Goldacre paper: Auxin: at the root of nodule development?
Ulrike MathesiusA School of Biochemistry and Molecular Biology, Australian National University and Australian Research Council Centre of Excellence for Integrative Legume Research, Linnaeus Way, Canberra, ACT 0200, Australia. Email: ulrike.mathesius@anu.edu.au
B This paper originates from the Peter Goldacre Award 2007 of the Australian Society of Plant Scientists, received by the author.
Functional Plant Biology 35(8) 651-668 https://doi.org/10.1071/FP08177
Submitted: 24 June 2008 Accepted: 14 August 2008 Published: 19 September 2008
Abstract
Root nodules are formed as a result of an orchestrated exchange of chemical signals between symbiotic nitrogen fixing bacteria and certain plants. In plants that form nodules in symbiosis with actinorhizal bacteria, nodules are derived from lateral roots. In most legumes, nodules are formed de novo from pericycle and cortical cells that are re-stimulated for division and differentiation by rhizobia. The ability of plants to nodulate has only evolved recently and it has, therefore, been suggested that nodule development is likely to have co-opted existing mechanisms for development and differentiation from lateral root formation. Auxin is an important regulator of cell division and differentiation, and changes in auxin accumulation and transport are essential for lateral root development. There is growing evidence that rhizobia alter the root auxin balance as a prerequisite for nodule formation, and that nodule numbers are regulated by shoot-to-root auxin transport. Whereas auxin requirements appear to be similar for lateral root and nodule primordium activation and organ differentiation, the major difference between the two developmental programs lies in the specification of founder cells. It is suggested that differing ratios of auxin and cytokinin are likely to specify the precursors of the different root organs.
Additional keywords: actinomycetes, auxin transport, cytokinin, flavonoids, lateral root, rhizobia, symbiosis.
General introduction
This review examines the questions of whether the phytohormone auxin is a regulator of both lateral root and symbiotic nodule development, and whether auxin has similar or divergent roles during the development of the two organs. Lateral roots and nodules are formed post-embryonically from endogenous cell types that are stimulated to divide, form an organ primordium, and later, differentiate and elongate (Fig. 1). In the case of lateral roots, cell divisions first occur in the pericycle. A lateral root primordium is formed after further divisions and the primordium differentiates into an organ with a central stele. An apical meristem becomes active and leads to lateral root elongation. Nodules usually arise from a combination of pericycle and cortical cell divisions, and after primordium formation a differentiated nodule forms, with an optional apical meristem and either central or peripheral localisation of vascular traces.
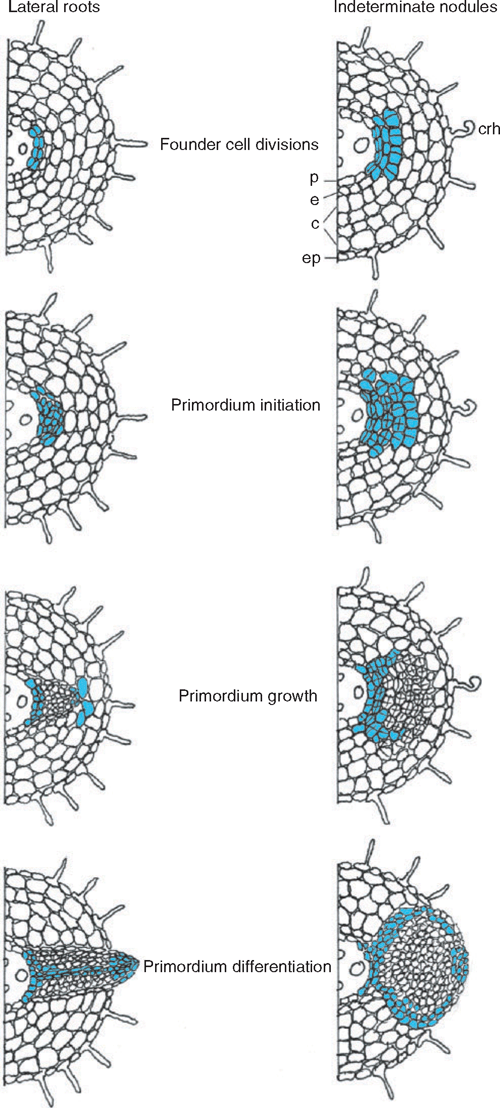
|
Lateral roots or branch roots have existed as parts of plant root systems for ~400 million years (Raven and Edwards 2001). In contrast, nodules only evolved relatively recently, ~60 million years ago, and their emergence could have been triggered by a lack of nitrogen in a CO2-rich environment (Sprent 2007, 2008). Nodules develop only on the roots (and sometimes stems) of certain plants that form a symbiosis with nitrogen fixing bacteria. It has been argued that because nodule development emerged recently during evolution, it is likely that the mechanisms that regulate nodule development were co-opted from existing processes, most likely those that regulate lateral root formation (Hirsch and LaRue 1997). Therefore, this review will first give an overview of the diversity of nodules in different host plants, highlighting the existence of types of nodules that resemble lateral roots.
