DAS in 2010–11) once again all the drought tolerant and locally adapted genotypes except ICC 14778 maintained an above average CUSM. Genotype ICC 14778 was a little late in maximising its water uptake; nevertheless, it managed a high CUSM at later stages beyond 75 DAS making it as a special genotype. The CUSM of genotypes ICC 4958 and ICC 8261 at these stages were average or below average as ICC 4958 was relatively advanced in its developmental stage and ICC 8261 was not utilising the soil water as effectively as that of other desi genotypes. The CUSM of the small rooting genotype ICC 1882 was maximum (12.15 mm) at the 45–60 cm soil but this superiority was not maintained further in the deeper soil layers. When the soil water uptake from the penultimate soil layers alone (90–120 cm) was considered, the drought tolerant ICC 867 and ICC 14799 and the best adapted Annigeri and ICCV 10 had used above average quantities and, the drought sensitive genotype ICC 7184 and the large rooting drought tolerant genotype ICC 4958 had used below average quantities of soil water. Also the CUSM from the penultimate soil depths displayed the highest range of variation among genotypes across growth stages, drought treatments and years indicating that these are the sites of origin for the genetic variation in soil water uptake. The trial mean of CUSM was the highest at the surface soil layers at 35 DAS, and with the increase in crop growth stage, it was found to be the highest at the soil depth 45–60 cm during the reproductive stage.
The results had conclusively revealed that drought tolerance or greater local adaptation can be explained in terms of the ability to maximise the crop utilised soil water within the available season. Though Annigeri, as a long standing variety for the peninsular India, and ICCV 10, as a wider adapted variety from South to Central India (Gowda et al. 1995), are known to be the best contenders for this region, nothing had been known about their water uptake ability and why it had taken so long to breed alternate varieties such as JG 11 suitable for replacement in this region. However, if variations in soil type, soil depth, profiles of water holding and the vapour pressure deficits are encountered, changes in soil water uptake and genotypic reactions to terminal DS can vary. Also, the timing of this water utilisation or efficient use of water across all the depths had also been important to maximise the crop utilised water. However, there are genotypes such as ICC 14778 and ICC 283 with a very late superiority in soil water uptake tend to produce much better grain yields by virtue of possessing other drought adaptive measures such as highest rate of partitioning into grains and the best shoot water status as indicated by the canopy temperature depressions at the final stages crop growth (Kashiwagi et al. 2008; Purushothaman et al. 2015).
The sowing time total profile available soil water, together with an on-season rainfall of 83 and 13 mm, accounted to 299 and 219 mm in these fields during 2009–10 and 2010–11 seasons. The water that remained unutilised particularly from 75 to 120 cm soil depths had been 90.0 mm and 95.5 mm (data not shown), but the maximum CUSM as seen under DS in ICCV 10 is 131 mm in 2009–10 and 126 mm in 2010–11 accounting for 83.0 mm in 2009–10 and 11.5 mm in 2010–11 towards soil evaporation. Occurrence of water loss in these experimental locations through evaporation had been found to be 25% (Kanwar et al. 1982). It seems likely that maximising transpiration over evaporation is a possibility and a fine-tuning the match of the soil water environment with that of the growth duration can increase the yield stability and drought tolerance. Again the soil water up to a depth 75 cm was completely used leaving part of the water from the lower depths unexploited. It took ~40 days to completely utilise all the water from the 60–75 soil zone (Fig. 4c–g). The root system also descended to the soil depths of 75–120 cm after 45 days of growth. Therefore it is likely that the roots of these soil zones did not have enough time to exploit the water fully and thus it is likely that this water cannot be utilised properly. Any efforts to utilise this soil water might improve the partitioning process to the grain yield (Krishnamurthy et al. 2010).
It is logical to believe that the quantum of water absorbed is directly proportional to the root prolificacy provided no other limitations such as soil water content operate. This study had clearly provided evidence that the RLD had a positive association with the soil water uptake and this relation had been linear. This linearity of relationship also provides conclusive evidence that the root production in chickpea is critically suboptimal.
Conclusions
Drought tolerance reactions and adaptation to DS mainly depended on the soil water utilisation efficiency of genotypes though these utilisation differences are marginal. Drought sensitivity can be explained by poor root growth and poor soil water utilisation alone but drought tolerance can be variable with variations in exploitation of moisture in various soil depths and growth stages. The ability to utilise soil water from surface soils and particularly from 15 to 30 cm soil depth is critical as it provides the priming effect for further root growth. Competent use of soil water from 90 to 120 cm soil depth had always been seen in the best adapted and drought tolerant genotypes most likely explaining the biomass partitioning success to filling grains. The amount of water left unused remained constant across all the genotypes and this explained that the water if not used will evaporate and the freshly descended roots did not have enough time to exploit the available water and also the root growth dynamics will require similar left overs.
Acknowledgements
This work was fully supported by Bill and Melinda Gates Foundation through a Generation Challenge Program grant (G4008–12. Linking genetic diversity with phenotype for drought tolerance traits through molecular and physiological characterisation of a diverse reference collection of chickpea). The technical support of Mr J Shankaraiah in managing the field experiments is gratefully acknowledged.
