Simulating daily field crop canopy photosynthesis: an integrated software package
Alex Wu A C D , Al Doherty A C , Graham D. Farquhar B C and Graeme L. Hammer A CA Centre for Plant Science, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Brisbane, Qld 4072, Australia.
B Research School of Biology, Australian National University, Canberra, ACT 2601, Australia.
C ARC Centre of Excellence for Translational Photosynthesis, Australia.
D Corresponding author. Email: c.wu1@uq.edu.au
Functional Plant Biology 45(3) 362-377 https://doi.org/10.1071/FP17225
Submitted: 9 August 2017 Accepted: 29 September 2017 Published: 13 November 2017
Abstract
Photosynthetic manipulation is seen as a promising avenue for advancing field crop productivity. However, progress is constrained by the lack of connection between leaf-level photosynthetic manipulation and crop performance. Here we report on the development of a model of diurnal canopy photosynthesis for well watered conditions by using biochemical models of C3 and C4 photosynthesis upscaled to the canopy level using the simple and robust sun–shade leaves representation of the canopy. The canopy model was integrated over the time course of the day for diurnal canopy photosynthesis simulation. Rationality analysis of the model showed that it simulated the expected responses in diurnal canopy photosynthesis and daily biomass accumulation to key environmental factors (i.e. radiation, temperature and CO2), canopy attributes (e.g. leaf area index and leaf angle) and canopy nitrogen status (i.e. specific leaf nitrogen and its profile through the canopy). This Diurnal Canopy Photosynthesis Simulator (DCaPS) was developed into a web-based application to enhance usability of the model. Applications of the DCaPS package for assessing likely canopy-level consequences of changes in photosynthetic properties and its implications for connecting photosynthesis with crop growth and development modelling are discussed.
Additional keywords: CO2 partial pressure, dry matter accumulation, modeling, modelling, radiation, temperature effects.
Introduction
The next advance in field crop productivity will likely need to come from improving crop use efficiency of resources (e.g. radiation, CO2, water and nitrogen), aspects of which are closely linked with overall crop photosynthetic efficiency (Long et al. 2015). For this, there is an emerging agenda focussed on genetic manipulation of the biochemical pathway of photosynthesis aiming to enhance photosynthesis for improved crop yield (Evans 2013; Long et al. 2015). However, progress is limited by the lack of connection between biochemical/leaf-level photosynthetic manipulation and crop performance, which is influenced by interactions between (photosynthetic) genetic controls, plant growth and development processes, and environmental effects. Crop models that can incorporate the interactions and integrate across scales of biological organisation might be the tool needed to accelerate progress in photosynthetic enhancement (Wu et al. 2016).
In many crop models that are used for seasonal simulation of crop growth, development and yield, daily biomass accumulation (which is determined by canopy photosynthesis) is a key driver of crop growth that has been used to simulate source-limited plant growth. Canopy photosynthesis modelling began with empirical models of leaf photosynthetic light response (PLR), which were upscaled and integrated to simulate diurnal canopy photosynthesis (Monsi and Saeki 1953; Hammer et al. 2009). There are multiple approaches for such upscaling, which focussed on modelling the heterogeneous light environment within the canopy. These can be classified into models with ‘simplified’ canopy representation, such as multi-layer models (each layer partitioned into sunlit and shade leaf fractions) (Duncan et al. 1967), single-layer big-leaf models (Sellers et al. 1992; Sands 1995) or (single-layer) sun–shade leaves models (Hammer and Wright 1994; de Pury and Farquhar 1997). Another type of approach is detailed models, such as static 3D and dynamic 3D (Vos et al. 2010) canopy architecture models. The respective (dis)advantages of these models have been discussed (Wu et al. 2016) and many have supported the simplicity and robustness of the sun–shade leaves approach. This approach can use either single or multiple layers with canopy leaf area index in each layer(s) partitioned into sunlit and shade leaf fractions. Another widely used type of canopy photosynthesis simulation, which avoids the need for photosynthesis modelling and upscaling, is to utilise a simple empirical linear relationship between daily crop (aboveground) biomass increment and intercepted solar radiation (or radiation use efficiency, RUE) (Sinclair and Muchow 1999). Theoretical derivations have shown consistencies between the PLR and RUE approaches to modelling (Hammer and Wright 1994). Both types underpin source-limited plant growth simulation, which can be connected with crop models that incorporate both source- and sink-limited crop growth (Hammer et al. 2010). For example, the APSIM crop models (Hammer et al. 2009) provide important effects that can regulate canopy photosynthesis via crop nitrogen status, which influences photosynthetic capacity. This is an effective and robust framework for connecting photosynthesis with crop growth, development and yield simulation (Wu et al. 2016).
Given the focus of photosynthetic enhancement at the biochemical level for field crop improvement, the PLR and RUE types of canopy photosynthesis modelling may not be adequate despite their apparent success in crop models. Their responses to variations at the biochemical level and to environment are difficult to predict due to the aggregated nature of the models. To overcome the limitations, canopy models based on more mechanistic photosynthesis models (e.g. C3 and C4 photosynthesis models; von Caemmerer 2000) have emerged (de Pury and Farquhar 1997) and have been incorporated into vegetation growth models (e.g. BioCro, http://biocro.r-forge.r-project.org/, accessed 16 October 2017; Ecosys, http://ecosys.ualberta.ca/, accessed 16 October 2017; GECROS, Yin and van Laar (2005); and WIMOVAC, Humphries and Long (1995)). Most of these have utilised the simple and robust sun–shade leaves approach. However, there are only a limited number of such canopy photosynthesis models being applied in crop models (Yin and Struik 2008). Despite a limited number, these examples of modelling work demonstrated the value of using biochemical based canopy photosynthesis models to expand the biological functionality of crop models, which could potentially aid progress in photosynthetic enhancement for field crop improvement.
Besides the eventual target of incorporating diurnal canopy photosynthesis into field crop performance prediction, there is also a need for developing a standalone diurnal canopy photosynthesis simulator. This is likely to stimulate and guide different approaches to leaf-level photosynthesis research and reinforces thinking at the canopy level. For example, correlating Rubsico carboxylation rate with leaf nitrogen content would be useful for simulation of instantaneous canopy photosynthetic rate (de Pury and Farquhar 1997). More examples of relationships between photosynthetic and plant attributes have also emerged (Braune et al. 2009). As discussed above, there are existing examples of canopy models; however, they have been developed as integrated modules in more extensive vegetation growth models. A standalone diurnal canopy photosynthesis simulator that informs canopy CO2 assimilation/biomass accumulation in terms of photosynthetic attributes and diurnal environment would be a desirable tool. Such a tool can be utilised to aid the wider community of photosynthesis experimentalists to understand consequences at a higher level over a longer simulation period, as well as providing a valuable teaching tool.
The rationale of extending crop modelling and aiding progress in photosynthesis research warrant the development for a standalone tool of diurnal canopy photosynthesis simulation. It will need to incorporate the biochemical models of photosynthesis as well as respond to diurnal environment effects for simulating canopy CO2 assimilation/biomass accumulation of a field crop over a day. To develop such a tool, three objectives have been identified:
-
develop a standalone C3 and C4 Diurnal Canopy Photosynthesis Simulator (DCaPS) for both C3 and C4 photosynthesis based on the concept of a cross-scale modelling framework that facilitates connection with crop growth and development dynamics (Wu et al. 2016),
-
present model rationality tests by simulating responses to key environmental factors (i.e. light, CO2 and temperature), canopy nitrogen status (i.e. specific leaf nitrogen and its profile through the canopy), and canopy attributes and architecture (i.e. canopy leaf area index and leaf angle), and
-
develop DCaPS into an interactive web-based application that can be accessed using internet browsers on any major platform for simulating likely canopy-level consequences of photosynthetic changes.
The implications of the DCaPS package for crop modelling and it applications for photosynthetic manipulation are also discussed.