The developmental mechanisms of lateral root formation have been studied in great detail, and auxin has emerged as a central regulator of lateral root development (Fukaki et al. 2007). Thus, this review will examine whether auxin might play a similar role in nodule development as it does in lateral root development. There is evidence that auxin is synthesised by the nitrogen fixing symbionts of host plants, but more importantly, that the symbiont indirectly alters auxin transport and localisation inside the host root. To understand how the symbiont can interfere with the auxin balance in the host, we will examine the known mechanism of auxin signalling and transport in the plant, followed by how these mechanisms play a part in the regulation of lateral root formation. This will be compared with the involvement of auxin during different stages of nodule development and in the regulation of nodule numbers. This comparison leads to the hypothesis that auxin requirements differ between lateral root and nodule development at the earliest stage of organ formation, the specification of founder cell types, whereas auxin is likely to have similar roles in regulating cell division and differentiation once the organ has been specified. This hypothesis could be tested in the future by genetically manipulating auxin synthesis or responses in the specific founder cell types of both organs.
Diversity of nodule types in nitrogen fixing symbioses
Nitrogen fixing symbioses occur between a range of plants and bacteria, and only a subset of these form root structures classified as nodules (Sprent and Sprent 1990). An example of an ancient nitrogen fixing symbiosis is the association of cycads with cyanobacteria, in which the symbiont induces the formation of so-called collaroid roots (Sprent and Sprent 1990). The more recent symbioses of higher plants with nitrogen fixing bacteria that lead to the formation of root (or stem) nodules only arose in plant families belonging to the Eurosid 1 clade (Soltis et al. 1995). Actinorhizal plants of eight Angiosperm families associate with actinomycetes of the genus Frankia. The most common nodulated plants are species of the Leguminosae, many of which form a symbiosis with α-proteobacteria called rhizobia, as well as certain β-proteobacteria (Sprent 2008). The only known nodulating non-legume is the tropical tree Parasponia of the Ulmacae family.
Studies of the diversity of legume and actinorhizal nodulation suggest that both the invasion process as well as the development of the nodule can occur in several ways (Hirsch and LaRue 1997; Gualtieri and Bisseling 2000; Sprent 2007). Invasion can be via crack entry of bacteria between epidermal and cortical cells, often at sites of lateral or adventitious root emergence, or via infection threads. Nodule development can be based on modifications of existing lateral or adventitious roots or involve de novo induction of cell divisions in pericycle and cortical cells. Nodule formation in the non-legume species shows similarities to the development of lateral roots. In actinorhizal plants, Frankia first cause cortical cell divisions to form a pre-nodule that is colonised by hyphae, and then stimulate the division of pericycle cells to form a lateral root-like nodule. Frankia invade the cortical cells of this nodule, which retains a central stele, similar to lateral roots (Pawlowski and Bisseling 1996). Similarly, in Parasponia, rhizobia trigger the initiation of a lateral root which they later invade (Trinick 1979). Nodule structures in legumes are diverse, and typically characterised by the initiation of a nodule from pericycle or cortical cells de novo, resulting in a nodule with peripheral vascular strands (Hirsch 1992).