References
Ahmad F, Gaur P, Croser J (2005) Chickpea (Cicer arietinum L.). In ‘Genetic resources, chromosome engineering and crop improvement – grain legumes’. (Eds R Singh, P Jauhar) pp. 185–214. (CRC Press: Boca Raton, FL, USA)Ali S, Maher AB, Anwar M, Haqqani AM (2002) Exploitation of genetic variability for grain yield improvement in chickpea. International Journal of Agriculture and Biology 4, 148–149.
Álvarez S, Navarro A, Bañón S, Sánchez-Blanco MJ (2009) Regulated deficit irrigation in potted dianthus plants: effects of severe and moderate water stress on growth and physiological responses. Scientia Horticulturae 122, 579–585.
| Regulated deficit irrigation in potted dianthus plants: effects of severe and moderate water stress on growth and physiological responses.Crossref | GoogleScholarGoogle Scholar |
Álvarez S, Navarro A, Nicolás E, Sánchez-Blanco MJ (2011) Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Scientia Horticulturae 129, 306–312.
| Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions.Crossref | GoogleScholarGoogle Scholar |
Álvarez S, Gómez-Bellot MJ, Castillo M, Bañón S, Sánchez-Blanco MJ (2012) Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environmental and Experimental Botany 78, 138–145.
| Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants.Crossref | GoogleScholarGoogle Scholar |
Bandyopadhyay PK (2014) Root distribution pattern of pulses in response to water availability. In ‘Resource conservation technology in pulses’. (Eds PK Ghosh, N Kumar, MS Venkatesh, KK Hazra, N Nadarajan) pp. 483–493. (Scientific Publishers: Jodhpur, India)
Bano A, Dorffling K, Bettin D, Hahn H (1993) Abscisic acid and cytokinins as possible root-to-shoot signals in xylem sap of rice plants in drying soil. Australian Journal of Plant Physiology 20, 109–115.
| Abscisic acid and cytokinins as possible root-to-shoot signals in xylem sap of rice plants in drying soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFWmt7Y%3D&md5=5c2868ffe4ed9398153b641484b25b6eCAS |
Bañon S, Ochoa JF, Sánchez-Blanco MJ, Alarcon JJ (2003) Influence of water deficit and low air humidity in the nursery on survival of Rhamnus alaternus seedlings following planting. Journal of Horticultural Science & Biotechnology 78, 518–522.
| Influence of water deficit and low air humidity in the nursery on survival of Rhamnus alaternus seedlings following planting.Crossref | GoogleScholarGoogle Scholar |
Bantilan MCS, Kumara Charyulu D, Gaur PM, Shyam Moses D, Davis J (2014) Short duration chickpea technology: enabling legumes revolution in Andhra Pradesh, India. Research report no. 23. ICRISAT Research Program Markets, Institutions and Policies. ICRISAT, Patancheru, India.
Beebe ST, Rao IM, Blair MW, Butare LO (2009) Breeding for abiotic stress tolerance in common bean: present and future challenges. SABRAO Journal of Breeding and Genetics 41, 1–11.
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Research 97, 248–253.
| Water deficit effects on root distribution of soybean, field pea and chickpea.Crossref | GoogleScholarGoogle Scholar |
Berger JD, Milroy SP, Turner NC, Siddique KHM, Imtiaz M, Malhotra R (2011) Chickpea evolution has selected for contrasting phenological mechanisms among different habitats. Euphytica 180, 1–15.
| Chickpea evolution has selected for contrasting phenological mechanisms among different habitats.Crossref | GoogleScholarGoogle Scholar |
Bernier J, Serraj R, Kumar A, Venuprasad R, Impa S, Gowda V, Oane R, Spaner D, Atlin G (2009) Increased water uptake explains the effect of qtl12.1, a large-effect drought-resistance QTL in upland rice. Field Crops Research 110, 139–146.
| Increased water uptake explains the effect of qtl12.1, a large-effect drought-resistance QTL in upland rice.Crossref | GoogleScholarGoogle Scholar |
Bishopp A, Lynch JP (2015) The hidden half of crop yields. Nature Plants 1, 15117
| The hidden half of crop yields.Crossref | GoogleScholarGoogle Scholar |
Blum A (2009) Effective use of water (EUW) and not water-use efficiency is the target of crop yield improvement under drought stress. Field Crops Research 112, 119–123.
| Effective use of water (EUW) and not water-use efficiency is the target of crop yield improvement under drought stress.Crossref | GoogleScholarGoogle Scholar |
Borrell AK, Mullet JE, George-Jaeggli B, van Oosterom EJ, Hammer GL, Klein PE, Jordan DR (2014) Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. Journal of Experimental Botany 65, 6251–6263.
| Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitl2gsbc%3D&md5=f0fb956421f7cf7433ed636792d481abCAS |
Chauhan YS, Nene YL, Johansen C, Haware MP, Saxena NP, Singh S, Sharma SB, Sahrawat KL, Burford JR, Rupela OP, Kumar Rao JVDK, Sithanantham S (1988) Effects of soil solarization on pigeonpea and chickpea. Research bulletin no. 11. ICRISAT, Patancheru, India.