Model overview
The Diurnal Canopy Photosynthesis Simulator (DCaPS) calculates diurnal (period from sunrise to sunset) canopy CO2 assimilation and daily (24 h) biomass increment for a crop under well watered conditions. A schematic diagram of the model is provided in Fig. 1, model detail in the next section, and a comprehensive description and list of model equations and parameters in Tables 1, 2 and the appendices. Daily values of incident solar radiation, air temperature (Ta) and air vapour pressure deficit (VPDa), commonly used in crop models, were used to derive instantaneous values at the start of each hour over the diurnal period. A single-layer sunlit and shade leaf modelling approach was used. Canopy leaf area index (LAIcan) was partitioned into sunlit and shade leaf fractions using the sun–shade leaves modelling approach (Hammer and Wright 1994; de Pury and Farquhar 1997) to calculate the amount of photosynthetically active radiation (PAR) absorbed by each fraction. Ta was assumed as a proxy for leaf temperature (Tl), which affects photosynthetic physiology. The canopy profile of leaf nitrogen on a leaf area basis (specific leaf nitrogen, SLN) was input and used to calculate the maximum rate of Rubisco carboxylation (Vcmax), the maximum rate of electron transport (Jmax) and the maximum phosphoenolpyruvate (PEP) carboxylase activity (Vpmax) (de Pury and Farquhar 1997), which are parameters of the C3 and C4 photosynthesis models (Farquhar et al. 1980; von Caemmerer 2000) used in DCaPS. The photosynthesis models were coupled with a CO2 diffusion model to calculate Cc and CO2 assimilation rate. Photosynthesis of both the sunlit and shade leaf fractions of the canopy were calculated, summed for the canopy, integrated hourly, and summed over the diurnal period to calculate total diurnal canopy photosynthesis, which was taken as the daily sum. This was converted to daily total biomass increment (BIOtotal,DAY) assuming a conversion ratio (B), which combines factors allowing for biochemical conversion and maintenance respiration (Sinclair and Horie 1989). A fraction of BIOtotal,DAY was partitioned to root and the remaining amount was taken as the daily aboveground canopy (shoot) biomass increment (BIOshoot,DAY).

|

|
|
Model detail
Absorbed photosynthetically active radiation (PAR)
In both the C3 and C4 photosynthesis models (not replicated here, but see Appendix 1 and 2, available as Supplementary Material to this paper), the potential electron transport rates (J, μmol e– m–2 s–1) of sunlit and shade leaf fractions are driven by absorbed PAR using a non-rectangular hyperbolic function (von Caemmerer 2000). Absorbed PAR for each of the sunlit and shade leaf fractions varies diurnally and its calculation requires diurnal total incident solar radiation, LAIcan, canopy architecture (in the form of canopy-average leaf angle), and optical properties (reflectance and transmittance) of leaves. Separation of absorbed PAR for the sunlit and shade leaf fractions of the canopy is a necessary detail to avoid errors in over estimation of photosynthetic rate (de Pury and Farquhar 1997).
The calculation of diurnal absorbed PAR depends on the radiation environment (Hammer and Wright 1994). First, diurnal extra-terrestrial radiation (So, MJ m–2 ground day–1) was calculated from latitude (Lat, radians) and day of year (DAY) (Eqn A5, see Supplementary Material). Then diurnal total incident solar radiation on the ground (Sg, MJ m–2 ground s–1) was calculated by multiplying So and the atmospheric transmission ratio (RATIO) (Eqn A4). Sg was then distributed sinusoidally over the diurnal period to derive instantaneous values for incident radiation (Io MJ m–2 ground s–1) (Eqn A3). Io consists of direct (Idir, MJ m–2 ground s–1) and diffuse (Idif, MJ m–2 ground s–1) radiation components. Diffuse radiation represents 17% of solar insolation (So) for any Lat, DAY and RATIO (Eqn A1). The diurnal pattern of atmospheric transmission of Idir is more complex, so was simply obtained by the difference between Io and Idif (Eqn A2). This approach allows the proportion of Idir and Idif to vary across a diurnal period giving, for example, higher proportion of Idif in early and later hours of the diurnal period and higher proportion of Idif if RATIO is low due to cloud cover.
The derived Idir and Idif (total incident solar radiation) from above were used to estimate direct and diffuse PAR (Idir_PAR and Idif_PAR respectively). It was assumed that 50% of the energy in Idir and Idif was PAR, which was converted to photosynthetic photon flux density by multiplying by 4.56 and 4.25 μmol PAR (J PAR)–1 respectively (Eqns A21 and A22). Units for Idir_PAR and Idif_PAR are μmol PAR m–2 ground s–1.
The PAR absorbed by either sunlit or shade leaves fractions (Iabs_sun and Iabs_sh, both with units of μmol PAR m–2 s–1) was calculated using the equations of de Pury and Farquhar (1997). This incorporated Idir_PAR and Idif_PAR, optical properties of leaves, such as reflectance and transmittance to PAR, LAIcan, and the proportion of intercepted radiation (dependent on canopy-average leaf angle and LAIcan). It was assumed that the sunlit leaf fraction received Idir_PAR, Idif_PAR and scattered radiation (caused by reflectance and transmittance of leaves), while the shade leaf fraction received only Idif_PAR and scattered radiation. Detailed equations and calculation procedures are given by Eqns A19–A32 in Appendix 1. In the current model, diurnal variations in leaf reflectance and transmittance are not considered. However, as a first approximation, it can be input into DCaPS for each diurnal simulation.
Air vapour pressure deficit (VPDa)
Air vapour pressure deficit (VPDa, kPa) was calculated as the difference between the saturated vapour pressure at air temperature (SVPa) and that at dew-point temperature (SVPd) (Eqns A16–A18). The minimum temperature (Ta,min; more below) for the day was assumed as the dew-point temperature, which has been shown to give robust estimates of VPDa (Lobell et al. 2015). Accordingly, VPDa varies diurnally with air temperature.
Specific leaf nitrogen (SLN) and photosynthetic physiology
Specific leaf nitrogen (SLN, g N m–2 leaf) influences key photosynthetic physiological parameters. Using the mathematical development by de Pury and Farquhar (1997), vertical variation through the canopy can be explicitly incorporated; the approach integrates the profile to give a total for the single-layer canopy, which is then partitioned into sunlit and shade leaves. Distribution of SLN in the canopy was assumed to follow an exponential decay with canopy depth (Eqn A34). The decay function was specified by SLN at the top layer of the canopy (SLNo, g N m–2 leaf) and the average SLN for the canopy (SLNav, g N m–2 leaf). To incorporate the effects of SLN on photosynthetic physiology, this model assumed that at the reference temperature of 25°C, the maximum rate of Rubisco carboxylation (Vcmax), the maximum rate of electron transport (Jmax), leaf day respiration (Rd), and the maximum PEP carboxylase activity (Vpmax) were all zero below a minimum SLN and increased linearly with slope of χV, χJ, χR and χP, respectively, above that threshold value (Eqns A37–A40). The minimum SLN values were 0.35 and 0.2 g N m–2 (leaf) for C3 and C4 respectively (Table 1).
Leaf temperature (Tl)
Estimation of air temperature (Ta, °C) is needed as it significantly influences leaf temperature (Tl, °C). A model of daily Ta (over the 24 h) was used (Eqn A15). Even though the majority of daylight hours can be modelled by the diurnal function of the model, the night time function is sometimes applicable for the early hours of the diurnal period. The diurnal period was modelled with a sine function and an exponential decay function was used during the night. The amplitude of the daily Ta fluctuation was specified by the maximum (Ta,max, °C) and minimum (Ta,min, °C) air temperature of the day. The phase shift of the sine function was determined by the lag coefficient for the maximum temperature (xlag), the night-time temperature coefficient (ylag), and the lag of minimum temperature from the time of sunrise (zlag) (Eqn A15). It was assumed here that Tl is approximated by Ta for both sunlit and shade leaf fractions. This is a reasonable assumption over a wide range of temperature under well watered conditions.