Two different nodule types have been studied in detail. Indeterminate nodules are formed on most temperate legumes, e.g. pea (Pisum sativum L.), clover (Trifolium sp.), alfalfa (Medicago sativa L.) and Barrel medic (Medicago truncatula Gaertn.), and are characterised by nodule initiation in the inner cortex and usually also the pericycle (Timmers et al. 1999). These nodules form a persistent nodule meristem, which allows continuous growth, and leads to the formation of elongated nodules. Determinate nodules are formed on many (sub)tropical plants, including soybean (Glycine max L.), bean (Phaseolus sp.) and Japanese trefoil (Lotus japonicus L.), and are initiated in the outer root cortex by cell enlargement and divisions. Cell divisions are later induced in the pericycle and inner cortex, and both cell division sites merge later on. These nodules are typically spherical because the nodule meristem differentiates (Rolfe and Gresshoff 1988). In some legumes, nodules arise at sites of lateral or adventitious root initiation and this is usually associated with crack entry invasion. For example, in peanut (Arachis hypogaea L.), nodules only arise from cortical cells adjacent to an emerging lateral root (Allen and Allen 1940). A similar pattern of nodule initiation occurs in many species of the Dalbergiae and Aeschynomeneae (Sprent 1989). In white clover (Trifolium repens L.), which usually forms indeterminate nodules after inoculation at the young root hair zone, the most susceptible zone for nodulation, nodules can be induced at sites of lateral root initiation when roots are inoculated in the mature root zone (Mathesius et al. 2000b ). In the aquatic legume Sesbania rostrata L., nodules can arise either de novo from the root cortex and pericycle or from adventitious or lateral root emergence sites. Under well-aerated conditions, root nodulation occurs via infection threads and is strictly dependent on nodulation (Nod) factor structure. Under water-logging conditions, adventitious root-based nodulation occurs, which is less stringent for Nod factor structure and invasion takes place via cracks through the epidermis (Goormachtig et al. 2004). S. rostrata can form both determinate and indeterminate nodules, depending on environmental conditions (Fernández-López et al. 1998). The switch between indeterminate and determinate nodules in Sesbania is likely to be regulated by ethylene (Fernández-López et al. 1998).
Nodulation – innovation by recruitment?
It is not clear what distinguishes plants forming nitrogen fixing symbioses from most other plants that do not form them, but it is likely that the presence of receptor kinases for bacterial Nod factors (lipochitin oligosaccarides) plays a key role in the ability to form symbioses (Spaink 2004; Zhang et al. 2007). The receptors that are necessary for the more ancient (~450 million year-old) symbiosis of mycorrhizal fungi with plants are thought to have been recruited for the more recent bacterial endosymbioses, as the same receptors are required for the interaction of legumes with rhizobia and of actinorhizal plants with Frankia (Gherbi et al. 2008; Markmann et al. 2008). A physiological characteristic of nodulating plants from a range of genera is an altered response to ABA: whereas ABA inhibits lateral root formation in non-nodulating plants, it stimulates their development in nodulating species (Liang and Harris 2005). Therefore, it is possible that changes in hormone response pathways are either a condition for or a consequence of the ability for nodulation.
Recently, it was found that the genomes of certain photosynthetic Bradyrhizobium species which infect the legume Aeschynomene via crack entry on the roots and stems do not contain any genes encoding the canonical Nod factor synthesis enzymes (Giraud et al. 2007). It is possible that nodulation may have started as a process involving infection of roots via crack entry and nodule formation based on a developmental program for lateral roots. Nodulation may have become more specific with the requirement of symbiosis for specific Nod factors, which allowed infection thread invasion (with the possible advantage of better selection of efficient rhizobial symbionts) and de novo formation of a nodule independent of lateral roots (Hirsch and LaRue 1997; Sprent 2007, 2008).
If nodule development has been recruited from lateral root formation, it could be expected that similar developmental signals regulate both processes. A major regulator of lateral root initiation, differentiation and meristem specification is auxin (Casimiro et al. 2003; Fukaki et al. 2007). In particular, auxin patterns in the plant determine subsequent developmental patterns (Heisler et al. 2005). Thus, auxin appears to be a pattern-determining global regulator, as well as a player in cell division, cell elongation and vascular tissue differentiation (Woodward and Bartel 2005; Teale et al. 2006). It has been suggested that auxin is also a regulator of nodule development (Thimann 1936; Hirsch 1992; Hirsch and Fang 1994). There are multiple ways by which symbiotic bacteria could alter root and nodule development through the involvement of auxin: via auxin synthesis by the microsymbiont, or through alteration of auxin synthesis, breakdown, signalling or transport in the host.