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology 30, 239–264.
| Understanding plant responses to drought: from genes to the whole plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtVKlt7o%3D&md5=0c43359463e985e3294c3b6104992db2CAS |
Chimungu JG, Brown KM, Lynch JP (2014a) Large root cortical cell size improves drought tolerance in maize (Zea mays L.). Plant Physiology 166, 2166–2178.
| Large root cortical cell size improves drought tolerance in maize (Zea mays L.).Crossref | GoogleScholarGoogle Scholar |
Chimungu JG, Brown KM, Lynch JP (2014b) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology 166, 1943–1955.
| Reduced root cortical cell file number improves drought tolerance in maize.Crossref | GoogleScholarGoogle Scholar |
Chyliński WK, Łukaszewska AJ, Kutnik K (2007) Drought response of two bedding plants. Acta Physiologiae Plantarum 29, 399–406.
| Drought response of two bedding plants.Crossref | GoogleScholarGoogle Scholar |
CIAT (2007) Annual report. Outcome line SBA-1. Improved beans for the developing world: 120. ICRISAT, Patancheru, India.
CIAT (2008) Annual report. Outcome line SBA-1. Improved beans for the developing world: 39–65. ICRISAT, Patancheru, India.
Comas LH, Anderson LJ, Dunst RM, Lakso AN, Eissenstat DM (2005) Canopy and environmental control of root dynamics in a long-term study of Concord grape. New Phytologist 167, 829–840.
| Canopy and environmental control of root dynamics in a long-term study of Concord grape.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2Mvjs1Ohtg%3D%3D&md5=bb84a26da54e17ea356ccf1eef751476CAS |
Comas LH, Becker SR, Cruz VM, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4, 442
| Root traits contributing to plant productivity under drought.Crossref | GoogleScholarGoogle Scholar |
Cutforth HW, Angadi SV, McConkey BG, Miller PR, Ulrich D, Gulden R, Volkmar KM, Entz MH, Brandt SA (2013) Comparing rooting characteristics and soil water withdrawal patterns of wheat with alternative oilseed and pulse crops grown in the semiarid Canadian prairie. Canadian Journal of Soil Science 93, 147–160.
| Comparing rooting characteristics and soil water withdrawal patterns of wheat with alternative oilseed and pulse crops grown in the semiarid Canadian prairie.Crossref | GoogleScholarGoogle Scholar |
Dardanelli JL, Ritchie JT, Calmon M, Andriani JM, Collino DJ (2004) An empirical model for root water uptake. Field Crops Research 87, 59–71.
| An empirical model for root water uptake.Crossref | GoogleScholarGoogle Scholar |
Davies FT, Olade-Portugal V, Aguilera-Gomez L, Alvarado MJ, Ferrera-Cerrato RC, Boutton TW (2002) Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorrhiza indigenous to Mexico. Scientia Horticulturae 92, 347–359.
| Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorrhiza indigenous to Mexico.Crossref | GoogleScholarGoogle Scholar |
De Sousa MA, Lima MDB (2010) Influence of suppression of the irrigation in stages of growth of bean cv. Carioca comum. Bioscience Journal 26, 550–557.
dos Santos TP, Lopes CM, Rodrigues ML, Souza CR, Silva JR, Maroco JP, Pereira JS, Chaves MM (2007) Effects of deficit irrigation strategies on cluster microclimate for improving fruit composition of Moscatel field-grown grapevines. Scientia Horticulturae 112, 321–330.
| Effects of deficit irrigation strategies on cluster microclimate for improving fruit composition of Moscatel field-grown grapevines.Crossref | GoogleScholarGoogle Scholar |
Drew MC, Webb J, Saker LR (1990) Regulation of K+ uptake and transport to the xylem in barley roots; K+ distribution determined by electron probe X-ray microanalysis of frozen-hydrated cells. Journal of Experimental Botany 41, 815–825.
| Regulation of K+ uptake and transport to the xylem in barley roots; K+ distribution determined by electron probe X-ray microanalysis of frozen-hydrated cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXjvFanug%3D%3D&md5=49ed05eb64c0ef736575e2cfffea069fCAS |
Eghball B, Maranville JW (1993) Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agronomy Journal 85, 147–152.
| Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXitFWqtL8%3D&md5=521e8955b511b886d171c6c1784e3c6fCAS |
El-Swaify SA, Pathak P, Rego TJ, Singh S (1985) Soil management for optimized productivity under rainfed conditions in the semi-arid tropics. In ‘Advances in soil science.’ pp. 1–64. (Springer-Verlag: New York)
Evans LT (1998) ‘Feeding the ten billion: plants and population growth.’ (Cambridge University Press: Cambridge, UK)
FAOSTAT (2014) ‘FAO statistics division.’ Available at http://faostat3.fao.org/compare/E [Verified 27 February 2016].
