Improvement of drought tolerance in white clover (Trifolium repens) by transgenic expression of a transcription factor gene WXP1
Qingzhen Jiang A , Ji-Yi Zhang B , Xiulin Guo A C , Mohamed Bedair B , Lloyd Sumner B , Joseph Bouton A and Zeng-Yu Wang A DA Forage Improvement Division, The Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA.
B Plant Biology Division, The Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA.
C Institute of Genetics and Physiology, Hebei Academy of Agricultural and Forestry Sciences, Shijiazhuang, Hebei 050051, China.
D Corresponding author. Email: zywang@noble.org
Functional Plant Biology 37(2) 157-165 https://doi.org/10.1071/FP09177
Submitted: 17 July 2009 Accepted: 26 September 2009 Published: 3 February 2010
Abstract
White clover (Trifolium repens L.) is an important pasture legume in many regions of the world. A commercial cultivar of white clover (cv. Patriot) was transformed with a Medicago truncatula L. transcription factor gene, WXP1, and a reporter gene, β-glucuronidase (GUS). The WXP1 gene and the GUS gene were placed under control of the Arabidopsis CER6 promoter. GUS staining and cross-section analysis revealed the CER6 promoter directed constitutive expression in leaves and epidermis preferential expression in petioles of white clover. Independent transgenic WXP1 lines, empty vector and wild-type controls were subjected to drought stress treatment. The plants were characterised by measuring several physiological parameters including gas exchange, chlorophyll fluorescence, relative water content and leaf water potential. The WXP1 transgenic lines had higher net photosynthetic rates, higher efficiency of PSII, higher relative water content and leaf water potential under drought-stressed conditions. Consistent with the results from physiological analyses, the transgenic white clover plants carrying WXP1 showed improved tolerance to drought stress.
Additional keyword: chlorophyll fluorescence, gas exchange, promoter, relative water content, transgenic plant, water potential.
Introduction
White clover (Trifolium repens L. 2n = 4X = 32) is a cool-season perennial forage legume that is adaptive to a wide range of climates in the world (Frame and Newbould 1986; Pederson 1995). It is of great value for livestock feed, soil improvement and reclaiming disturbed land because of its nitrogen fixing capability (Seker et al. 2003). However, white clover is less drought tolerant than some other perennial temperate forage legumes (e.g. alfalfa (Medicago sativa L.)) owing to its shallow root system and inability to effectively control transpiration (Hart 1987; Annicchiarico and Piano 2004). As white clover is often grown together with a companion grass in cattle grazing systems, competition with associated grasses such as perennial ryegrass (Lolium perenne L.) or tall fescue (Festuca arundinacea Schreb.) places white clover under additional water stress.
Drought is one of the most adverse environmental factors that affect plant growth and production (Bhatnagar-Mathur et al. 2008). Plants have many characteristics that are adaptive to environments. For example, plant surfaces are covered with a cuticle layer that protects plants from biotic and abiotic stresses and limits gas and water exchange between the surrounding air and the intercellular spaces (Li et al. 2007). Plants respond to drought stress by displaying complex physiological and biochemical changes that involve the function of many genes. Transcription factor genes have received much attention in recent years because the transfer of a single such gene may activate many stress tolerance genes under normal growing conditions and result in improved stress tolerance (Zhang 2003; Shinozaki and Yamaguchi-Shinozaki 2007). WXP1 (wax production) is a transcription factor gene identified from the model legume Medicago truncatula L. (Zhang et al. 2005). Transgenic expression of the WXP1 gene under the control of CaMV35S promoter enhanced wax production and drought tolerance in transgenic Arabidopsis and alfalfa (Zhang et al. 2005, 2007). Overexpression of another wax-related transcription factor gene, WIN1/SHN1, also led to increased wax accumulation and improved drought tolerance in Arabidopsis (Aharoni et al. 2004; Broun et al. 2004).
The side effect of overexpression of these genes is slower growth of the transgenics when compared with wild-type (WT) plants. We have been investigating the use of the CER6 (eceriferum6) promoter to drive the WXP1 gene to mitigate the side effect. It was reported that CER6 promoter directed high levels of gene expression in epidermal cells of Arabidopsis (Hooker et al. 2002). Overexpression of the CER6 gene under control of the CER6 promoter resulted in increased wax deposition on Arabidopsis stems (Hooker et al. 2002).