Importance of auxin synthesis by the microsymbiont
Auxin is known as a plant hormone and is synthesised by all higher plants (Ljung et al. 2002). The most abundant form of auxin in plants is indole-3-acetic acid (IAA). However, many plant-associated soil bacteria are also known to synthesise auxin, in particular IAA, and this could be part of a strategy to manipulate the growth of host plants (Spaepen et al. 2007). Auxin synthesis has been demonstrated in non-symbiotic plant growth-promoting bacteria (Dobbelaere et al. 1999), in symbiotic nitrogen-fixing cyanobacteria (Sergeeva et al. 2002), in the actinomycete Frankia (Wheeler et al. 1984) and in rhizobia (Kefford et al. 1960). The exudation of various compounds from plants has been shown to stimulate IAA synthesis in bacteria. Most importantly, bacteria are likely to use tryptophan exuded by plant roots as a precursor for auxin synthesis (Kefford et al. 1960). Flavonoids, which are exuded in particular from legume roots to stimulate Nod factor synthesis, have also been shown to stimulate IAA synthesis in Rhizobium sp. (Theunis et al. 2004).
There is evidence that auxin synthesis by bacteria alters root architecture in non-nodulating plants. For example, auxin synthesis by Pantoea (Erwinia) agglomerans pv. gypsophila stimulates the formation of tumours in its plant host Gypsophila paniculata L. (Clark et al. 1993). Auxin synthesis by plant growth-promoting rhizobacteria can partially explain some of the growth-promoting effects that these bacteria have on plants, including stimulation of root growth in wheat by Azospirillum brasilense (Dobbelaere et al. 1999), and stimulation of root elongation in canola by Pseudomonas putida (Xie et al. 1996).
Auxin synthesis in cyanobacteria that associate with cycads and certain Angiosperms was found to be more commonly the case in symbiotic than in free-living species (Sergeeva et al. 2002). It is possible that the auxin synthesised by these cyanobacteria is involved in the activation of mitotic divisions in the infection structures of cycads. Auxin synthesis by actinomycetes that form symbioses with actinorhizal plants could be involved in infection. In Casuarina glauca Sieber, the auxin import protein AUX1 is specifically induced in root cells colonised by Frankia (Peret et al. 2007). The authors of this study suggested that Frankia synthesise IAA which is transported into colonised host cells via AUX1 and that this is a necessary step in plant cell infection. Similarly, the synthesis of IAA by rhizobia can contribute towards successful nodulation in legumes (Kefford et al. 1960). Although the early steps of nodule initiation can be induced by Nod factors alone, synthesis of IAA by rhizobia could be important at later stages of nodulation. Studies with Rhizobium mutants deficient in IAA synthesis have shown that nitrogen fixation can be impaired by a lack of rhizobial auxin, whereas increased nodulation efficiency can be reached with IAA overproducing strains, although this might differ between determinate and indeterminate legumes (Pii et al. 2007). It has been noted that non-legumes can also be stimulated to form nodule-like structures after application of auxin and the resulting structures can be colonised by diazotrophs, including Azospirillum and Rhizobium sp. which appear to infect via crack entry (Christiansen-Weniger 1998). Therefore, it could be hypothesised that auxin production by microsymbionts is a general and maybe ancient mechanism to alter root architecture and induce nodule-like structures in plants. However, in most studies it has not yet been demonstrated whether bacterial mutants deficient in auxin synthesis would also be deficient in symbiosis or other interactions. As discussed below, a more refined strategy of rhizobia to control nodule development is likely to be the indirect manipulation of auxin transport or turnover in the plant host.
Evidence for altered auxin content and distribution in host plants during nodulation
Auxin was first connected with nodulation with the discovery of increased auxin levels in legume nodules (Thimann 1936), and this has subsequently been confirmed in several legumes and actinorhizal plants. To examine spatial and temporal changes in auxin accumulation during nodulation, the auxin responsive promoters GH3 and DR5 have been monitored in legumes forming determinate and indeterminate nodules. In the legume white clover, which forms indeterminate nodules, rhizobia caused a reduction in GH3 activation at and below the site of infection within 10 h (Mathesius et al. 1998b ). This decrease was followed by an increase in expression at the site of nodule initiation ~24 h after inoculation. Similarly, DR5 expression appeared to be interrupted below the site of nodule initiation in M. truncatula, but induced in the forming nodule (Huo et al. 2006). GH3 expression could then be observed in the first dividing pericycle and cortical cells of a forming nodule in white clover (Mathesius et al. 1998b ) and in M. truncatula (van Noorden et al. 2006) (Fig. 1). GH3 expression was high in the early nodule primordium of white clover, but then disappeared from the centre of a differentiating nodule and remained only in the nodule meristem and the vascular bundles (Fig. 1; Mathesius et al. 1998b ). Similarly, high GH3 expression was found in the first dividing outer cortical cells in the determinate legume L. japonicus after Mesorhizobium loti infection (Pacios-Bras et al. 2003). During later stages of determinate nodule development, GH3 expression was similarly present in peripheral vascular tissue and meristematic cells.