The response of photosynthesis to Tl was modelled through responses of the C3 and C4 photosynthesis model parameters to Tl (i.e. Kc, Ko, Vomax/Vcmax, Vcmax, Jmax and Rd for C3 plus Kp and Vpmax for C4; Table 1). There is a growing availability of these temperature responses, in particular, for model species. The most comprehensive dataset for the C3 Nicotiana tabacum L. have been used effectively for simulating temperature responses of leaf photosynthesis (Bernacchi et al. 2002). Temperature responses of Kc, Ko and Vomax/Vcmax are usually assumed to be similar among C3 species (von Caemmerer 2013), so here we used parameters from N. tabacum for C3 crop species. It was reported that Kc of Triticum aestivum L. is significantly different to N. tabacum (Sharwood et al. 2016), but whether or not this has significant implications for diurnal canopy photosynthesis will require sensitivity analysis when other wheat parameters also become available. Parameter availability for C4 crop species is not as comprehensive so here we used parameters for the C4 model species Setaria viridis (L.) P.Beauv. (Boyd et al. 2015). These default values can be readily changed as parameters of C4 crop species become better known.
Temperature responses of Kc, Ko, Vcmax, Rd, Kp and Vpmax were modelled using an exponential type function (Eqn 1, adapted from Sharkey et al. (2007)), whereas Jmax, due to its apparent optimum in temperature response (Farquhar et al. 1980), was modelled via a normal distribution function (Eqn 2, adapted from June et al. (2004)). Vcmax/Vomax and its temperature response were not available from Bernacchi et al. (2002), where Kc and Ko were reported, but can be back calculated from Kc, Ko and Γ* with Eqns A52 and A53 (assuming a chloroplastic oxygen partial pressure (Oc) of 210000 μbar). Its temperature response can be modelled with Eqn 1. In summary, temperature responses of the C3 and C4 photosynthesis model parameters to Tl were modelled with Eqn 1 or 2 with parameter values given in Table 2.
Expression of the exponential type function used to describe temperature response of certain photosynthesis model parameters (adapted from Sharkey et al. (2007)):

where P25 is the modelled value of parameter at 25°C, c and b are empirical constants, which are balanced to give the factor after P25 unity at 25°C. Expression of the normal distribution function (adapted from June et al. (2004)):

where Topt is the optimum temperature and Ω is the difference in temperature from Topt at which P falls to e–1 (0.37).
Chloroplastic CO2 partial pressure (Cc)
Air CO2 (Ca, μbar) has to diffuse into leaves to reach the carboxylating site of Rubisco inside the chloroplasts for photosynthesis. The best practice for expressing CO2 levels is in partial pressure (Sharkey et al. 2007). To convert from the usual unit of ppm to μbar, it was multiplied by the air pressure (e.g. at sea level, CO2 of 400 ppm is (400 × 10–6 × 1013 250 μbar = 405.3 μbar). Leaf boundary-layer and stomatal conductance have significant effects on the drawdown of intercellular airspace CO2 partial pressure (Ci) relative to Ca (Leuning 1995) and mesophyll conductance has significant effects on the drawdown of CO2 partial pressure at the carboxylating site of Rubisco (Cc) relative to Ci (Flexas et al. 2012). Diffusional conductance, the reciprocal of resistance, of these three components (i.e. leaf boundary-layer (glb), stomatal (gs) and mesophyll (gm) conductance) are incorporated in Cc estimation (Eqn A47) based on Fick’s first law of diffusion. To model crop canopies, the turbulent resistance through the canopy boundary layer, which would affect CO2 partial pressure, air temperature and vapour pressure deficit (relative to those above the canopy), needs to be considered (Leuning et al. 1995). However, in their modelling work, Leuning et al. (1995) showed simulated canopy photosynthesis reproduced features in data so the omission of the turbulent resistance is a reasonable approximation.
However, there are uncertainties in the estimation of glb and gs. The model that is commonly used for glb estimation relies on leaf width and local wind speed (Goudriaan and van Laar 1994), which cannot be assigned a priori. Numerous types of leaf stomatal conductance (gs) models have been developed over the years (Damour et al. 2010). Two particular types are widely used. These are the empirical multiplicative models of environmental influences such as light, Ca and VPDa (e.g. the Jarvis model; Jarvis (1976)) and the semi-empirical models relating gs to photosynthesis with VPDa (e.g. the BWB model (abbreviated using authors’ names); Ball et al. (1987)), whereas more mechanistic models with plant physiology considerations based on abscisic acid or hydraulic control have also been developed (Damour et al. 2010). The limitation of the multiplicative type models is the lack of interactions between plant physiology and among the environmental factors; while the models relating gs to photosynthesis rely on empirical parameters, which cannot be assigned a priori. These empirical coefficients can vary greatly between C3 species (Li et al. 2012) and so cannot be generalised for C3 crop species, whereas the coefficients are rarely reported for C4 crop species. Given that there are limited data available to calibrate the empirical coefficients of the Jarvis or the BWB models for C3 and especially C4 crop species, an alternative approach to estimate Ci is to use the ratio of Ci/Ca, which is based on stomatal optimisation theory in that stomata respond to maintain a constant Ci under a given Ca to maximise CO2 assimilation. This ratio (~0.7 for C3 and ~0.4 for C4) has been found to be stable with Ca between 100 μbar and 400 μbar in combination with any PPFD between 250 μmol m–2 s–1 and 2000 μmol m–2 s–1 (Wong et al. 1979); further, Ci/Ca does not appear to change under elevated Ca (Ainsworth and Long 2005). The consistency in Ci/Ca in a wide range of conditions makes it an efficient and robust modelling approach. It is not clear how Ci/Ca would respond to PPFD lower than 250 μmol m–2 s–1, but such conditions only apply to the very early and late hours in a diurnal period, which amount to less than ~5% of diurnal canopy photosynthesis and so any changes under such conditions would have limited effect on diurnal total estimation. However, like gs, Ci/Ca is influenced by VPDa. It was found to decrease linearly with VPDa in various species including C3 Oryza sativa and C4 Zea mays (Zhang and Nobel 1996). Ci/Ca response to VPDa can be given by:

where a and b are empirical constants. For C3 they are –0.12 and 0.90, respectively; for C4, they are –0.19 and 0.84 respectively. At VPDa between 1 to 2 kPa, Eqn 3 gives ~0.7 and 0.5 for C3 and C4, respectively, which are similar to those reported by Wong et al. (1979). Here, we used the simpler Ci/Ca ratio approach for Cc estimation (Eqn A49), which avoided the need for glb and gs.
The importance of mesophyll conductance (gm) has been recognised only recently. gm in model C3 species is known to vary with temperature and there is evidence that gm may also respond to the other key environmental factors (e.g. irradiance and CO2), but this variation is not yet completely certain (Pons et al. 2009). So here we included only the effect of temperature and modelled this by using the normal distribution function (Eqn 2). At the reference temperature (i.e. 25°C) gm (per leaf area) in C3 wheat is assumed to be 0.55 mol CO2 m–2 s–1 bar–1. No values for C4 species have been reported, so the C3 value was used as a default value.
