The mixotrophic nature of photosynthetic plants
Susanne Schmidt A , John A. Raven B and Chanyarat Paungfoo-Lonhienne A CA School of Agriculture and Food Sciences, The University of Queensland, St Lucia, Qld 4072, Australia.
B Division of Plant Sciences, University of Dundee at the James Hutton Institute, Invergowrie, Dundee DD2 5DA, UK.
C Corresponding author. Email: chanyarat@uq.edu.au
This review originates from the Peter Goldacre Award 2011 of the Australian Society of Plant Scientists that was received by the last author.
Functional Plant Biology 40(5) 425-438 https://doi.org/10.1071/FP13061
Submitted: 15 February 2013 Accepted: 22 March 2013 Published: 18 April 2013
Abstract
Plants typically have photosynthetically competent green shoots. To complement resources derived from the atmospheric environment, plants also acquire essential elements from soil. Inorganic ions and molecules are generally considered to be the sources of soil-derived nutrients, and plants tested in this respect can grow with only inorganic nutrients and so can live as autotrophs. However, mycorrhizal symbionts are known to access nutrients from organic matter. Furthermore, specialist lineages of terrestrial photosynthetically competent plants are mixotrophic, including species that obtain organic nutrition from animal prey (carnivores), fungal partners (mycoheterotrophs) or plant hosts (hemi-parasites). Although mixotrophy is deemed the exception in terrestrial plants, it is a common mode of nutrition in aquatic algae. There is mounting evidence that non-specialist plants acquire organic compounds as sources of nutrients, taking up and metabolising a range of organic monomers, oligomers, polymers and even microbes as sources of nitrogen and phosphorus. Plasma-membrane located transporter proteins facilitate the uptake of low-molecular mass organic compounds, endo- and phagocytosis may enable the acquisition of larger compounds, although this has not been confirmed. Identifying the mechanisms involved in the acquisition of organic nutrients will provide understanding of the ecological significance of mixotrophy. Here, we discuss mixotrophy in the context of nitrogen and phosphorus nutrition drawing parallels between algae and plants.
Additional keywords: endocytosis, mixotrophy, organic nutrients, plant nutrition, root hairs.
Introduction
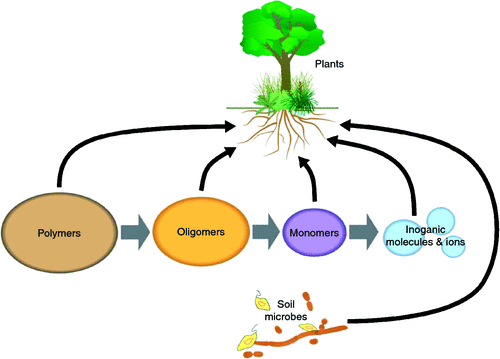
Terrestrial primary production and the resulting vast phytomass is fuelled by the photosynthetic power of the plant shoot, complemented by the root’s acquisition of water and nutrients from soil. Although most terrestrial organisms live predominantly either above- or belowground, plants, with the notable exception of epiphytes, span both. Green plants are considered autotrophs that are capable of converting inorganic material into organic matter using the electromagnetic energy of light. The atmosphere provides the substrates for photosynthesis, whereas all other essential elements are predominantly derived from soil (Marschner 1995). A plant is truly autotrophic when its roots acquire only inorganic nutrients, including nitrate, ammonium, base cations, ortho- and polyphosphates and sulfate. Autotrophy has been shown in hydroponic experiments on the essentiality of particular elements in which plants complete their life cycle in the absence of exogenous organic compounds (Arnon and Stout 1939; Marschner 1995; Epstein and Bloom 2005). However, roots in soil are exposed to numerous organic compounds, and much of the soil’s nitrogen (N) and phosphorus (P) is organic. Soil N occurs predominantly as amino acid-based molecules (Schulten and Schnitzer 1997), organic P, for example, as phytate and nucleotides, and organic sulfur as a variety of organosulfur compounds (Dyer and Wrenshall 1941; Schulte and Kelling 1999). In addition, soils contain a large range of carbon compounds such as organic acids and sugars, including those released by roots, and there are bidirectional fluxes (soil–roots) of organic matter (Jones et al. 2009).
There is broad agreement that plants acquire N and P in inorganic form. Inorganic N is relatively mobile in soil, although orthophosphate has significant restrictions on its mobility, but has greater mobility than phytate or polyphosphate. The acquisition of inorganic ions and molecules by roots involves plasma membrane located transporters that catalyse selective uptake, and accumulation, of inorganic N and P. It is generally assumed that plants are net exporters of organic compounds into soil and importers of inorganic ions and molecules, but recently the role of organic compounds as plant nutrients is being re-evaluated (reviewed by Paungfoo-Lonhienne et al. 2012).
It therefore seems timely to explore mixotrophy – the combined nutritional modes of autotrophy and heterotrophy – in ecological and mechanistic contexts. We focus here on N and P because they occur as organic and inorganic compounds in soils, and their supply is a major determinant of plant growth in natural ecosystems and bioproduction systems (reviewed by Lambers et al. 2008; Peltzer et al. 2010; Ma et al. 2012). We briefly outline nutrient acquisition strategies to highlight root specialisations, and discuss mixotrophy in algae and in plants. The last section discusses how larger organic molecules may enter roots, and the function of organic compounds as signalling molecules that increase root branching and the presence of root tips and associated root hair zones.
Overview of nutrient acquisition strategies
Root specialisations: mycorrhizal fungi, N2 fixing procaryotes and cluster roots
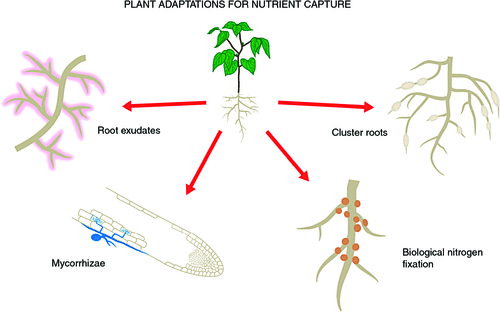
The four main specialisations for nutrient acquisition are root exudates and their extreme occurrence in the functioning of cluster roots, and symbioses with mycorrhizal fungi and N2 fixing bacteria (Fig. 1). Although exudates universally contribute to root function, their amount and composition varies among species and growth situations, the other specialisations are species specific. The mycorrhizal symbioses are most common and formed by most of plant species with a range of fungal partners, different extent of mycorrhizal colonisation, and varying benefits derived from the symbiosis. The dependence of plants on mycorrhizal fungi for accessing nutrients from organic matter and soil minerals is well established (Smith and Read 2008). Mycorrhizas are not the focus here, although we discuss mycoheterotrophy in the context of green orchids.
|
An estimated 10% of plant taxa have N2 fixing symbioses (Franche et al. 2009), providing an alternative N source to soil N at the cost of photosynthates required to operate N2 fixation by the bacterial symbionts and, especially, the production and maintenance of the specialised structures (nodules, rhizothamnia and coralloid toots) housing the symbiont. Far fewer plant species have N2 fixing symbioses than mycorrhizas, although most ecosystems contain N2 fixing plants. N2 fixing symbioses are beyond the scope of this review (see Sprent 2001).
Several thousand plant species do not form mycorrhizal symbioses, including all species of the Proteaceae, many sedges, and species in several other plant families (Tester et al. 1987; Lambers et al. 2008), including the model plant Arabidopsis thaliana and other Brassicaceae. However, the recently discovered symbiotic associations with endophytic dark septate fungi are quite common (Caldwell et al. 2000), and occur in Arabidopsis thaliana (Mandyam et al. 2013). Most Proteaceae, many sedges, some N2 fixing species (perhaps most actinorhizal species and some legumes) and other species produce cluster roots in response to low nutrient availability. Cluster roots are considered particularly beneficial in P-depauperate soils (reviewed by Dinkelaker et al. 1995; Neumann and Martinoia 2002; Lambers et al. 2006). Cluster roots impact a small volume of soil by exuding protons, organic acids, secondary compounds and enzymes (Dinkelaker et al. 1995; Shane and Lambers 2005; Paungfoo-Lonhienne et al. 2009), and, less well characterised, through interaction with microbes (Marschner et al. 2002; Weisskopf et al. 2011).
Ecosystems have been classified along a gradient of ‘organic-to-inorganic nutrients dominated’, with the former requiring greater specialisation by the plant to access nutrients than the latter. In slow-mineralisation organic-nutrient rich ecosystems, such as heathlands and boreal forests, specialised ecto- and ericoid mycorrhizal fungi depolymerise organic matter via extracellular enzymes, and supply nutrients to plants (Read and Perez-Moreno 2003; Lambers et al. 2008). In rapid-mineralisation ecosystems such as grasslands, plants are have mostly arbuscular mycorrhizas and are thought to acquire inorganic nutrients that are derived from breakdown of organic matter by soil microbes (Read and Perez-Moreno 2003).
In light of all root specialisations, it remains uncertain what proportion of N and P is acquired by roots as inorganic or organic compounds. The difficulties associated with studying rhizosphere processes, including chemical characterisation of nutrients and quantification of their fluxes, have limited progress in understanding the importance of organic N and P for plant nutrition. However, roots take up a variety of organic compounds (Näsholm et al. 2009; Paungfoo-Lonhienne et al. 2012). An important consideration for the discussion on mixotrophy is that irrespective of root specialisation, a significant proportion of the root surface is free of fungi or other microbes (Bais et al. 2006).