Most of the transgenic work aimed at improving stress tolerance employed model species or model cultivars/genotypes. Although such concept testing is valuable, proof of the functionality of such genes in elite cultivars is essential for the development of commercially viable materials. In this study, the WXP1 gene was placed under the control of the CER6 promoter and the gene construct was introduced into an elite white clover, cv. Patriot. The transgenic plants were analysed under water-stressed and water-recovery conditions. To date, there has been no report on effective enhancement of drought tolerance of white clover through transgenic approaches and the evaluation of drought response of transgenics in other species has largely relied on visual phenotype observation. In the present study, in addition to phenotype observation, physiological performance was evaluated by analysing gas-exchange and chlorophyll fluorescence parameters. Since these parameters reflect the plant stomatal responses and the integrity of photosystem II (PSII) upon exposure to stress, the physiological analyses provide useful information in evaluation of drought responses of the transgenic white clover plants.
Materials and methods
Construction of CER6-GUS, CER6-WXP1 vectors and generation of transgenic white clover plants
The CER6 promoter was obtained from Arabidopsis by PCR amplification (5′-AAGCTTCGATATCGGTTGTTG-3′ and 5′-CCATGGTCGGAGAGTTTTAATG-3′) of genomic DNA. The CER6-GUS binary vector was constructed by replacing the CaMV35S promoter in pCAMBIA3301 (Zhang et al. 2005). The CER6-WXP1 binary vector was constructed by replacing the CaMV35S promoter in pC35S-WXP1 (Zhang et al. 2005). The resulting binary vectors, CER6-GUS and CER6-WXP1, were transferred into Agrobacterium tumefaciens strain AGL1 using freeze–thaw method (Chen et al. 1994).
The commercial white clover (Trifolium repens L.) cv. Patriot (Bouton et al. 2005) was used for Agrobacterium-mediated transformation. Transgenic plants were obtained by following the protocol described by Mouradov et al. (2006). White clover lines transformed with the original pCAMBIA3301 vector were used as empty vector control (CK).
Histochemical and cross-section analysis of CER6 promoter in transgenic white clover
Histochemical GUS staining was carried out on trifoliates and petioles of regenerated white clover plants carrying CER6-GUS. GUS expression pattern was visualised after incubating samples in 100 mM sodium phosphate, pH 7.0, 10 mM Na-EDTA, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 0.3% (w/v) X-Gluc and 0.1% (v/v) Triton X-100 at 37°C overnight (Schnurr et al. 2004). For cross-section analysis, tissues were fixed in 4% (v/v) formaldehyde and 0.2% (v/v) glutaraldehyde in sample buffer (0.05 M potassium phosphate buffer, pH 7.2) for 2 h. The samples were then washed twice for 10 min in sample buffer, dehydrated in an ethanol series and embedded in paraffin wax (Xiao et al. 2006). Samples were then sectioned and visualised under a microscope.
PCR and RT–PCR analyses of regenerated CER6-WXP1 white clover plants
After phosphinothricin (PPT) selection, regenerated plants were subjected to PCR screening to identify transgenic lines. Genomic DNA was extracted from newly developed leaves of each regenerated plant following the manufacturer’s instructions (DNeasy Plant Mini Kit, Qiagen, Valencia, CA, USA). DNA concentration was determined by a spectrophotometer. For the identification of CER6-WXP1 plants, the following primers were used for PCR amplification in a reaction volume of 25 µL: 5′-TTGCTCCCATCACTTGCTTTTGT-3′ and 5′-GCTTTGTGGGCTTTGGAGGTA-3′. The forward primer targets to the CER6 promoter and the reverse primer is specific to the WXP1 region.
Semiquantitative RT–PCR was used to detect transcript levels of WXP1. Total RNA was extracted from young leaves of the transgenic lines and controls using MRC Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH, USA). After determination of RNA concentration by a spectrophotometer, two micrograms of total RNA were used for cDNA synthesis in a total volume of 20 µL reaction by Omniscript RT Kit (Qiagen). Two microlitres of cDNA were used in each PCR (25 µL) to amplify WXP1 and the actin gene. Primers for amplifying the WXP1 gene were 5′-AATGGGTTGCTGAGATAAGACTAC-3′ and 5′-CAAGACCGGCAACAGGATT-3′ (this primer is located in the NOS terminator region). Primers for amplifying the actin gene were 5′-GATATGGAAAAGATCTGGCATCAC-3′ and 5′-TCATACTCGGCCTTGGAGATCCAC-3′ (Pang et al. 2007). PCR conditions were 95°C for 2 min for denaturing of template cDNAs, 23–35 cycles of 94°C for 1 min, 50°C for 1 min and 72°C for 1 min, followed by 72°C for 10 min. Transcript levels were detected by electrophoresis of amplification products in 1% (w/v) agarose/ethidium bromide gels.
