Evolution of halophytes: multiple origins of salt tolerance in land plants
Timothy J. Flowers A B E , Hanaa K. Galal A C and Lindell Bromham DA Biology and Environmental Science, School of Life Sciences, John Maynard Smith Building, University of Sussex, Falmer, Brighton, BN1 9QG, UK.
B School of Plant Biology, Faculty of Natural and Agricultural Sciences, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Faculty of Science, Assiut University, Assiut 71516, Egypt.
D Centre for Macroevolution and Macroecology, Research School of Biology, Australian National University, Canberra, ACT 0200, Australia.
E Corresponding author. Email: t.j.flowers@sussex.ac.uk
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 37(7) 604-612 https://doi.org/10.1071/FP09269
Submitted: 6 November 2009 Accepted: 3 March 2010 Published: 2 July 2010
Abstract
The evolution of salt tolerance is interesting for several reasons. First, since salt-tolerant plants (halophytes) employ several different mechanisms to deal with salt, the evolution of salt tolerance represents a fascinating case study in the evolution of a complex trait. Second, the diversity of mechanisms employed by halophytes, based on processes common to all plants, sheds light on the way that a plant’s physiology can become adapted to deal with extreme conditions. Third, as the amount of salt-affected land increases around the globe, understanding the origins of the diversity of halophytes should provide a basis for the use of novel species in bioremediation and conservation. In this review we pose the question, how many times has salt tolerance evolved since the emergence of the land plants some 450–470 million years ago? We summarise the physiological mechanisms underlying salt-tolerance and provide an overview of the number and diversity of salt-tolerant terrestrial angiosperms (defined as plants that survive to complete their life cycle in at least 200 mM salt). We consider the evolution of halophytes using information from fossils and phylogenies. Finally, we discuss the potential for halophytes to contribute to agriculture and land management and ask why, when there are naturally occurring halophytes, it is proving to be difficult to breed salt-tolerant crops.
Additional keywords: phylogeny, saline agriculture, salinity.
The freshwater origin of land plants
Water relations
All organisms are faced with balancing their water relations with those of their environment. Marine photosynthetic organisms achieve this balance at the water potential of seawater, about –2.3 MPa (Harvey 1966), using a mixture of ions and organic compounds (Bisson and Kirst 1995; Iwamoto and Shiraiwa 2005). Terrestrial plants must also adjust their water potential to be at least as low as that of the soil in which they are growing. For plants living in saline environments, the Na+ concentration can range from ~100 to 2380 mM Na (Flowers 1985), equivalent to a water potential range from –0.5 to –11 MPa. This means that plants growing in saline soils must adjust osmotically to water potentials that are commonly in the range –2 to –3 MPa; they do this, as do marine plants, by accumulating a mixture of ions and organic solutes (e.g. Flowers et al. 1977). For all terrestrial plants, however, whether living in a saline or non-saline environment, the major factor affecting their water relations is the large difference in concentration of water vapour between their stubstomatal pores and the atmosphere – the driving force for the movement of water through the soil–plant–atmosphere continuum (even at 90% RH the atmospheric water potential is –14.2 MPa). The consequent flux of water through the plant carries essential nutrients to the shoots, but in a saline environment, any dissolved solutes from the soil that enter the transpiration stream are also carried to the leaves where they must be accommodated, re-circulated or excreted.
The first land plants
Before the evolution of embryophytes (terrestrial plants that are not algae; viz. bryophytes, pteridophytes and spermatophytes), green plants were composed of the Chlorophyta and Streptophyta, which split ~1000 million years ago (Becker and Marin 2009). Palaeontological and molecular evidence suggests embryophytes arose from the Streptophyta rather than the Chlorophyta; from charophycean green algae (Graham 1993; Kenrick and Crane 1997; Raven and Edwards 2001; Waters 2003) in the Ordovician period, some 450 million years ago. This date is supported by molecular data (Sanderson 2003), although the transition to terrestrial habitats happened on several occasions (Lewis and McCourt 2004). While there is some uncertainty concerning the origins of the embryophytes, with support for the Charales, Coleochaetales and the Zygnematales (Becker and Marin 2009) being sister to the embryophytes, recent evidence favours the Charales (Becker and Marin 2009).