Fenta BA, Beebe SE, Kunert KJ, Burridge JD, Barlow KM, Lynch JP, Foyer CH (2014) Field phenotyping of soybean roots for drought stress tolerance. Agronomy 4, 418–435.
| Field phenotyping of soybean roots for drought stress tolerance.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475.
| Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies.Crossref | GoogleScholarGoogle Scholar |
Franco JA, Arreola J, Vicente MJ, Martinez-Sanchez JJ (2008) Nursery irrigation regimes affect the seedling characteristics of Silene vulgaris as they relate to potential performance following transplanting into semi-arid conditions. Journal of Horticultural Science & Biotechnology 83, 15–22.
| Nursery irrigation regimes affect the seedling characteristics of Silene vulgaris as they relate to potential performance following transplanting into semi-arid conditions.Crossref | GoogleScholarGoogle Scholar |
Franco JA, Bañón S, Vicente MJ, Miralles J, Martínez-Sánchez JJ (2011) Root development in horticultural plants grown under abiotic stress conditions – a review. Journal of Horticultural Science & Biotechnology 86, 543–556.
| Root development in horticultural plants grown under abiotic stress conditions – a review.Crossref | GoogleScholarGoogle Scholar |
Gaur PM, Jukanti AK, Samineni S, Chaturvedi SK, Basu PS, Babbar A, Jayalakshmi V, Nayyar H, Devasirvatham V, Mallikarjuna N, Krishnamurthy L (2014) Climate change and heat stress tolerance in chickpea. In ‘Climate change and plant abiotic stress tolerance’. (Eds N Tuteja, SS Gill) pp. 837 –856. (Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany)
Gowda CLL, Singh O, Sethi SC, Singh KB, Rao BV, Rahman MM, Kumar J, Rahman MA (1995) Registration of ‘ICCV 10’ chickpea. Crop Science 35, 589
| Registration of ‘ICCV 10’ chickpea.Crossref | GoogleScholarGoogle Scholar |
Gowda VR, Henry A, Vadez V, Shashidhar HE, Serraj R (2012) Water uptake dynamics under progressive drought stress in diverse accessions of the OryzaSNP panel of rice (Oryza sativa). Functional Plant Biology 39, 402–411.
| Water uptake dynamics under progressive drought stress in diverse accessions of the OryzaSNP panel of rice (Oryza sativa).Crossref | GoogleScholarGoogle Scholar |
Gregory PJ (1994) Resource capture by root networks. In ‘Resource capture by crops’. (Eds JL Monteith, RK Scott, MH Unsworth) pp. 77–97. (Nottingham University Press: Nottingham, UK)
Guswa AJ (2008) The influence of climate on root depth: a carbon cost‐benefit analysis. Water Resources Research 1, 44
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Science 49, 299–312.
| Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt?Crossref | GoogleScholarGoogle Scholar |
Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. Journal of Experimental Botany 61, 2185–2202.
| Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGnsr0%3D&md5=90c0f43966d4bb18e2740adb745f5548CAS |
Henry A, Gowda VRP, Torres RO, McNally KL, Serraj R (2011) Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Research 120, 205–214.
| Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields.Crossref | GoogleScholarGoogle Scholar |
ICRISAT (1992) ‘ICC 4958 A drought resistant chickpea. Plant material description no. 33.’ (ICRISAT: Patancheru, India)
Itoh H, Hayashi S, Nakajima T, Hayashi T, Yoshida H, Yamazaki K, Komatsu T (2009) Effects of soil type, vertical root distribution and precipitation on grain yield of winter wheat. Plant Production Science 12, 503–513.
| Effects of soil type, vertical root distribution and precipitation on grain yield of winter wheat.Crossref | GoogleScholarGoogle Scholar |
Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP (2013) Root cortical burden influences drought tolerance in maize. Annals of Botany 112, 429–437.
| Root cortical burden influences drought tolerance in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSitbbK&md5=edfd402ae015084bfc1393663a040a69CAS |
Johansen C, Singh DN, Krishnamurthy L, Saxena NP, Chauhan YS, Kumar Rao JVDK (1997) Options for alleviating moisture stress in pulse crops. In ‘Recent advances in pulses research’. (Eds AN Asthana, A Masood) pp. 425–442. (Indian Society of Pulses Research and Development: Kanpur, India)
Jones JW, Zur B (1984) Simulation of possible adaptive mechanisms in crops subjected to water stress. Irrigation Science 5, 251–264.
| Simulation of possible adaptive mechanisms in crops subjected to water stress.Crossref | GoogleScholarGoogle Scholar |
Jordan WR, Dugas WA, Shouse PJ (1983) Strategies for crop improvement drought-prone region (Sorghum bicolor, Triticum aestivum, wheat plant breeding). Agricultural Water Management 7, 281–299.
| Strategies for crop improvement drought-prone region (Sorghum bicolor, Triticum aestivum, wheat plant breeding).Crossref | GoogleScholarGoogle Scholar |
Kamoshita A, Wade L, Yamauchi A (2000) Genotypic variation in response of rainfed lowland rice to drought and rewatering: 3. Water extraction during the drought period. Plant Production Science 3, 189–196.
| Genotypic variation in response of rainfed lowland rice to drought and rewatering: 3. Water extraction during the drought period.Crossref | GoogleScholarGoogle Scholar |
Kanwar JS, Kampen J, Virmani SM (1982) ‘Management of Vertisols for maximising crop production-ICRISAT experience.’ In ‘Vertisols and rice soils of the tropics. Transacctions of the 12th international congress of soil science. Vol. 3’. pp. 94–118. (Indian Society of Soil Science: New Delhi)
Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Krishna H, Chandra S, Vadez V, Serraj R (2005) Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 146, 213–222.
