LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum
Jie He A B , Dominic J. Q. Koh A and Lin Qin A
A B , Dominic J. Q. Koh A and Lin Qin A
A Natural Sciences and Science Education Academic Group, National Institute of Education, Nanyang Technological University, 1 Nanyang Walk, Singapore 637616.
B Corresponding author. Email: jie.he@nie.edu.sg
Functional Plant Biology - https://doi.org/10.1071/FP20375
Submitted: 3 December 2020 Accepted: 16 April 2021 Published online: 11 May 2021
Journal Compilation © CSIRO Publishing 2021 Open Access CC BY-NC-ND
Abstract
The edible halophyte Mesembryanthemum crystallinum L. was grown at different NaCl salinities under different combined red and blue light-emitting diode (LED) light treatments. High salinity (500 mM NaCl) decreased biomass, leaf growth, and leaf water content. Interactions between LED ratio and salinity were detected for shoot biomass and leaf growth. All plants had Fv/Fm ratios close to 0.8 in dark-adapted leaves, suggesting that they were all healthy with similar maximal efficiency of PSII photochemistry. However, measured under the actinic light near or above the growth light, the electron transport rate (ETR) and photochemical quenching (qP) of M. crystallinum grown at 100 and 250 mM NaCl were higher than at 500 mM NaCl. Grown under red/blue LED ratios of 0.9, M. crystallinum had higher ETR and qP across all salinities indicating higher light energy utilisation. Crassulacean acid metabolism (CAM) was induced in M. crystallinum grown at 500 mM NaCl. CAM-induced leaves had much higher non-photochemical quenching (NPQ), suggesting that NPQ can be used to estimate CAM induction. M. crystallinum grown at 250 and 500 mM NaCl had higher total chlorophyll and carotenoids contents than at 100 mM NaCl. Proline, total soluble sugar, ascorbic acid, and total phenolic compounds were higher in plants at 250 and 500 mM NaCl compared with those at 100 mM NaCl. An interaction between LED ratio and salinity was detected for proline content. Findings of this study suggest that both salinity and light quality affect productivity, photosynthetic light use efficiency, and proline accumulation of M. crystallinum.
Keywords: common ice plant, Mesembryanthemum crystallinum, halophyte, leaf growth, photosynthetic light use efficiency, water relations, Crassulacean acid metabolism, light-emitting diode, electron transport rate, photochemical quenching, non-photochemical quenching.
Introduction
Food production depends upon the availability of land and water. Singapore is one of the world’s most land- and water-constrained countries. Thus, Singapore imports more than 90% of food consumed. Because of the increasing world population, maintaining food security in terms of quantity and quality is an increasing challenge for Singapore. Furthermore, the COVID-19 pandemic has placed unprecedented stresses on the global food supply chains. In March 2019, the Singapore government launched plans such as the ‘30 by 30’ goal to have 30% of Singapore’s nutritional needs to be met locally by 2030 (Chang 2019). To step up food security within the constraints of limited land, the use of high-technology farming such as vertical farming under light-emitting diode (LED) lighting has been growing in Singapore since 2012. Further, depletion of fresh water resources also poses serious worldwide constraints to crop productivity. Agricultural yield can be enhanced by growing halophytic vegetables, in which seawater can be used instead of fresh water.
Salt-loving halophytes can tolerate a wide range of salinities even above seawater salinity (~500 mM NaCl) through compartmentalisation, water use efficiency and ion selectivity (Waisel 1972), thus providing a basis to develop halophytes as gourmet vegetables (Yensen 2006; Castañeda-Loaiza et al. 2020). Mesembryanthemum crystallinum L. (common ice plant) is native to Africa and naturalised in Australia, Mediterranean, the Americas and the Caribbean (Adams et al. 1998). According to El-Gawad and Shehata (2014), the leaves of M. crystallinum can be eaten raw, cooked as a spinach substitute or pickled. M. crystallinum has been successfully cultivated as a vegetable crop in Japan, India, California, Australia and New Zealand (Herppich et al. 2008; Agarie et al. 2009), including Singapore (He et al. 2017). M. crystallinum is also a potential source of bioactive compounds that can be beneficial to human health (Agarie et al. 2009).
M. crystallinum possesses epidermal bladder cells that sequester Na+ ions from metabolically active tissues (Shabala et al. 2014), act as water storage reservoirs, and aid in ion homeostasis (Agarie et al. 2007). M. crystallinum can perform crassulacean acid metabolism (CAM) under stress conditions such as salinity stress (Cushman et al. 2008). However, under well-watered conditions, M. crystallinum performs C3 photosynthesis (Winter and Holtum 2005; He et al. 2017). Salinity perturbs plant water relations and photosynthetic performance by reducing leaf gas exchange, the content of photosynthetic pigments, distorting chloroplast ultrastructure, and PSII (Betzen et al. 2019; Pan et al. 2021). However, moderate salinity stress can be beneficial as it induces production of secondary metabolites without adversely affecting growth of M. crystallinum (Herppich et al. 2008; Atzori et al. 2017).
Under natural conditions, light level also affects the physiological performance of halophytes such as seed germination, seedling growth (Lázaro-Lobo et al. 2020), and stomatal and non-stomatal limitation of photosynthesis (Pan et al. 2021). Our recent studies also showed that productivity and photosynthetic characteristics of M. crystallinum grown indoors were affected by salinity (He and Qin 2020b) as well as LED spectral quality when plants were grown with fresh water (He et al. 2017). Different LED spectra play different roles in plant growth and photosynthesis. Biomass accumulation under red light was smaller than red light supplemented with blue light (Dou et al. 2017; He et al. 2017, 2019). Red light alone reduces photosynthetic rate whereas blue light maintains photosystem complexes and increases Rubisco content (Muneer et al. 2014; He et al. 2017, 2019). Red- and blue-light combinations can increase plant productivity (Sabzalian et al. 2014; Wang et al. 2016; He et al. 2019). Red-light supplemented with optimal level of blue light also enhanced photosynthetic performance including higher photosynthetic capacity, stomatal conductance, PSII photochemistry, and photosynthetic pigments compared with red-light alone (Hogewoning et al. 2010; Savvides et al. 2012; Hernández and Kubota 2016; Wang et al. 2016; He et al. 2019).
Globally, there is a major paradigm shift of how we perceive food; from the traditional concept of carbohydrate, protein, fat, and calories towards critical functional molecules such as the diverse variety of phytochemicals in vegetables. Phytochemicals such as chlorophyll (Chl), carotenoids (Car), phenolic compounds and ascorbic acid (ASC), are bioactive plant chemicals in vegetables that have health promoting properties (Hounsome et al. 2008; Boestfleisch et al. 2014). To protect against oxidative stress caused by salinity, antioxidants such as phenolic compounds and ASC are produced (Dat et al. 2000). Salinity also causes hyperosmotic stress in halophytic plants. Osmolytes such as proline and total soluble sugars (TSS), which can be utilised in functional food, are produced for protection against hyperosmotic stress (Hasegawa et al. 2000; Flowers and Colmer 2008; Agarie et al. 2009; Ben Hsouna et al. 2020). LEDs provide narrow-bandwidth light treatments, so may modulate medicinal and crop plant metabolomes to enhance antioxidant properties (Carvalho et al. 2016; Holopainen et al. 2018). Our recent studies have shown that drought stress enhanced the concentrations of phytochemicals such as phenolic compounds, ASC, and proline of M. crystallinum grown indoors under a combination of red- and blue-LED lighting (He et al. 2020). However, there is very little information on the effects of light quality on M. crystallinum grown under different saline conditions. Past studies have focussed on a single factor, whereas both factors simultaneously influence plant growth of halophytes (Lázaro-Lobo et al. 2020; Pan et al. 2021). Thus, this project aimed to investigate how changes in both salinity and light quality affect growth, physiology, and nutritional quality of M. crystallinum when grown indoors. The findings of this study could also help M. crystallinum growers to raise productivity and nutritional quality through optimal selections of LED lighting and salinity.