Root exudates
Root exudates affect the availability of nutrients directly or indirectly (reviewed by Walker et al. 2003; Bais et al. 2006; Jones et al. 2009). Exudates are complex chemical mixtures of low and high molecular weight compounds, including organic acids, phytosiderophores (only in the Poaceae), sugars, vitamins, amino acids, purines, nucleosides, inorganic ions, dissolved gases, high molecular weight carbohydrates (mucilage), enzymes and root border cells (reviewed by Dakora and Phillips 2002). Exudates are communication agents and facilitate nutrient acquisition through various mechanisms (see reviews by Walker et al. 2003; Bais et al. 2006). The mechanisms that plants use to interpret the chemical signals in the rhizosphere that originate from soil organisms and other plants, remain largely unknown (Bais et al. 2006). This has relevance to mixotrophy, and is discussed below.
Root-derived enzymes released into the apoplast and rhizosphere may contribute to mixotrophy if they generate organic compounds that are subsequently acquired by roots. Root exoenzymes have been studied for their role in catalysing the breakdown of large organic molecules as well as other functions. High-molecular mass inorganic compounds (e.g. poly-phosphates) and organic compounds (e.g. protein, phytate, DNA) can be degraded by root-derived phosphatases, proteases, phytases and DNAses (reviewed by Paungfoo-Lonhienne et al. 2012). Phosphatases are exuded, for example, from cluster roots of Proteaceae and Fabaceae (Dinkelaker et al. 1995; Yamamura et al. 2002; Wasaki et al. 2003). In legumes, root phosphatase activity is higher than in other forbs regardless of the presence of nodules (Venterink 2011), and soil associated with N2 fixing plants has higher extracellular phosphatase activity than soil without N2 fixers (Houlton et al. 2008). Root-derived proteases have been described in Hakea (Proteaceae), carnivorous plants, Arabidopsis and other species including crops (Adlassnig et al. 2012). Proteases are present in the root apoplast (Tornero et al. 1996; Hamilton et al. 2003), and the presence of protein degradation products in the apoplast of inner root cortex suggests that proteases are active in the apoplast and at the root surface (Paungfoo-Lonhienne et al. 2008).
From an evolutionary viewpoint, the release of enzymes into the rhizosphere is advantageous only if the nutrient gain outweighs the nutrient loss in the exudates, or alternatively, if breakdown products have important signalling roles (Paungfoo-Lonhienne et al. 2008). The presence of enzymes in the apoplast may catalyse the degradation of larger molecules that enter roots via transpiration-driven movement of the soil solution and diffusion, but this is not well characterised. Overall, there has been comparatively little research on root exoenzymes for plant nutrition, as it was generally assumed that microbes drive the breakdown of soil organic compounds. The rhizosphere was treated as a ‘black box’ and researchers were satisfied to consider microbial activity in general terms (Silberbush 2013). Recent advances in molecular methods characterising microbial communities in soil, rhizosphere and roots promise to advance knowledge of rhizosphere dynamics (Bulgarelli et al. 2012; Lundberg et al. 2012).
The positive relationship between the release of enzymes and access to organic nutrients that has been observed in plants tested in controlled conditions has not always been confirmed in soil. For example, wheat genotypes with greater exudation of phosphatase acquired more P from organic P when grown axenically, but not when grown in soil (George et al. 2008). Similarly, transgenically-enhanced root exudation of phytase improved plant growth with phytate in controlled conditions, but to a lesser extent in soil (reviewed by Richardson et al. 2009). Root-derived exoenzymes may have numerous roles, although how much they contribute to the breakdown of organic compounds in the apoplast relative to the rhizosphere, and subsequent uptake of the resulting compounds, is unknown.
Mixotrophy in phytoplankton, other aquatic organisms and terrestrial plants
Mixotrophy, as phagomixotrophy, is a common and biogeochemically important strategy in eukaryotic phytoplankton and a range of planktonic and benthic protists and metazoans which have acquired photosynthesis by endosymbiosis of cyanobacterial or microalgal cells or through kleptoplasty (Raven 1997; Stoecker 1998; Raven et al. 2009; Esteban et al. 2010; Hartmann et al. 2012; Flynn et al. 2013). In most planktonic habitats the strategy predicted to maximise the abundance of mixotrophs is a predominantly photosynthetic mode of energy gain in combination with consuming bacteria and other microbes which increases acquisition of essential elements (Crane and Grover 2010), thereby outcompeting planktonic phototrophs and phagotrophs (Tittel et al. 2003; Hartmann et al. 2012).
Is mixotrophy an ancestral state in algae? Endocytosis, itself a synapomorphy of eukaryotes, was how all extant algae and hence embryophytic (‘higher’ plants), regardless of whether or not they exhibit mixotrophy, obtained their plastids by primary, secondary or tertiary endosymbiosis followed by genetic integration (Raven 1997; Raven et al. 2009). It is likely that phagomixotrophy was retained in some clades after the genetic integration of plastids (Raven et al. 2009). Phagotrophy also underlies kleptoplasty, where plastids from an algal food item are retained in a functional form in an otherwise heterotrophic cell (Raven et al. 2009; Flynn et al. 2013). Such kleptoplastids are capable of long-term functionality if the nucleus from the plastid source is retaining a functional state with the plastids which occurs in the ciliate Myrionecta rubra (Johnson et al. 2007). However, in some cases, plastid longevity occurs without retention of food alga nuclei or prior horizontal gene transfer from the algal nucleus to the grazer nucleus (Wagele et al. 2011; Pillet and Pawlowski 2013).
In addition to phagomixotrophy, most algae are also capable of osmomixotrophy, i.e. uptake of individual soluble organic molecules by transporters in the plasmalemma (Raven et al. 2009; Flynn et al. 2013) and perhaps, of soluble macromolecules by fluid-phase endocyotosis, so-called pinocytosis. The significance of osmomixotrophy in phytoplankton under natural conditions is unclear; it is possible that the net flux of low molecular mass organic solutes is outwards under many conditions (see Flynn et al. 2013). Osmomixotrophy also occurs in photosynthetic bacteria such as cyanobacteria, as well as in proteobacterial anoxygenic photosynthetic bacteria. Particularly widespread in the surface ocean are anoxygenic aerobic photosynthetic proteobacteria, and the otherwise heterotrophic proteobacteria with proteorhodopsin (Zubkov 2009; Raven and Donnelly 2013). Here the photochemical reactions do not power autotrophic CO2 assimilation, but spare the use of respiratory processes in energising solute transport and ADP phosphorylation, and so may legitimately be considered mixotrophs since the cells use two sources of energy, i.e. electromagnetic radiation and dissolved organic compounds (Zubkov 2009; Raven and Donnelly 2013).
Osmomixotrophy as a source of organic carbon permitting growth (in the dark) or increasing growth (in the light) is not universal in autotrophs. Obligate photoautotrophy, i.e. the inability to grow other than with light as the energy source and inorganic nutrients supplying essential elements, has been reported for several algae (including cyanobacteria) (Raven 2012). As with algae that cannot be grown in laboratory culture under any conditions so far used, so with obligate photoautotrophic algae: perhaps the next culture attempt with different conditions will allow growth (for so far unculturable organisms) or growth with a contribution from external organic carbon (for obligate photoautotrophs). However, for the moment at least there are obligately photolithotrophic algae (Raven 2012). It is important to note that the obligate photoautotrophy does not preclude the assimilation of exogenous organic compounds, just their involvement in growth (Raven 2012). A final point about obligate photoautotrophy is that technical complications mean that it is not readily tested for embryophytic, and especially vascular plants.
An endocytosis-like uptake of protein by the planktonic heterotrophic planctomycete bacterium Gemmata obscuriglobus was recently reported (Lonhienne et al. 2010). This process is probably not involved in mixotrophy in anoxygenic photosynthetic bacteria, since the planctomycetes have a uniquely complex cell structure for a bacterium, and no planctomycete is known to have photosynthesis (Fuerst et al. 1993). However, a planctomycete contains proteorhodopsin (McCarren and DeLong 2007), so the endocytosis-like means of protein uptake could be involved in this variant on mixotrophy. In summary, the categorisation of autotrophic phytoplankton and heterotrophic microzooplankton does not account for the dual mode of nutrition and warrants revision of the dichotomous concept (Flynn et al. 2013).
A related process, which overlaps with mixotrophy phylogenetically and functionally, is auxotrophy – the inability of some algae to make all enzyme cofactors known as vitamin in metazoan nutrition. The most widespread vitamin requirement is that of cobalamin (vitamin B12), with 155 of the 306 algae examined needing an external supply of vitamin B12 (Croft et al. 2006). Where the vitamin B12 auxotrophs are also phagomixotrophs the prey is presumably a source of the vitamin, bearing in mind that only archaea and bacteria are able to synthesise vitamin B12. Auxotrophic algae that are not phagomixotrophs must obtain the vitamin from associated bacteria or archaea or from the growth medium. Vitamin B12 dependence comes about from loss of an alternative, vitamin B12-independent, pathway of methionine synthesis (Helliwell et al. 2011). For other vitamins, auxotrophy comes about from loss of the capacity to synthesise the vitamin. Since embryophytic plants have no known requirement for exogenous vitamins, the assumption is that they evolved from a charophycean green alga with no requirements for exogenous vitamins. This is the most economical hypothesis, but it does not rule out the alternative of an auxotrophic algal ancestor with subsequent horizontal gene transfer that overcame the need for external vitamins.