Kashiwagi J, Krishnamurthy L, Crouch JH, Serraj R (2006) Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crops Research 95, 171–181.
| Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress.Crossref | GoogleScholarGoogle Scholar |
Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Gaur PM (2008) Rapid screening technique for canopy temperature status and its relevance to drought tolerance improvement in chickpea. Journal of SAT Agricultural Research 6, 1–4.
Kashiwagi J, Krishnamurthy L, Gaur PM, Upadhyaya HD, Varshney RK, Tobita S (2013) Traits of relevance to improve yield under terminal drought stress in chickpea (C. arietinum L.). Field Crops Research 145, 88–95.
| Traits of relevance to improve yield under terminal drought stress in chickpea (C. arietinum L.).Crossref | GoogleScholarGoogle Scholar |
Kashiwagi J, Krishnamurthy L, Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, Ito O, Varshney RK (2015) Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crops Research 170, 47–54.
| Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.).Crossref | GoogleScholarGoogle Scholar |
Kell DB (2011) Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108, 407–418.
| Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVygsLjM&md5=04b690547d43406d5e43a0f3b88189a0CAS |
Koike T, Kitao M, Quoreshi AM, Matsuura Y (2003) Growth characteristics of root–shoot relations of three birch seedlings raised under different water regimes. Plant and Soil 255, 303–310.
| Growth characteristics of root–shoot relations of three birch seedlings raised under different water regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotV2js7Y%3D&md5=d4afc6a3bc49d5c3ee85c1ecd17c22c5CAS |
Konings H, Jackson MB (1979) A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Zeitschrift für Pflanzenphysiologie 92, 385–397.
| A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktFGit7w%3D&md5=ff24b4ecb27110715b72dcf353f93312CAS |
Kramer PJ, Boyer JS (1995) ‘Water relations of plants and soils.’ (Academic Press: San Diego, CA, USA)
Krishnamurthy L, Johansen C, Sethi SC (1999) Investigation of factors determining genotypic differences in seed yield of non-irrigated and irrigated chickpeas using a physiological model of yield determination. Journal Agronomy & Crop Science 183, 9–17.
| Investigation of factors determining genotypic differences in seed yield of non-irrigated and irrigated chickpeas using a physiological model of yield determination.Crossref | GoogleScholarGoogle Scholar |
Krishnamurthy L, Kashiwagi J, Gaur PM, Upadhyaya HD, Vadez V (2010) Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Research 119, 322–330.
| Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm.Crossref | GoogleScholarGoogle Scholar |
Krishnamurthy L, Kashiwagi J, Upadhyaya HD, Gowda CLL, Gaur PM, Singh S, Purushothaman R, Varshney RK (2013) Partitioning coefficient – a trait that contributes to drought tolerance in chickpea. Field Crops Research 149, 354–365.
| Partitioning coefficient – a trait that contributes to drought tolerance in chickpea.Crossref | GoogleScholarGoogle Scholar |
Kulkarni M, Deshpande U (2007) Gradient in vitro testing of tomato (Solanum lycopersicon L.) cultivars by inducing water deficit – a new approach to screen germplasm for drought tolerance. Asian Journal of Plant Science 6, 934–940.
| Gradient in vitro testing of tomato (Solanum lycopersicon L.) cultivars by inducing water deficit – a new approach to screen germplasm for drought tolerance.Crossref | GoogleScholarGoogle Scholar |
Kumar J, Abbo S (2001) Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments. Advances in Agronomy 2, 122
Kumar J, Rao BV (2001) Registration of ICCV 96029, super early and double podded chickpea germplasm. Crop Science 41, 605–606.
| Registration of ICCV 96029, super early and double podded chickpea germplasm.Crossref | GoogleScholarGoogle Scholar |
Kumar J, Haware MP, Smithon JB (1985) Registration of four short-duration Fusarium wilt-resistant kabuli (Garbanzo) chickpea germplasm. Crop Science 25, 576–577.
| Registration of four short-duration Fusarium wilt-resistant kabuli (Garbanzo) chickpea germplasm.Crossref | GoogleScholarGoogle Scholar |
Kumar B, Abdel‐Ghani AH, Reyes‐Matamoros J, Hochholdinger F, Lübberstedt T (2012) Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breeding 131, 465–478.
| Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines.Crossref | GoogleScholarGoogle Scholar |
Lafitte HR, Champoux MC, McLaren G, O’Toole JC (2001) Rice root morphological traits are related to isozyme group and adaptation. Field Crops Research 71, 57–70.
| Rice root morphological traits are related to isozyme group and adaptation.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Atkin OK, Millenaar FF (2002) Respiratory patterns in roots in relation to their functioning. In ‘Plant roots: the hidden half’. (Eds Y Waisel, A Eshel, K Kafkaki) pp. 521–552. (Marcel Dekker Inc.: New York)
Liao MT, Fillery IRP, Palta JA (2004) Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Functional Plant Biology 31, 121–129.
| Early vigorous growth is a major factor influencing nitrogen uptake in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsl2gtrk%3D&md5=132d4a2fb4c0b031026c7444b6a80fa9CAS |
Liu L, Gan Y, Bueckert R, Van Rees K (2011) Rooting systems of oilseed and pulse crops. II: Vertical distribution patterns across the soil profile. Field Crops Research 122, 248
| Rooting systems of oilseed and pulse crops. II: Vertical distribution patterns across the soil profile.Crossref | GoogleScholarGoogle Scholar |
Lopes MS, Reynolds MP (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology 37, 147–156.
| Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat.Crossref | GoogleScholarGoogle Scholar |
Ludlow MM, Muchow RC (1990) A critical evaluation of traits for improving crop yields in water-limited environments. Advances in Agronomy 43, 107–153.
| A critical evaluation of traits for improving crop yields in water-limited environments.Crossref | GoogleScholarGoogle Scholar |
Lush JL (1940) Intra-sire correlation and regression of offspring on dams as a method of estimating heritability of characters. Proceedings of the American Society of Animal Nutrition 33, 293–301.
Lynch JP (1995) Root architecture and plant productivity. Plant Physiology 109, 7–13.
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357.
| Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSitbfJ&md5=03958de958e36d07f3233d52724a7053CAS |
Lynch JP, Chimungu JG, Brown KM (2014) Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany 65, 6155–6166.
| Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitl2gsbw%3D&md5=74ab7a97947fb65dcaa8aa44071691caCAS |
Ma SC, Li FM, Xu BC, Huang ZB (2010) Effect of lowering the root/shoot ratio by pruning roots on water use efficiency and grain yield of winter wheat. Field Crops Research 115, 158–164.
| Effect of lowering the root/shoot ratio by pruning roots on water use efficiency and grain yield of winter wheat.Crossref | GoogleScholarGoogle Scholar |
Manavalan LP, Musket T, Nguyen HT (2011) Natural genetic variation for root traits among diversity lines of maize (Zea mays L.). Maydica 56, 1707
Manschadi AM, Christopher JT, Hammer GL, deVoil P (2010) Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosystems 144, 458–462.
| Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Mishra HS, Rathore TR, Savita US (2001) Water use efficiency of irrigated winter maize under cool weather conditions of India. Irrigation Science 21, 27–33.
| Water use efficiency of irrigated winter maize under cool weather conditions of India.Crossref | GoogleScholarGoogle Scholar |
Molz FJ, Remson I (1970) Extraction term models of soil moisture use by transpiring plants. Water Resources Research 6, 1346–1356.
| Extraction term models of soil moisture use by transpiring plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXjtFWiug%3D%3D&md5=6748e99c6d53b78374838e344c66b8f7CAS |
Moroke TS, Schwartz RC, Brown KW, Juo ASR (2005) Soil water depletion and root distribution of three dryland crops. Soil Science Society of America Journal 69, 197–205.
| Soil water depletion and root distribution of three dryland crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXnvFGltA%3D%3D&md5=8832c2688830b0ed6807ec99f55fd17bCAS |
Navarro A, Álvarez S, Castillo M, Bañón S, Sánchez-Blanco MJ (2009) Changes in tissue-water relations, photosynthetic activity, and growth of Myrtus communis plants in response to different conditions of water availability. Journal of Horticultural Science & Biotechnology 84, 541
| Changes in tissue-water relations, photosynthetic activity, and growth of Myrtus communis plants in response to different conditions of water availability.Crossref | GoogleScholarGoogle Scholar |
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. Journal of Experimental Botany 60, 1927–1937.
| Plant phenology: a critical controller of soil resource acquisition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFSjur8%3D&md5=8b9860ad01c9c8f75d65bc3cf3ae923bCAS |
Okada K, Kondo M, Ando H, Kakuda K (2002) Water uptake under water stress at panicle initiation stage in upland rice as affected by previous soil water regimes. Soil Science and Plant Nutrition 48, 151–158.
| Water uptake under water stress at panicle initiation stage in upland rice as affected by previous soil water regimes.Crossref | GoogleScholarGoogle Scholar |
Pandey RK, Maranville JW, Admou A (2000a) Deficit irrigation and nitrogen effects on maize in a Sahelian environment: I. Grain yield and yield components. Agricultural Water Management 46, 1–13.