Studies of the localisation of GH3 and DR5 reporters during lateral root development in white clover and M. truncatula, respectively, found high expression in early dividing pericycle cells, whereas expression decreased in the forming primordium and was retained only in the apical meristem and central vascular tissue of a differentiated lateral root (Fig. 1) (Mathesius et al. 1998b ; Huo et al. 2006). Studies in Arabidopsis using the DR5 promoter also demonstrated high activity in the first dividing pericycle cells of a lateral root, with disappearing staining in the forming primordium (Benkovà et al. 2003). Therefore, changes in auxin distribution are likely to shape organ development during both nodule and lateral root development.
There are indications that the observed changes in auxin accumulation during nodulation are regulated by the plant upon Nod factor perception and are most likely due to changes in auxin transport. It was observed in several legumes that synthetic auxin transport inhibitors can induce nodules spontaneously in the absence of rhizobia (Allen et al. 1953; Wu et al. 1996), and this was accompanied by similar expression of nodulation genes as in normal nodules (Hirsch et al. 1989). In addition, the reduction of GH3 expression observed during the early stages of indeterminate nodule formation can be mimicked by Nod factors and the synthetic auxin transport inhibitor 1-N-naphthylphalamic acid (NPA) (Mathesius et al. 1998b ). The next section, therefore, examines possible mechanisms of auxin transport regulation in the plant.
How can auxin patterns be altered in the plant?
Auxin synthesis and translocation
Auxin is synthesised mainly in young shoot tissues and distributed from there to other tissues and organs via transport, although most other tissues can also synthesise auxin (Ljung et al. 2002). Auxin can occur as the free, active form, or be conjugated for storage. Tracking of radiolabelled auxin showed that there are a transport of auxin from the shoot to the root tip through the vascular tissue, and a transport in the root from its tip to its elongation zone through epidermal cells (Mitchell and Davies 1975; Tsurumi and Ohwaki 1978). In addition to long distance auxin transport, local transport of auxin along and across tissues is important for auxin localisation in small groups of cells, for example in an emerging lateral root or in the root cap during gravitropism (Jones 1998). Although auxin can be transported within the plant via the phloem from source to sink tissues, polar auxin transport can be regulated specifically by active polar auxin transport (PAT) through auxin transport proteins (Fig. 2).
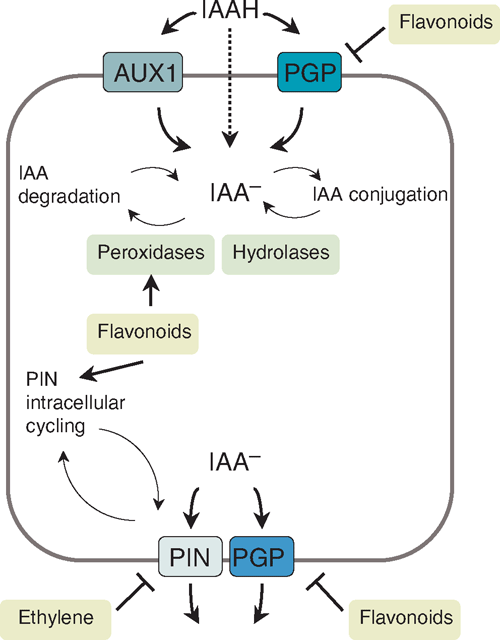
|
Auxin import and export
Auxin is a weak acid; when present in the acidic cell wall environment, it takes a protonated form (IAAH) and can enter cells to a certain degree by diffusion. It can also enter into cells by auxin importers of the amino acid permease families AUX1 (Auxin resistant 1), LAX (like-AUX1) and PGP4, a member of the MDR/PGP (Multidrug resistance/P-glycoprotein) families (Terasaka et al. 2005; Yang et al. 2006). This involves proton symport for AUX1 and ATP-driven uptake for PGPs (Fig. 2). One expression site of AUX1 is in protophloem cells, and AUX1 has, therefore, been suggested to play a role in auxin unloading from the phloem and loading into the PAT system (Swarup et al. 2001).