Materials and methods
Plant materials and experimental design
Mesembryanthemum crystallinum L. seeds were germinated on filter paper before being inserted into polyurethane cubes and incubated under a photosynthetic photon flux density (PPFD) of 100 μmol m–2 s–1 provided by high-pressure sodium lamps for 4–5 weeks. Seedlings were then transplanted into an indoor hydroponic system. They were grown under three different LED lamps with red/blue (R/B) ratios of 0.9, 2.0 and 2.8 (defined as R/B 0.9, R/B 2.0 and R/B 2.8, see Supplementary material Fig. S1, WR-16W, Beijing Lighting Valley Technology Co. Ltd) and exposed to the same level of PPFD of 300 µmol m–2 s–1, 12 h photoperiod. Under each LED spectrum, plants were grown under three NaCl salinities by adding 100, 250, and 500 mM NaCl, respectively, to a full-strength Netherlands Standard Composition with 2.2 ± 0.2 mS cm–1 conductivity and pH 6.0 ± 0.2. The room temperature and relative humidity were 24.5/23°C and 56/82% (day/night) respectively.
Measurements of productivity, leaf growth and leaf water status
Plants from each treatment were harvested 15 days after transplanting. Leaf number was recorded. Shoots and roots were separated for FW measurements. The youngest fully expanded leaves were also weighed separately before measuring their areas using a leaf area meter (WinDIAS3 Image Analysis System) to obtain total leaf area (TLA). Leaves and roots were then dried separately at 80°C for 4 days before re-weighing them to obtain DW. Specific leaf area (SLA) was determined as La/LDW where La is the leaf area (cm2) and LDW is the leaf dry weight (g) (Hunt et al. 2002). Leaf succulence (LS) was estimated as LFW/La where LFW is the leaf FW (Agarie et al. 2007). Leaf dry matter content (LDMC) was determined by LDW/LFW (Garnier et al. 2001). Leaf water content (LWC) was determined as (LFW − LDW)/LFW.
Analysis of root morphology
Ten days after transplanting, the roots of each plant were placed in a tray of water and scanned with a WIN MAC RHIZO scanner. Total root length, total number of root tips and total root surface area were determined by WIN MAC RHIZO ver. 3.9 program.
Measurement of Chl fluorescence Fv/Fm ratio
Maximum photochemical efficiency of PSII was estimated in leaf samples adapted to darkness for 15 min by the Fv/Fm ratio during mid-photoperiod using the Plant Efficiency Analyser (Hansatech Instruments). Plants cultivated for 15 days were used for the measurements of Fv/Fm ratio and all other parameters described in the following sections.
Measurement of CAM acidity
Leaf disks (1 cm diameter) were punched and placed in microtitre plate wells before the beginning and the end of photoperiod. The Milli-Q water (1 mL) was added to each well before heating in 95°C water bath for 15 min. The extracts in the wells were titrated against 0.005 M NaOH, using three drops of phenolphthalein for indicator until end-point was reached. Final volume of NaOH used to reach end-point was used to calculate CAM acidity as μmol H+ g–1 FW (He and Teo 2007).
Measurements of Chl and Car
Fresh leaf disks of 0.1 g cut from the youngest fully expanded leaves were soaked in 5 mL of N,N-dimethylformamide (Sigma chemical Co.) in the dark for 48 h at 4°C before measuring the absorptions at 647, 664, and 480 nm respectively using a spectrophotometer (UV-2550 Shimadzu). Chl a, Chl b, and Car concentrations were calculated according to Wellburn (1994).
Measurements of electron transport rate (ETR), photochemical quenching (qP) and non-photochemical quenching (NPQ)
The youngest fully expanded leaves were harvested and electron transport rate (ETR), photochemical quenching (qP), and non-photochemical quenching (NPQ) were determined at 25°C in the laboratory. Prior to measurements, the leaves were pre-darkened for 15 min. By using the IMAGING PAM MAXI (Walz), images of fluorescence emission were digitised within the camera and transferred via ethernet interface (GigEVision®) to the PC for storage and analysis. Measurements and calculations of ETR, qP, and NPQ were determined as described previously (He et al. 2011).
Measurement of proline
Proline was measured as described by Bates et al. (1973) with modification. The youngest fully expanded leaf samples were rapidly frozen in liquid nitrogen and stored at –80°C. Frozen tissue of 0.5 g was ground with 6 mL of 3% sulfosalicylic acid and centrifuged at 8422g for 10 min at 4°C. The supernatant (1 mL) was mixed with 1 mL of acid-ninhydrin and acetic acid and the mixture was heated in a water bath at 95°C for 1 h. The reaction was stopped by placing the mixture on ice. The reaction mixture was extracted with 2 mL of toluene and vortexed for 30 s. The absorbance was read at 520 nm using toluene as a blank (UV-2550 spectrophotometer, Shimadzu). The proline concentration was determined from a standard curve.
Measurement of total soluble sugar (TSS)
Dried tissue was used to determine TSS concentration by colourimetric method established by DuBois et al. (1956) and modified by He et al. (2020).
Measurement of ASC
Total ASC was assayed from 0.5 g of frozen leaves by the reduction of 2,6-dichlorophenolindophenol (DCPIP) according to Leipner et al. (1997) and modified by He et al. (2020). The ASC concentration was spectrophotometrically assayed by measuring the absorbance at 524 nm using a spectrophotometer (UV-2550 Shimadzu). L-ascorbic acid was used as a standard. Results were expressed as µg ASC g–1 FW of leaves.
Measurements of total phenolic compounds
The concentration of total phenolic compounds was determined from 0.5 g of fresh samples based on the Folin-Ciocalteu method according to Kang and Saltveit (2002) and Ragaee et al. (2006) with modification (He et al. 2020). Concentrations of total phenolic compounds were spectrophotometrically assayed by measuring the absorbance at 740 nm using a spectrophotometer (UV-2550 Shimadzu). Gallic acid was used as a standard. Total phenolic compounds of the samples were expressed as gallic acid equivalents in micrograms per gram of tissue.
Statistical analysis
Data was first checked for homoscedasticity and data transformation was performed as necessary. Once data was confirmed to be homoscedastic, two-way ANOVA was performed to detect interaction between LED ratio and NaCl salinity for the different parameters studied (see Supplementary material Table S1). For this work, when interaction between LED ratios and NaCl concentration ([NaCl]) was found to be significant, post-hoc tests were not performed, but trends of those parameters are discussed. If no statistically significant interaction between LED ratios and [NaCl] was detected, the main effects were checked via one-way ANOVA for significant differences (P < 0.05) and Tukey’s test was performed to discriminate the means among the levels of the corresponding factor. Statistical analysis was performed using Minitab (MINITAB Inc., Release 17, 2013).
Results
Productivity, leaf growth and leaf water status
An interaction between LED ratio and [NaCl] on shoot FW was significant (Table S1, F4,27 = 3.62, P < 0.05), indicating the effect of salinity on shoot FW was influenced by light quality. Shoot FW declined with increasing [NaCl] for each LED ratio (Fig. 1a). M. crystallinum grown at 100 mM NaCl had higher shoot FW than those grown at 250 and 500 mM NaCl. An interaction between LED ratio and [NaCl] was also detected for shoot DW (Table S1, F4,27 = 5.30, P < 0.05). Shoot DW (Fig. 1d) showed similar trends as shoot FW. For root FW (Fig. 1b), no interaction between LED ratio and [NaCl] was detected but only [NaCl] had a significant effect (Table S1, F2,27 = 82.64, P < 0.05). Root FW declined significantly with increasing [NaCl] for each LED ratio (Fig. 1b). Root FW of plants grown in the three [NaCl] conditions was significantly different from one another. Although root DW exhibited similar trends as those of root FW (Fig. 1e), an interaction between LED ratio and [NaCl] was found (Table S1, F4,27 = 3.97, P < 0.05). For shoot : root FW ratio, no interaction between LED ratio and [NaCl] (Table S1) was detected but both main effects were significant ([NaCl] F2,27 = 27.90, LED F2,27 = 3.38, P < 0.05). Shoot : root FW ratio of M. crystallinum grown at 100 mM NaCl was significantly higher than at 250 mM and 500 mM NaCl (Fig. 1c). For plants grown at 250 mM NaCl, shoot : root FW ratio for plants under R/B 2.8 was significantly higher than under R/B 2.0. However, there were no significantly differences in shoot : root FW ratio between plants grown under R/B 0.9 and R/B2.8 at 250 mM [NaCl]. No interaction between LED ratios and [NaCl] for shoot : root DW ratio was detected (Table S1), but [NaCl] had a significant effect (F2,27 = 18.65, P < 0.05). Shoot : root DW ratio (Fig. 1f) showed a trend opposite to shoot : root FW ratio (Fig. 1c) with M. crystallinum grown at 500 mM NaCl being significantly higher than those grown at 100 mM and 250 mM NaCl.