The first records of land plants are microfossils consisting of spores and plant parts, which appeared in the mid-Ordovician (~470 million years ago); they are found through to the Early Silurian period (Wellman and Gray 2000). The earliest clearly recognisable land plants date from ~40 million years later, in the Early Late Silurian (Wellman and Gray 2000), in which plants such as Cooksonia can be seen in the fossil record (Graham 1993). The origin of these early embryophytes is crucial to the origin of salt tolerance in land plants as it could have evolved from species that lived in fresh or salt water: by the time the first transition of plants to a terrestrial environment occurred, the seas were saline (Railsback et al. 1990), even if a little less so (26–29 g per 1000 g seawater) than today’s oceans (30–40 g per 1000 g seawater).
Did terrestrial plants derive from charophycean green algae living in fresh or salt water? Since Characean algae occupied both freshwater and marine habitats in the mid-Paleozoic (Kenrick and Crane 1997), early embryophytes could have arisen from marine or freshwater species. Any marine organism making the transition to the land on the edge of a saline pool would have to cope not only with water loss from its cells to the atmosphere, but also with hyper-saline conditions, which would have occurred as water evaporated from the pool. If, however, the first land plants evolved at the edges of freshwater pools (Raven and Edwards 2001), then land plants would have arisen from an organism (or organisms) that had adapted to living in fresh water.
A particularly important adaptation to freshwater life is the development of systems for acquiring nutrients from the low concentrations of minerals present in fresh water rather than the high concentrations of the nutrient rich (eutrophic) marine environment (Rodriguez-Navarro and Rubio 2006). This ability would be equally important for growth on land, where plants need to acquire nutrients in a nutrient poor (oligotrophic) environment. Any such adaptation to oligotrophy might have left intact those systems required for osmotic adjustment and which are necessary for growth in seawater or in fluctuating salinity. If embryophytes originated from freshwater algae, then, as suggested by Becker and Marin (2009), the streptophytes were ‘physiologically pre-adapted to terrestrial existence by their primary freshwater lifestyle in contrast to their marine sister lineage, i.e. Chlorophyta’ (perhaps because their adaptation to an oligotrophic lifestyle resulted in evolution of specific mechanisms for dealing with fluctuating ion concentration in the environment; see also Ion compartmentation below). Analysis of fossils from Rhynie in Aberdeenshire in Scotland suggests that by the Early Devonian, ~400 million years ago, plants were drought and probably salt tolerant (Channing and Edwards 2009). The question, then, is how many times salt tolerance has evolved since the emergence of the land plants some 450–470 million years ago.
Physiology of salt tolerance
Although there are many aspects of the physiology of salt tolerance that are yet to be understood, it is clear that the trait is complex in that, at a minimum, it requires the combination of several different traits: the accumulation and compartmentation of ions for osmotic adjustment; the synthesis of compatible solutes; the ability to accumulate essential nutrients (particularly K) in the presence of high concentrations of the ions generating salinity (Na); the ability to limit the entry of these saline ions into the transpiration stream; and the ability to continue to regulate transpiration in the presence of high concentrations of Na+ and Cl– (Flowers and Colmer 2008).
Commonality of traits
All plants, not just halophytes, have the main characteristics necessary for salt tolerance – the ability to acquire ions that are then compartmentalised in vacuoles (fundamental to turgor generation in plants and algae) and discrimination in favour of K over Na. Given that the physiological foundations of salt tolerance are found in all plants, it is not surprising that plant species show a continuum of salt tolerance with glycophytes such as chickpea (Flowers et al. 2010) at one extreme (where just 25 mM NaCl can be toxic) and halophytes (which can tolerate salt concentrations as high as 500–1000 mM) at the other. Furthermore, salt tolerance is often part of a multi-faceted syndrome that may include, for example, tolerance of flooding (Colmer and Voesenek 2009) and drought. Indeed, physiological responses to salinity are often similar to responses to other environmental stresses (Munns and Tester 2008) and may rely on common stress-tolerance pathways (Tuteja 2007).
Ion compartmentation