Northern blot hybridisation analysis
Total RNA was extracted with MRC Tri-Reagent (Molecular Research Center Inc.). A total of 20 μg of RNA were loaded in each lane of 1.2% agarose gels with formaldehyde. WXP1 cDNA were 32P-labelled using the RanPrime DNA Labelling System (Invitrogen, Carlsbad, CA, USA) as instructed by the manufacturer. Northern hybridisation was conducted using a high efficiency hybridisation system and washing/pre-hyb solution (Molecular Research Center) following the manufacturer’s instructions.
Plant material and growth conditions
After two preliminary screenings, three independent transgenic white clover lines were selected for further study together with a WT and a CK. The T0 transgenic plants and the controls were propagated using stolon cuttings from healthy and young branches. Well established plants were transferred into 4.5-inch pots filled with Turface MVP clay (Profile Products LLC, Buffalo Grove, IL, USA). Plants were well watered in the growth chamber under the following conditions: day/night length 16/8 h, light intensity ~250–300 µmol m–2 s–1, and day/night temperature 23/19°C. After 2 weeks of growth, plants of similar size were grouped into at least five replicates and water was stopped for 2 days for physiological measurements before re-watering. Plants were also subjected to drought stress for up to a week until the control plants became almost dried or dead-like if not intended for physiological measurement after re-watering. Experiments were repeated at least three times for all measurements. Two separate experiments were conducted for phenotypic characterisation.
Gas-exchange and chlorophyll fluorescence measurement
Leaf gas exchange and chlorophyll fluorescence were measured in situ on the centre leaflets from at least five fully developed uppermost and also penultimate trifoliates on the main stem of white clover using Li-6400 coupled with the 6400–40 leaf chamber fluorometer (Li-6400, Li-Cor, Lincoln, NE, USA). The leaf chamber fluorometer was used as a light source. Environmental conditions in the leaf chamber consisted of a photon flux density of 500 µmol m–2 s–1 with 10% blue light, a flow rate of 400 mmol m–2 s–1 and an ambient CO2 concentration of 400 µmol mol–1 air. Ambient temperature and relative humidity were ~25°C and 50% during all measurements. Data were manually logged when gas-exchange and chlorophyll fluorescence parameters became stable. Measurements were conducted at well watered conditions (WW) before water was stopped, then 2 days after water was withdrawn (representing water-stressed (WS) status) and the next day after re-watering (representing re-watered (RW) status).
Measurement of relative water content and leaf water potential
After finishing gas-exchange measurements, leaflets were collected into ziplock bags and kept on ice before leaf area measurements. Leaf area (LA) was recorded using an area meter (LI-3000, Li-Cor). Fresh weight (FW) was measured right after leaf area measurement and then leaflets were set in water for 48 h at 4°C to achieve full turgor. After weighing the samples for turgid weight (TW), samples were oven-dried at 65°C for 48 h to obtain dry weight (DW). Plant water status was evaluated by relative water content (RWC). RWC was calculated as RWC = (FW – DW)/(TW – DW) × 100 (Ritchie et al. 1990).
Mid-day leaf water potential was measured using Wescor Thermocouple Psychrometer (Wescor Inc., South Logan, UT, USA) at day 0 (plants were just watered), 24 and 48 h after watering was ceased, and 24 h after plants were re-watered. Center and side leaflets from the uppermost fully developed trifoliates were measured from at least five plants (clones) for each of the transgenic lines and the controls.