For leaf number and TLA, interactions between LED ratio and [NaCl] were detected (Table S1, F4,27 = 3.56, P < 0.05 for leaf number and F4,27 = 3.05, P < 0.05 for TLA). M. crystallinum grown at 500 mM NaCl had the lowest leaf number while those grown at 100 mM NaCl had highest number (Fig. 2a). The downward trend seen in leaf number with increasing [NaCl] was also observed in TLA for all LED ratios (Fig. 2b). However, no interaction between LED ratio and [NaCl] was detected for SLA (Table S1). Both LED ratio and [NaCl] had significant effects on SLA (LED F2,27 = 3.50, P < 0.05, [NaCl] F2,27 = 308.22, P < 0.05). At 100 mM NaCl, SLA of plants grown under R/B 0.9 was significantly lower than under R/B 2.0. At 250 and 500 mM NaCl, there were no significant differences in SLA among the plants grown under the three LED ratios (Fig. 2c).
No interaction between LED ratio and [NaCl] was detected for LS, LDMC, and LWC (Table S1). However, only [NaCl] had a significant effect on LS (F2,27 = 23.10, P < 0.05), LMDC (F2,27 = 152.35, P < 0.05) and LWC (F2,27 = 152.35, P < 0.05). M. crystallinum grown at 100 and 250 mM NaCl generally had similar LS values under all LED ratios (Fig. 2d) and were not significantly different from each other. Plants grown at 500 mM NaCl had significantly lower LS than those of plants grown at 100 and 250 mM NaCl. The trend for LWC (Fig. 2f) paralleled that of LS (Fig. 2d). LWC of M. crystallinum significantly decreased with increasing [NaCl] and they were significantly different from one another. LDMC increased significantly with increasing [NaCl]. LDMC of plants in each of the three salinities were significantly different from one another (Fig. 2e).
Fv/Fm ratio and CAM acidity
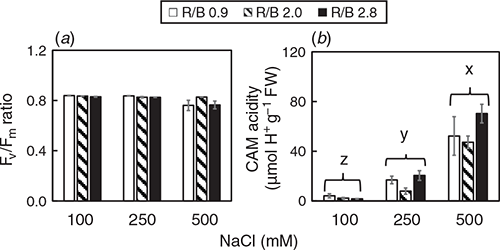
There was an interaction detected for Fv/Fm ratio (Table S1, F4,63 = 3.29, P < 0.05). Fv/Fm ratios of M. crystallinum grown at different conditions were close to 0.8 except for those grown at 500 mM under R/B 0.9 and R/B 2.8 had Fv/Fm ratios slightly below 0.8 (Fig. 3a), indicating the plants were healthy. There was no interaction between LED ratio and [NaCl] for CAM acidity (Table S1), but [NaCl] had a significant effect on this parameter (F2,18 = 87.89, P < 0.05). CAM acidity rose significantly with increasing [NaCl] for all LED ratios (Fig. 3b). M. crystallinum grown at 100 mM NaCl was the lowest. CAM acidity of plants grown at 500 mM NaCl was four times higher than those at 100 mM NaCl.
|
Photosynthetic pigments
Table S1 shows that there is an interactive effect between LED ratio and [NaCl] for total Chl (F4,18 = 6.20, P < 0.05) and Chl a/b ratio (F4,27 = 4.30, P < 0.05). M. crystallinum grown at 100 mM NaCl had lower total Chl compared with those grown at 250 and 500 mM NaCl, regardless of LED ratio (Fig. 4a). When grown at 100 and 250 mM NaCl, total Chl under R/B 0.9 and 2.0 seemed to be higher than those under R/B 2.8. However, plants grown at 500 mM NaCl showed opposite results. No clear trend was observed for Chl a/b ratio among the different treatments (Fig. 4b). Total Car showed a similar trend as total Chl (Fig. 4c). However, no interaction between LED ratio and [NaCl] was detected for total Car (Table S1). Instead, only [NaCl] had a significant effect (F2,27 = 16.08, P < 0.05). M. crystallinum grown at 250 and 500 mM [NaCl] had significantly higher total Car than those grown at 100 mM NaCl (Fig. 4c). For Chl/Car ratio, no interaction between LED ratio and [NaCl] was detected (Table S1). Only [NaCl] had a significant effect (F2,27 = 12.27, P < 0.05). Although statistically, Chl/Car ratios of M. crystallinum were significantly higher at 100 and 500 mM than at 250 mM, there were no large differences in Chl/Car ratios among M. crystallinum grown under the three [NaCl] conditions and LED (Fig. 4d).
ETR, qP, and NPQ
The light response curves of ETR, qP, and NPQ only showed for plants subjected to the extremes of each factor to demonstrate the effect of both factors on the overall responses (Fig. 5). ETR (Fig. 5a) and NPQ (Fig. 5c) increased whereas qP (Fig. 5b) decreased with increasing PPFD for all plants. The light response curves of ETR and qP for M. crystallinum grown at 500 mM NaCl were generally below those at 100 mM NaCl. However, the light response curves of NPQ of M. crystallinum grown at 500 mM NaCl were above those grown at 100 mM NaCl, especially under R/B 0.9. Fig. 6 shows the mean values of ETR, qP, and NPQ, measured at the actinic light which was near the growth light level. Interactions between LED ratio and [NaCl] were detected (Table S1) for ETR (F4,27 = 5.18, P < 0.05), qP (F4,27 = 4.24, P < 0.05), and NPQ (F4,27 = 6.51, P < 0.05). ETR of M. crystallinum grown at 100 and 250 mM NaCl seemed to be higher than at 500 mM NaCl, for all LED ratios (Fig. 6a). Within each [NaCl] condition, plants grown under R/B 0.9 had higher ETR values compared with those under R/B 2.0 or R/B 2.8. Plants grown under R/B 0.9 generally had higher qP values than those under R/B 2.0 or 2.8 for each [NaCl] condition (Fig. 6b). Plants grown at 500 mM NaCl displayed slightly lower qP values than those at 100 or 250 mM NaCl across all LED ratios. NPQ values of M. crystallinum grown at 500 mM NaCl were almost double of those at 100 250 mM NaCl, across all LED ratios (Fig. 6c).

|
Phytochemicals
An interaction between LED ratio and [NaCl] was detected for proline (Table S1, F4,18 = 307.18, P < 0.05). M. crystallinum grown at 100 mM NaCl had very low proline content compared with those of plants grown at 250 and 500 mM NaCl. Proline content in plants grown at 500 mM under R/B 0.9 and R/B 2.8 were at least three times higher than other plants (Fig. 7a). No interaction was detected for TSS (Table S1), but both [NaCl] and LED ratio had significant effects ([NaCl] F2,18 = 87.47 P < 0.05, LED F2,18 = 9.50, P < 0.05). For plants growth under each given LED ratio, TSS content rose significantly with increasing [NaCl]. Plants grown at 500 mM NaCl under R/B 0.9 had significantly higher TSS content than those under R/B 2.0 and 2.8 (Fig. 7b). No interactions between LED ratio and [NaCl] were detected for ascorbic acid and total phenolic compounds (Table S1), but only [NaCl] had a significant effect on both parameters (ascorbic acid F2,45 = 16.84, P < 0.05; total phenolic compounds F2,18 = 81.92, P < 0.05). Ascorbic acid and total phenolic compounds were significantly lower in M. crystallinum grown at 100 mM NaCl than those grown at 250 and 500 mM NaCl. However, there were no significant differences in these phytochemical concentrations between plants grown at 250 and 500 mM NaCl (Fig. 7c, d). LED ratio had no significant impact on ascorbic acid and total phenolic compounds under each [NaCl] condition (Fig. 7c, d).