Most commonly, the ions dominating saline environments are those of Na and Cl, as they predominate in seawaters: halophytes use these ions for most of their osmotic adjustment (Yeo 1983). It is clear, however, that in the process of ion accumulation for osmotic adjustment, some ions can ‘leak’ into the transpiration stream (Yeo et al. 1987) and that such leaks must be minimised if the aerial parts are not to be swamped by ions (Munns 2005). Consequently, tight regulation of ion transport from roots to shoots is vital for halophytes. Sufficient Na and Cl must be accumulated to achieve osmotic adjustment but excess must be avoided as both ions are toxic at the concentrations required for osmotic adjustment. In practice, Na+ and Cl– are largely compartmentalised in vacuoles in halophytes (Flowers et al. 1977, 1986). A range of metabolically inert organic compounds is also present and utilised to adjust the osmotic potential of the cytoplasm (Flowers and Colmer 2008). In some species, excess ions are secreted through glands that have evolved on the surface of the aerial parts. Recirculation of ions from shoots back to the roots is unlikely to be an important aspect of salt tolerance as the ions would have to be carried in the symplasm of the phloem where they would be toxic (Flowers et al. 1986; Munns and Tester 2008). Furthermore, phloem transport delivers solutes to growing regions where ions would cause metabolic damage unless re-exported to the environment. The latter would not remove ions from the rhizosphere. So, ions reaching the leaves must be accommodated in vacuoles in the leaf cells, a process that involves the transport of ions across both plasma and vacuolar membranes.
Land plants, including mosses and ferns, regulate their internal Na+ concentrations through the control of influx and efflux. Within the eukaryotes, Na+ efflux is mediated by Na+/H+ antiporters and Na+-pump ATPases, of which there are two types: the Na+/K+-ATPases of animal cells and the fungal Na+-ATPases. Higher plants do not appear to express Na+-ATPases (Garciadeblas et al. 2001) meaning that they must rely on Na+/H+ antiporters to transport Na+ out of their cytoplasm, whether it be into the apoplast or the vacuoles. Benito and Rodriguez-Navarro (2003) argued that the absence of the Na+-ATPases in higher plants was evidence for their evolution in non-saline oligotrophic environments where Na+ concentrations were low and there was no need for the system(s) than mediated extensive Na+ efflux. This would presumably have occurred during the early development of land organisms in non-saline environments (Horodyski and Knauth 1994) or through the loss of such a system(s) following the migration of marine organisms to freshwater. Interestingly, the moss Physcomitrella patens (Bryopsida) expresses two ATPases similar to fungal ENA-Na+-ATPases (Benito and Rodriguez-Navarro 2003; Lunde et al. 2007). The presence of the Na+-ATPases in a moss suggests these enzymes were lost during early evolution of land plants (Benito and Rodriguez-Navarro 2003), which then rely on the Na+/H+ antiporters (also present in P. patens, Benito and Rodriguez-Navarro 2003) to regulate cytosolic Na+. The question of how plants living in seawater achieve this function remains. The Na+/H+ antiporters in plants are electroneutral (Munns and Tester 2008), which means they would not facilitate Na+ efflux at the alkaline pH values of seawater (Benito and Rodriguez-Navarro 2003). However, seagrasses do presumably efflux Na+ but how their Na+/H+ antiporters function in this respect is unclear (Garciadeblás et al. 2007; Touchette 2007). The same question can be posed for halophytes, although it is possible that the rhizosphere pH could be lower than that of seawater; the operation of Na-efflux through antiporters has been recently questioned (Britto and Kronzucker 2009).
K/Na selectivity
The selectivity of halophytes for K over Na varies between families of flowering plants (Flowers et al. 1986). Net selectivity (net SK : Na), calculated as the ratio of K concentration in the plant to that in the medium divided by the ratio of Na concentration in the plant to that in the medium, ranges between average values of 9 and 60 (Flowers and Colmer 2008) with an overall mean of 19; it is only in the Poales that net SK : Na values of the order of 60 are found. Within the monocots (Fig. 1), there are three orders with halophytes, but no data are available for the net SK : Na values of species within the Arecales. In the Alismatales, the average net SK : Na (across just three species) is 16 (range 10 to 22), suggesting that high selectivity has evolved only in the Poales (for halophytes within this order, average selectivities of are 58 in the Juncaginaceae (two species) and 60 in the Poaceae (nine species)). There is too little data to analyse the net SK : Na values within the dicots, but the average value is 11 compared with 60 in the Poales (Flowers and Colmer 2008).