| Deficit irrigation and nitrogen effects on maize in a Sahelian environment: I. Grain yield and yield components.Crossref | GoogleScholarGoogle Scholar |
Pandey RK, Maranville JW, Chetima MM (2000b) Deficit irrigation and nitrogen effects on maize in a Sahelian environment: II. Shoot growth, nitrogen uptake and water extraction. Agricultural Water Management 46, 15–27.
| Deficit irrigation and nitrogen effects on maize in a Sahelian environment: II. Shoot growth, nitrogen uptake and water extraction.Crossref | GoogleScholarGoogle Scholar |
Pinheiro HA, DaMatta FM, Chaves ARM, Loureiro ME, Ducatti C (2005) Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Annals of Botany 96, 101–108.
| Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora.Crossref | GoogleScholarGoogle Scholar |
Puangbut D, Jogloy S, Vorasoot N, Akkasaeng C, Kesmala T, Rachaputi RCN, Wright GC, Patanothai A (2009) Association of root dry weight and transpiration efficiency of peanut genotypes under early season drought. Agricultural Water Management 96, 1460–1466.
| Association of root dry weight and transpiration efficiency of peanut genotypes under early season drought.Crossref | GoogleScholarGoogle Scholar |
Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, Krishnamurthy L (2014) Kabuli and desi chickpeas differ in their requirement for reproductive duration. Field Crops Research 163, 24–31.
| Kabuli and desi chickpeas differ in their requirement for reproductive duration.Crossref | GoogleScholarGoogle Scholar |
Purushothaman R, Thudi M, Krishnamurthy L, Upadhyaya HD, Kashiwagi J, Gowda CLL, Varshney RK (2015) Association of mid-reproductive stage canopy temperature depression with the molecular markers and grain yields of chickpea (Cicer arietinum L.) germplasm under terminal drought. Field Crops Research 174, 1–11.
| Association of mid-reproductive stage canopy temperature depression with the molecular markers and grain yields of chickpea (Cicer arietinum L.) germplasm under terminal drought.Crossref | GoogleScholarGoogle Scholar |
Purushothaman R, Krishnamurthy L, Upadhyaya HD, Vadez V, Varshney RK (2016) Shoot traits and their relevance in terminal drought tolerance of chickpea (Cicer arietinum L.). Field Crops Research 197, 10–27.
Ratnakumar P, Vadez V, Nigam SN, Krishnamurthy L (2009) Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought using a lysimetric system. Plant Biology 11, 124–130.
| Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought using a lysimetric system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlslajsrc%3D&md5=98e96a8bb795f4561c4883aa81982344CAS |
Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 40, 943–950.
| A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments.Crossref | GoogleScholarGoogle Scholar |
Ritchie JT (1981) Soil water availability. Plant and Soil 58, 327–338.
| Soil water availability.Crossref | GoogleScholarGoogle Scholar |
Robertson WK, Hammond LC, Johnson JT, Boote KJ (1980) Effects of plant-water stress on root distribution of corn, soybeans, and peanuts in sandy soil. Agronomy Journal 72, 548–550.
| Effects of plant-water stress on root distribution of corn, soybeans, and peanuts in sandy soil.Crossref | GoogleScholarGoogle Scholar |
Ryan JG (1997) A global perspective on pigeonpea and chickpea sustainable production systems: present status and future potential. In ‘Recent advances in pulses research’. (Eds AN Asthana, M Ali) pp. 1–31. (Indian Society of Pulses Research and Development: Kanpur, India)
Sabaghpour SH, Kumar J, Rao TN (2003) Inheritance of growth vigour and its association with other characters in chickpea. Plant Breeding 122, 542–544.
| Inheritance of growth vigour and its association with other characters in chickpea.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Blanco MJ, Rodríguez P, Morales MA, Ortuño MF, Torrecillas A (2002) Comparative growth and water relation of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Science 162, 107–113.
| Comparative growth and water relation of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery.Crossref | GoogleScholarGoogle Scholar |
Sauter A, Davies WJ, Hartung W (2001) The long‐distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. Journal of Experimental Botany 52, 1991–1997.
| The long‐distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXns1Wkt78%3D&md5=c698b4b143d441e4d2bda0fdaa2900acCAS |
Saxena NP, Krishnamurthy L, Johansen C (1993) Registration of a drought-resistant chickpea germplasm. Crop Science 33, 1424
| Registration of a drought-resistant chickpea germplasm.Crossref | GoogleScholarGoogle Scholar |
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annual Review of Plant Biology 58, 47–69.
| Nutrient sensing and signaling: NPKS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsVahs74%3D&md5=6a0bf403a79acf4fc1ca111869ce59a1CAS |
Schoppach RM, Wauthelet D, Jeanguenin L, Sadok W (2014) Conservative water use under high evaporative demand associated with smaller root metaxylem and limited trans-membrane water transport in wheat. Functional Plant Biology 41, 257–269.
| Conservative water use under high evaporative demand associated with smaller root metaxylem and limited trans-membrane water transport in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXisF2rs7s%3D&md5=8a6d2bb792bf56ce5ccc071a9cba0730CAS |
Searle SR (1961) Phenotypic, genetic and environmental correlations. Biometrics 17, 474–480.