Discussion
Productivity, leaf growth and leaf water status
Most halophytes require saline conditions to attain optimal growth. M. crystallinum shows optimal growth within 50–250 mM NaCl (Flowers et al. 1986). Our previous study confirmed that M. crystallinum grown at 100 mM NaCl had the highest shoot FW and largest leaf area compared with those grown at 0, 250, and 500 mM NaCl. However, M. crystallinum grown at 500 mM NaCl had the lowest shoot and leaf area (He and Qin 2020b). In this study, plants grown at 500 mM NaCl also had the lowest shoot and root shoot FW (Fig. 1a, b). Suboptimal salinities negatively affect growth by decreasing carbon fixation or re-allocating energy and resources towards osmotic adjustment through synthesising osmolytes (Flowers and Colmer 2008; Flowers et al. 2010; Hamed et al. 2013; Benjamin et al. 2019; Ben Hsouna et al. 2020) such as proline and TSS (Fig. 7a, b). Different LED spectral quality may also affect biomass accumulation in M. crystallinum. We have recently reported that LED R : B ratio of 9 : 1 promoted highest growth for M. crystallinum (He et al. 2017). Enhanced growth under combined red- and blue-LED has also been observed in spinach, radish, and lettuce (Muneer et al. 2014; Wang et al. 2016). As both salinity and light quality can affect biomass, it was not surprising to find statistically significant interactions between LED ratio and [NaCl] for shoot FW and DW (Table S1, Fig. 1). This implies that light quality influences salinity effects on M. crystallinum and vice versa. Thus, it appears necessary to control both factors in order to optimise yield.
It has been reported that shoot biomass accumulation was due to increases in leaf number and leaf area (Wang et al. 2016; He and Qin 2020a). The reductions of these two parameters (Fig. 2a, b) might partly account for the lower biomass under higher salinity. As interactions between light quality and salinity were detected for both parameters, the effects of light quality on both parameters are likely influenced by salinity. M. crystallinum grown at 100 mM NaCl had the significantly higher SLA than when grown at 250 and 500 mM NaCl (Fig. 2c). Although red- and blue-light combinations enhance leaf growth (Christophe et al. 2006; He et al. 2019), light quality seemed to impact SLA in this study only where R/B 2.0 promote thinner leaves compared with the other LED ratios. However, this appears restricted to only low salinity conditions of 100 mM NaCl (Fig. 2c).
M. crystallinum accumulates Na+ and Cl– in the bladder cells of leaves and stems, preventing their excessive accumulation in photosynthetic tissues (Agarie et al. 2007; Castañeda-Loaiza et al. 2020). Leaf extension and water status might have been depressed by bladder cells and vacuoles reaching maximum capacity and unable to sequester more Na+, resulting in excess Na accumulating in the leaves (Munns 1993). The LS of plants measured on a leaf area basis was significantly lower when grown at 500 mM NaCl than at 100 and 250 mM NaCl (Fig. 2d). This result suggests that there was less water in the leaves grown under the highest salinity of 500 mM NaCl regardless of different leaf thickness measured by SLA (Fig. 2c). This is further supported by the trends observed for LWC (Fig. 2f), which is related to the maximum water content that can potentially be achieved by the leaf.
The depression of LS and LWC in M. crystallinum grown at 500 mM NaCl could be attributed to the stunted root architecture which might have limited water uptake. For instance, plants grown at 500 mM NaCl had the shortest total root length with smallest number of root tip and total root surface area while the greatest values of these parameters belonged to plants grown at 100 mM NaCl (Fig. S2). Herppich et al. (2008) reported no significant effect of salinity on LS and LWC when M. crystallinum was grown at 150 mM NaCl and harvested at much later growth stage. It is possible that reductions in LS and LWC observed in this study are the early effects of salinity stress, and are evident only at salinities >250 mM NaCl. LDMC is the growth trait which has been proposed as an indicator of plant resource use (Garnier et al. 2001). LDMC (mg g–1) is the proportion of the leaf matter content without water related to the mass of the leaf with the maximum water content. In this study, LDMC was significantly higher in plants grown at 500 mM NaCl than at 100 and 250 mM NaCl (Fig. 2e), indicating the former accumulated more biomass for the same amount of FW. However, the higher LDMC at higher [NaCl] condition was more likely due to the low water content as biomass was clearly lower at higher salinities (Fig. 1). Lowest LWC (Fig. 2f) and highest LDMC (Fig. 2e) could explain why shoot : root FW ratio (Fig. 1c) and DW (Fig. 1f) were respectively the lowest and the highest in M. crystallinum grown at 500 mM NaCl.
Photosynthetic light use efficiency and photosynthetic pigments
The Fv/Fm ratio is an early indicator of salt stress and provides important information on maximal (potential) efficiency of PSII photochemistry (Kalaji et al. 2011; Matsuoka et al. 2018). The Fv/Fm ratios in dark-adapted leaves among all plants were close to 0.8 (Fig. 3a), indicating that there were no evidence of damage to PSII (James et al. 2002; Barker et al. 2004; Broetto et al. 2007). However, M. crystallinum grown at different [NaCl] exhibited different photochemical light use efficiency measured by ETR, qP, and NPQ in light-adapted leaves (Figs 5, 6). Measured under the actinic light which was either near (Fig. 6) or above (Fig. 5) their growth light, the ETR values of M. crystallinum grown at 100 mM NaCl were higher than at 500 mM NaCl. Broetto et al. (2007) reported that the maximal quantum efficiency of PSII, Fv/Fm measured at predawn always remaining at 0.8, showing that there was no acute photoinhibition, when M. crystallinum with 400 mM NaCl under both high light (1000 μmol m–2 s–1, HLSA) and low light (200 μmol m–2 s–1, LLSA) for 13 days. Broetto et al. (2007) also found that ETRmax (ETR at saturated light) of M. crystallinum grown at 400 mM NaCl under both high light and low light declined during the daily courses. Furthermore, in the present study, plants grown under R/B 0.9 had higher ETR values across all salinities. M. crystallinum grown under higher blue light utilised more light energy indicated by the high ETR. The higher ETR could be due to higher cyclic electron transport around photosystem I to avoid photodamage (Shikanai 2007; Takahashi and Badger 2011). Different ETRs for M. crystallinum grown under different light sources could also be due to the variability in the PSII/PSI stoichiometry. It is likely that photosystem stoichiometry was adjusted (Chow et al. 1990), leading to a change in light partitioning coefficient. This is a plausible strategy for plants to cope with the higher energy associated with blue light under R/B 0.9 than under R/B 2.0 or 2.8. This further explains why all plants were relatively healthy with Fv/Fm ratios around 0.8. qP, the proportion of PSII reaction centres that remained open, showed similar responses as those of ETR to LED quality (Fig. 6b). This result further supports that M. crystallinum grown under high blue light (R/B 0.9) exhibited higher photosynthetic light use efficiency compared with those grown under R/B 2.0 or 2.8. However, the light source for actinic light illumination when fluorescence kinetics were analysed was different from LED lights under which M. crystallinum were grown. Thus, the absorptance of M. crystallinum leaves to actinic light (fixed at 0.84) may not be the same due to light spectrum acclimation.
Blue light has been reported to increase total Chl pool, leading to increases in ETR (Wang et al. 2016; He et al. 2017). Under salinity stress, total Chl might reduce due to increased Chl degradation and reduced Chl synthesis (Santos 2004). However, in this study, M. crystallinum grown at 250 and 500 mM NaCl had higher total Chl content compared with those grown at 100 mM (Fig. 4a). Similarly, in the study with two obligate halophytes – Sesuvium portulacastrum and Tecticornia indica – Rabhi et al. (2012) reported that under saline conditions (200 and 400 mM NaCl), total Chl content was significantly enhanced in both species. The aforementioned studies only investigated the effects of light quality or salinity separately. In this study, the interactions between the two factors were statistically significant (Table S1) for both total Chl and ETR (Table S1; Fig. 6), the effect of salinity on photosynthetic light use efficiency of light-adapted leaves are possibly influenced by light quality under which they were grown. Li et al. (2020) reported hybrid Pennisetum grown under NaCl salinity condition had lower Chl a/b ratios compared with those grown without NaCl. Rabhi et al. (2012) found that the Chl a/b ratio was slightly modified by salinity and, in both S. portulacastrum and T. indicia only at 400 mM NaCl; it was found that Chl a/b ratio increased in S. portulacastrum and decreased in T. indica. However, in this study, there were no obvious difference in Chl a/b ratio among all M. crystallinum (Fig. 4b). Matsuoka et al. (2018) also found that after 2 weeks of treatment with 500 mM NaCl, M. crystallinum had little variation in the Chl a/b ratio, suggesting a constant antenna size.