|
Salt glands
Glandular structures are not uncommon on plants; they can secrete a range of organic compounds (Wagner 1991; Wagner et al. 2004). However, the ability to secrete salt appears to have evolved less frequently than salt tolerance. Salt glands, epidermal appendages of one to a few cells that secrete salt to the exterior of a plant (Thomson et al. 1988) have been described in just a few orders of flowering plants – the Poales (e.g. in Aeluropus littoralis and Chloris gayana), Myrtales (e.g. the mangrove Laguncularia racemosa), Caryophyllales (e.g. Mesembryanthemum crystallinum and the saltbush Atriplex halimus), Lamiales (e.g. the mangroves Avicennia marina and Avicennia germinans) and the Solanales (e.g. Cressa cretica). Their distribution across the orders of flowering plants suggests at least three origins, although there may have been more independent origins within orders (see for example within the Caryophyllales, Fig. 2b). Whether salt glands evolved from glands that originally performed some other function is unclear, but it is difficult, at least in the Poaceae, to get glandular hairs on non-halophytes (such as Zea mays L.) to secrete salt (Ramadan and Flowers 2004).

|
Compatible solutes
The compatible solutes synthesised by halophytes range from quaternary ammonium compounds through sulfonium analogues to proline and sugar alcohols: most are widely distributed through the orders of flowering plants, reflecting both phylogeny and functional needs (Flowers and Colmer 2008). There is no clear pattern of particular solutes being exclusive to particular orders, other than of sorbitol to the Plantaginaceae, so that it appears that although the ability to synthesise and compartmentalise a compatible solute is a requirement in halophytes, the nature of that solute depends on the habitat and the availability of N. Compatible solutes comprise a small proportion of the total solutes accumulated by halophytes (Yeo 1983).
How many halophytes are there?
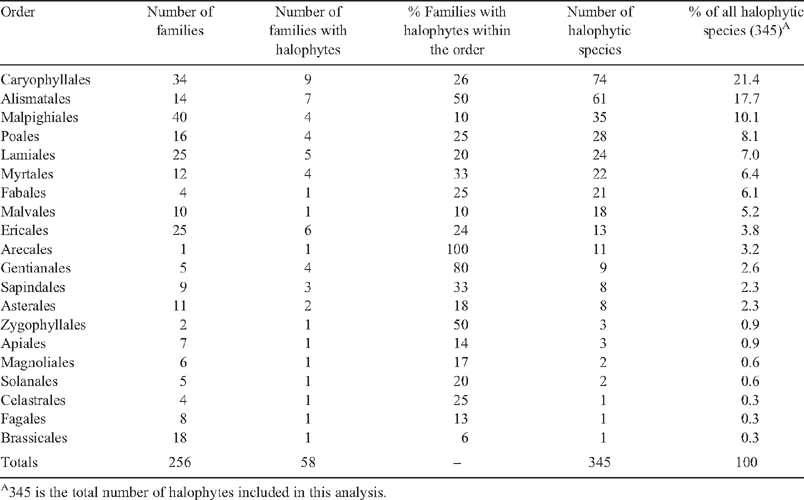
As a trait, salt tolerance has been recognised in plants for more than 200 years (Flowers et al. 1986) and there have been several definitions of what constitutes a ‘halophyte’. Angiosperm species show a wide range of levels of salt tolerance from inhibition of growth at low salt concentrations (e.g. sensitive chickpea genotypes dying in 25 mM NaCl, Flowers et al. 2010) to tolerance of salt concentrations as high or higher than seawater (e.g. the halophyte Arthrocnemum macrostachyum survives in up to 1000 mM NaCl, Khan et al. 2005). Any definition of a ‘halophyte’ must include a threshold value of salt concentration and the number of halophytes clearly depends on the salt concentration used in that definition. By setting a dividing line between halophytes and glycophytes (salt sensitive plants) at a salt concentration of ~80 mM NaCl (a conductivity in the soil solution of 7.8 dS m–1), Aronson (1989) listed ~1550 salt-tolerant plants. Using the same definition, Menzel and Lieth (2003) recorded over 2600 species. These comprehensive lists define salt tolerant plants using a salt concentration that is much lower than that present in seawaters (an average of ~480 mM Na+ and 580 mM Cl–). Since there are species that have evolved tolerance to seawater salinities, we have chosen to use a salt concentration of ‘~200 mM salt’ as our defining limit (as did Flowers and Colmer 2008): precision is not required as salt concentrations in natural habitats fluctuate with evaporation and precipitation of water. Applying the definition of ‘plants that survive to complete their life cycle in at least 200 mM salt’ to Aronson’s (1989) database generates a list of some 350 species, which we call ‘halophytes’ (whether this group of the most salt tolerant of plant species should be given a separate name, such as ‘euhalophyte’, is an issue that could be further debated). Our halophytes are not uniformly distributed across the taxa of flowering plants listed by Stevens (2001). The orders with the greatest number of halophytes (Table 1) are the Caryophyllales (which includes those species that were formerly in the Chenopodiaceae such as the saltbushes in the genus Atriplex, genera with reduced leaves such Salicornia and Tectomia and succulent members of the genus Suaeda) and the Alismatales (which includes marine flowering plants belonging to genera such as Zostera and Thalassia). Although our list of halophytes is not comprehensive as the limits of tolerance for many species are not known, our analysis suggests that halophytes are not more than ~0.25% of the known species of angiosperms.
|
Salt tolerance in bryophytes and pteridophytes
Halophytes are uncommon among the higher plants and this situation is also true for bryophytes and pteridophytes. There are some mosses that occur in areas that are inundated by tides (Table 2); for example, four species in Northern England (Adam 1976) and five species in Nova Scotia, one of which has been shown to survive immersion in seawater for 24 h (Garbary et al. 2008). Aronson (1989) lists only one genus of a salt resistant fern. This low frequency of salt tolerance in bryophytes and pteridophytes indicates that they also have rarely evolved the ability to tolerate salt concentrations approaching those of seawater, although some are able to grow in lower concentrations (e.g. Physcomitrella patens in 100 mM NaCl, see Lunde et al. 2007).