Diurnal canopy photosynthesis, daily respiration, root and canopy biomass accumulation, and RUE
Diurnal canopy CO2 assimilation, daily respiration and conversion losses, and allowance for root biomass were used to calculate daily aboveground canopy (shoot) biomass increment (BIOshoot,DAY). This model assumes that photosynthesis during the diurnal period results in carbon assimilation for the entire day so we use the symbol Acan,DAY. Acan,DAY was calculated by summing the CO2 assimilation of the sunlit (Asun) and shaded (Ash) leaf fractions of the canopy at the start of each hour over the diurnal period, integrated hourly and summed over the diurnal period (Eqn A73). Using the C3 and C4 photosynthesis models, leaf respiration during the diurnal period can be implicitly accounted for at the leaf level with the parameter Rd (Eqns A51, A54, A55 and A59). However, this lacks consideration of respiration from other plant organs and during the night period. A common approach is to omit the leaf-level Rd (by setting χR to zero) and consider respiration at the plant level on a daily basis, which can be accounted for within a conversion ratio (B) that combines factors allowing for biochemical conversion of CO2 to biomass and CO2 loss due to maintenance respiration (Sinclair and Horie 1989). This approach is consistent with the conservative respiration : photosynthesis ratio approach (Gifford 2003), by which plant respiration is taken as a fraction of total canopy photosynthesis. The conversion ratio, B, is 0.41 g biomass (g CO2)–1 for cereal crops such as rice and maize (Sinclair and Horie 1989). Therefore, daily whole-plant biomass increment (BIOtotal,DAY) was calculated by multiplying Acan,DAY with the molecular weight of CO2 (= 44 g (mol CO2)–1) and B. To calculate shoot biomass increment (BIOshoot,DAY), BIOtotal,DAY is multiplied by the fraction of aboveground (shoot) biomass to total biomass (shoot + root), denoted by Pshoot (Eqn A74). In effect, this simulates partitioning of a fraction of BIOtotal,DAY to root. Here, Pshoot is given a default of 1 assuming a mature canopy around flowering. The RUE for the day (RUEDAY, g biomass MJ–1) was then calculated by dividing BIOshoot,DAY by the total amount of intercepted solar radiation (Eqns A75 and A76 respectively).
Model rationality analysis
Environmental parameters
Default environmental parameters were set for C3 wheat (winter crop) and C4 sorghum (summer crop), with a canopy leaf area index (LAIcan) = 6, growing in the southern hemisphere spring (DAY = 298) and summer (DAY = 15), respectively, at locations with Lat = –35° and –27.5° respectively. Clear sky with atmospheric transmission ratio (RATIO) of 0.75 was assumed unless otherwise stated. The average maximum and minimum air temperatures at these times of year were 21 and 7°C for Lat = –35° and 30 and 15°C for Lat = –27.5°.
Diurnal canopy photosynthesis in relation to canopy architecture
Diurnal patterns of net C3 and C4 canopy photosynthesis for a range of canopy LAI (LAIcan) and canopy-average leaf inclination relative to the horizontal (β) were simulated as a qualitative test of the DCaPS. The C4 simulations (Fig. 2c, d) were consistent in the diurnal pattern and magnitude with those reported by Duncan et al. (1967) and Hammer et al. (2009), who found that the canopy with erect leaves (β = 80°) continued to increase canopy photosynthetic rate beyond LAIcan = 4 at high LAIcan (= 8) due to better light distribution throughout the canopy. There was ~40% increase in midday canopy photosynthetic rate at LAIcan = 8 compared with LAIcan = 4, which was comparable to that simulated by Duncan et al. (1967) and Hammer et al. (2009). In the canopy with less erect leaves (β = 40°), there was little increase in canopy photosynthetic rate with increase in LAIcan beyond 4. In terms of the magnitude, the simulated canopy photosynthetic rate at LAIcan = 4 (Fig. 2c, d) was comparable to that observed in a similar size maize canopy (~70 μmol CO2 m–2 s–1) by Grant et al. (1989). The simulation also indicated that for a canopy with low LAIcan (= 2), less erect leaves (β = 40°) offered greater PAR absorption by both sunlit and shade leaf fractions consistent with greater radiation interception as found by Hammer et al. (2009). Even though the sunlit LAI (LAIsun) can be reduced up to 30% with less erect leaves, its photosynthetic rate was not affected due to associated increase in absorbed PAR. For the case of the shade leaf fraction, the increase in both the absorbed PAR and shade LAI (LAIsh) significantly increased shade leaf Aj,sh. These consequences for the two leaf fractions translate to a greater canopy photosynthesis with less erect leaves at low LAIcan. The effects of leaf erectness were mostly analogous for the C3 simulations (Fig. 2a, b). However, the greater radiation interception offered by less erect leaves at low LAIcan did not translate to greater canopy photosynthesis. Unlike C4, reduction in LAIsun significantly reduced the Rubisco-limited photosynthetic rate (Ac,sun) resulting in reduced photosynthetic rate in the sunlit leaf fraction. This more than offset the increase (because of increase in both the absorbed PAR and LAIsh) in Aj,sh. So in the case of C3, Rubisco limitation can reduce photosynthetic rate of small (low LAIcan) canopies with less erect leaves.

|
Diurnal canopy photosynthesis in relation to CO2 with varying temperature
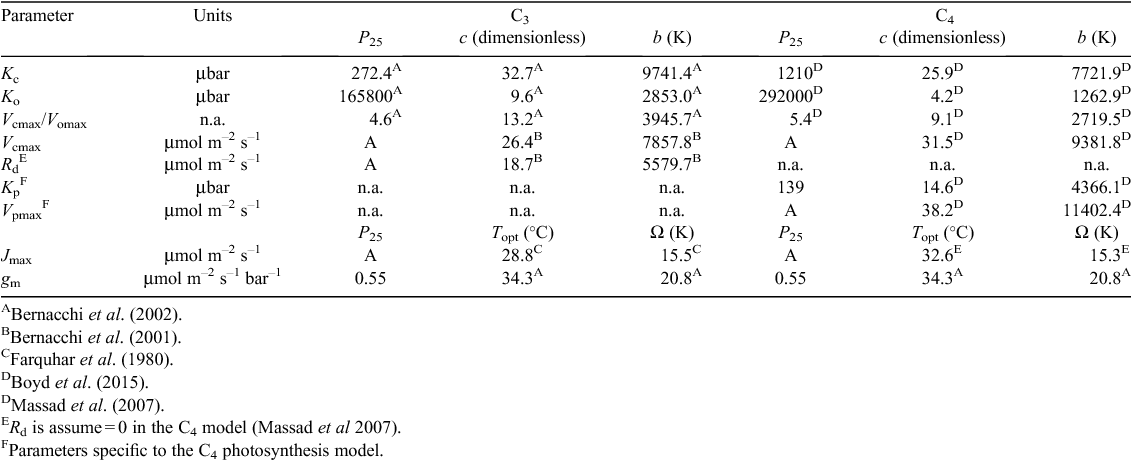
A simulation of net C3 and C4 diurnal canopy photosynthesis (Acan,DAY) for a range of air CO2 partial pressures (Ca) and temperatures (Ta) was undertaken as a qualitative test. Based on various large-scale free-air CO2 enrichment (FACE) studies, elevating Ca to 475–600 μbar (or an average of 540 μbar) increased C3 Acan,DAY by an average of 28% relative to Ca = 360 μbar (Ainsworth and Long 2005). This increase was reproduced when Ta_min and Ta_max were set to 14 and 28°C, respectively, simulating hot days for winter wheat crops (Fig. 3). Ainsworth and Long (2005) noted that when the large-scale free-air CO2 enrichment (FACE) studies were categorised by temperature, the relative increase in light saturated photosynthesis was lower (an average of 19%) at lower temperatures (<25°C). Simulation results for average days with Ta_min and Ta_max of 7 and 21°C, respectively, is consistent with this CO2 response at lower temperatures (Fig. 3). The increase in Acan,DAY can be as high as 50% at Ca = 1000 μbar. This result is discussed further below in regards to daily radiation use efficiency. In the case of C4 canopy photosynthesis, the response of net Acan,DAY to Ca and temperature was significantly less, which is consistent with the finding that C4 maize photosynthesis is not significantly affected by elevated Ca (Leakey et al. 2006). This simulation suggested that canopy photosynthesis of C3 crops can significantly benefit from elevated CO2, while C4 crops do not.