Gas chromatography-mass spectrometry (GC-MS) analysis of cuticular wax content
Leaf cuticular wax samples were collected from one leaflet of the top of two expanded trifoliates excised from the major stems of well watered and water-stressed white clover plants. The two leaflets were combined as one leaf sample. Each sample was added to 10 mL of GC-MS grade hexane (Sigma-Aldrich Inc., St Louis, MO, USA). Tissues were agitated for 2 min and the solvent was decanted into new glass tubes. The same amount of hexane was used to rinse the tissues and tubes for 10 s and was pooled to the sample tube. Hexane was evaporated to ~1 mL under a nitrogen stream, transferred into 2 mL autosampler vials and then evaporated completely. The extracts were resuspended and derivatised with 60.0 µL of 70% pyridine and 30% MSTFA solution containing 10.0 ng µL–1 of cholesterol as internal standard. Derivatisation was performed for 60 min at 50°C. One microlitre of the solution was injected onto an Agilent 6890 gas chromatograph (Agilent, Palo Alto, CA, USA) using a splitless injection. The injector was held at 280°C and the transfer arm held at 250°C. Separation was achieved using an oven programmed at an initial temperature of 120°C for 1 min, ramped linearly to 260°C at 8°C min–1 then to 315°C at 5°C min–1, and held at 315°C for 15 min. The GC was coupled to an Agilent 5973 MSD using electron impact ionisation while scanning 50–650 m/z. Mass spectral deconvolution and metabolite identifications were done using AMDIS and NIST spectral library. Peak picking and alignment were done using MET-IDEA software. Standard calibration curves were constructed using 1-triacontanol, octacosanoic acid, heintriacontane and tridecanal, and were used for the quantifications of fatty alcohols, fatty acids, alkanes and aldehydes, respectively. Cholesterol was used for the quantification of triterpenes and unknowns.
The amount of total wax composition and each cuticular wax constituent was expressed per unit of leaf area. Leaf areas were determined using a leaf area meter (LI-3000, Li-Cor). All values represent averages of five replicates ± s.e.
Statistical analysis
Experimental data were analysed by SAS (SAS Institute, Cary, NC, USA) using completely randomised design with at least five replications for each transgenic line and the controls. Since the trends in individual experiments were similar, all experimental data for gas-exchange and chlorophyll fluorescence, RWC and water potential were pooled and analysed together taking experiment as a random effect. Differences between lines were analysed by general linear model (GLM) of the SAS program, with P < 0.05 as significant level according to least significant difference (l.s.d.).
Results
GUS staining of transgenic white clover lines with CER6-GUS
To analyse the activity of CER6 promoter in white clover, CER6-GUS was introduced into white clover by Agrobacterium-mediated transformation. Ten independent transgenic lines were stained and GUS activity was detected in both trifoliates and petioles (Fig. 1b, d). Cross-section analysis of the tissues revealed different GUS expression patterns in leaves and petioles. In the leaves, almost all cells were stained blue, but in the petioles, GUS activity was preferentially strong in epidermal cells (Fig. 1e, f).
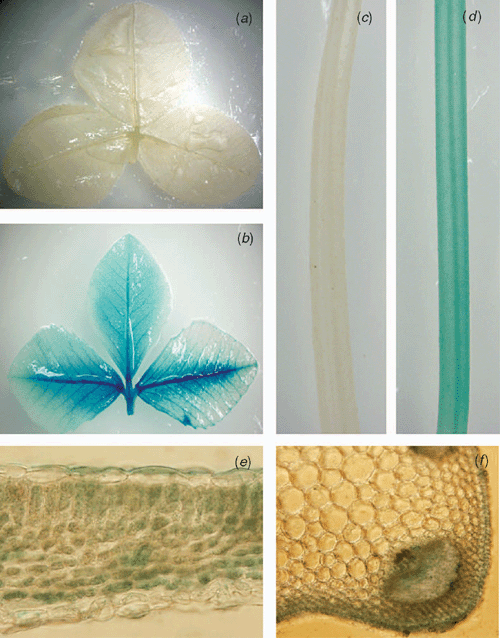
|
Molecular characterisation of WXP1 transgenic plants
PCR analysis of the regenerated white clover plants identified more than 20 CER6-WXP1 positive lines (data not shown). Preliminary experiments were conducted to detect transcript levels and to check the plants’ responses to water withdrawal. Based on the preliminary experiments, three independent transgenic lines (45, 56 and 63) were selected for further and more detailed analyses. Northern blot hybridisation analysis (Fig. 2) and RT–PCR analysis (data not shown) revealed variable levels of WXP1 gene expression in the selected transgenic lines of white clover.