|
Repeated evolution of salt-tolerance in angiosperms
In an earlier analysis based on the salt tolerance criterion used by Aronson (1989) and a phylogeny constructed by Takhtajan (1969), Flowers et al. (1977) concluded that salt tolerance was widely distributed amongst the families of flowering plants and suggested a polyphyletic origin, something more recently established for other complex traits such as C4 photosynthesis (Sage 2004) and crassulacean acid metabolism (Crayn et al. 2004). In Fig. 1, we have plotted the distribution of clades containing halophytes (as defined above) on an order-level phylogeny of angiosperms based on the main tree published by Stevens (Stevens 2001). From this order-level tree we can see that salt tolerance seems to have evolved multiple times.
We cannot determine the exact number of origins of salt tolerance from this phylogeny for several reasons. First, without information on the number of halophytes within each order, and their relationships to each other, we cannot know whether each family containing halophytes represents a single origin or multiple origins (see below). Second, if a clade contains both halophytes and non-halophytes, we cannot be sure whether salt tolerance was gained independently in multiple lineages, or whether the condition is ancestral to the clade and then lost in some families. Third, the evidence for the presence of halophytes in some orders is based on incomplete data (e.g. there are just two species reported as able to grow in seawater in the Magnoliales (Annonaceae) and without any physiological data). In most orders that contain families with halophytes, the percentage of halophytes is 1% or less (Table 1), which makes it unlikely that the condition is ancestral and the non-halophytes lost salt-tolerance. Therefore it seems likely that virtually each of the red boxes in Fig. 1 represents at least one independent origin of salt tolerance.
Many of the orders marked in Fig. 1 may contain multiple origins of halophytes. For example, within the Asterales (Fig. 2a), we have recorded eight halophytes in two families (seven in the Asteraceae and one in the Goodeniaceae), but the remaining families do not have any halophytes. It is parsimonious to assume that at least two lineages within the Asterales evolved salt tolerance independently. Examining the presences of particular traits associated with salt tolerance may also reveal some interesting evolutionary patterns. For example, five of the nine salt-tolerant lineages within the Caryophyllales use salt glands to excrete excess salt from the leaves. This illustrates how even related lineages may develop different strategies for dealing with environmental salt. The distribution of salt glands within the phylogeny (Fig. 2b) suggests that even within this single clade of flowering plants, a key salt tolerance mechanism has evolved independently several times.
It also seems likely that the distribution of halophyte-containing families is not random over the phylogeny, with some clades containing more putative origins of salt tolerance than others. If true, this would seem to offer support for the idea that some clades are more likely to give rise to halophytes than others. It may be profitable to look at the physiology of members of those clades to see if they share certain characteristics that make adapting to environmental salt easier than in other clades. Mapping key components of salt tolerance on phylogenies may help to reveal whether some lineages are predisposed to evolving particular solutions to salinity, whether some traits that confer salt tolerance are more easily acquired than others, or even whether the component traits of salt tolerance are usually acquired in a particular order. Looking at the patterns of origination within families will be important for answering the questions of whether there can be an ancestral condition that favours the evolution of salt tolerance.
Importance of halophytes to agriculture and land management
Salinity is an expanding problem. More than 800 million ha of land is salt-affected, which is over 6% of the world’s land area (Flowers and Yeo 1995). Salt-affected land is increasing worldwide through vegetation clearance and irrigation, both of which raise the watertable bringing dissolved salts to the surface. It is estimated that up to half of irrigation schemes worldwide are affected by salinity (Flowers and Yeo 1995). Although irrigated land is a relatively small proportion of the total global area of food production, it produces a third of the food (Munns and Tester 2008). Salt stress has been identified as one of the most serious environmental factors limiting the productivity of crop plants (Flowers and Yeo 1995; Barkla et al. 1999), with a huge impact on agricultural productivity. The global annual cost of salt-affected land is likely to be well over US$12 billion (Qadir et al. 2008). Future agricultural production will rely increasingly on our ability to grow food and fibre plants in salt-affected land (Rozema and Flowers 2008; Qadir et al. 2008).
Breeding for salt tolerance
Although it is abundantly clear that, at some level, salinity reduces the growth of plants, the reason is generally not clear. Both Na+ and Cl– can reach toxic concentrations if not compartmentalised in vacuoles, but within the complexity of the whole plant, the exact cause of any damage is generally unknown. However, the consequences are that salinity reduces plant growth and enhances senescence of mature leaves, resulting in a reduction in functional leaf area: in crop plants, this decreases yield (Munns et al. 2006; Munns and Tester 2008). There is, therefore, a great incentive to increase the salt tolerance of crop plants to allow greater yields in salt-affected soils. However, breeding of salt-tolerant crops is hampered by the complexity of salt tolerance as seen in halophytes (see above). So, despite a large amount of effort that has been directed towards increasing the salt tolerance of crops, ranging from wide-crossing to transgenics, few productive salt-tolerant varieties have been produced (Flowers and Yeo 1995; Flowers 2004; Flowers and Flowers 2005; Witcombe et al. 2008). The realisation that halophytes have evolved several times should encourage attempts to generate new salt-tolerant crops and may assist plant breeders in several ways. First, it may illuminate specific traits associated with salt tolerance that may be selected in established crop plants (e.g. Huang et al. 2008); second, it may lead to the domestication of naturally salt tolerant species as crop plants.
Halophytes as crops