|
RUEDAY in relation to CO2 with varying temperature
A simulation of C3 and C4 daily canopy radiation use efficiency (RUEDAY) for a range of air CO2 partial pressures (Ca) and air temperatures (Ta) was also undertaken as a qualitative test. Elevated Ca is known to increase the net photosynthetic rate of C3 plants resulting in increased biomass accumulation and RUE (Kimball et al. 2002) and there is also an enhanced effect on RUE at higher Ta (Reyenga et al. 1999). The general consensus is that RUE of C3 crops increases almost linearly from Ca of 300 to 660 μbar, reaches ~30% increase at double the ambient Ca and plateaus at ~50% beyond Ca of 1000 μbar (Lobell et al. 2015). O’Leary et al. (2015) found a ~22% increase in wheat crop biomass in response to elevated Ca from 365 to 550 μmol mol–1. These known responses of C3 RUE were reproduced with the model for winter wheat crops experiencing average to hot temperatures (Fig. 3). The response relative of RUEDAY to Ca and temperature reflect that of net Acan,DAY because of the linear relationship between RUEDAY and Acan,DAY (Eqns A74 and A75). In the case of the C4 canopy, elevated Ca had only a small effect on the simulated RUEDAY (Fig. 3), which was consistent with a lack of response to Ca observed in C4 sorghum. This simulation suggested that elevated CO2 can significantly benefit canopy biomass accumulation of C3 crops, but not C4 crops.
RUEDAY in relation to average temperature
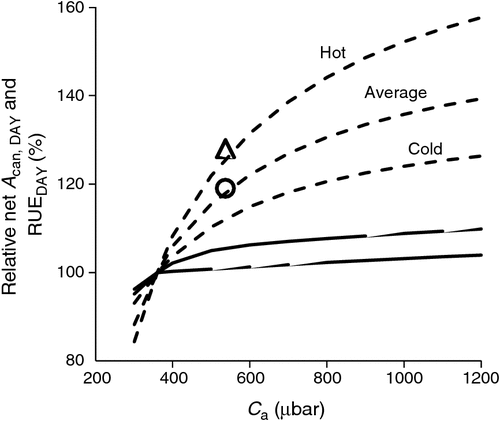
A simulation of C3 and C4 daily canopy radiation use efficiency (RUEDAY) for a range of average air temperature was undertaken as a qualitative test. Air temperature (Ta) varies diurnally between the daily maximum (Ta,max) and minimum (Ta,min) temperature and the diurnal pattern can be modelled with Eqn A15. A 15°C difference between Ta,max and Ta,min was assumed and a range of temperature used so that temperatures ranging from 0−35°C for C3 and 10−40°C for C4 were included. When plotted against average daily temperature the simulated RUEDAY was relatively insensitive between average temperatures of 14−23°C for C3 and 21−28°C for C4 (Fig. 4). This is consistent with known insensitivities of crop biomass accumulation and RUE to temperatures around optimal values (Yan and Hunt 1999). These temperature ranges for C3 and C4 responses were associated with the response of leaf photosynthesis to temperature, which is also insensitive within a broad range (e.g. C3, rice and wheat (Nagai and Makino 2009); C4, various grasses (Ludlow 1981), maize (response curve was derived from picking a typical Ci (e.g. 150 μbar) in A/Ci curves measured at different temperature in Massad et al. (2007)). Further, RUE under optimum growth conditions has been reported as 1.2–1.5 g MJ–1 for wheat (Fischer et al. 2014) and 1.2–1.4 g MJ–1 for dwarf sorghum (George-Jaeggli et al. 2013). The simulated maximum RUEDAY for C3 and C4 corresponded with these reported ranges (Fig. 4).
|
The comparison here between C3 wheat and C4 dwarf sorghum RUE does not reveal differences in magnitude between C3 and C4 crops, where the latter is typically higher. RUE of some tall hybrid sorghum varieties (George-Jaeggli et al. 2013) and maize (Sinclair and Muchow 1999) was found to be as high as 1.6–1.8 g MJ–1, or possibly even higher (2.0–2.2 g MJ–1) during the rapid stem elongation and maximum biomass accumulation phase (Olson et al. 2012). The typical high C4 RUE could be ascribed to higher photosynthetic rate (Hammer et al. 2010) and/or differences in canopy architecture, which may affect diurnal canopy photosynthesis (Fig. 2). These scenarios (and their combinations) can be simulated with the model. As a demonstration of this capability, we have assumed the first case by increasing the slope of the linear relationship between the maximum rate of Rubisco carboxylation (χVc), maximum rate of electron transport (χJ), maximum PEP carboxylase activity (χVP) and specific leaf nitrogen; these gave greater Vcmax, Jmax and Vpmax, respectively, and simulated the typical high RUE in C4 crops (Fig. 4).
RUEDAY in relation to SLNav with varying direct : diffuse radiation
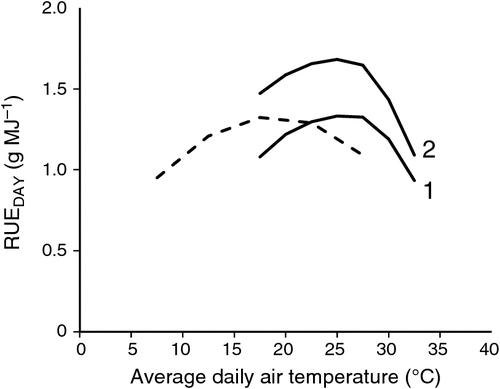
A simulation of C3 and C4 RUEDAY for a range of canopy-average specific leaf nitrogen (SLNav) with varying direct : diffuse radiation was undertaken as a qualitative test. Direct : diffuse radiation was varied by changing the atmospheric transmission ratio (RATIO) in a similar manner to the simulation study by Hammer and Wright (1994). High values (RATIO = 0.75) reflect clear sky with high transmission of direct radiation and a low fraction of diffuse radiation. Massignam (2003) found that RUE of the C3 crop sunflower responded asymptotically to SLNav with RUE of ~1 and 1.5 g MJ–1 at SLNav of 1.5 and 2 g N m–2 respectively. This was closely predicted by the model with clear sky conditions (Fig. 5a). Muchow and Sinclair (1994) found that RUE of field-grown dwarf sorghum responded asymptotically to SLNav, but did not approach the asymptote because sorghum SLNav maximised at 1.3 g N m–2 giving RUE of 1.26 g MJ–1. This was also closely predicted by the model with clear sky conditions (Fig. 5b). The simulated RUEDAY response of typical dwarf sorghum was not significantly different from that for wheat, but when the model was parameterised for the greater photosynthetic rate of the tall hybrid sorghums, the response was higher at all SLNav (Fig. 5b). These responses were comparable to that of maize crops (RUE of ~1.5 and 2 g MJ–1 at SLNav of 1 and 2 g N m–2 respectively) (Massignam 2003). In general, direct radiation level was higher with higher RATIO, while the absolute level of diffuse radiation was insensitive to RATIO, leading to a greater fraction of diffuse at low RATIO (cloudy days). Simulated results of increasing diffuse radiation fraction on C3 species (Fig. 5a) were consistent with Tubiello et al. (1997), who found a significant increase (~40%) in wheat RUE when grown under high diffuse radiation conditions due to the fact that diffuse radiation penetrates deeper into the crop canopy and increases photosynthetic rate of the lower leaves. Hammer and Wright (1994) used a simpler canopy photosynthesis model to show that decreases in RATIO caused RUE to increase. This response of RUEDAY to RATIO was reproduced (Fig. 5). Although both C3 and C4 types responded similarly to the increased fraction of diffuse radiation, the standard C4 types achieved higher RUEDAY at much lower SLNav as a result of their greater photosynthetic rate (Fig. 5b). This is consistent with the comparison of responses reported by Sinclair and Horie (1989).
|
Applications for photosynthesis manipulation – a tool for assessing consequences of photosynthetic changes
The Diurnal Canopy Photosynthesis Simulator (DCaPS) enables simulation of likely canopy-level consequences of photosynthetic manipulation and canopy structural attributes in C3 and C4 field crops. It integrates many non-linear responses of leaf photosynthesis to environment and processes involved in upscaling to the canopy level (see ‘Model detail’).