NPQ values of M. crystallinum grown at 500 mM NaCl were close to 2, whereas those at 100 and 250 mM NaCl were around 1.0–1.08 across all LED ratios (Fig. 6c), indicating an increase in the thermal dissipation of excess energy via the xanthophyll cycle that involves Car under higher [NaCl] (Koyro 2006; Broetto et al. 2007; Jahns and Holzwarth 2012). This was supported by the fact that total Car of plants grown at 500 mM NaCl, across the LED ratios, were significantly higher than at 100 mM (Fig. 4c). However, it was also noted that total Car content was similar for plants grown at 250 and 500 mM NaCl (Fig. 4c), whereas NPQ was much higher in plants grown at 500 than at 250 mM NaCl (Fig. 6c). Therefore, there was no clear relationship between NPQ and Car in the present study. Similar to the total Chl content, Rabhi et al. (2012) found that higher total Car content in both S. portulacastrum and T. indica grown at 200 and 400 mM NaCl. Koyro (2006) reported that salt-induced increase of the Car content in leaves of halophyte, Plantago coronopus, could function to dissipate the excess energy in the PSI and PSII. In this study, although interaction between LED ratio and [NaCl] was not detected (Table S1) for Chl/Car ratio only [NaCl] had a significant effect on Chl/Car ratios among M. crystallinum grown under the three [NaCl] conditions. However, the impacts of LED on Chl/Car ration did not vary greatly (Fig. 4d).
CAM acidity
M. crystallinum demonstrates a substantial reversion to C3 photosynthesis following the removal of stress (Winter and Holtum 2014; He et al. 2017; He and Qin 2020b). In our recent studies, M. crystallinum grown indoors with fresh water had high light-saturated CO2 assimilation rate (Asat) and stomatal conductance (gs sat) but very low CAM acidity during light period (He et al. 2017). We have also found that simulating drought stress causes water deficit of M. crystallinum but does not induce CAM (He et al. 2020). According to Cushman et al. (2008), CAM acidity levels of at least 40 μmol H+ g–1 FW were deemed to be performing CAM under saline conditions. In this study, M. crystallinum grown at 500 mM NaCl was most likely engaging CAM as all plants at 500 mM NaCl had CAM acidity of >40 μmol H+ g–1 FW (Fig. 3b), indicating the mode of photosynthesis was switched from C3 to crassulacean acid metabolism (CAM) upon high salt stress. Matsuoka et al. (2018) reported that high salinity induced CAM photosynthesis in M. crystallinum, which apparently resulted in photoinhibition measured by the decreased Fv/Fm ratio during the first 3 days of CAM induction. However, in this study, Fv/Fm ratio were close to 0.8 in all plants. Matsuoka et al. (2018) also found that Fv/Fm ratios of CAM-induced leaves did not change diurnally, but NPQ showed a clear diurnal change. Under actinic illumination near the growth light level, NPQ values of CAM-induced leaves in the dark period gradually increased during CAM induction. Based on the study of Matsuoka et al. (2018), there was no close relationship between Fv/Fm ratio and the induction of CAM photosynthesis under salt stress. However, many researchers (Keiller et al. 1994; Broetto et al. 2007; Niewiadomska et al. 2011; Matsuoka et al. 2018) reported that in the CAM-inducible M. crystalline under high salt stress, lower qP and higher NPQ were observed during the dark period than during the light period, under the same actinic light. Thus, NPQ can be used to estimate the degree of CAM induction. Higher NPQ in M. crystallinum grown at 500 mM NaCl was also observed in the present study when chlorophyll fluorescence parameters were analysed under an actinic light which is near the growth light level in the middle of light period (Fig. 6c). However, for M. crystallinum grown at 100 and 250 mM NaCl, CAM induction doesn’t seem to occur (Fig. 3b) and their NPQ values were much lower than those of plants grown at 500 mM NaCl across all LED ratios (Fig. 6c). It has been reported that blue light induces higher NPQ (Hemming 2011; He et al. 2015, 2017; Hamdani et al. 2019). In our previous study, it was found that higher blue-LEDs resulted in higher NPQ in M. crystallinum grown with freshwater (He et al. 2017). However, this was not observed in the present study with M. crystallinum grown with saline water when the measurements were carried out under the actinic light which is near the growth light (Fig. 6c). There is an interaction between LED quality and salinity for NPQ (Table S1), the results of NPQ could be the impact of one factor depending on the level of the other factor. In other words, the effect of blue light on NPQ was attenuated under saline conditions. However, when measured under higher actinic lights, NPQ of M. crystallinum grown at 500 mM NaCl was much higher under R/B 0.9 (higher blue-LED) than under R/B 2.8 (Fig. 5c). The induction of CAM when M. crystallinum grown at 500 mM NaCl could potentially account for its low biomass accumulation (Fig. 1) as CAM is an energetically expensive process. CAM requires a ready supply of organic intermediates and to pump malate across the tonoplast, all of which require high amounts of ATP. As the interaction between light quality and salinity was not significant for CAM acidity (Table S1), it seemed that changing salinity would be sufficient to induce CAM in M. crystallinum.
Phytochemicals
Proline and TSS are well known osmolytes that enable plants to avoid the consequences of hyperosmotic stress caused by high salinity (Ashraf and Harris 2004; Flowers and Colmer 2008; Agarie et al. 2009; Hamed et al. 2013; Benjamin et al. 2019; Ben Hsouna et al. 2020). Our study also showed that high proline and TSS accumulation were observed in M. crystallinum grown at 250 and 500 mM NaCl (Fig. 7a, b). Sanadhya et al. (2015) also reported the similar results which proline, total amino acids and TSS increased with increasing salt concentrations in halophyte Aeluropus lagopoides. In the perennial halophyte Sesuvium portulacastrum, Nikalje et al. (2018) revealed an increase in the proline content in its leaves and roots subjected to both low and high salt treatments. Thus, the increased proline and TSS content could be one of the strategies for halophyte to prevent salt-induced damage. In the present study, M. crystallinum grown at 250 and 500 mM had significantly lower LWC compared with those grown at 100 mM NaCl (Fig. 2f). This result indicates that accumulation of proline and TSS could also be one of the strategies for M. crystallinum grown under higher salinities to prevent the effects of water deficit on its physiological process (Paul and Cockburn 1989; Lokhande and Suprasanna 2012; Kumari et al. 2015; He et al. 2020). Paul and Cockburn (1989) reported that CAM was induced in M. crystallinum plants grown at 400 mM NaCl, which was accompanied by the accumulation of proline and pinitol to constitute 71% of the soluble carbohydrate. Kumari et al. (2015) suggested that proline is the important metabolite involved in salt tolerance of halophytes. In our previous study, we found that grown under simulated drought stress, M. crystallinum accumulated much higher amounts of proline and TSS compared with well-watered plants (He et al. 2020).
Proline accumulation was also associated with light when plants subjected to salt stress (Goas et al. 1982; Hayashi et al. 2000). Proline content of Arabidopsis increased in light and decreased in darkness (Hayashi et al. 2000). However, there is very little work published on the effect of light quality on proline accumulation. In this study, light quality seemed to affect proline similarly to salinity stress but its effects cannot be considered without salinity and vice versa (Table S1). Although no interaction between LED ratio and [NaCl] was detected for TSS, both salinity and light quality affected TSS levels separately. Light quality affects photosynthetic rate (Muneer et al. 2014; He et al. 2017), which affects sugar production with R/B 0.9 promoting high TSS accumulation under high salinity conditions. Muneer et al. (2014) reported that photosynthetic performance of lettuce leaves increased with an increasing light intensity under blue LED illumination. In the study with M. crystallinum grown with fresh water, we previously demonstrated that blue LED enhance photosynthetic CO2 assimilation rate (He et al. 2017). In the present study, M. crystallinum grown at 500 mM NaCl had high CAM acidity across all LED ratios (Fig. 3c). High TSS accumulation in M. crystallinum grown under R/B 0.9 at 500 mM NaCl is very unlikely due to its high photosynthetic rate. Hyperosmotic stress may have occurred in M. crystallinum grown at 500 mM NaCl, and thus it is likely that high blue promoted TSS accumulation for protection against hyperosmotic stress (Hasegawa et al. 2000; Flowers and Colmer 2008; Agarie et al. 2009; Ben Hsouna et al. 2020).