| Phenotypic, genetic and environmental correlations.Crossref | GoogleScholarGoogle Scholar |
Serraj R, Krishnamurthy L, Kashiwagi J, Kumar J, Chandra S, Crouch JH (2004) Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. Field Crops Research 88, 115–127.
| Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought.Crossref | GoogleScholarGoogle Scholar |
Sharp RE, Davies WJ (1985) Root growth and water uptake by maize plants in drying soils. Journal of Experimental Botany 36, 1441–1456.
| Root growth and water uptake by maize plants in drying soils.Crossref | GoogleScholarGoogle Scholar |
Silim SN, Saxena MC (1993) Adaptation of spring-sown chickpea to the Mediterranean basin: I. Response to moisture supply. Field Crops Research 34, 121–136.
| Adaptation of spring-sown chickpea to the Mediterranean basin: I. Response to moisture supply.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR (1994) Limits to crop yield? In ‘Physiology and determination of crop yield’. (Ed. KJ Boote) pp. 509–532. (ASA, CSSA and SSSA: Madison, WI, USA)
Soltani A, Ghassemi-Golezani K, Khooie FR, Moghaddam M (1999) A simple model for chickpea growth and yield. Field Crops Research 62, 213–224.
| A simple model for chickpea growth and yield.Crossref | GoogleScholarGoogle Scholar |
Soltani A, Khooie FR, Ghassemi-Golezani K, Moghaddam M (2000) Thresholds for chickpea leaf expansion and transpiration response to soil water deficit. Field Crops Research 68, 205–210.
| Thresholds for chickpea leaf expansion and transpiration response to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Sponchiado BN, White JW, Castillo JA, Jones PG (1980) Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture 25, 249–257.
| Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types.Crossref | GoogleScholarGoogle Scholar |
Steele KA, Price AH, Witcombe JR, Shrestha R, Singh BN, Gibbons JM, Virk DS (2013) QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theoretical and Applied Genetics 126, 101–108.
| QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bmtFylsQ%3D%3D&md5=057fd07dbd31ca03c3fe41c46cc32063CAS |
Suji KK, Prince KS, Mankhar PS, Kanagaraj P, Poornima R, Amutha K, Kavitha S, Biji KR, Gomez SM, Babu RC (2012) Evaluation of rice (Oryza sativa L.) near iso-genic lines with root QTLs for plant production and root traits in rainfed target populations of environment. Field Crops Research 137, 89–96.
| Evaluation of rice (Oryza sativa L.) near iso-genic lines with root QTLs for plant production and root traits in rainfed target populations of environment.Crossref | GoogleScholarGoogle Scholar |
Trachsel S, Stamp P, Hund A (2010) Growth of axile and lateral roots of maize: response to desiccation stress induced by polyethylene glycol 8000. Maydica 55, 101–109.
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil 341, 75–87.
| Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjsrc%3D&md5=290d705e20e1a515b744831364899db4CAS |
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102.
| Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1WgsrnM&md5=e30633885a519fbf67b1d41ca339c4f8CAS |
Vadez V, Soltani A, Sinclair TR (2012) Modelling possible benefits of root related traits to enhance terminal drought adaptation of chickpea. Field Crops Research 137, 108–115.
| Modelling possible benefits of root related traits to enhance terminal drought adaptation of chickpea.Crossref | GoogleScholarGoogle Scholar |
Wang X, Gan Y, Hamel C, Lemke R, McDonald C (2012) Water use profiles across the rooting zones of various pulse crops. Field Crops Research 134, 130–137.
| Water use profiles across the rooting zones of various pulse crops.Crossref | GoogleScholarGoogle Scholar |
Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63, 3485–3498.
| Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVSit7c%3D&md5=8ca794a1ea3b5795ff5bc20412c2692dCAS |
Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M (2014) Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. Journal of Experimental Botany 65, 6231–6249.
| Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitl2gsbY%3D&md5=dcfb209a8c1835b48a4cea3afa8fac34CAS |
White JW, Castillo JA (1992) Evaluation of diverse shoot genotypes on selected root genotypes of common bean under soil water deficits. Crop Science 32, 762–765.
| Evaluation of diverse shoot genotypes on selected root genotypes of common bean under soil water deficits.Crossref | GoogleScholarGoogle Scholar |
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell & Environment 25, 195–210.
| ABA-based chemical signalling: the co-ordination of responses to stress in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhslaktbY%3D&md5=2a09a62a6ca64bb64b171adad021cfd9CAS |
Yu GR, Zhuang J, Nakayama K, Jin Y (2007) Root water uptake and profile soil water as affected by vertical root distribution. Plant Ecology 189, 15–30.
| Root water uptake and profile soil water as affected by vertical root distribution.Crossref | GoogleScholarGoogle Scholar |
Zaman-Allah M, Jenkinson DM, Vadez V (2011) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. Journal of Experimental Botany 62, 4239–4252.
| A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVeit7jJ&md5=92e474048222fd32771055d9f1286ec5CAS |
Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment 33, 740–749.