Mixotrophy has been a recent focus of phytoplankton research (e.g. Bronk et al. 2007; Gómez-Baena et al. 2008; Zubkov et al. 2008; Poretsky et al. 2010; Flynn et al. 2013), and photosynthetic plants are also investigated for their ability to satisfy energy and nutritional needs with organic carbon (C) compounds. An obvious parallel to algal phagomixotrophy in green plants is the carnivorous mode of nutrition (Fig. 2; Raven et al. 2009). We do not consider carnivorous angiosperms in detail here, but note that they are photosynthetic (Juniper et al. 1989; Raven et al. 2009; Król et al. 2012), can take up organic C in amino acids and peptides via transporters (Schulze et al. 1999) and via endocytosis (Adlassnig et al. 2012), but have a significant C cost in trap construction and maintenance and in enzyme secretion (Ellison and Adamec 2011; Sirová et al. 2011).

|
Another variant on mixotrophy is the mycoheterotrophy in photosynthetically competent plants. It was originally thought that mycoheterotrophy only occurs in specialist achlorophyllous plants that obtain all their C and N from mycorrhizal fungi (Leake 2004; Leake and Cameron 2010). More recently it was discovered that some green orchids are mixotrophs, gaining organic C and N via a combination of mycoheterotrophy and photosynthesis (Fig. 2; reviewed by Selosse and Roy 2009). Substantial fungus-mediated mixotrophy occurs in the photosynthesising ericaceous tribe Pyroleae (Tedersoo et al. 2007) and the orchid Cephalanthera damasonium (Julou et al. 2005) with an estimated 10–67% of carbon obtained from fungal partners. Selosse and Roy (2009) draw analogies between mixotrophy of plants and algae, and suggest that partial mycoheterotrophy may be more common in light-limited environments than currently acknowledged. The authors also highlight the dearth of knowledge of cellular mechanisms enabling the transfer of nutrients between fungi and partially mycoheterotrophic plants.
Analogous to mycoheterotrophy by photosynthetically competent plants, but without a fungal link, is hemiparasitism (Fig. 2; Marshall and Ehleringer 1990). Here a photosynthetically competent vascular (usually angiosperm) plant obtains all water and nutrient elements which cannot be supplied by its own photosynthesis from another (usually angiosperm) vascular plant (Raven 1983). Hemiparasites are generally linked exclusively to the host xylem and acquire N mostly as organic N as all root-acquired, including organic N, ammonium and N2 fixed by root nodules or rhizothamnia, move in the xylem as organic N, although only nitrate and most P move as inorganic constituents (Raven 1983). Acquisition of organic C and N by hemiparasites from the xylem stream of the host involves the parallel acquisition of two or more atoms of organic C per atom N when dicarboxylic amino acid and their amides are the form in which N is moved, and one C per N (with not all C recoverable as organic C), when ureides such as allantoin are the main N transport molecules.
Mixotrophy is deemed the exception in terrestrial plants, excepting carnivorous, partial mycoheterotrophic or hemi-parasitic plants, but a recent study using 13C-labelled litter showed that Quercus petraea trees in north-eastern France are mixotrophic in spring (Bréda et al. 2013).
Organic nutrients as sources of essential nutrients for plants
In recent decades it has been ‘rediscovered’ that nitrate and ammonium are not the sole N sources for plants; early plant nutrition researchers considered a surprising variety of organic compounds as nutrient sources (reviewed by Paungfoo-Lonhienne et al. 2012). Amino acids are present in soil, and plants use or even prefer amino acids over other N forms (Chapin et al. 1993; Chapin 1995; Näsholm et al. 1998; Schimel and Bennett 2004), and amino acid transporters catalysing the uptake of amino acids into roots have been identified (Hirner et al. 2006; Lee et al. 2007; Svennerstam et al. 2007, 2011). The difficulties associated with quantifying N conversions in soils, detecting simultaneous uptake and release of N by soil and symbiotic organisms and roots, and identifying the chemical form in which N is acquired by plants have prevented unequivocal assessment of the contribution of organic N to the plants’ N uptake (reviewed by Näsholm et al. 2009). Ecological and physiological studies have evaluated amino acids for their role as organic N and C sources, but debate continues as to whether these compounds are generated at sufficient rates in soil to be significant N sources, and whether plants can compete with microbes for their uptake. Larger organic N compounds in soils include peptides and proteins. Membrane transporters catalysing the uptake of di- and tri-peptides into roots have now been identified (Komarova et al. 2008). It has been suggested that use of peptides as N and C source contributes to the success of the endemic angiosperm Deschampsia antarctica with increasing temperatures on the Antarctic Peninsula (Hill et al. 2011). We found that green fluorescent protein enters root hairs and supplements inorganic N in low-N supplied Arabidopsis but have not identified the mechanisms that permit such uptake (Paungfoo-Lonhienne et al. 2008).
Experimental and technical innovations are advancing knowledge on organic nutrients and include microdialysis as tool to quantify N flux via passive diffusion (Inselsbacher and Näsholm 2012). Soil microdialysis showed that amino acids account for 80% of the soil N supply in undisturbed boreal forest soil, whereas conventional extraction techniques detected inorganic N as the dominant soil N form. Microdialysis and analysis of a larger range of soil organic compounds have great potential to improve knowledge of N and other nutrients (Inselsbacher et al. 2011; Warren 2013), as widely used soil extraction methods were established to identify fertiliser needs in agriculture focussed on inorganic nutrients. Promising techniques include high resolution research techniques of soil, roots and soil microorganisms including confocal and nano-SIMS imaging with fluorescent and stable isotope labelled N compounds (Paungfoo-Lonhienne et al. 2008; Clode et al. 2009; Whiteside et al. 2009; Kilburn et al. 2010).
Similar questions as for N arise about the role of organic P for plant nutrition. Inorganic P (orthophosphate, Pi) is considered the main source for plants and is acquired via membrane transporters (Mudge et al. 2002; Smith et al. 2003). Organic P forms are generally considered unavailable for plant uptake, but have been more recently investigated as sources for plants. As discussed above, exoenzymes cleaving phosphate esters have been described in several plants species, and the ability of axenically cultivated Arabidopsis and wheat to grow with nucleic acid and other organic P forms as sole P sources was thought to be due to exuded DNAases, phytases and phosphatases (Chen et al. 2000; Richardson et al. 2000). We showed that organic P consisting of 25-nucleotide DNA, that was protected from enzymatic degradation, entered root cells (Fig. 3; Paungfoo-Lonhienne et al. 2010a), indicating that organic P compounds can enter roots. So far there have been no reports that angiosperms can use the uptake system of some cyanobacteria and certain other bacteria which acquire and then cleave organic phosphonates (Dyhrman et al. 2006).
|
Possible mechanisms of the uptake of large organic compounds into roots
Adding to the uncertainties of the role of organic nutrients is the scant mechanistic understanding of how large organic compounds enter roots. The membrane-spanning proteins that catalyse selective passage of ions or molecules across the plasma membrane cannot transport large macromolecules such as proteins, polynucleotides or polysaccharides. Eukaryotic cells ingest macromolecules via endocytosis by progressively enclosing the substance in a section of the plasma membrane, followed by invagination and pinching off to form an intracellular vesicle containing the ingested substance (Alberts et al. 1989). Endocytosis retrieves membrane material and associated cargo from the plasma membrane for internal utilisation or destruction, and for processing and recycling to the plasma membrane (Robinson 2005). Endocytosis is common to all eukaryotes and enabled by cytoskeleton-forming proteins, but was thought not to occur in prokaryotes due to missing cellular components and thick and rigid cell walls. However, uptake of proteins by bacterium Gemmata obscuriglobus (Planctomycetes) occurs as an energy-dependent process analogous to eukaryotic endocytosis (Lonhienne et al. 2010), and some bacteria incorporate other bacteria as endosymbionts (McCutcheon and von Dohlen 2011).
Endocytosis serves multiple purposes in plants including membrane recycling, protein transport, cell-to-cell communication, cell signalling and cell wall morphogenesis (Holstein 2002; Šamaj et al. 2004; Šamaj et al. 2005; Geldner and Jurgens 2006). The role of endocytosis for nutrient uptake remains a controversial topic. In the 1960s pinocytosis, a type of endocytosis that involves invagination of the plasma membrane around a fluid phase and subsequent formation of a small versicle within the cytoplasm, was considered by some researchers as a main route of entry of nutrients into root cells (Jensen and McLaren 1960; Bradfute et al. 1964; Bradfute and McLaren 1964; reviewed by Cram 1980). Cram (1980) critically reviewed this hypothesis, pointing out major problems such as the required implausibly high density of selective binding sites in the membrane and the thermodynamic and mechanistic difficulties of creating a fluid-filled spaces by invagination of the plasmalemma in a turgid plant cell. The points raised by Cram (1980) were reiterated and developed by Raven (1987) and Robinson et al. (2008). However, FM dyes, the most reliable endocytosis tracer (Emans et al. 2002; Bolte et al. 2004), have unequivocally demonstrated that plant cells perform endocytosis (Emans et al. 2002) and contain endosomes (Geldner 2004; Voigt et al. 2005). Membrane internalisation in plant cells occurs principally via clathrin-coated vesicles similar to most animal cells (Pérez-Gómez and Moore 2007). Even though the analysis of the Arabidopsis genome has revealed considerable conservation of components of the endocytotic machinery among eukaryotes, endocytosis research in plants has lagged behind that of animals. A proposed reason has been the lack of obvious candidates for endocytotic cargo molecules (Robinson et al. 2008).