Because of the higher pH inside the cell, deprotonated auxin (IAA–) cannot diffuse back out of a plant cell; it requires active export (Fig. 2). Auxin is exported by transporters of the PIN (Pin-formed) and PGP families, including PIN1 to PIN7, PGP1 and PGP19 from Arabidopsis (Geisler et al. 2005; Petrasek et al. 2006). In addition to their individual auxin transport activities, it is likely that PIN and PGP form complexes that enhance each other’s activities (Blakeslee et al. 2007). The polarity of auxin transport is established by the polar localisation of PIN proteins on either the basal or apical side of the cell (Wisniewska et al. 2006). Different members of the PIN family are localised in a cell-and developmental-specific pattern, for example PIN1 is localised on the apical side of vascular cells in the root and mediates acropetal auxin flow, whereas PIN2 is localised at the basal side of epidermal cells in the root tip where it mediates basipetal auxin flow. Mutations or mis-expression of PIN genes causes changes in auxin accumulation and plant development (Friml 2003; Vieten et al. 2007).
Regulation of auxin transport proteins
Auxin transport can be altered by the regulation of the activity, localisation, and internalisation of auxin transport proteins. The expression and localisation of PIN proteins are regulated by PINOID, a serine-threonine receptor kinase that can direct PIN proteins to either side of the cell through changes in phosphorylation (Friml et al. 2004). Dynamic cycling of PIN and AUX1 proteins between the plasma membrane and internal vesicles leads to changes in transport protein availability. The cycling of PIN proteins involves transport via actin filaments and is regulated by GNOM, a GDP/GTP exchange factor for small G proteins (Geldner et al. 2003). The internalisation of AUX1 by vesicle cycling is regulated by ARX4 (Auxin resistant 4) but not by GNOM (Dharmasiri et al. 2006), suggesting two independent internalisation mechanisms. Auxin export can be inhibited by synthetic and natural auxin efflux inhibitors (AEIs), including NPA and TIBA (2,3,5-triiodobenzoic acid), which bind to the so-called NPA-binding proteins (NBPs). The NBPs have been suggested to interfere with PIN activity through a possible third protein (Muday and DeLong 2001). Although no NBPs have been identified with certainty, the tir3 mutant of Arabidopsis, which shows reduced NPA binding, is defective in the large protein BIG, which mediates the effect of NPA on PIN trafficking within the cell (Gil et al. 2001). AEIs also affect auxin transport by inhibiting actin dynamics, which are required for PIN cycling (Dhonukshe et al. 2008). In addition, NPA inhibits auxin export by binding to MDR/PGPs (Noh et al. 2001; Murphy et al. 2002; Geisler et al. 2005).