|
Phenotype of the transgenic white clover plants
No obvious morphological and developmental differences were observed between the transgenic lines and the controls under a normal watering regime (Fig. 3a, WW). Two days after watering was stopped, empty-vector control (CK) and WT plants started to wilt (Fig. 3b, WS), but the three WXP1 transgenic lines were still growing normally (Fig. 3b, WS). When all the plants were re-watered after 2 days of water stress, the WXP1 transgenic lines recovered better and more quickly than the controls (Fig. 3c, RW). Some wilted leaves of the control plants were not able to recover after watering was restored (Fig. 3c, RW).

|
Gas-exchange parameters under different watering conditions
Under well watered conditions, no significant difference was found between the transgenic lines and the empty vector control in gas-exchange parameters, including net photosynthetic rate (An), stomatal conductance (gs) and transpiration rate (E) (Fig. 4a–c, WW).
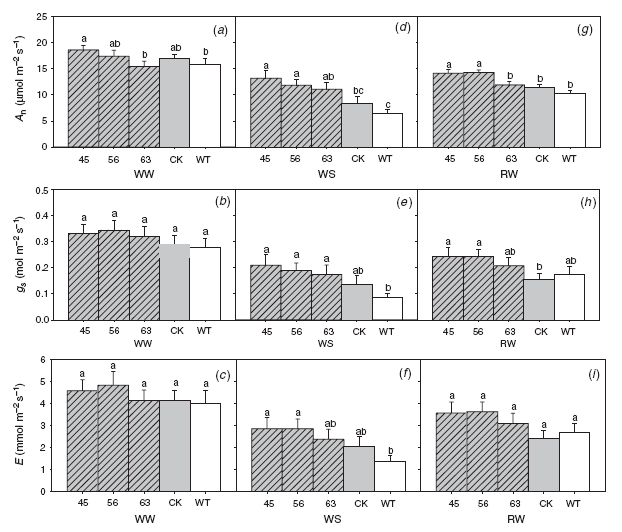
|
Two days after the last watering, all lines tested showed lower photosynthetic rates, stomatal conductance and transpiration rates than those values at well watered conditions (Fig. 4d–f, WS; 4a–c, WW). However, values of the WXP1 transgenic lines (45 and 56) decreased to a lesser extent and remained at higher means of net photosynthesis, stomatal conductance and transpiration than both controls under water-stressed conditions (Fig. 4d–f, WS). The transgenic line 63 had higher net photosynthetic rates and stomatal conductance than the WT control, but not significantly different from the empty vector control (Fig. 4d, e, WS).
After watering was resumed, transgenic lines 45 and 56 had higher photosynthetic rates and stomatal conductance than the controls (Fig. 4g, h, RW). There was no difference in transpiration rate between all the tested lines under re-watered conditions (Fig. 4i, RW).
Chlorophyll fluorescence parameters under different watering conditions
Chlorophyll fluorescence parameters were recorded simultaneously with gas exchange; the parameters included efficiency of excitation energy captured by open PSII reaction centers (Fv′/Fm′), quantum yield (ΦPSII), coefficient of photochemical quenching (qP) and apparent electron transport rate (ETR). There was no significant difference in these parameters between the transgenic lines and the empty vector control under well watered conditions (Table 1, WW).
Under water-stressed conditions, the transgenic lines showed higher levels of quantum efficiency, quantum yield, coefficient of photochemical quenching and electron transport rate than the controls (Table 1, WS). After watering was resumed, values of Fv′/Fm′, ΦPSII and ETR were still higher in the transgenics compared with the controls (Table 1, RW). Values of qP were higher than the WT control, however, no difference was detected between the transgenics and the empty vector control (Table 1, RW).
In agreement with what was observed in gas-exchange parameters, throughout the water treatment cycle, the changes of chlorophyll fluorescence parameters in the transgenic WXP1 lines were less than the controls (Table 1, WW, WS and RW). This indicated that PSII of these transgenic lines was not affected as much as the controls by drought stress.
Leaf relative water content (RWC) and water potential (ψ) under different watering conditions
After watering was withheld for 2 days, all the WXP1 transgenic white clover lines had significantly higher values of leaf RWC than the controls, indicating their improved ability to withstand drought stress (Fig. 5). No differences between transgenic lines and controls were observed under re-watered conditions (data not shown).