Considerable effort has been invested to develop DCaPS into an interactive web-based application (www.dcaps.net.au), which can be run with internet browsers on any major platform without prior installation of DCaPS (DCaPS v1.0 source code is available at https://github.com/QAAFI/DCaPS.git, accessed 16 October 2017). This web-based application is conveniently available for experimentalists working on photosynthetic research and/or as a teaching tool. DCaPS can be parameterised for a range of environments, canopy attributes and photosynthetic physiology. The online application reports diurnal patterns of environmental variables, diurnal canopy photosynthesis and daily canopy biomass increment.
Here we present two examples of using DCaPS to simulate consequences of changing photosynthetic attributes in both C3 and C4. These are simplified examples to demonstrate the capacity of DCaPS to capture complex dynamic interactions between photosynthetic physiology and diurnal variations in environment, which are not mechanistically included in, for example, the RUE type of canopy photosynthesis models. Users need to be aware of possible concomitant changes associated with changing model parameters. However, knowledge generated from exercising this model could inform photosynthetic manipulation efforts for assisting field crop improvement.
Relative CO2/O2 specificity of Rubisco
Increasing the relative CO2/O2 specificity of Rubisco (Sc/o) is a strategy for increasing CO2 assimilation in isolated leaves (Evans 2013). There are likely concomitant changes associated with changing Sc/o (Evans 2013). However, in this simulation, we have minimised complexity by assuming all other parameters are kept at default values (Tables 1, 2).
Using DCaPS, it was estimated that a significant (25%) increase in Sc/o, could increase C3 and C4 diurnal canopy photosynthetic rate (Acan,DAY) by ~6.0% and 2.5%, respectively, assuming all other parameters were kept at default values (Tables 1, 2). When Sc/o was set to increase by 25%, much of the enhancement was not translated to increase in photosynthetic rate as Rubisco-limited photosynthesis is less sensitive to changes in Sc/o than electron transport limited photosynthesis. In addition, differential effects on sunlit and shaded leaves associated with the canopy light environment contributed to this overall outcome when integrated to the canopy level. Fig. 6a–f shows the changes to instantaneous photosynthesis of the sunlit and shade leaf fractions throughout the day.

|
In the case of C3, Rubisco-limited (Ac,sun) and electron-transport-limited (Aj,sun) photosynthetic rates of the sunlit leaf fraction increased by an average of 2.6 and 7.2% over the diurnal cycle, respectively (Fig. 6b). However, between 11 : 00 and 15 : 00 hours, when the canopy had a high photosynthetic rate, the sunlit leaf fraction was Rubisco (Ac,sun) limited. This interplay between Ac and Aj limitation throughout the day resulted in only 5.5% increase as opposed to the potential 7.2%. On the other hand, the shade leaf fraction increased by 7.7% over the diurnal cycle (all contributed by effects on electron-transport-limited rate (Aj)) (Fig. 6c). Altogether, compared with a potential 7.2% increase in Acan,DAY, Ac limitation around noon reduced the potential increase in Acan,DAY to 6.0%.
C4 canopy photosynthesis was less responsive to changes in Sc/o than C3, which was consistent with the notion that increasing Sc/o in C4 plants has less effect on photosynthesis as they have evolved CO2-concentrating mechanisms for enhanced photosynthesis. In the case of C4 canopy photosynthesis, there was no interplay between Rubisco and electron transport limitations with all effects related to the latter (i.e. Aj) (Fig. 6e, f), and totalling to a 2.5% increase in Acan,DAY.
Rubisco activity and electron-transport rate
There is evidence that Rubisco activity and electron transport capacity can vary among species, can respond to the prevailing environment, and be bioengineered (reviewed by Evans (2013)). Putative changes in Rubisco activity and electron transport capacity can be implemented in the C3 and C4 photosynthesis models of DCaPS through changing the slope of the linear relationship between the maximum rate of Rubisco carboxylation (χVc), the maximum rate of electron transport (χJ) and specific leaf nitrogen; giving greater Vcmax and Jmax, respectively. Here we examine diurnal canopy photosynthesis consequences of such variations.
The simulation of consequences on diurnal canopy photosynthesis of changes in Vcmax and Jmax for C3 and C4 types are presented in Fig. 6g–n. Acan,DAY did not respond to increase in Vcmax for C3 types because both the sunlit and shade leaf fractions were mostly electron transport (Aj) limited in the reference scenario (Fig. 6g) and any increase in Rubisco activity (Ac) was not useful (Fig. 6h). However, increasing Jmax could increase Acan,DAY by 4.5%, which was attributed to higher electron-transport limited photosynthetic rate of the sunlit leaf fraction (Aj,sun) during early and late hours of the day (Fig. 6i). The largest effect was when Vcmax and Jmax were both increased by 20%, which gave a 9.5% increase in Acan,DAY. This shifted the whole diurnal photosynthetic rate higher (Fig. 6j). It was apparent that the sunlit fraction of the canopy was more sensitive to these changes and contributed most to the higher canopy photosynthesis.
For C4 canopy photosynthesis, there was less interplay between Rubisco- and electron-transport-limited photosynthetic rate. C4 canopy photosynthesis was always electron-transport limited (Fig. 6k). Hence, increase in Rubisco activity (Vcmax) had little or no effect on Acan,DAY (Fig. 6l). However, a 20% increase in maximum rate of electron transport (Jmax) increased Acan,DAY significantly (6%) (Fig. 6m) and increasing both Jmax and Vcmax simultaneously, increased Acan,DAY by 8% (Fig. 6n).
Implications for crop performance prediction – connecting biochemical photosynthesis models with crop models for seasonal simulations
Many crop models incorporate canopy photosynthesis as a key driver for crop growth for seasonal simulation. In some of these models, under well watered conditions, canopy CO2 assimilation/biomass accumulation is based on the empirical RUE approach, while others incorporate more detailed models of photosynthetic light response (PLR). Depending on the detail required for canopy photosynthesis simulation, either type of model can be used. However, the intrinsic empirical nature of these approaches makes it difficult to realistically model responses to manipulation of photosynthetic processes and environmental effects and so that often simple empirical indices are invoked to generate possible effects (Wu et al. 2016).
In this study, we have shown that the DCaPS can rationally simulate canopy photosynthetic rate responses to photosynthetic physiology, key environmental factors and crop status (e.g. light, Ca, Ta and SLNav). This provides confidence in incorporating DCaPS into crop growth and development models to drive aboveground canopy biomass accumulation in seasonal simulations. The capacity to connect with photosynthetic attributes makes DCaPS a valuable tool to improve the biological functionality of crop models in terms of aboveground canopy biomass accumulation under well watered conditions.
At first inspection, it may seem unduly complicated to introduce DCaPS into a crop model due to the parameterisation requirements at the biochemical/leaf level (Table 1). However, many are related to a small subset of key parameters, while others (e.g. temperature response parameters, Table 2) can be assigned a priori depending on the application of DCaPS. For example, the parameter values for kinetic properties of Rubisco (i.e. Kc, Ko, Vcmax/Vomax) and their temperature responses are relatively conserved within C3 species (von Caemmerer 2013). This means parameter values obtained from extensively studied model species, such as Arabidopsis and tobacco, can be used for C3 crop species. Further, more comprehensive parameter values for C3 (Braune et al. 2009) and C4 (von Caemmerer 2000; Massad et al. 2007) crop species are also emerging. This leaves a small set of parameters (three and four parameters for C3 and C4 respectively) to be assigned as indicated in Table 1.