There is evidence for a vesicle-mediated transport system between vacuole and plasma membrane (Echeverria 2000). Internalised lucifer yellow, an endocytosis marker, occurs in transition zone cells of the inner cortex of intact maize root apices indicating that large molecules are transported across cortex cells (Baluska et al. 2004, who acknowledged that results from the use of lucifer yellow can be compromised by the occurrence of carrier-mediated movement of this tracer across plant membranes). That larger molecules enter roots via endocytosis has long been proposed as exogenously supplied macromolecules such as DNA were detected in roots (reviewed by Paungfoo-Lonhienne et al. 2012). Sucrose and glucose enter cells by membrane transporters (Sauer 2007) and are also incorporated into intact walled plant cells via endocyctosis (Etxeberria et al. 2005a, 2005b). Endocytosis and an additional unknown mechanism have been proposed to facilitate entry of peptides into walled plant cells (Eggenberger et al. 2011).
It was assumed that carnivorous plants first digest organic compounds in animal-catching organs such as pitchers into inorganic and smaller organic compounds (amino acids, peptides), before transport into plant cells by membrane transporters (Schulze et al. 1999). Recently, endocytosis was identified as a mechanism facilitating entry of proteins into tissues of carnivorous plants. Proteins are absorbed by endosomes which fuse with primary lysosomes which results in larger compartments where degradation takes place (Adlassnig et al. 2012). Endocytosis also plays an integral role in the endocytic uptake of Rhizobium bacteria in legume roots, involving internalisation into plant cells (reviewed by Brewin 2004).
Microbes occur in much higher density in the rhizosphere than bulk soil and are attracted by root-derived compounds and debris, and it is conceivable that plants acquire and digest microbes as sources of nutrients. We tested this possibility and presented the first evidence that root cells incorporate non-pathogenic and non-symbiotic microbes as nutrient sources (Fig. 3; Paungfoo-Lonhienne et al. 2010b). As microbes compete with plants for soil nutrients, uptake and digestion of microbes could be an adaptation for securing nutrients (Paungfoo-Lonhienne et al. 2010b).
Effect of organic nutrients on root morphology
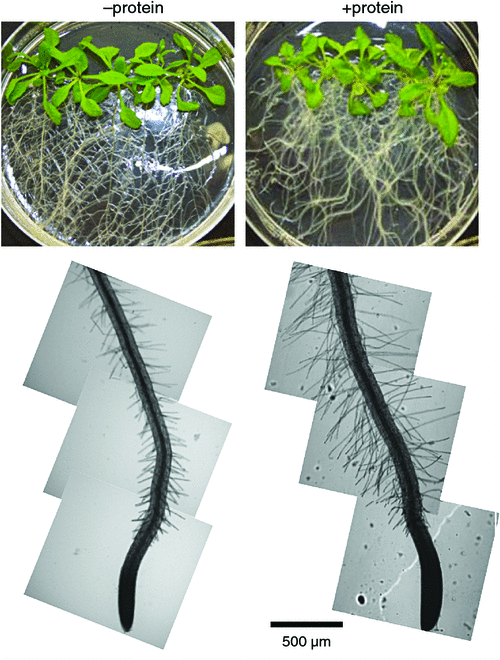
It is possible that the effects of external organic compounds on root function are related to mixotrophy. For example, organic compounds that trigger increased root branching by increasing the number of root tips and extent of root hair production, may stimulate processes that rely on the presence of these structures especially nutrient uptake. Indeed, the observed effects of organic polymers on root morphogenesis include enhanced root branching and root hair growth (Fig. 4) and are congruent with the notion that organic polymers facilitate root proliferation in sites that are rich in organic matter (Paungfoo-Lonhienne et al. 2008; Paungfoo-Lonhienne et al. 2010a).
|
An extension of the root system allows exploration of soil for the continued absorption of inorganic and organic nutrients. The effect of inorganic nutrients on root morphology depends on the nutrient solute and its concentration. For example, supply of ammonium stimulates lateral root initiation and higher-order lateral root branching, while nitrate stimulates lateral root elongation (Forde and Walch-Liu 2009; Lima et al. 2010). Although there is a vast literature on the quantitative effects of inorganic nutrients on root growth and morphology (reviewed by Hermans et al. 2006), much less is known about the effects of organic forms on these characteristics. Amino acids and peptides alter root morphology and biomass, root length, thickness, surface area and root hairs (Walch-Liu et al. 2006a, 2006b; Cambui et al. 2011; Soper et al. 2011), providing indirect evidence that root structure and uptake of these compounds may be linked. Extension of the root system is particularly important for the acquisition of less mobile and less soluble nutrients such as most P-compounds (Bates and Lynch 1996). Root hairs have a large effect on the uptake of P-compounds as evidenced by morphologically different root hairs of crop species and varieties and their P-compound acquisition. Length and surface area of root hairs were significantly correlated with the depletion of P-compounds in the rhizosphere in low P growth condition (Bates and Lynch 1996; Gahoonia and Nielsen 1996; Gahoonia et al. 1997; Ma et al. 2001). Long root hairs are considered a beneficial trait for sustaining crop yields in low P soil (Gahoonia and Nielsen 2004). Although root hairs are under genetic control, their growth is modulated by environmental conditions. Increased root hair length under P-limitation is an adaptation that aids P-compound acquisition. Root colonisation with arbuscular mycorrhizal fungi is more pronounced in species and genotypes that have few or short root hairs (Smith and Read 2008). Root hair length increased in the presence of DNA in plants grown in P-replete medium (Paungfoo-Lonhienne et al. 2010a), pointing to a role of organic compounds as signalling agents. Similarly, phytate as a P source in axenic growth conditions increased root growth but dramatically decreased shoot growth (Richardson et al. 2000). The responsiveness of plants to organic compounds can be interpreted as a further indicator for their involvement in plant nutrient relations.
Do root hairs provide a path for large molecule into roots?
Root hairs are specialised epidermal cells and the outermost interface between roots and soil. Root hairs can constitute up to 70% of the root surface. They are tip-growing extensions originating from root epidermal cells, so-called trichoblasts, which play an important role in water and nutrient uptake (reviewed by Datta et al. 2011). Root hairs are the major site of inorganic P uptake (Pi, phosphate) (Gahoonia and Nielsen 1998) containing the high affinity phosphate transporters responsible for Pi uptake (Mudge et al. 2002; Schunmann et al. 2004), and transporters for ammonium and nitrate (Lauter et al. 1996).
Tip growth in plant cells is an actin-based process that requires targeted exocytosis and compensatory endocytosis to occur at the growth cone and involves polarised membrane trafficking and the presence of endosomal compartments at the tip of root hairs (Ovečka et al. 2005; Voigt et al. 2005). Endocytosis occurs at the tip of growing root hairs and subsequent trafficking of the incorporated membrane. Mercado-Blanco and Prieto (2012) speculated that this process facilitates passive entry of bacteria into root hairs. Root hairs of N2 fixing species facilitate the endophytic colonisation of roots, and recent studies indicate that root hairs of non-N2 fixing angiosperm incorporate microbes (Fig. 5d; Prieto et al. 2011; Mercado-Blanco and Prieto 2012). Our research indicates that uptake of protein, DNA and microbes appears to be restricted to root-hair-producing trichoblasts (Paungfoo-Lonhienne et al. 2008, 2010a, 2010b) (Fig. 5a–c). These observations support the argument that root hairs are one of the entry routes of large molecule into root cells.

|
Conclusions
Mixotrophy is currently considered an exception in higher plants, and restricted to carnivorous, hemi-parasitic and partially hetero-mycotrophic species. However, similar to other mixotrophic organisms, notably phytoplankton, plants seem to be able to complement photosynthetic energy gain with essential elements from organic compounds and microbes. Although the contribution of organic carbon is likely to be less important for plants that are not light-limited, organic N and P compounds could supplement essential elements acquired as inorganic compounds. Knowledge of whether mixotrophy is important for plants only in certain ecosystems or whether it is a more general feature of plants is lacking. Similar to the uncertainty surrounding organic C fluxes in the rhizosphere (Jones et al. 2009), we have little quantitative information on how much organic versus inorganic N and P compounds contribute to plant nutrition. In semi-controlled growth conditions, several studies have shown a preference of plants for organic N monomers over inorganic N (e.g. Stoelken et al. 2010). Similarly, in highly artificial axenic growth conditions, Arabidopsis satisfied ~10% of N demand with protein (Paungfoo-Lonhienne et al. 2008). A knowledge gap remains in our understanding of how microbes, especially procaryotes, act in the rhizosphere, apoplast and as root endophytes. The rapid advances in microbial research will shed light into these questions, and more powerful analyses techniques will decipher the chemical nature and fluxes of organic compounds at much finer resolution than what has been possible in the past.
Approaches that span from molecular to ecological techniques, the use of mutants, sophisticated microscopy and modelling can be used to address the questions raised here. We argue that a new framework that considers plants as mixotrophs has numerous benefits including advancing the sustainable use of soils and efficient nutrient supply to plants with nutrients contained in organic materials. This is pertinent as the use of fertilisers is accelerating, much of which is applied as inorganic substances (or in the case of urea is rapidly converted to inorganics), and nutrient inefficiencies characterise many modern crop systems (Tilman et al. 2002; Davidson et al. 2012). Illustrating the dramatic discrepancy in fertiliser use is food production in China that increased 3.4-fold from 1961 to 2009, but was accompanied by 37- and 91-fold increases in N and P fertiliser application (Zhang et al. 2013). The resultant pollution is a problem of global significance (Gruber and Galloway 2008), motivating research and development on improving nutrient supply, uptake and assimilation by plants.