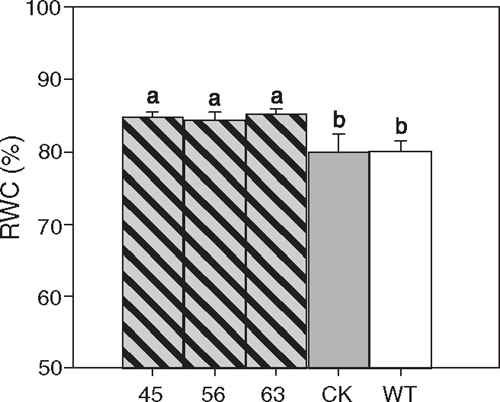
|
Leaf water potential under well watered conditions was between –0.93 MPa and –1.00 MPa for all the lines tested (Table 2, WW). No apparent decrease was noticed in the transgenic lines 24 h after water had ceased, but a gradual decrease was observed in the controls (data not shown). After 48 h leaf water potential of the controls declined sharply while values in transgenic lines decreased slowly (Table 2, WS). At this time point, the transgenic lines maintained statistically higher leaf water potential (–1.28 MPa to –1.50 MPa) than the controls (–2.00 MPa) (Table 2, WS). One day after watering was resumed, water potential of all lines recovered, with the transgenic lines having higher values than the controls (Table 2, RW).
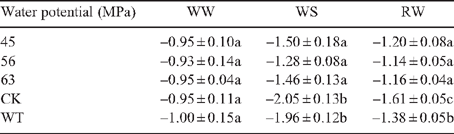
|
Cuticular wax content and constituents under different watering conditions
GC-MS analysis of the transgenic and control plants detected cuticular wax constituents including long-chain fatty acids, primary alcohols, alkanes, aldehydes and terpenes. No obvious difference in wax content was found between the transgenic lines and the controls under normal or drought conditions (Fig. 6). However, there was a clear trend that total wax content was elevated in all the lines (both transgenic and non-transgenic) under water-stressed conditions (Fig. 6).
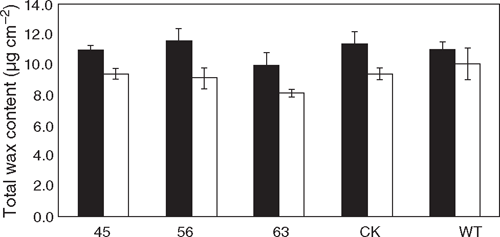
|
Discussion
Drought tolerance is a complex trait for genetic improvement. Perennial crops like white clover possess higher endogenous tolerance to environmental stresses than the widely used model species Arabidopsis, thus, it is more challenging to detect the impact of stress related transgenes. Furthermore, commercial cultivars such as ‘Patriot’ have already shown improved persistence (Bouton et al. 2005) and set a high standard for further improvement. There has been no report on improvement of abiotic stress tolerance of white clover by genetic engineering. By introducing a single transcription factor gene, WXP1, into an elite cultivar ‘Patriot’, transgenic white clover plants with enhanced drought tolerance were obtained.
Constitutive transgenic expression of some stress-related transcription factor genes (e.g. CBF/DREB, WIN1/SHN1) negatively affects plant growth (Jaglo-Ottosen et al. 1998; Kasuga et al. 1999; Aharoni et al. 2004; Broun et al. 2004). The use of suitable promoters may alleviate this problem. In this study, the WXP1 gene was driven by the Arabidopsis CER6 promoter. Hooker et al. (2002) reported the CER6 promoter as highly effective in directing epidermis-specific expression in Arabidopsis and tobacco. In white clover, however, epidermis-specific expression was observed only in petioles. The expression pattern in leaves was constitutive. The results indicated that some of the tissue-specific regulatory elements in the promoter region may not be recognised in white clover. Nevertheless, the use of the CER6 promoter led to high levels of expression of the WXP1 gene in white clover. It is noteworthy that the transgenic plants had normal growth and development. Therefore, the CER6 promoter is beneficial for genetic modification of white clover.