To facilitate connection with crop growth and development simulation models, DCaPS, which operates on a daily timescale, needs to be connected with environmental and crop canopy attribute data that vary throughout the growing season. These data, already used and output by some crop models, can be input on a daily frequency into DCaPS at the start of each daily simulation. Recall that DCaPS incorporates four key environmental parameters (radiation, Ta, VPDa, Ca) and the three parameters for canopy attributes (LAIcan, β (canopy-average leaf inclination relative to the horizontal) and SLNav). Radiation, Ta, VPDa, LAIcan and SLNav can be connected with daily values supplied by crop models such as APSIM (Hammer et al. 2009, 2010). This leaves Ca and β to be assigned. It would be reasonable to assume Ca as a constant, while β can be reasonably estimated if a spherical leaf-angle distribution is assumed for field crops (Eqn A26). The design of DCaPS, which accepts daily values of environmental parameters and crop attributes allows convenient connection with crop models for seasonal simulation.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The work is financially supported by the ARC Centre of Excellence for Translational Photosynthesis, funded by the Australian Research Council’s Centre of Excellence funding program. The authors acknowledge helpful discussions with Dr Erik van Oosterom in relation to the model schematic, Professor John Evans and Professor Susanne von Caemmerer in relation to the C3 and C4 photosynthesis models as well as providing useful references, Dr Enli Wang in relation to temperature response of leaf photosynthesis as well as providing useful references, and the National eResearch Collaboration Tools and Resources (NECTAR) for providing a free web server for hosting dcaps.net.au.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–372.| What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2.Crossref | GoogleScholarGoogle Scholar |
Ball JT, Woodrow I, Berry J (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In ‘Progress in photosynthesis research’. (Ed. J Biggins) pp. 221–224. (Martinus Nijhoff Publishers: Dordrecht, The Netherlands)
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment 24, 253–259.
| Improved temperature response functions for models of Rubisco-limited photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFGrt7k%3D&md5=6409d4c27cdeb7d2ab0dc2ffcf1f98a7CAS |
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998.
| Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktlOj&md5=1a29283064c28fa71fde6bd9c92cb31dCAS |
Boyd RA, Gandin A, Cousins AB (2015) Temperature response of C4 photosynthesis: biochemical analysis of Rubisco, phosphoenolpyruvate carboxylase and carbonic anhydrase in Setaria viridis. Plant Physiology 169, 1850–1861.
Braune H, Mueller J, Diepenbrock W (2009) Integrating effects of leaf nitrogen, age, rank, and growth temperature into the photosynthesis-stomatal conductance model LEAFC3-N parameterised for barley (Hordeum vulgare L.). Ecological Modelling 220, 1599–1612.
| Integrating effects of leaf nitrogen, age, rank, and growth temperature into the photosynthesis-stomatal conductance model LEAFC3-N parameterised for barley (Hordeum vulgare L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntVyltrs%3D&md5=dd29306a87d1652eb4bb02225720be9bCAS |
Damour G, Simonneau T, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant, Cell & Environment 33, 1419–1438.
de Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell & Environment 20, 537–557.
| Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models.Crossref | GoogleScholarGoogle Scholar |
Duncan WG, Loomis RS, Williams WA, Hanau R (1967) A model for simulating photosynthesis in plant communities. Hilgardia 38, 181–205.
| A model for simulating photosynthesis in plant communities.Crossref | GoogleScholarGoogle Scholar |
Evans JR (2013) Improving photosynthesis. Plant Physiology 162, 1780–1793.
| Improving photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlWlt7zE&md5=7d83be5dba892356e03ac61919ba57c5CAS |
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
| A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXksVWrt7w%3D&md5=6db882a9dac8dc5a797c6c3206d6450cCAS |
Fischer T, Byerlee D, Greg E (2014) Crop yields and global food security: will yield increase continue to feed the world? ACIAR Monograph 158. Australian Centre for International Agricultural Research, Canberra, Australia.
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriqui M, Diaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Galle A, Galmes J, Kodama N, Medrano H, Niinemets U, Peguero-Pina JJ, Pou A, Ribas-Carbo M, Tomas M, Tosens T, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194, 70–84.
| Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis.Crossref | GoogleScholarGoogle Scholar |
George-Jaeggli B, Jordan DR, van Oosterom EJ, Broad IJ, Hammer GL (2013) Sorghum dwarfing genes can affect radiation capture and radiation use efficiency. Field Crops Research 149, 283–290.
| Sorghum dwarfing genes can affect radiation capture and radiation use efficiency.Crossref | GoogleScholarGoogle Scholar |
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Functional Plant Biology 30, 171–186.
| Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research.Crossref | GoogleScholarGoogle Scholar |
Goudriaan J, van Laar HH (1994) ‘Modelling potential crop growth processes: textbook with exercises.’ (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Grant RF, Peters DB, Larson EM, Huck MG (1989) Simulation of canopy photosynthesis in maize and soybean. Agricultural and Forest Meteorology 48, 75–92.
| Simulation of canopy photosynthesis in maize and soybean.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, Wright GC (1994) A theoretical-analysis of nitrogen and radiation effects on radiation use efficiency in peanut. Australian Journal of Agricultural Research 45, 575–589.
| A theoretical-analysis of nitrogen and radiation effects on radiation use efficiency in peanut.Crossref | GoogleScholarGoogle Scholar |
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Science 49, 299–312.
| Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt?Crossref | GoogleScholarGoogle Scholar |
Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. Journal of Experimental Botany 61, 2185–2202.
| Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGnsr0%3D&md5=7da0847d4bd8ddd66075e8d8b8d38b46CAS |
Humphries SW, Long SP (1995) WIMOVAC – a software package for modeling the dynamics of plant leaf and canopy photosynthesis. Computer Applications in the Biosciences 11, 361–371.
Jarvis PG (1976) Interpretation of variations in leaf water potential and stomatal conductance found in canopies in field. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 273, 593–610.
| Interpretation of variations in leaf water potential and stomatal conductance found in canopies in field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XhslKlt70%3D&md5=ac664590527ab722e127870458b59622CAS |
June T, Evans JR, Farquhar GD (2004) A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Functional Plant Biology 31, 275–283.
| A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjt1ejurs%3D&md5=60323fd262d736493ea757b7386f60f3CAS |
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. In ‘Advances in agronomy. Vol. 77’. (Ed. LS Donald) pp. 293–368. (Academic Press: Cambridge, MA, USA)
Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiology 140, 779–790.
| Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsV2iu7k%3D&md5=71af44347fbcb0ab5233defbdacb0b4aCAS |
Leuning R (1995) A critical-appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant, Cell & Environment 18, 339–355.
| A critical-appraisal of a combined stomatal-photosynthesis model for C3 plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlslCksbs%3D&md5=17b66cdd361087ceb56b8c2ddfc65c92CAS |
Leuning R, Kelliher FM, De Pury DGG, Schulze ED (1995) Leaf nitrogen, photosynthesis, conductance and transpiration: scaling from leaves to canopies. Plant, Cell & Environment 18, 1183–1200.
| Leaf nitrogen, photosynthesis, conductance and transpiration: scaling from leaves to canopies.Crossref | GoogleScholarGoogle Scholar |
Li G, Lin L, Dong Y, An D, Li Y, Luo W, Yin X, Li W, Shao J, Zhou Y, Dai J, Chen W, Zhao C (2012) Testing two models for the estimation of leaf stomatal conductance in four greenhouse crops cucumber, chrysanthemum, tulip and lilium. Agricultural and Forest Meteorology 165, 92–103.
| Testing two models for the estimation of leaf stomatal conductance in four greenhouse crops cucumber, chrysanthemum, tulip and lilium.Crossref | GoogleScholarGoogle Scholar |
Lobell DB, Hammer GL, Chenu K, Zheng B, McLean G, Chapman SC (2015) The shifting influence of drought and heat stress for crops in northeast Australia. Global Change Biology 21, 4115–4127.
| The shifting influence of drought and heat stress for crops in northeast Australia.Crossref | GoogleScholarGoogle Scholar |
Long SP, Marshall-Colon A, Zhu X-G (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66.
| Meeting the global food demand of the future by engineering crop photosynthesis and yield potential.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXls1ags7g%3D&md5=0f52bae8cd18fe3840a531dbee8e58d2CAS |
Ludlow MM (1981) Effect of temperature on light utilization efficiency of leaves in C3 legumes and C4 grasses. Photosynthesis Research 1, 243–249.
| Effect of temperature on light utilization efficiency of leaves in C3 legumes and C4 grasses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2czps1ygtg%3D%3D&md5=b2e9366f0e2602b0260a61cf94349533CAS |
Massad R-S, Tuzet A, Bethenod O (2007) The effect of temperature on C4-type leaf photosynthesis parameters. Plant, Cell & Environment 30, 1191–1204.
| The effect of temperature on C4-type leaf photosynthesis parameters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeiurrF&md5=07aadfeec5ae3a75ca7f11d9ec54ad54CAS |
Massignam AM (2003) Quantifying nitrogen effects on crop growth processes in maize and sunflower. PhD thesis. School of Land, Crop and Food Sciences, University of Queensland, St Lucia, Qld, Australia.