Naturally salt-tolerant species are now being promoted in agriculture, particularly to provide forage, medicinal plants, aromatic plants (Qadir et al. 2008) and for forestry (Marcar and Crawford 2004). For example, Barrett-Lennard (2002) identified 26 salt-tolerant plant species capable of producing products (or services) of value to agriculture in Australia. Examples of useful halophytes include the potential oil-seed crops Kosteletzkya virginica (e.g. Ruan et al. 2008); Salvadora persica (e.g. Reddy et al. 2008); Salicornia bigelovii (Glenn et al. 1991) and Batis maritima (Marcone 2003); fodder crops such as Atriplex spp. (e.g. El Shaer 2003), Distichlis palmeri (e.g. Masters et al. 2007) and biofuels (Y. Waisel, pers. comm.; Qadir et al. 2008). Growing salt-tolerant biofuel crops on marginal agricultural land would help to counter concerns that the biofuel industry reduces the amount of land available for food production (Qadir et al. 2008). At the extreme, plants that can grow productively at very high salt levels could be irrigated with brackish water or seawater (Rozema and Flowers 2008). Although plants that put resources (c.f. Yeo 1983) into developing salt-tolerance mechanisms (e.g. the production of compatible solutes to maintain osmotic balance is an energetic cost) may do so at the expense of other functions, many halophytes show optimal growth in saline conditions (Flowers and Colmer 2008) and salt marshes have high productivity (Colmer and Flowers 2008; Flowers and Colmer 2008). The fact that dicolytedonous halophytes can grow at similar rates to glycophytes (see Flowers and Colmer 2008 for a more detailed discussion) suggests that salt tolerance per se will not limit productivity. Here the contrast with drought tolerance is stark: without water plants do not grow, but may survive; with salt water, some plants can grow well.
Apart from direct use as crops, we may increasingly need to rely on halophytes for revegetation and remediation of salt-affected land. Over the last 200 years, industrialisation in Europe and elsewhere has lead to an enormous increase of production, use and release of traces of heavy metals into the environment. A large portion of these toxic materials, including Cd, Cu, Pb and Zn, accumulate in sediments, including the soils of tidal marshes. Recent studies showed that some seagrasses and salt marsh plants are capable of extracting heavy metals from sediments and accumulating them in belowground or aboveground tissues (Weis and Weis 2004; Cambrolle et al. 2008; Lewis and Devereux 2009). The processes and potential application of these aquatic halophytes merits much greater research and development. Growing salt-tolerant plants, including species of Kochia, Bassia, Cynodon, Medicago, Portulaca, Sesbania, and Brachiaria, may also improve other soil properties, such as increasing water conductance or increasing soil fertility (Qadir et al. 2008). Halophytes may also lower the watertable, thereby allowing growth of salt-sensitive species in salt-affected land (Barrett-Lennard 2002).
Finally, conservation and land management in salt-affected regions will benefit from improved knowledge of the salt tolerance of local species. Halophytes are being targeted for ‘ecosystem engineering’ projects, such as stabilising wetlands to increase the success of revegetation programs (Fogel et al. 2004). Halophytes are also being used to develop sustainable agricultural practise, for example, using indigenous halophytes to restore overgrazed pasture and to reduce reliance on irrigation (Peacock et al. 2003). Salt-tolerant species are increasingly being considered for landscape remediation. For example, well planned revegetation can reduce both the in situ salt concentration at mine sites and also reduce the movement of salts from the mine sites in runoff (Carroll and Tucker 2000). However, care must be taken in introducing species for bioremediation as they may become an environmental problem in their own right. For example, Kochia planted for forage and bioremediation in Western Australia in 1990 soon spread and was declared a weed in 1992 (Qadir et al. 2008).
The halophyte paradox
In this review, we have shown that salt tolerance has evolved many different times in angiosperms and in different ways. We have also noted that, despite increasing salinisation of agricultural land, few salt-tolerant crops have been produced. This raises an important paradox: if all plants contain the basic physiological mechanisms on which salt tolerance is based and if halophytes have evolved in many different lineages using a variety of adaptations, then why is it so difficult to breed salt-tolerant crops?
The reasons for the slow progress in changing the salt tolerance of plants were discussed by Flowers and Yeo (1995), who argued that this was because salinity had not become a problem of sufficient priority that the necessary resource was being applied to its solution. Moreover, they also concluded that treating salinity resistance as a single character could account for the limited success. We have already established that the salt tolerance of halophytes is a multi-genic trait and the same is true for non-halophytes (Flowers 2004). It is not our intention here to rehearse the arguments over approaches to breeding for complex traits. However, it is clear that breeding for yield has not delivered many salt-resistant varieties of the common crops (Flowers 2004; Witcombe et al. 2008). The probability of combining two complex traits – yield and salt tolerance – to maximise yield under saline conditions is evidentially very low. As a consequence, Yeo et al. (1990) advocated understanding the physiology of tolerance and pooling a range of traits an approach that had some success for rice (Dedolph and Hettel 1997). A similar approach has been advocated for durum wheat combining traits for regulating sodium accumulation and osmotic adjustment (Munns et al. 2002; James et al. 2006, 2008). The traits that necessarily need to be pooled for wheat (and barley) include Na ‘exclusion’, K/Na discrimination, the retention of ions in sheaths, tissue tolerance, ion partitioning into different leaves, osmotic adjustment, enhanced vigour, water use efficiency and early flowering (Colmer et al. 2005). In addition other traits such as tolerance to water–logging (see Colmer and Flowers 2008 for a discussion of flooding tolerance in halophytes) are vitally important, further complicating any breeding program (Colmer et al. 2005). Given the complexity of the task, it is not surprising that the transfer of one or two genes has generally failed to change the tolerance of transgenic plants; what is surprising is that there are instances where such simple gene transfers appear to have altered yield (Flowers 2004).
Evolutionary studies of halophytes, which reconstruct the origin and development of salt tolerance in a variety of plant lineages, may help us to understand why artificial breeding has failed to produce robust and productive salt tolerant crops. Such studies may also help us develop new salt-tolerant lines by revealing the order of acquisition of components of salt tolerance, or indicating favourable genetic backgrounds on which salt tolerance may be developed. By examining the repeated evolution of this complex trait we may identify particular traits or conditions that predispose species to evolve a complex, multi-faceted trait such as salt tolerance and give rise to halophyte lineages. More generally, this may shed light on the adaptation of angiosperm lineages to extreme environments. In order to achieve these ends, more information is required on, at a minimum, the effects of salt on the growth and ion relations of a wider range of plant species that may prove to be halophytes.
Acknowledgements
HKG would like to than the Egyptian Government for financial support while at the University of Sussex and our thanks also go to Jessica Thomas and Katherine Byron for assistance in earlier stages of this project.
Adam P
(1976) Occurrence of bryophytes on British salt marshes. Journal of Bryology 9, 265–274.