Many physiological processes are involved in plant drought stress responses. Characterisation of physiological changes of transgenic materials is important for understanding the mechanism of drought tolerance. However, there has been a lack of physiological characterisation and analysis of transgenic materials under stress conditions. Evaluation of transgene impact on abiotic stress tolerance has been largely based on visual observation of phenotypes (Jaglo-Ottosen et al. 1998; Kasuga et al. 1999; Aharoni et al. 2004; Broun et al. 2004; Zhang et al. 2007). In this study, physiological responses of the transgenic white clover under both water deficit and recovery conditions were studied in details.
Under drought stress conditions as in this experimental work, photosynthetic activity is negatively regulated through stomatal (closure of stomata) and/or non-stomatal factors (including damage to photosynthetic apparatus). Stomatal limitations are typically evaluated by gas-exchange measurements (Jiang et al. 2006) while measurement of chlorophyll fluorescence has been used to evaluate the integrity of PSII (Shabala 2002). Under mild water-stressed conditions, stomata play a dominant role in controlling net CO2 uptake (Cornic 2000; Souza et al. 2004). The transgenic white clover plants showed a higher net photosynthetic rate under water stress and re-watered conditions. Transpiration rate and stomatal conductance of the transgenics were similar to empty vector control. In agreement with these gas-exchange parameters, leaf water potential and relative water content were higher in the transgenics. Less reduction in water potential may help to maintain relatively higher levels of photosynthesis in the transgenics. All the data indicated that transgenic lines had improved capacity to withstand water withdrawal compared with the controls.
PSII is sensitive to water stress (van Rensburg and Kruger 1993) and plays an important role in the response of leaf photosynthesis to environmental disruptions (Baker 1991; Lal et al. 2008). Inhibition of photosynthesis by drought stress could result from photochemical and/or energy-dependent quenching (De Ronde et al. 2004). Combined analyses of chlorophyll fluorescence and gas exchange can lead to an overall picture of plant response to the environment (Maxwell and Johnson 2000). Compared with gas exchange parameters, variations in chlorophyll fluorescence between different water statuses were less prominent in white clover, indicating that gas-exchange parameters An and gs are more sensitive to water stress. Photoinhibition occurs when the rate of damage of PSII is larger than that of repair in chloroplasts (Lal et al. 2008). The transgenics suffered less damage to their PSII because the plants had significantly higher values of Fv′/Fm′, ΦPSII, qP and ETR than the controls, and this made it possible for the transgenics to maintain relatively higher photosynthetic activity under stress conditions. The physiological data showed that transgenic lines with higher gas exchange rates also had less change in chlorophyll fluorescence parameters. The consistent results of gas exchange and chlorophyll fluorescence confirmed that the transgenic lines experienced less damage under stress conditions and were more tolerant to drought than the controls.
Overexpression of WXP1 in Arabidopsis and alfalfa led to increased wax accumulation in the leaves, and it was believed that WXP1 is involved in wax biosynthesis (Zhang et al. 2005, 2007). Unexpectedly, no superior wax accumulation was observed in the transgenic WXP1 lines of white clover. Growth conditions might account for this because wax accumulation in higher plants is influenced by a variety of environmental factors, including light (von Wettstein-Knowles et al. 1979) and water deficit (Bengtson et al. 1979; Hooker et al. 2002). Another possible explanation is that the function of the WXP1 gene is species-specific. The results showed enhanced drought tolerance in the transgenic white clover plants was not due to increased wax accumulation. Therefore, the function of the WXP1 gene and its mechanism in conferring drought tolerance is far more complicated than previously thought. Because transcription factors are capable of activating or suppressing transcription of target genes as switches of the regulatory cascade, it is important to identify downstream target genes of WXP1 in future studies to provide a better understanding of gene regulation in plant stress tolerance.
In summary, the transcription factor gene WXP1 was placed under the control of the CER6 promoter and introduced into a commercial cultivar (‘Patriot’) of white clover. The transgenic plants showed improved physiological performance and enhanced tolerance to drought stress. Although the mechanism of WXP1 requires further study, it can be concluded that the gene is useful for improving plant drought tolerance.
Acknowledgements
The work was supported by The Samuel Roberts Noble Foundation. The authors thank Rhonda Walker and Jackie Kelly for critical reading of the manuscript.