Monsi M, Saeki T (1953) Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14, 22–52.
Muchow RC, Sinclair TR (1994) Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum. Crop Science 34, 721–727.
| Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum.Crossref | GoogleScholarGoogle Scholar |
Nagai T, Makino A (2009) Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant & Cell Physiology 50, 744–755.
| Differences between rice and wheat in temperature responses of photosynthesis and plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkslKhuro%3D&md5=228c2b63ead328cc73968f01a6500544CAS |
O’Leary GJ, Christy B, Nuttall J, Huth N, Cammarano D, Stöckle C, Basso B, Shcherbak I, Fitzgerald G, Luo Q, Farre-Codina I, Palta J, Asseng S (2015) Response of wheat growth, grain yield and water use to elevated CO2 under a free-air CO2 enrichment (FACE) experiment and modelling in a semi-arid environment. Global Change Biology 21, 2670–2686.
| Response of wheat growth, grain yield and water use to elevated CO2 under a free-air CO2 enrichment (FACE) experiment and modelling in a semi-arid environment.Crossref | GoogleScholarGoogle Scholar |
Olson SN, Ritter K, Rooney W, Kemanian A, McCarl BA, Zhang Y, Hall S, Packer D, Mullet J (2012) High biomass yield energy sorghum: developing a genetic model for C4 grass bioenergy crops. Biofuels, Bioproducts & Biorefining 6, 640–655.
| High biomass yield energy sorghum: developing a genetic model for C4 grass bioenergy crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlKjurvL&md5=5199cf04791891500323058941754a9bCAS |
Parton WJ, Logan JA (1981) A model for diurnal variation in soil and air temperature. Agricultural Meteorology 23, 205–216.
| A model for diurnal variation in soil and air temperature.Crossref | GoogleScholarGoogle Scholar |
Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany 60, 2217–2234.
| Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlyitbk%3D&md5=0f0dce6b6cd5b91a3270d419e64a7a03CAS |
Reyenga PJ, Howden SM, Meinke H, McKeon GM (1999) Modelling global change impacts on wheat cropping in south-east Queensland, Australia. Environmental Modelling & Software 14, 297–306.
| Modelling global change impacts on wheat cropping in south-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Sands P (1995) Modelling Canopy Production. II. From single-leaf photosynthesis parameters to daily canopy photosynthesis. Functional Plant Biology 22, 603–614.
Sellers PJ, Berry JA, Collatz GJ, Field CB, Hall FG (1992) Canopy reflectance, photosynthesis, and transpiration. 3. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sensing of Environment 42, 187–216.
| Canopy reflectance, photosynthesis, and transpiration. 3. A reanalysis using improved leaf models and a new canopy integration scheme.Crossref | GoogleScholarGoogle Scholar |
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment 30, 1035–1040.
| Fitting photosynthetic carbon dioxide response curves for C3 leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeiur3F&md5=6e09ee70f54fef63acd184d4920e8411CAS |
Sharwood RE, Ghannoum O, Whitney SM (2016) Prospects for improving CO2 fixation in C3-crops through understanding C4-Rubisco biogenesis and catalytic diversity. Current Opinion in Plant Biology 31, 135–142.
| Prospects for improving CO2 fixation in C3-crops through understanding C4-Rubisco biogenesis and catalytic diversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XmslKqt7s%3D&md5=74bd6921192cf36162dd5b2c4c4a8aedCAS |
Sinclair TR, Horie T (1989) Leaf nitrogen, photosynthesis, and crop radiation use efficiency – a review. Crop Science 29, 90–98.
| Leaf nitrogen, photosynthesis, and crop radiation use efficiency – a review.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Muchow RC (1999) Radiation use efficiency. Advances in Agronomy 65, 215–265.
| Radiation use efficiency.Crossref | GoogleScholarGoogle Scholar |
Tubiello F, Volk T, Bugbee B (1997) Diffuse light and wheat radiation-use efficiency in a controlled environment. Life Support & Biosphere Science 4, 77–85.
van Oosterom EJ, Borrell AK, Chapman SC, Broad IJ, Hammer GL (2010) Functional dynamics of the nitrogen balance of sorghum: I. N demand of vegetative plant parts. Field Crops Research 115, 19–28.
| Functional dynamics of the nitrogen balance of sorghum: I. N demand of vegetative plant parts.Crossref | GoogleScholarGoogle Scholar |
von Caemmerer S (2000) ‘Biochemical models of leaf photosynthesis. Vol. 2.’ (CSIRO Publishing: Melbourne)
von Caemmerer S (2013) Steady-state models of photosynthesis. Plant, Cell & Environment 36, 1617–1630.
| Steady-state models of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1Cht7fN&md5=4de7a1493d1534fa7b10a67151a24a9aCAS |
Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, de Visser PHB (2010) Functional–structural plant modelling: a new versatile tool in crop science. Journal of Experimental Botany 61, 2101–2115.
| Functional–structural plant modelling: a new versatile tool in crop science.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGgu7g%3D&md5=a1f35a31d3e9a6460499de7af45b651fCAS |
Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282, 424–426.
| Stomatal conductance correlates with photosynthetic capacity.Crossref | GoogleScholarGoogle Scholar |
Wu A, Song Y, van Oosterom EJ, Hammer GL (2016) Connecting biochemical photosynthesis models with crop models to support crop improvement. Frontiers in Plant Science 7, 1518
| Connecting biochemical photosynthesis models with crop models to support crop improvement.Crossref | GoogleScholarGoogle Scholar |
Yan W, Hunt LA (1999) An equation for modelling the temperature response of plants using only the cardinal temperatures. Annals of Botany 84, 607–614.
| An equation for modelling the temperature response of plants using only the cardinal temperatures.Crossref | GoogleScholarGoogle Scholar |
Yin X, Struik PC (2008) Applying modelling experiences from the past to shape crop systems biology: the need to converge crop physiology and functional genomics. New Phytologist 179, 629–642.
| Applying modelling experiences from the past to shape crop systems biology: the need to converge crop physiology and functional genomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVKns7zJ&md5=71af747d6b4e8fd8db75ff7fad708fcaCAS |
Yin X, Struik PC (2009) C3 and C4 photosynthesis models: an overview from the perspective of crop modelling. NJAS Wageningen Journal of Life Sciences 57, 27–38.
| C3 and C4 photosynthesis models: an overview from the perspective of crop modelling.Crossref | GoogleScholarGoogle Scholar |
Yin X, van Laar HH (2005) ‘Crop systems dynamics: an ecophysiological simulation model for genotype-by-environment interactions.’ (Wageningen Academic Publishers: Wageningen, The Netherlands)
Zhang H, Nobel P (1996) Dependency of C i/C a and leaf transpiration efficiency on the vapour pressure deficit. Functional Plant Biology 23, 561–568.