Salinity and drought stress usually induce oxidative damage, forming reactive oxygen species (ROS) in both glycophytes and halophytes (Chaparzadeh et al. 2004; Bose et al. 2014; Wang et al. 2004). Apart from the accumulation of proline which has antioxidant functions (Bose et al. 2014), halophytes are also able to synthesise certain natural antioxidants such as ascorbic acid and total phenolic compounds under saline and drought conditions (Ksouri et al. 2007; Dat et al. 2000; He et al. 2020). Ascorbic acid is involved in the Mehler reaction (Smirnoff 1996), which scavenges ROS. Phenolic compounds possess antioxidative properties and confer various physiological responses to stresses in plants (Cheynier et al. 2013). As salt stress induces oxidative stress (Ozgur et al. 2013), accumulation of both compounds was expected. Ben Hsouna et al. (2020) reported that both phenolic contents and the antioxidant activity of leaves of the halophyte Lobularia maritima were increased at 200 mM salinity stress. Although the antioxidant activity was not determined in this study, ascorbic acid and total phenolic compounds of M. crystallinum were similarly and significantly higher when grown at 250 and 500 mM NaCl than at 100 mM NaCl (Fig. 7c, d), suggesting that levels of these substances can be controlled by adjusting salinity levels. Separately, red- and blue-light combinations also promote ascorbic acid and phenolic compounds (Holopainen et al. 2018). However, this could be species-dependent since there were no significant differences in the two compounds among the three LED ratios in this study.
In conclusion, this study aimed to investigate the interactive effects between salinity and light quality on growth, photosynthesis and phytochemical production of M. crystallinum. The results revealed a highly complex picture as there were no distinct patterns in which interactions were found for the parameters studied. However, the findings did show that M. crystallinum grown with high salinity of 500 mM NaCl was unfavourable although higher accumulations of phytochemicals such as proline, TSS, ascorbic acid, and total phenolic compounds were observed in those plants. The findings of this study provide the M. crystallinum growers with information to enhance productivity and nutritional quality through optimal selections of LED lighting and salinity. It would be feasible to first grow M. crystallinum under low salinity such as 100 mM NaCl to achieve high biomass before transferring to high salinity conditions to enhance phytochemical production. Furthermore, the interaction between salinity and light quality may depend on the light intensity, which merits further study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research project is supported by the Ministry of Education, Singapore, under its Academic Research Fund Tier 1 (2018-T1–001–008) and teaching materials’ vote of National Institute of Education, Nanyang Technological University, Singapore.
Acknowledgements
We are indebted to Professor Fred Wah Soon Chow, Australian National University, for the opportunities he made widely available to establish and refine a system to effectively study the efficiency of photosynthetic light utilisation under different LED spectral quality.
References
Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum. New Phytologist 138, 171–190.| Growth and development of Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar |
Agarie S, Shimoda T, Shimizu Y, Baumann K, Sunagawa H, Kondo A, Ueno O, Nakahara T, Nose A, Cushman JC (2007) Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. Journal of Experimental Botany 58, 1957–1967.
| Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar | 17452753PubMed |
Agarie S, Kawaguchi A, Kodera A, Sunagawa H, Kojima H, Nose A, Nakahara T (2009) Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Production Science 12, 37–46.
| Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation.Crossref | GoogleScholarGoogle Scholar |
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Science 166, 3–16.
| Potential biochemical indicators of salinity tolerance in plants.Crossref | GoogleScholarGoogle Scholar |
Atzori G, de Vos AC, van Rijsselberghe M, Vignolini P, Rozema J, Mancuso S, van Bodegom PM (2017) Effects of increased seawater salinity irrigation on growth and quality of the edible halophyte Mesembryanthemum crystallinum L. under field conditions. Agricultural Water Management 187, 37–46.
| Effects of increased seawater salinity irrigation on growth and quality of the edible halophyte Mesembryanthemum crystallinum L. under field conditions.Crossref | GoogleScholarGoogle Scholar |
Barker DH, Marszalek J, Zimpfer JF, Adams WW (2004) Changes in photosynthetic pigment composition and absorbed energy allocation during salt stress and CAM induction in Mesembryanthemum crystallinum. Functional Plant Biology 31, 781–787.
| Changes in photosynthetic pigment composition and absorbed energy allocation during salt stress and CAM induction in Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar | 32688949PubMed |
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205–207.
| Rapid determination of free proline for water-stress studies.Crossref | GoogleScholarGoogle Scholar |
Ben Hsouna AB, Ghneim-Herrera T, Romdhane WB, Dabbous A, Saad RB, Brini F, Abdelly C, Hamed KB (2020) Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Functional Plant Biology 47, 912–924.
| Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima.Crossref | GoogleScholarGoogle Scholar |
Benjamin JJ, Lucini L, Jothiramshekar S, Parida A (2019) Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiology and Biochemistry 135, 528–545.
| Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes.Crossref | GoogleScholarGoogle Scholar | 30442441PubMed |
Betzen BM, Smart CM, Maricle KL, MariCle BR (2019) Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Transactions of the Kansas Academy of Science 122, 49–58.
| Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species.Crossref | GoogleScholarGoogle Scholar |
Boestfleisch C, Wagenseil NB, Buhmann AK, Seal CE, Wade EM, Muscolo A, Papenbrock J (2014) Manipulating the antioxidant capacity of halophytes to increase their cultural and economic value through saline cultivation. AoB Plants 6, plu046
| Manipulating the antioxidant capacity of halophytes to increase their cultural and economic value through saline cultivation.Crossref | GoogleScholarGoogle Scholar | 25125698PubMed |
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany 65, 1241–1257.
| ROS homeostasis in halophytes in the context of salinity stress tolerance.Crossref | GoogleScholarGoogle Scholar | 24368505PubMed |
Broetto F, Duarte HM, Lüttge U (2007) Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3–CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress. Journal of Plant Physiology 164, 904–912.
| Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3–CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress.Crossref | GoogleScholarGoogle Scholar | 16781797PubMed |
Carvalho SD, Schwieterman ML, Abrahan CE, Colquhoun TA, Folta KM (2016) Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Frontiers in Plant Science 7, 1328
| Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum).Crossref | GoogleScholarGoogle Scholar | 27635127PubMed |
Castañeda-Loaiza V, Oliveira M, Santos T, Schüler L, Lima AR, Gama F, Salazar M, Neng NR, Nogueira JMF, Varela J, Barreira L (2020) Wild vs cultivated halophytes: nutritional and functional differences. Food Chemistry 333, 127536
| Wild vs cultivated halophytes: nutritional and functional differences.Crossref | GoogleScholarGoogle Scholar | 32707417PubMed |
Chang A-L (2019) Singapore sets 30% goal for home-grown food by 2030 [online]. Available at https://www.straitstimes.com/singapore/spore-sets-30-goal-for-home-grown-food-by-2030 [accessed 1 January 2020].
Chaparzadeh N, D’Amico ML, Khavari-Nejad RA, Izzo R, Navari-Izzo F (2004) Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiology and Biochemistry 42, 695–701.
| Antioxidative responses of Calendula officinalis under salinity conditions.Crossref | GoogleScholarGoogle Scholar | 15474374PubMed |
Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiology and Biochemistry 72, 1–20.
| Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology.Crossref | GoogleScholarGoogle Scholar | 23774057PubMed |
Chow WS, Melis A, Anderson JM (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proceedings of the National Academy of Sciences of the United States of America 87, 7502–7506.
| Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 11607105PubMed |
Christophe A, Moulia B, Varlet-Grancher C (2006) Quantitative contributions of blue light and PAR to the photocontrol of plant morphogenesis in Trifolium repens (L.). Journal of Experimental Botany 57, 2379–2390.
| Quantitative contributions of blue light and PAR to the photocontrol of plant morphogenesis in Trifolium repens (L.).Crossref | GoogleScholarGoogle Scholar | 16798853PubMed |
Cushman JC, Agarie S, Albion RL, Elliot SM, Taybi T, Borland AM (2008) Isolation and characterisation of mutants of common ice plant deficient in crassulacean acid metabolism. Plant Physiology 147, 228–238.
| Isolation and characterisation of mutants of common ice plant deficient in crassulacean acid metabolism.Crossref | GoogleScholarGoogle Scholar | 18326789PubMed |
Dat J, Vandenabeele S, Vranova ÂE, Van Montagu M, Inze ÂD, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences 57, 779–795.
| Dual action of the active oxygen species during plant stress responses.Crossref | GoogleScholarGoogle Scholar | 10892343PubMed |
Dou H, Niu G, Gu M, Masabni JG (2017) Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 3(2), 36.