Acknowledgements
We are grateful to Dr Jozef Visser for discussions about mixotrophy and comments that have improved the manuscript, Jessica Vogt for helping with the figures. Our research is funded by the Australian Research Council (Discovery Grant DP0986495) and The University of Queensland. Research facilities are kindly provided by the ARC Centre of Excellence for Integrative Legume Research. John Raven’s understanding of mixotrophy in photosynthetic organisms has been significantly improved by involvement in a series of workshops on mixotrophy in phytoplankton funded through a Leverhulme Trust International Network, which facilitated discussion with Professor Kevin Flynn, Professor Diane Stoecker, Dr Aditee Mitra, Professor Pat Glibert, Professor Per-Jule Hansen, Professor Edna Granéli, Professor Joann Burkholder and Professor Michael Zubkov. John Raven also acknowledges very helpful discussion with Professor Stephen Maberly. The University of Dundee is a registered Scottish charity (No: SC015096).
References
Adlassnig W, Koller-Peroutka M, Bauer S, Koshkin E, Lendl T, Lichtscheidl IK (2012) Endocytotic uptake of nutrients in carnivorous plants. The Plant Journal 71, 303–313.| Endocytotic uptake of nutrients in carnivorous plants.Crossref | GoogleScholarGoogle Scholar | 22417315PubMed |
Alberts B, Bray D, Lewis JMR, Roberts K, Watson JD (1989) The plasma membrane. In ‘The cell’. pp. 275–340. (Garland Publishing Inc.: New York)
Arnon DI, Stout PR (1939) The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiology 14, 371–375.
| The essentiality of certain elements in minute quantity for plants with special reference to copper.Crossref | GoogleScholarGoogle Scholar | 16653564PubMed |
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57, 233–266.
| The role of root exudates in rhizosphere interactions with plants and other organisms.Crossref | GoogleScholarGoogle Scholar | 16669762PubMed |
Baluska F, Samaj J, Hlavacka A, Kendrick-Jones J, Volkmann D (2004) Actin-dependent fluid-phase endocytosis in inner cortex cells of maize root apices. Journal of Experimental Botany 55, 463–473.
| Actin-dependent fluid-phase endocytosis in inner cortex cells of maize root apices.Crossref | GoogleScholarGoogle Scholar | 14739268PubMed |
Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment 19, 529–538.
| Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability.Crossref | GoogleScholarGoogle Scholar |
Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. Journal of Microscopy 214, 159–173.
| FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells.Crossref | GoogleScholarGoogle Scholar | 15102063PubMed |
Bradfute OE, McLaren AD (1964) Entry of protein molecules into plant roots. Physiologia Plantarum 17, 667–675.
| Entry of protein molecules into plant roots.Crossref | GoogleScholarGoogle Scholar |
Bradfute OE, Chapman-Andresen C, Jensen WA (1964) Concerning morphological evidence for pinocytosis in higher plants. Experimental Cell Research 36, 207–210.
| Concerning morphological evidence for pinocytosis in higher plants.Crossref | GoogleScholarGoogle Scholar |
Bréda N, Maillard P, Montpied P, Bréchet C, Garbaye J, Courty P-E (2013) Isotopic evidence in adult oak trees of a mixotrophic lifestyle during spring reactivation. Soil Biology & Biochemistry 58, 136–139.
| Isotopic evidence in adult oak trees of a mixotrophic lifestyle during spring reactivation.Crossref | GoogleScholarGoogle Scholar |
Brewin NJ (2004) Plant cell wall remodelling in the rhizobium-legume symbiosis. Critical Reviews in Plant Sciences 23, 293–316.
| Plant cell wall remodelling in the rhizobium-legume symbiosis.Crossref | GoogleScholarGoogle Scholar |
Bronk DA, See JH, Bradley P, Killberg L (2007) DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences 4, 283–296.
| DON as a source of bioavailable nitrogen for phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488, 91–95.
| Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota.Crossref | GoogleScholarGoogle Scholar | 22859207PubMed |
Caldwell BA, Jumpponen A, Trappe JM (2000) Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia 92, 230–232.
| Utilization of major detrital substrates by dark-septate, root endophytes.Crossref | GoogleScholarGoogle Scholar |
Cambui CA, Svennerstam H, Gruffman L, Nordin A, Ganeteg U, Näsholm T (2011) Patterns of plant biomass partitioning depend on nitrogen source. PLoS ONE 6, e19211
| Patterns of plant biomass partitioning depend on nitrogen source.Crossref | GoogleScholarGoogle Scholar | 21544211PubMed |
Chapin FS (1995) Ecology – new cog in the nitrogen-cycle. Nature 377, 199–200.
| Ecology – new cog in the nitrogen-cycle.Crossref | GoogleScholarGoogle Scholar |
Chapin FS, Moilanen L, Kielland K (1993) Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 361, 150–153.
| Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge.Crossref | GoogleScholarGoogle Scholar |
Chen DL, Delatorre CA, Bakker A, Abel S (2000) Conditional identification of phosphate-starvation-response mutants in Arabidopsis thaliana. Planta 211, 13–22.
| Conditional identification of phosphate-starvation-response mutants in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 10923699PubMed |
Clode PL, Kilburn MR, Jones DL, Stockdale EA, Cliff JB, Herrmann AM, Murphy DV (2009) In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry. Plant Physiology 151, 1751–1757.
| In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 19812187PubMed |
Cram WJ (1980) Pinocytosis in plants. New Phytologist 84, 1–17.
| Pinocytosis in plants.Crossref | GoogleScholarGoogle Scholar |
Crane KW, Grover JP (2010) Coexistence of mixotrophs, autotrophs, and heterotrophs in planktonic microbial communities. Journal of Theoretical Biology 262, 517–527.
| Coexistence of mixotrophs, autotrophs, and heterotrophs in planktonic microbial communities.Crossref | GoogleScholarGoogle Scholar | 19878684PubMed |
Croft MT, Warren MJ, Smith AG (2006) Algae need their vitamins. Eukaryotic Cell 5, 1175–1183.
| Algae need their vitamins.Crossref | GoogleScholarGoogle Scholar | 16896203PubMed |
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil 245, 35–47.
| Root exudates as mediators of mineral acquisition in low-nutrient environments.Crossref | GoogleScholarGoogle Scholar |
Datta S, Kim C, Pernas M, Pires N, Proust H, Tam T, Vijayakumar P, Dolan L (2011) Root hairs: development, growth and evolution at the plant–soil interface. Plant and Soil 346, 1–14.
| Root hairs: development, growth and evolution at the plant–soil interface.Crossref | GoogleScholarGoogle Scholar |
Davidson EA, David MB, Galloway JN, Goodale CL, Haeuber R, Harrison JA, Howarth RW, Jaynes DB, Lowrance RR, Nolan BT, et al (2012) Excess nitrogen in the US environment: trends, risks and solution. Issues in Ecology 15, 1–16.
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid roots and other root clusters. Botanica Acta 108, 183–200.
Dyer B, Wrenshall CL (1941) Organic P in soils: I. The extraction and separation of organic phosphorus compounds from soils. Soil Science 51, 159–170.
| Organic P in soils: I. The extraction and separation of organic phosphorus compounds from soils.Crossref | GoogleScholarGoogle Scholar |
Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB, Webb EA (2006) Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439, 68–71.
| Phosphonate utilization by the globally important marine diazotroph Trichodesmium.Crossref | GoogleScholarGoogle Scholar | 16397497PubMed |
Echeverria E (2000) Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiology 123, 1217–1226.
| Vesicle-mediated solute transport between the vacuole and the plasma membrane.Crossref | GoogleScholarGoogle Scholar | 10938341PubMed |
Eggenberger K, Mink C, Wadhwani P, Ulrich AS, Nick P (2011) Using the peptide Bp100 as a cell-penetrating tool for the chemical engineering of actin filaments within living plant cells. ChemBioChem 12, 132–137.
| Using the peptide Bp100 as a cell-penetrating tool for the chemical engineering of actin filaments within living plant cells.Crossref | GoogleScholarGoogle Scholar | 21154994PubMed |
Ellison AM, Adamec L (2011) Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same? Oikos 120, 1721–1731.
| Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same?Crossref | GoogleScholarGoogle Scholar |
Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to Brefeldin A and Wortmannin. The Plant Cell 14, 71–86.
| Uptake of a fluorescent marker in plant cells is sensitive to Brefeldin A and Wortmannin.Crossref | GoogleScholarGoogle Scholar | 11826300PubMed |
Epstein E, Bloom AJ (2005) ‘Mineral nutrition of plants: principles and perspectives. 2nd edn. (SinauerAssociates Inc.: Sunderland, MA, USA)
Esteban GF, Fenchel T, Finlay BJ (2010) Mixotrophy in ciliates. Protist 161, 621–641.
| Mixotrophy in ciliates.Crossref | GoogleScholarGoogle Scholar | 20970377PubMed |
Etxeberria E, Baroja-Fernandez E, Munoz FJ, Pozueta-Romero J (2005a) Sucrose-inducible endocytosis as a mechanism for nutrient uptake in heterotrophic plant cells. Plant & Cell Physiology 46, 474–481.