Aharoni A,
Dixit S,
Jetter R,
Thoenes E,
van Arkel G, Pereira A
(2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. The Plant Cell 16, 2463–2480.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Annicchiarico P, Piano E
(2004) Indirect selection for root development of white clover and implications for drought tolerance. Journal Agronomy & Crop Science 190, 28–34.
| Crossref | GoogleScholarGoogle Scholar |

Baker NR
(1991) Possible role of photosystem II in environmental perturbations of photosynthesis. Physiologia Plantarum 81, 563–570.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bhatnagar-Mathur P,
Vadez V, Sharma KK
(2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Reports 27, 411–424.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bouton JH,
Woodfield DR,
Caradus JR, Wood DT
(2005) Registration of ‘Patriot’ white clover. Crop Science 45, 797–798.
| Crossref |

Broun P,
Poindexter P,
Osborne E,
Jiang C-Z, Riechmann JL
(2004) WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 101, 4706–4711.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Chen H,
Nelson RS, Sherwood JL
(1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze–thaw transformation and drug selection. BioTechniques 16, 664–670.
|
CAS |
PubMed |

Cornic G
(2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture – not by affecting ATP synthesis. Trends in Plant Science 5, 187–188.
| Crossref | GoogleScholarGoogle Scholar |

De Ronde JA,
Cress WA,
Kruger GHJ,
Strasser RJ, Van Staden J
(2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. Journal of Plant Physiology 161, 1211–1224.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Frame J, Newbould P
(1986) Agronomy of white clover. Advances in Agronomy 40, 1–88.
| Crossref | GoogleScholarGoogle Scholar |

Hooker TS,
Millar AA, Kunst L
(2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiology 129, 1568–1580.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jaglo-Ottosen KR,
Gilmour SJ,
Zarka DJ,
Schabenberger O, Tomashow MF
(1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Jiang Q,
Roche D,
Monaco T, Durham S
(2006) Gas exchange, chlorophyll fluorescence parameters, and carbon isotope discrimination of fourteen barley genetic lines in response to salinity. Field Crops Research 96, 269–278.
| Crossref | GoogleScholarGoogle Scholar |

Kasuga M,
Liu Q,
Miura S,
Yamaguchi-Shinozaki K, Shinozaki K
(1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology 17, 287–291.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lal S,
Gulyani V, Khurana P
(2008) Overexpression of HVA1 gene from barley generates tolerance to salinity and water stress in transgenic mulberry (Morus indica). Transgenic Research 17, 651–663.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Li Y,
Beisson F,
Ohlrogge J, Pollard M
(2007) Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiology 144, 1267–1277.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Maxwell K, Johnson GN
(2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51, 659–668.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Pang Y,
Peel GJ,
Wright E,
Wang Z-Y, Dixon RA
(2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiology 145, 601–615.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

van Rensburg L, Kruger GHJ
(1993) Differential inhibition of photosynthesis (in vivo and in vitro), and changes in chlorophyll a fluorescence induction kinetics of four tobacco cultivars under drought stress. Journal of Plant Physiology 141, 357–365.
|
CAS |

Ritchie SW,
Nguyen HT, Holaday AS
(1990) Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Science 30, 105–111.

Schnurr J,
Shockey J, Browse J
(2004) The Acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. The Plant Cell 16, 629–642.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Seker H,
Rowe DE, Brink GE
(2003) White clover morphology changes with stress treatments. Crop Science 43, 2218–2225.

Shinozaki K, Yamaguchi-Shinozaki K
(2007) Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany 58, 221–227.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Souza RP,
Machado EC,
Silva JAB,
Lagoa AMMA, Silveira JAG
(2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environmental and Experimental Botany 51, 45–56.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Xiao K,
Lui JR,
Dewbre GR,
Harrison MJ, Wang Z-Y
(2006) Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula. Plant Biology 8, 439–449.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhang JZ
(2003) Overexpression analysis of plant transcription factors. Current Opinion in Plant Biology 6, 430–440.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhang J-Y,
Broeckling CD,
Blancaflor EB,
Sledge MK,
Sumner LW, Wang Z-Y
(2005) Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). The Plant Journal 42, 689–707.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Zhang J-Y,
Broeckling CD,
Sumner LW, Wang Z-Y
(2007) Heterologous expression of two Medicago truncatula putative ERF transcription factor genes, WXP1 and WXP2, in Arabidopsis led to increased leaf wax accumulation and improved drought tolerance, but differential response in freezing tolerance. Plant Molecular Biology 64, 265–278.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |