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28, 350–356.
| Colorimetric method for determination of sugars and related substances.Crossref | GoogleScholarGoogle Scholar |
El-Gawad AMA, Shehata HS (2014) Ecology and development of Mesembryanthemum crystallinum L. in the deltaic Mediterranean coast of Egypt. Egyptian Journal of Basic and Applied Sciences 1, 29–37.
| Ecology and development of Mesembryanthemum crystallinum L. in the deltaic Mediterranean coast of Egypt.Crossref | GoogleScholarGoogle Scholar |
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar |
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. The Quarterly Review of Biology 61, 313–337.
| Halophytes.Crossref | GoogleScholarGoogle Scholar |
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology 37, 604–612.
| Evolution of halophytes: multiple origins of salt tolerance in land plants.Crossref | GoogleScholarGoogle Scholar |
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15, 688–695.
| A standardized protocol for the determination of specific leaf area and leaf dry matter content.Crossref | GoogleScholarGoogle Scholar |
Goas G, Goas M, Larher F (1982) Accumulation of free proline and glycine betaine in Aster tripolium subjected to a saline shock: a kinetic study related to light period. Physiologia Plantarum 55, 383–388.
| Accumulation of free proline and glycine betaine in Aster tripolium subjected to a saline shock: a kinetic study related to light period.Crossref | GoogleScholarGoogle Scholar |
Hamdani S, Khan N, Perveen S, Qu M, Jiang J, Govindjee , Zhu X-G (2019) Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynthesis Research 139, 107–121.
| Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light.Crossref | GoogleScholarGoogle Scholar | 30456488PubMed |
Hamed KB, Ellouzi H, Talbi OZ, Hessini K, Slama I, Ghnaya T, Bosch SM, Savouré A, Abdelly C (2013) Physiological response of halophytes to multiple stresses. Functional Plant Biology 40, 883–896.
| Physiological response of halophytes to multiple stresses.Crossref | GoogleScholarGoogle Scholar | 32481158PubMed |
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51, 463–499.
| Plant cellular and molecular responses to high salinity.Crossref | GoogleScholarGoogle Scholar | 15012199PubMed |
Hayashi F, Ichino T, Osanai M, Wada K (2000) Oscillation and regulation of proline content by P5CS and proDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant & Cell Physiology 41, 1096–1101.
| Oscillation and regulation of proline content by P5CS and proDH gene expressions in the light/dark cycles in Arabidopsis thaliana L.Crossref | GoogleScholarGoogle Scholar |
He J, Qin L (2020a) Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. Journal of Plant Physiology 252, 153239
| Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse.Crossref | GoogleScholarGoogle Scholar | 32763651PubMed |
He J, Qin L (2020b) Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities. Acta Horticulturae 219–226.
| Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities.Crossref | GoogleScholarGoogle Scholar |
He J, Teo LCD (2007) Susceptibility of CAM Dendrobium Burana Jade green leaves and green flower petals to high light under tropical natural conditions. Photosynthetica 45, 214–221.
| Susceptibility of CAM Dendrobium Burana Jade green leaves and green flower petals to high light under tropical natural conditions.Crossref | GoogleScholarGoogle Scholar |
He J, Tan BHG, Qin L (2011) Source-to-sink relationship between green leaves and green pseudobulbs of C3 orchid in regulation of photosynthesis. Photosynthetica 49, 209–218.
| Source-to-sink relationship between green leaves and green pseudobulbs of C3 orchid in regulation of photosynthesis.Crossref | GoogleScholarGoogle Scholar |
He J, Qin L, Liu YM, Choong TW (2015) Photosynthetic capacities and productivity of indoor hydroponically grown Brassica alboglabra Bailey under different light sources. American Journal of Plant Sciences 6, 554–563.
| Photosynthetic capacities and productivity of indoor hydroponically grown Brassica alboglabra Bailey under different light sources.Crossref | GoogleScholarGoogle Scholar |
He J, Qin L, Chong ELC, Choong TW, Lee SK (2017) Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs. Frontiers in Plant Science 8, 361
| Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs.Crossref | GoogleScholarGoogle Scholar | 28367156PubMed |
He J, Qin L, Chow WS (2019) Impacts of LED spectral quality on leafy vegetables: productivity closely linked to photosynthetic performance or associated with leaf traits? International Journal of Agricultural and Biological Engineering 12, 16–25.
| Impacts of LED spectral quality on leafy vegetables: productivity closely linked to photosynthetic performance or associated with leaf traits?Crossref | GoogleScholarGoogle Scholar |
He J, Chua EL, Qin L (2020) Drought does not induce crassulacean acid metabolism (CAM) but regulates photosynthesis and enhances nutritional quality of Mesembryanthemum crystallinum. PLoS One 15, e0229897
| Drought does not induce crassulacean acid metabolism (CAM) but regulates photosynthesis and enhances nutritional quality of Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar | 33232385PubMed |
Hemming S (2011) Use of natural and artificial light in horticulture – interaction of plant and technology. Acta Horticulturae 25–35.
| Use of natural and artificial light in horticulture – interaction of plant and technology.Crossref | GoogleScholarGoogle Scholar |
Hernández R, Kubota C (2016) Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environmental and Experimental Botany 121, 66–74.
| Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs.Crossref | GoogleScholarGoogle Scholar |
Herppich WB, Huyskens-Keil S, Schreiner M (2008) Effects of saline irrigation on growth, physiology and quality of Mesembryanthemum crystallinum L., a rare vegetable crop. Journal of Applied Botany and Food Quality 82, 47–54.
Hogewoning SW, Trouworst G, Maljaars H, Poorter H, van leperen W, Harbinson J (2010) Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Journal of Experimental Botany 61, 3107–3117.
| Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light.Crossref | GoogleScholarGoogle Scholar | 20504875PubMed |
Holopainen JK, Kivimäenpää M, Julkunen-Tiitto R (2018) New light for phytochemicals. Trends in Biotechnology 36, 7–10.
| New light for phytochemicals.Crossref | GoogleScholarGoogle Scholar | 28939181PubMed |
Hounsome N, Hounsome B, Tomos D, Edwards-Jones G (2008) Plant metabolites and nutritional quality of vegetables. Journal of Food Science 73, R48–R65.
| Plant metabolites and nutritional quality of vegetables.Crossref | GoogleScholarGoogle Scholar | 18460139PubMed |
Hunt R, Causton DR, Shipley B, Askew AP (2002) A modern tool for classical plant growth analysis. Annals of Botany 90, 485–488.
| A modern tool for classical plant growth analysis.Crossref | GoogleScholarGoogle Scholar | 12324272PubMed |
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochimica et Biophysica Acta 1817, 182–193.
| The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II.Crossref | GoogleScholarGoogle Scholar | 21565154PubMed |
James RA, Rivelli AR, von Caemmerer S, Munns R (2002) Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Functional Plant Biology 29, 1393–1403.
| Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat.Crossref | GoogleScholarGoogle Scholar | 32688739PubMed |
Kalaji HM, Govindjee , Bosa K, Kościelniak J, Źuk-Gołaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environmental and Experimental Botany 73, 64–72.
| Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces.Crossref | GoogleScholarGoogle Scholar |
Kang HM, Saltveit ME (2002) Antioxidant capacity of lettuce leaf tissue increases after wounding. Journal of Agricultural and Food Chemistry 50, 7536–7541.
| Antioxidant capacity of lettuce leaf tissue increases after wounding.Crossref | GoogleScholarGoogle Scholar | 12475267PubMed |
Keiller DR, Slocombe SP, Cockburn W (1994) Analysis of chlorophyll a fluorescence in C3 and CAM forms of Mesembryanthemum crystallinum. Journal of Experimental Botany 45, 325–334.
| Analysis of chlorophyll a fluorescence in C3 and CAM forms of Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar |
Koyro H-W (2006) Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.) Environmental and Experimental Botany 56, 136–146.
| Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.)Crossref | GoogleScholarGoogle Scholar |
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiology and Biochemistry 45, 244–249.
| Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima.Crossref | GoogleScholarGoogle Scholar | 17408958PubMed |
Kumari A, Das P, Parida AK, Agarwal PK (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Frontiers in Plant Science 6, 537
| Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 26284080PubMed |
Lázaro-Lobo A, Herrera M, Campos JA, Ervin GN (2020) Influence of local adaptations, transgenerational effects and changes in offspring’s saline environment on Baccharis halimifolia L. under different salinity and light levels. Environmental and Experimental Botany 177, 104134
| Influence of local adaptations, transgenerational effects and changes in offspring’s saline environment on Baccharis halimifolia L. under different salinity and light levels.Crossref | GoogleScholarGoogle Scholar |
Leipner J, Fracheboud Y, Stamp P (1997) Acclimation by suboptimal temperature diminishes photooxidative damage in maize leaves. Plant, Cell & Environment 20, 366–372.
| Acclimation by suboptimal temperature diminishes photooxidative damage in maize leaves.Crossref | GoogleScholarGoogle Scholar |
Li P, Zhu Y, Song X, Song F (2020) Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiology and Biochemistry 155, 93–104.
| Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum.Crossref | GoogleScholarGoogle Scholar | 32745934PubMed |
Lokhande VH, Suprasanna P (2012) Prospects of Halophytes in understanding and managing abiotic stress tolerance. In ‘Environmental adaptations and stress tolerance of plants in the era of climate change’. (Eds P Ahmad, MNV Prasad) pp. 26–59. (Springer: Berlin)
Matsuoka T, Onozawa A, Sonoike K, Kore-Eda S (2018) Crassulacean acid metabolism induction in Mesembryanthemum crystallinum can be estimated by non-photochemical quenching upon actinic illumination during the dark period. Plant & Cell Physiology 59, 1966–1975.
| Crassulacean acid metabolism induction in Mesembryanthemum crystallinum can be estimated by non-photochemical quenching upon actinic illumination during the dark period.Crossref | GoogleScholarGoogle Scholar |
Muneer S, Kim EJ, Park JS, Lee JH (2014) Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). International Journal of Molecular Sciences 15, 4657–4670.
| Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.).Crossref | GoogleScholarGoogle Scholar | 24642884PubMed |
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell & Environment 16, 15–24.
| Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses.Crossref | GoogleScholarGoogle Scholar |
Niewiadomska E, Bilger W, Gruca M, Mulisch M, Miszalski Z, Krupinska K (2011) CAM-related changes in chloroplastic metabolism of Mesembryanthemum crystallinum L. Planta 233, 275–285.
| CAM-related changes in chloroplastic metabolism of Mesembryanthemum crystallinum L.Crossref | GoogleScholarGoogle Scholar | 21046147PubMed |
Nikalje GC, Variyar PS, Joshi MV, Nikam TD, Suprasanna P (2018) Temporal and spatial changes in ion homeostasis, antioxidant defense and accumulation of flavonoids and glycolipid in a halophyte Sesuvium portulacastrum (L.) L. PLoS One 13, e0193394
| Temporal and spatial changes in ion homeostasis, antioxidant defense and accumulation of flavonoids and glycolipid in a halophyte Sesuvium portulacastrum (L.) L.Crossref | GoogleScholarGoogle Scholar | 29641593PubMed |
Ozgur R, Uzilday B, Sekmen AH, Turkan I (2013) Reactive oxygen species regulation and antioxidant defence in halophytes. Functional Plant Biology 40, 832–847.
| Reactive oxygen species regulation and antioxidant defence in halophytes.Crossref | GoogleScholarGoogle Scholar | 32481154PubMed |
Pan T, Liu M, Kreslavski VD, Zharmukhamedov SK, Nie C, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S (2021) Non-stomatal limitation of photosynthesis by soil salinity. Critical Reviews in Environmental Science and Technology 51, 791–825.
| Non-stomatal limitation of photosynthesis by soil salinity.Crossref | GoogleScholarGoogle Scholar |
Paul ML, Cockburn W (1989) Pinitol, a compatible solute in Mesembryanthemum crystallinum L.? Journal of Experimental Botany 40, 1093–1098.
| Pinitol, a compatible solute in Mesembryanthemum crystallinum L.?Crossref | GoogleScholarGoogle Scholar |
Rabhi M, Castagna A, Remorini D, Scattino C, Smaoui A, Ranieri A, Abdelly C (2012) Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. South African Journal of Botany 79, 39–47.
| Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica.Crossref | GoogleScholarGoogle Scholar |
Ragaee S, Abdel-Aal EM, Noaman M (2006) Antioxidant activity and nutrient composition of selected cereals for food use. Food Chemistry 98, 32–38.
| Antioxidant activity and nutrient composition of selected cereals for food use.Crossref | GoogleScholarGoogle Scholar |
Sabzalian MR, Heydarizadeh P, Zahedi M, Boroomand A, Agharokh M, Sahba MR, Schoefs B (2014) High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agronomy for Sustainable Development 34, 879–886.
| High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production.Crossref | GoogleScholarGoogle Scholar |
Sanadhya P, Agarwal P, Agarwal PK (2015) Ion homeostasis in a salt-secreting halophytic grass. AoB Plants 7, plv055
| Ion homeostasis in a salt-secreting halophytic grass.Crossref | GoogleScholarGoogle Scholar | 25990364PubMed |
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Scientia Horticulturae 103, 93–99.
| Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves.Crossref | GoogleScholarGoogle Scholar |
Savvides A, Fanourakis D, van Ieperen W (2012) Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. Journal of Experimental Botany 63, 1135–1143.
| Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves.Crossref | GoogleScholarGoogle Scholar | 22121201PubMed |
Shabala S, Bose J, Hedrich R (2014) Salt bladders: do they matter? Trends in Plant Science 19, 687–691.
| Salt bladders: do they matter?Crossref | GoogleScholarGoogle Scholar | 25361704PubMed |
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology 58, 199–217.
| Cyclic electron transport around photosystem I: genetic approaches.Crossref | GoogleScholarGoogle Scholar | 17201689PubMed |
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Annals of Botany 78, 661–669.
| The function and metabolism of ascorbic acid in plants.Crossref | GoogleScholarGoogle Scholar |
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends in Plant Science 16, 53–60.
| Photoprotection in plants: a new light on photosystem II damage.Crossref | GoogleScholarGoogle Scholar | 21050798PubMed |
Waisel Y (1972) ‘Biology of halophytes.’ (Academic Press: New York)
Wang B, Lüttge U, Ratajczak R (2004) Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte Suaeda salsa L. Journal of Plant Physiology 161, 285–293.
| Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte Suaeda salsa L.Crossref | GoogleScholarGoogle Scholar | 15077627PubMed |
Wang J, Lu W, Tong Y, Yang Q (2016) Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Frontiers in Plant Science 7, 250
| Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light.Crossref | GoogleScholarGoogle Scholar | 27014285PubMed |
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144, 307–313.
| The spectral determination of chlorophylls a and b, as well as carotenoids, using various solvents with spectrophotometers of different resolution.Crossref | GoogleScholarGoogle Scholar |
Winter K, Holtum JAM (2005) The effects of salinity, crassulacean acid metabolism and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species. Planta 222, 201–209.
| The effects of salinity, crassulacean acid metabolism and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species.Crossref | GoogleScholarGoogle Scholar | 15968514PubMed |
Winter K, Holtum JAM (2014) Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 65, 3425–3441.
| Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis.Crossref | GoogleScholarGoogle Scholar | 24642847PubMed |
Yensen NP (2006) Halophyte uses for the twenty-first century. In ‘Ecophysiology of high salinity tolerant plants’. (Eds MA Khan, DJ Weber) pp. 367–397. (Springer-Verlag: Berlin)