| Sucrose-inducible endocytosis as a mechanism for nutrient uptake in heterotrophic plant cells.Crossref | GoogleScholarGoogle Scholar |
Etxeberria E, Gonzalez P, Tomlinson P, Pozueta-Romero J (2005b) Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. Journal of Experimental Botany 56, 1905–1912.
| Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells.Crossref | GoogleScholarGoogle Scholar | 15911561PubMed |
Flynn KJ, Stoecker DK, Mitra A, Raven JA, Glibert PM, Hansen PJ, Granéli E, Burkholder JM (2013) Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research 35, 3–11.
| Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types.Crossref | GoogleScholarGoogle Scholar |
Forde BG, Walch-Liu P (2009) Nitrate and glutamate as environmental cues for behavioural responses in plant roots. Plant, Cell & Environment 32, 682–693.
| Nitrate and glutamate as environmental cues for behavioural responses in plant roots.Crossref | GoogleScholarGoogle Scholar |
Franche C, Lindstrom K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant and Soil 321, 35–59.
| Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants.Crossref | GoogleScholarGoogle Scholar |
Fuerst JA, Hawkins JA, Holmes A, Sly LI, Moore CJ, Stackebrandt E (1993) Porphyrobacter neustonensis gen. nov., sp. nov., an aerobic bacteriochlorophyll-synthesizing budding bacterium from fresh water. International Journal of Systematic Bacteriology 43, 125–134.
| Porphyrobacter neustonensis gen. nov., sp. nov., an aerobic bacteriochlorophyll-synthesizing budding bacterium from fresh water.Crossref | GoogleScholarGoogle Scholar | 8427804PubMed |
Gahoonia TS, Nielsen NE (1996) Variation in acquisition of soil phosphorus among wheat and barley genotypes. Plant and Soil 178, 223–230.
| Variation in acquisition of soil phosphorus among wheat and barley genotypes.Crossref | GoogleScholarGoogle Scholar |
Gahoonia TS, Nielsen NE (1998) Direct evidence on participation of root hairs in phosphorus (^32P) uptake from soil. Plant Soil 198, 147–152.
Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant and Soil 262, 55–62.
| Barley genotypes with long root hairs sustain high grain yields in low-P field.Crossref | GoogleScholarGoogle Scholar |
Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191, 181–188.
| Root hairs and phosphorus acquisition of wheat and barley cultivars.Crossref | GoogleScholarGoogle Scholar |
Geldner N (2004) The plant endosomal system – its structure and role in signal transduction and plant development. Planta 219, 547–560.
| The plant endosomal system – its structure and role in signal transduction and plant development.Crossref | GoogleScholarGoogle Scholar | 15221385PubMed |
Geldner N, Jurgens G (2006) Endocytosis in signalling and development. Current Opinion in Plant Biology 9, 589–594.
| Endocytosis in signalling and development.Crossref | GoogleScholarGoogle Scholar | 17011816PubMed |
George TS, Gregory PJ, Hocking P, Richardson AE (2008) Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environmental and Experimental Botany 64, 239–249.
| Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils.Crossref | GoogleScholarGoogle Scholar |
Gómez-Baena G, López-Lozano A, Gil-Martínez J, Lucena JM, Diez J, Candau P, Garcia-Fernández JM (2008) Glucose uptake and its effect on gene expression in Prochlorococcus. PLoS ONE 3, e3416
| Glucose uptake and its effect on gene expression in Prochlorococcus.Crossref | GoogleScholarGoogle Scholar | 18941506PubMed |
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296.
| An Earth-system perspective of the global nitrogen cycle.Crossref | GoogleScholarGoogle Scholar | 18202647PubMed |
Hamilton JMU, Simpson DJ, Hyman SC, Ndimba BK, Slabas AR (2003) Ara12 subtilisin-like protease from Arabidopsis thaliana: purification, substrate specificity and tissue localization. Biochemical Journal 370, 57–67.
| Ara12 subtilisin-like protease from Arabidopsis thaliana: purification, substrate specificity and tissue localization.Crossref | GoogleScholarGoogle Scholar |
Hartmann M, Grob C, Tarran GA, Martin AP, Burkill PH, Scanlan DJ, Zubkov MV (2012) Mixotrophic basis of Atlantic oligotrophic ecosystems. Proceedings of the National Academy of Sciences of the United States of America 109, 5756–5760.
| Mixotrophic basis of Atlantic oligotrophic ecosystems.Crossref | GoogleScholarGoogle Scholar | 22451938PubMed |
Helliwell K, Wheeler G, Goldstein R, Smith A (2011) Insights into the evolution of vitamin B12 auxotrophy from sequences algal genomes. Molecular Biology and Evolution 28, 2921–2933.
| Insights into the evolution of vitamin B12 auxotrophy from sequences algal genomes.Crossref | GoogleScholarGoogle Scholar | 21551270PubMed |
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science 11, 610–617.
| How do plants respond to nutrient shortage by biomass allocation?Crossref | GoogleScholarGoogle Scholar | 17092760PubMed |
Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Climate Change 1, 50–53.
| Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition.Crossref | GoogleScholarGoogle Scholar |
Hirner A, Ladwig F, Stransky H, Okumoto S (2006) Arabidopsis LHT1 Is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. The Plant Cell 18, 1931–1946.
| Arabidopsis LHT1 Is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll.Crossref | GoogleScholarGoogle Scholar | 16816136PubMed |
Holstein SEH (2002) Clathrin and plant endocytosis. Traffic 3, 614–620.
| Clathrin and plant endocytosis.Crossref | GoogleScholarGoogle Scholar |
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454, 327–330.
| A unifying framework for dinitrogen fixation in the terrestrial biosphere.Crossref | GoogleScholarGoogle Scholar | 18563086PubMed |
Inselsbacher E, Näsholm T (2012) A novel method to measure the effect of temperature on diffusion of plant-available nitrogen in soil. Plant and Soil 354, 251–257.
| A novel method to measure the effect of temperature on diffusion of plant-available nitrogen in soil.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E, Ohlund J, Jamtgard S, Huss-Danell K, Näsholm T (2011) The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil. Soil Biology & Biochemistry 43, 1321–1332.
| The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil.Crossref | GoogleScholarGoogle Scholar |
Jensen WA, McLaren AD (1960) Uptake of proteins by plant cells: the possible occurrence of pinocytosis in plants. Experimental Cell Research 19, 414–417.
| Uptake of proteins by plant cells: the possible occurrence of pinocytosis in plants.Crossref | GoogleScholarGoogle Scholar | 14407138PubMed |
Johnson MD, Oldach D, Delwiche CF, Stoecker DK (2007) Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature 445, 426–428.
| Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra.Crossref | GoogleScholarGoogle Scholar | 17251979PubMed |
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant and Soil 321, 5–33.
| Carbon flow in the rhizosphere: carbon trading at the soil–root interface.Crossref | GoogleScholarGoogle Scholar |
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA (2005) Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist 166, 639–653.
| Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium.Crossref | GoogleScholarGoogle Scholar | 15819926PubMed |
Juniper BE, Robins RJ, Joel DM (1989) ‘The carnivorous plants.’ (Academic Press Limited: London)
Kilburn MR, Jones DL, Clode PL, Cliff JB, Stockdale EA, Herrmann AM, Murphy DV (2010) Application of nanoscale secondary ion mass spectrometry to plant cell research. Plant Signaling & Behavior 5, 760–762.
| Application of nanoscale secondary ion mass spectrometry to plant cell research.Crossref | GoogleScholarGoogle Scholar |
Komarova NY, Thor K, Gubler A, Meier S, Dietrich D, Weichert A, Suter Grotemeyer M, Tegeder M, Rentsch D (2008) AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiology 148, 856–869.
| AtPTR1 and AtPTR5 transport dipeptides in planta.Crossref | GoogleScholarGoogle Scholar | 18753286PubMed |
Król E, Płachno B, Adamec L, Stolarz M, Dziubińska H, Trębacz K (2012) Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany 109, 47–64.
| Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’.Crossref | GoogleScholarGoogle Scholar | 21937485PubMed |
Lambers H, Shanes MW, Cramer MD, Perse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98, 693–713.
| Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits.Crossref | GoogleScholarGoogle Scholar | 16769731PubMed |
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends in Ecology & Evolution 23, 95–103.
| Plant nutrient-acquisition strategies change with soil age.Crossref | GoogleScholarGoogle Scholar |
Lauter FR, Ninnemann O, Bucher M, Riesmeier JW, Frommer WB (1996) Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato. Proceedings of the National Academy of Sciences of the United States of America 93, 8139–8144.
| Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato.Crossref | GoogleScholarGoogle Scholar | 8755617PubMed |
Leake JR (2004) Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Current Opinion in Plant Biology 7, 422–428.
| Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar | 15231265PubMed |
Leake JR, Cameron DD (2010) Physiological ecology of mycoheterotrophy. New Phytologist 185, 601–605.
| Physiological ecology of mycoheterotrophy.Crossref | GoogleScholarGoogle Scholar | 20356334PubMed |
Lee Y-H, Foster J, Chen J, Voll LM, Weber APM, Tegeder M (2007) AAP1 transports uncharged amino acids into roots of Arabidopsis. The Plant Journal 50, 305–319.
| AAP1 transports uncharged amino acids into roots of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 17419840PubMed |
Lima JE, Kojima S, Takahashi H, von Wiren N (2010) Ammonium triggers lateral root branching in Arabidopsis in an ammonium transporter1;3-dependent manner. The Plant Cell 22, 3621–3633.
| Ammonium triggers lateral root branching in Arabidopsis in an ammonium transporter1;3-dependent manner.Crossref | GoogleScholarGoogle Scholar | 21119058PubMed |
Lonhienne TGA, Sagulenko E, Webb RI, Lee KC, Franke J, Devos DP, Nouwens A, Carrolla BJ, Fuerst JA (2010) Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proceedings of the National Academy of Sciences of the United States of America 107, 12883–12888.
| Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus.Crossref | GoogleScholarGoogle Scholar |
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, Glavina del Rio T, et al (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488, 86–90.
| Defining the core Arabidopsis thaliana root microbiome.Crossref | GoogleScholarGoogle Scholar | 22859206PubMed |
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell & Environment 24, 459–467.
| Regulation of root hair density by phosphorus availability in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Ma L, Velthof GL, Wang FH, Qin W, Zhang WF, Liu Z, Zhang Y, Wei J, Lesschen JP, Ma WQ, Oenema O, Zhang FS (2012) Nitrogen and phosphorus use efficiencies and losses in the food chain in China at regional scales in 1980 and 2005. Science of the Total Environment 434, 51–61.
| Nitrogen and phosphorus use efficiencies and losses in the food chain in China at regional scales in 1980 and 2005.Crossref | GoogleScholarGoogle Scholar | 22542299PubMed |
Mandyam KG, Roe J, Jumpponen A (2013) Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biology
| Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization.Crossref | GoogleScholarGoogle Scholar | in press
Marschner H (1995) ‘Mineral nutrition of higher plants.’ (Academic Press Ltd.: London)
Marschner P, Neumann G, Kania A, Weiskopf L, Lieberei R (2002) Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant and Soil 246, 167–174.
| Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.).Crossref | GoogleScholarGoogle Scholar |
Marshall JD, Ehleringer JR (1990) Are xylem-tapping mistletoes partially heterotrophic? Oecologia 84, 244–248.
McCarren J, DeLong EF (2007) Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla. Environmental Microbiology 9, 846–858.
| Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla.Crossref | GoogleScholarGoogle Scholar | 17359257PubMed |
McCutcheon JP, von Dohlen CD (2011) An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Current Biology 21, 1366–1372.
| An interdependent metabolic patchwork in the nested symbiosis of mealybugs.Crossref | GoogleScholarGoogle Scholar | 21835622PubMed |
Mercado-Blanco J, Prieto P (2012) Bacterial endophytes and root hairs. Plant and Soil 361, 301–306.
| Bacterial endophytes and root hairs.Crossref | GoogleScholarGoogle Scholar |
Mudge S, Rae A, Diatloff E, Smith F (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. The Plant Journal 31, 341–353.
| Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 12164813PubMed |
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bioassays with tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar |
Näsholm T, Ekblad A, Nordin A, Giesler R, Hogberg M, Hogberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392, 914–916.
| Boreal forest plants take up organic nitrogen.Crossref | GoogleScholarGoogle Scholar |
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytologist 182, 31–48.
| Uptake of organic nitrogen by plants.Crossref | GoogleScholarGoogle Scholar | 19210725PubMed |
Neumann G, Martinoia E (2002) Cluster roots – an underground adaptation for survival in extreme environments. Trends in Plant Science 7, 162–167.
| Cluster roots – an underground adaptation for survival in extreme environments.Crossref | GoogleScholarGoogle Scholar | 11950612PubMed |
Ovečka M, Lang I, Baluška F, Ismail A, Illeš P, Lichtscheidl IK (2005) Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226, 39–54.
| Endocytosis and vesicle trafficking during tip growth of root hairs.Crossref | GoogleScholarGoogle Scholar | 16231100PubMed |
Paungfoo-Lonhienne C, Lonhienne TGA, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S (2008) Plants can use protein as a nitrogen source without assistance from other organisms. Proceedings of the National Academy of Sciences of the United States of America 105, 4524–4529.
| Plants can use protein as a nitrogen source without assistance from other organisms.Crossref | GoogleScholarGoogle Scholar | 18334638PubMed |
Paungfoo-Lonhienne C, Schenk PM, Lonhienne TGA, Brackin R, Meier S, Rentsch D, Schmidt S (2009) Nitrogen affects cluster root formation and expression of putative peptide transporters. Journal of Experimental Botany 60, 2665–2676.
| Nitrogen affects cluster root formation and expression of putative peptide transporters.Crossref | GoogleScholarGoogle Scholar | 19380419PubMed |
Paungfoo-Lonhienne C, Lonhienne TGA, Mudge SR, Schenk PM, Christie M, Carroll BJ, Schmidt S (2010a) DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth. Plant Physiology 153, 799–805.
| DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth.Crossref | GoogleScholarGoogle Scholar | 20388669PubMed |
Paungfoo-Lonhienne C, Rentsch D, Robatzek S, Webb R, Sagulenko E, Näsholm T, Schmidt S, Lonhienne T (2010b) Turning the table: plants consume microbes as a source of nutrients. PLoS ONE 5, e11915
| Turning the table: plants consume microbes as a source of nutrients.Crossref | GoogleScholarGoogle Scholar | 20689833PubMed |
Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S (2012) Past, present and future of organic nutrients. Plant and Soil 359, 1–18.
| Past, present and future of organic nutrients.Crossref | GoogleScholarGoogle Scholar |
Peltzer D, Wardle D, Allison V, Baisden W, Bardgett R, Chadwick O, Condron L, Parfitt R, Porder S, Richardson S, Turner B, Vitousek P, Walker J, Walker L (2010) Understanding ecosystem retrogression. Ecological Monographs 80, 509–529.
| Understanding ecosystem retrogression.Crossref | GoogleScholarGoogle Scholar |
Pérez-Gómez J, Moore I (2007) Plant endocytosis: it is clathrin after all. Current Biology 17, R217–R219.
| Plant endocytosis: it is clathrin after all.Crossref | GoogleScholarGoogle Scholar | 17371763PubMed |
Pillet L, Pawlowski J (2013) Transcriptome analysis of foraminiferan Elphidium margaritaceum questions the role of gene transfer in kleptoplastidy. Molecular Biology and Evolution 30, 66–69.
| Transcriptome analysis of foraminiferan Elphidium margaritaceum questions the role of gene transfer in kleptoplastidy.Crossref | GoogleScholarGoogle Scholar | 22993235PubMed |
Poretsky RS, Sun SL, Mou XZ, Moran MA (2010) Transporter genes expressed by coastal bacterioplankton in response to dissolved organic carbon. Environmental Microbiology 12, 616–627.
| Transporter genes expressed by coastal bacterioplankton in response to dissolved organic carbon.Crossref | GoogleScholarGoogle Scholar | 19930445PubMed |
Prieto P, Schiliro E, Maldonado-Gonzalez MM, Valderrama R, Barroso-Albarracin JB, Mercado-Blanco J (2011) Root hairs play a key role in the endophytic colonization of olive roots by Pseudomonas spp. with biocontrol activity. Microbial Ecology 62, 435–445.
| Root hairs play a key role in the endophytic colonization of olive roots by Pseudomonas spp. with biocontrol activity.Crossref | GoogleScholarGoogle Scholar | 21347721PubMed |
Raven JA (1983) Phytophages of xylem and phloem: a comparison of animal and plantsap-feeders. Advances in Ecological Research 13, 135–234.
| Phytophages of xylem and phloem: a comparison of animal and plantsap-feeders.Crossref | GoogleScholarGoogle Scholar |
Raven JA (1987) The role of vacuoles. New Phytologist 106, 357–422.
Raven JA (1997) Phagotrophy in phototrophs. Limnology and Oceanography 42, 198–205.
| Phagotrophy in phototrophs.Crossref | GoogleScholarGoogle Scholar |
Raven JA (2012) Carbon. In ‘Ecology of cyanobacteria’. 2nd edn. (Ed. B Whitton) pp. 443–460. (Springer: Berlin)
Raven JA, Donnelly S (2013) Brown dwarfs and black smokers. The potential for photosynthesis using the radiation from low-temperature black bodies. In ‘Habitability of other planets and satellites’. (Ed. J-P Devera) (Springer: Berlin) (In press)
Raven JA, Beardall J, Flynn KJ, Maberly SC (2009) Phagotrophy in the origins of photosynthesis in eukaryotes and as a complementary mode of nutrition in phototrophs: relation to Darwin’s insectivorous plants. Journal of Experimental Botany 60, 3975–3987.
| Phagotrophy in the origins of photosynthesis in eukaryotes and as a complementary mode of nutrition in phototrophs: relation to Darwin’s insectivorous plants.Crossref | GoogleScholarGoogle Scholar | 19767306PubMed |
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytologist 157, 475–492.
| Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance?Crossref | GoogleScholarGoogle Scholar |
Richardson AE, Hadobas PA, Hayes JE (2000) Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant, Cell & Environment 23, 397–405.
| Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture.Crossref | GoogleScholarGoogle Scholar |
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop and Pasture Science 60, 124–143.
| Plant mechanisms to optimise access to soil phosphorus.Crossref | GoogleScholarGoogle Scholar |
Robinson DG (2005) Plant and fungal endocytosis. Protoplasma 226, 1
Robinson DG, Jiang LW, Schumacher K (2008) The endosomal system of plants: charting new and familiar territories. Plant Physiology 147, 1482–1492.