Barkla BJ,
Vera Estrella R, Pantoja O
(1999) Towards the production of salt-tolerant crops. Advances in Experimental Medicine and Biology 464, 77–89.
|
CAS |
PubMed |

Barrett-Lennard EG
(2002) Restoration of saline land through revegetation. Agricultural Water Management 53, 213–226.
| Crossref | GoogleScholarGoogle Scholar |

Becker B, Marin B
(2009) Streptophyte algae and the origin of embryophytes. Annals of Botany 103, 999–1004.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Benito B, Rodriguez-Navarro A
(2003) Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. The Plant Journal 36, 382–389.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bisson MA, Kirst GO
(1995) Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82, 461–471.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Britto DT, Kronzucker HJ
(2009) Ussing’s conundrum and the search for transport mechanisms in plants. New Phytologist 183, 243–246.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Cambrolle J,
Redondo-Gomez S,
Mateos-Naranjo E, Figueroa ME
(2008) Comparison of the role of two Spartina species in terms of phytostabilization and bioaccumulation of metals in the estuarine sediment. Marine Pollution Bulletin 56, 2037–2042.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Carroll C, Tucker A
(2000) Effects of pasture cover on soil erosion and water quality on central Queensland coal mine rehabilitation. Tropical Grasslands 34, 254–262.

Channing A, Edwards D
(2009) Yellowstone hot spring environments and the paleo-ecophysiology of Rhynie chert plants: towards a synthesis. Plant Ecology & Diversity 2, 111–143.
| Crossref | GoogleScholarGoogle Scholar |

Colmer TD, Flowers TJ
(2008) Flooding tolerance in halophytes. New Phytologist 179, 964–974.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Colmer TD, Voesenek L
(2009) Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36, 665–681.
| Crossref | GoogleScholarGoogle Scholar |

Colmer TD,
Munns R, Flowers TJ
(2005) Improving salt tolerance of wheat and barley: future prospects. Australian Journal of Experimental Agriculture 45, 1425–1443.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Crayn DM,
Winter K, Smith JAC
(2004) Multiple origins of crassulacean acid metabolism and the epiphytic habit in the neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences of the United States of America 101, 3703–3708.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Flowers TJ
(1985) Physiology of halophytes. Plant and Soil 89, 41–56.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Flowers TJ
(2004) Improving crop salt tolerance. Journal of Experimental Botany 55, 307–319.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Flowers TJ, Colmer TD
(2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Flowers TJ, Flowers SA
(2005) Why does salinity pose such a difficult problem for plant breeders? Agricultural Water Management 78, 15–24.
| Crossref | GoogleScholarGoogle Scholar |

Flowers TJ, Yeo AR
(1995) Breeding for salinity resistance in crop plants – where next. Australian Journal of Plant Physiology 22, 875–884.
| Crossref | GoogleScholarGoogle Scholar |

Flowers TJ,
Troke PF, Yeo AR
(1977) The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology 28, 89–121.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Flowers TJ,
Hajibagheri MA, Clipson NJW
(1986) Halophytes. The Quarterly Review of Biology 61, 313–337.
| Crossref | GoogleScholarGoogle Scholar |

Flowers TJ,
Gaur PM,
Laxmipathi Gowda CL,
Krishnamurthy L,
Samineni S,
Siddique KHM,
Turner NC,
Vadez V,
Varshney RK, Colmer TD
(2010) Salt sensitivity in chickpea. Plant, Cell & Environment 33, 490–509.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Fogel BN,
Crain CM, Bertness MD
(2004) Community level engineering effects of Triglochin maritima (seaside arrowgrass) in a salt marsh in northern New England, USA. Journal of Ecology 92, 589–597.
| Crossref | GoogleScholarGoogle Scholar |

Garbary DJ,
Miller AG,
Scrosati R,
Kim KY, Schofield WB
(2008) Distribution and salinity tolerance of intertidal mosses from Nova Scotian salt marshes. The Bryologist 111, 282–291.
| Crossref | GoogleScholarGoogle Scholar |

Garciadeblas B,
Benito B, Rodríguez-Navarro A
(2001) Plant cells express several stress calcium ATPases but apparently no sodium ATPase. Plant and Soil 235, 181–192.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Garciadeblás B,
Haro R, Benito B
(2007) Cloning of two SOS1 transporters from the seagrass Cymodocea nodosa. SOS1 transporters from Cymodocea and Arabidopsis mediate potassium uptake in bacteria. Plant Molecular Biology 63, 479–490.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Glenn EP,
Oleary JW,
Watson MC,
Thompson TL, Kuehl RO
(1991) Salicornia-bigelovii Torr – an oilseed halophyte for seawater irrigation. Science 251, 1065–1067.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Horodyski RJ, Knauth LP
(1994) Life on land in the Precambrian. Science 263, 494–498.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Huang S,
Spielmeyer W,
Lagudah ES, Munns R
(2008) Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. Journal of Experimental Botany 59, 927–937.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Iwamoto K, Shiraiwa Y
(2005) Salt-regulated mannitol metabolism in algae. Marine Biotechnology 7, 407–415.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

James RA,
Davenport RJ, Munns R
(2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiology 142, 1537–1547.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

James RA,
von Caemmerer S,
Condon AGT,
Zwart AB, Munns R
(2008) Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat. Functional Plant Biology 35, 111–123.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kenrick P, Crane PR
(1997) The origin and early evolution of plants on land. Nature 389, 33–39.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Khan MA,
Ungar IA, Showalter AM
(2005) Salt stimulation and tolerance in an intertidal stem-succulent halophyte. Journal of Plant Nutrition 28, 1365–1374.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lewis MA, Devereux R
(2009) Nonnutrient anthropogenic chemicals in seagrass ecosystems: fate and effects. Environmental Toxicology and Chemistry 28, 644–661.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lewis LA, McCourt RM
(2004) Green algae and the origin of land plants. American Journal of Botany 91, 1535–1556.
| Crossref | GoogleScholarGoogle Scholar |

Lunde C,
Drew DP,
Jacobs AK, Tester M
(2007) Exclusion of Na+ via sodium ATPase (PpENA1) ensures normal growth of Physcomitrella patens under moderate salt stress. Plant Physiology 144, 1786–1796.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Marcone MF
(2003) Batis maritima (Saltwort/Beachwort): a nutritious, halophytic, seed bearings, perennial shrub for cultivation and recovery of otherwise unproductive agricultural land affected by salinity. Food Research International 36, 123–130.
| Crossref | GoogleScholarGoogle Scholar |

Masters DG,
Benes SE, Norman HC
(2007) Biosaline agriculture for forage and livestock production. Agriculture Ecosystems & Environment 119, 234–248.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Munns R
(2005) Genes and salt tolerance: bringing them together. New Phytologist 167, 645–663.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Munns R, Tester M
(2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Munns R,
Husain S,
Rivelli AR,
James RA,
Condon AG,
Lindsay MP,
Lagudah ES,
Schachtman DP, Hare RA
(2002) Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant and Soil 247, 93–105.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Munns R,
James RA, Lauchli A
(2006) Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany 57, 1025–1043.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Peacock JM,
Ferguson ME,
Alhadrami GA,
McCann IR,
Al Hajoj A,
Saleh A, Karnik R
(2003) Conservation through utilization: a case study of the indigenous forage grasses of the Arabian Peninsula. Journal of Arid Environments 54, 15–28.
| Crossref | GoogleScholarGoogle Scholar |

Qadir M,
Tubeileh A,
Akhtar J,
Larbi A,
Minhas PS, Khan MA
(2008) Productivity enhancement of salt-affected environments through crop diversification. Land Degradation & Development 19, 429–453.
| Crossref | GoogleScholarGoogle Scholar |

Railsback LB,
Ackerly SC,
Anderson TF, Cisne JL
(1990) Paleontological and isotope evidence for warm saline deep waters in ordovician oceans. Nature 343, 156–159.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Ramadan T, Flowers JJ
(2004) Effects of salinity and benzyl adenine on development and function of microhairs of Zea mays L. Planta 219, 639–648.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Raven JA, Edwards D
(2001) Roots: evolutionary origins and biogeochemical significance. Journal of Experimental Botany 52, 381–401.
|
CAS |
PubMed |

Reddy MP,
Shah MT, Patolia JS
(2008) Salvadora persica, a potential species for industrial oil production in semiarid saline and alkali soils. Industrial Crops and Products 28, 273–278.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Rodriguez-Navarro A, Rubio F
(2006) High-affinity potassium and sodium transport systems in plants. Journal of Experimental Botany 57, 1149–1160.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rozema J, Flowers T
(2008) Crops for a salinized world. Science 322, 1478–1480.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Ruan CJ,
Li H,
Guo YQ,
Qin P,
Gallagher JL,
Seliskar DM,
Lutts S, Mahy G
(2008) Kosteletzkya virginica, an agroecoengineering halophytic species for alternative agricultural production in China’s east coast: ecological adaptation and benefits, seed yield, oil content, fatty acid and biodiesel properties. Ecological Engineering 32, 320–328.
| Crossref | GoogleScholarGoogle Scholar |

Sage RF
(2004) The evolution of C4 photosynthesis. New Phytologist 161, 341–370.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Sanderson MJ
(2003) Molecular data from 27 proteins do not support a Precambrian origin of land plants. American Journal of Botany 90, 954–956.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Touchette BW
(2007) Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology 350, 194–215.
| Crossref | GoogleScholarGoogle Scholar |

Wagner GJ
(1991) Secreting glandular trichomes – more than just hairs. Plant Physiology 96, 675–679.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wagner GJ,
Wang E, Shepherd RW
(2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Annals of Botany 93, 3–11.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Waters ER
(2003) Molecular adaptation and the origin of land plants. Molecular Phylogenetics and Evolution 29, 456–463.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Weis JS, Weis P
(2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International 30, 685–700.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wellman CH, Gray J
(2000) The microfossil record of early land plants. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 355, 717–732.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Witcombe JR,
Hollington PA,
Howarth CJ,
Reader S, Steele KA
(2008) Breeding for abiotic stresses for sustainable agriculture. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 703–716.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Yeo AR
(1983) Salinity resistance: physiologies and prices. Physiologia Plantarum 58, 214–222.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Yeo AR,
Yeo ME, Flowers TJ
(1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. Journal of Experimental Botany 38, 1141–1153.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Yeo AR,
Yeo ME,
Flowers SA, Flowers TJ
(1990) Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theoretical and Applied Genetics 79, 377–384.
| Crossref | GoogleScholarGoogle Scholar |



 Indicates a node with rather weak support according to
Indicates a node with rather weak support according to 