| The endosomal system of plants: charting new and familiar territories.Crossref | GoogleScholarGoogle Scholar | 18678740PubMed |
Šamaj J, Baluška F, Voigt B, Schlicht M, Volkmann D, Menzel D (2004) Endocytosis, actin cytoskeleton, and signaling. Plant Physiology 135, 1150–1161.
| Endocytosis, actin cytoskeleton, and signaling.Crossref | GoogleScholarGoogle Scholar | 15266049PubMed |
Šamaj J, Read ND, Volkmann D, Menzel D, Baluska F (2005) The endocytic network in plants. Trends in Cell Biology 15, 425–433.
| The endocytic network in plants.Crossref | GoogleScholarGoogle Scholar | 16006126PubMed |
Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Letters 581, 2309–2317.
| Molecular physiology of higher plant sucrose transporters.Crossref | GoogleScholarGoogle Scholar | 17434165PubMed |
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85, 591–602.
| Nitrogen mineralization: challenges of a changing paradigm.Crossref | GoogleScholarGoogle Scholar |
Schulte EE, Kelling KA (1999) ‘Understand plant nutrients – soil and applied sulfur A2525.’ (University of Wisconsin-Extension) Available at www.soils.wisc.edu/extension/pubs/A2525.pdf
Schulten HR, Schnitzer M (1997) The chemistry of soil organic nitrogen: a review. Biology and Fertility of Soils 26, 1–15.
| The chemistry of soil organic nitrogen: a review.Crossref | GoogleScholarGoogle Scholar |
Schulze W, Frommer WB, Ward JM (1999) Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. The Plant Journal 17, 637–646.
| Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes.Crossref | GoogleScholarGoogle Scholar | 10230062PubMed |
Schunmann P, Richardson A, Vickers C, Delhaize E (2004) Promoter analysis of the barley Pht1;1 phosphate transporter gene identifies regions controlling root expression and responsiveness to phosphate deprivation. Plant Physiology 136, 4205–4214.
| Promoter analysis of the barley Pht1;1 phosphate transporter gene identifies regions controlling root expression and responsiveness to phosphate deprivation.Crossref | GoogleScholarGoogle Scholar | 15542491PubMed |
Selosse MA, Roy M (2009) Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science 14, 64–70.
| Green plants that feed on fungi: facts and questions about mixotrophy.Crossref | GoogleScholarGoogle Scholar | 19162524PubMed |
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant and Soil 274, 101–125.
| Cluster roots: a curiosity in context.Crossref | GoogleScholarGoogle Scholar |
Silberbush M (2013) Root study: why is it behind other plant studies? American Journal of Plant Sciences 4, 198–203.
| Root study: why is it behind other plant studies?Crossref | GoogleScholarGoogle Scholar |
Sirová D, Borovec J, Picek T, Adamec L, Nedbalová L, Vrba J (2011) Ecological implications of organic carbon dynamics in the traps of aquatic carnivorous Utricularia plants. Functional Plant Biology 38, 583–593.
| Ecological implications of organic carbon dynamics in the traps of aquatic carnivorous Utricularia plants.Crossref | GoogleScholarGoogle Scholar |
Smith SE, Read DJ (2008) ‘Mycorrhizal symbiosis.’ (Academic Press: Amsterdam)
Smith FW, Mudge SR, Rae AL, Glassop D (2003) Phosphate transport in plants. Plant and Soil 248, 71–83.
| Phosphate transport in plants.Crossref | GoogleScholarGoogle Scholar |
Soper FM, Paungfoo-Lonhienne C, Brackin R, Rentsch D, Schmidt S, Robinson N (2011) Arabidopsis and Lobelia anceps access small peptides as a nitrogen source for growth. Functional Plant Biology 38, 788–796.
| Arabidopsis and Lobelia anceps access small peptides as a nitrogen source for growth.Crossref | GoogleScholarGoogle Scholar |
Sprent JI (2001) ‘Nodulation in legumes.’ (The Cromwell Press: Royal Botanical Gardens, Kew)
Stoecker DK (1998) Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications. European Journal of Protistology 34, 281–290.
| Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications.Crossref | GoogleScholarGoogle Scholar |
Stoelken G, Simon J, Ehlting B, Rennenberg H (2010) The presence of amino acids affects inorganic N uptake in non-mycorrhizal seedlings of European beech (Fagus sylvaticus). Tree Physiology 30, 1118–1128.
| The presence of amino acids affects inorganic N uptake in non-mycorrhizal seedlings of European beech (Fagus sylvaticus).Crossref | GoogleScholarGoogle Scholar | 20595637PubMed |
Svennerstam H, Ganeteg U, Bellini C, Näsholm T (2007) Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiology 145, 1853–1860.
Svennerstam H, Jämtgård S, Ahmad I, Huss-Danell K, Näsholm T, Ganeteg U (2011) Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytologist 191, 459–467.
Tedersoo L, Pellet P, Koljalg U, Selosse MA (2007) Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151, 206–217.
| Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae.Crossref | GoogleScholarGoogle Scholar | 17089139PubMed |
Tester M, Smith SE, Smith FA (1987) The phenomenon of nonmycorrhizal plants. Canadian Journal of Botany 65, 419–431.
| The phenomenon of nonmycorrhizal plants.Crossref | GoogleScholarGoogle Scholar |
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418, 671–677.
| Agricultural sustainability and intensive production practices.Crossref | GoogleScholarGoogle Scholar | 12167873PubMed |
Tittel J, Bissinger V, Zippel B, Gaedke U, Bell E, Lorke A, Kamjunke N (2003) Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proceedings of the National Academy of Sciences of the United States of America 100, 12776–12781.
| Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs.Crossref | GoogleScholarGoogle Scholar | 14569026PubMed |
Tornero P, Conejero V, Vera P (1996) Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: similarity of functional domains to subtilisin-like endoproteases. Proceedings of the National Academy of Sciences of the United States of America 93, 6332–6337.
| Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: similarity of functional domains to subtilisin-like endoproteases.Crossref | GoogleScholarGoogle Scholar | 8692815PubMed |
Venterink HO (2011) Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply. Plant and Soil 347, 137–146.
| Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply.Crossref | GoogleScholarGoogle Scholar |
Voigt B, Timmers ACJ, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H (2005) Actin-based motility of endosomes is linked to the polar tip growth of root hairs. European Journal of Cell Biology 84, 609–621.
| Actin-based motility of endosomes is linked to the polar tip growth of root hairs.Crossref | GoogleScholarGoogle Scholar | 16032929PubMed |
Wagele H, Deusch O, Handeler K, Martin R, Schmitt V, Christa G, Pinzger B, Gould SB, Dagan T, Klussmann-Kolb A, Martin W (2011) Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes. Molecular Biology and Evolution 28, 699–706.
| Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes.Crossref | GoogleScholarGoogle Scholar | 20829345PubMed |
Walch-Liu P, Ivanov II, Filleur S, Gan YB, Remans T, Forde BG (2006a) Nitrogen regulation of root branching. Annals of Botany 97, 875–881.
| Nitrogen regulation of root branching.Crossref | GoogleScholarGoogle Scholar | 16339770PubMed |
Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG (2006b) Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant & Cell Physiology 47, 1045–1057.
| Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiology 132, 44–51.
| Root exudation and rhizosphere biology.Crossref | GoogleScholarGoogle Scholar | 12746510PubMed |
Warren CR (2013) High diversity of small organic N observed in soil water. Soil Biology & Biochemistry 57, 444–450.
| High diversity of small organic N observed in soil water.Crossref | GoogleScholarGoogle Scholar |
Wasaki J, Yamamura T, Shinano T, Osaki M (2003) Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant and Soil 248, 129–136.
| Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency.Crossref | GoogleScholarGoogle Scholar |
Weisskopf L, Heller S, Eberl L (2011) Burkholderia species are major inhabitants of white lupin cluster roots. Applied and Environmental Microbiology 77, 7715–7720.
| Burkholderia species are major inhabitants of white lupin cluster roots.Crossref | GoogleScholarGoogle Scholar | 21908626PubMed |
Whiteside MD, Treseder KK, Atsatt PR (2009) The brighter side of soils: quantum dots track organic nitrogen through fungi and plants. Ecology 90, 100–108.
| The brighter side of soils: quantum dots track organic nitrogen through fungi and plants.Crossref | GoogleScholarGoogle Scholar | 19294917PubMed |
Yamamura T, Wasaki J, Shinano T, Osaki M (2002) Expression of acid phosphatase gene in cluster roots of white lupin. Plant & Cell Physiology 43, S112–S112.
Zhang F, Cui Z, Chen X, Ju X, Shen J, Chen Q, Liu X, Zhang W, Mi G, Fan M, Jiang R (2013) Integrated nutrient management for food security and environment quality in China. In ‘Advances in agronomy. Vol. 116’. (Ed. DL Sparks) pp. 1–40. (Academic Press) (In Press)
Zubkov MV (2009) Photoheterotrophy in marine prokaryotes. Journal of Plankton Research 31, 933–938.
| Photoheterotrophy in marine prokaryotes.Crossref | GoogleScholarGoogle Scholar |
Zubkov MV, Tarran GA, Mary I, Fuchs BM (2008) Differential microbial uptake of dissolved amino acids and amino sugars in surface waters of the Atlantic Ocean. Journal of Plankton Research 30, 211–220.
| Differential microbial uptake of dissolved amino acids and amino sugars in surface waters of the Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Glossary
|