Flavonoids are natural auxin transport regulators
Flavonoids are a class of natural AEIs, some of which can regulate PIN activity and localisation (Peer and Murphy 2007). Flavonoids are synthesised by all plants. They have diverse structures and many functions, e.g. they can act as antioxidants, enzyme regulators, molecular signals for rhizobial nod gene expression, flower pigments, UV protectants and antimicrobials (Winkel-Shirley 2001). Flavonoids with specific structures, especially flavonols, were found to inhibit auxin transport by competing with synthetic AEIs for plasma membrane and microsomal binding sites (Stenlid 1976; Jacobs and Rubery 1988; Bernasconi 1996). Flavonoids are likely to have several targets in plant cells, as they have been shown to interact with PGP auxin transport proteins (Bernasconi 1996) as well as with an aminopeptidase (Murphy and Taiz 1999). The flavonol quercetin enhanced auxin uptake by PGP4 in a heterologous system (Terasaka et al. 2005) and reduced auxin export by PGP1 in a manner similar to that of NPA (Geisler et al. 2005). The action of flavonoids on MDR/PGPs in plants is similar to the modulation of many members of MDR/PGPs by flavonoids in animals (Morris and Zhang 2006). In addition to regulating PGPs, a lack of flavonoids in Arabidopsis altered the expression and localisation of certain PIN proteins, and it was suggested that flavonoids could act by targeting PIN intracellular cycling, at least in the root tip (Peer et al. 2004). However, it is likely that PIN protein localisation is not directly regulated by flavonoids but by auxin localisation itself in a positive feedback loop (Peer et al. 2004). This could be regulated at the level of vesicle cycling as auxin was shown to inhibit internalisation of PIN proteins mediated by BIG, thus, auxin could increase its own transport (Paciorek et al. 2005), a phenomenon known as the ‘canalisation hypothesis’ (Sachs 1981). Auxin was also shown to increase PIN gene expression in a positive feedback loop (Vieten et al. 2005). Studies in flavonoid-deficient Arabidopsis mutants confirmed that these plants had higher rates of auxin transport whereas mutants over-accumulating flavonols show decreased auxin transport rates (Murphy et al. 2000; Brown et al. 2001; Peer et al. 2004). Flavonoids could be an ideal link between auxin transport and the environment because flavonoids are accumulated in response to a variety of environmental stimuli (Buer and Muday 2004; Taylor and Grotewold 2005). The co-localisation of flavonoids at sites of high auxin concentration supports their role in auxin transport control (Murphy et al. 2000; Peer et al. 2001; Buer and Muday 2004; Buer et al. 2006).
Auxin transport and response regulate lateral root development
The polar auxin transport system has been shown to be necessary for setting up plant developmental patterns (Friml 2003) and, not surprisingly, the correct auxin localisation and subsequent auxin response are crucial for lateral root development (Casimiro et al. 2003; De Smet et al. 2006; Fukaki et al. 2007).
Lateral root initiation
Lateral roots usually emerge from pericycle cells opposite xylem poles behind the root differentiation zone. Lateral root initiation is regulated developmentally, leading to an acropetal sequence of lateral root initiation, but environmental influences (e.g. drought, impedence, nutrient availability) can modify this pattern (Dubrovsky et al. 2000). During root development, lateral root initials (‘founder cells’) are probably specified in the root meristem. It is thought that pericycle founder cells of lateral roots remain in a meristematic state after emerging from the root apical meristem, i.e. they remain competent to divide in an otherwise differentiated part of the root. The founder cells, i.e. the pericycle cells opposite xylem poles, are mostly found in the G2 phase of the cell cycle, whereas pericycle cells not forming founder cells are mainly found in the G1 phase (Beeckman et al. 2001; Roudier et al. 2003). Following an asymmetric division, a small primordium forms from a specified number of cell divisions. The primordium later differentiates into different tissue types, after which it emerges from the root and continues to elongate (Fig. 1) (Malamy and Benfey 1997).
The role of auxin transport in founder cell specification
Studies in Arabidopsis have correlated the strict temporal and spatial pattern of lateral root initiation, with an oscillation of auxin activity occurring in two files of protoxylem cells in the root basal meristem, i.e. the zone between the root apical meristem and the elongation zone (De Smet et al. 2007). The source of this oscillation is not known, but could be due to auxin that is recycled by the basal meristem from the root tip through the root cap via AUX1-mediated auxin transport. Pericycle founder cells then require activation through auxin signalling to undergo cell cycling. This activation requires the action of auxin response proteins, especially members of the AUX/IAA (auxin/indole-3-acetic acid) family, which act as repressors of ARFs (auxin response factors), the transcriptional regulators of other auxin responsive genes (Badescu and Napier 2006; Parry and Estelle 2006). Degradation of AUX/IAA proteins occurs through the SCFTIR1 (SKP1, Cullin and F-box protein, in this case TIR1) complex after binding of auxin to its receptor TIR1 (transport inhibitor response 1), and leads to auxin-induced gene expression changes (Fig. 3). Auxin is directly involved in activating the cell cycle during lateral root initiation (Himanen et al. 2002) and the expression of downstream genes (Himanen et al. 2004; Vanneste et al. 2005). Mutants which overproduce auxin, like the Arabidopsis superroot mutant, have increased numbers of lateral roots (Boerjan et al. 1995) and similarly exogenous application of auxin increases lateral root numbers (Wightman et al. 1980; Laskowski et al. 1995). In contrast, mutants resistant to auxin show reduced numbers of lateral roots (De Smet et al. 2006).
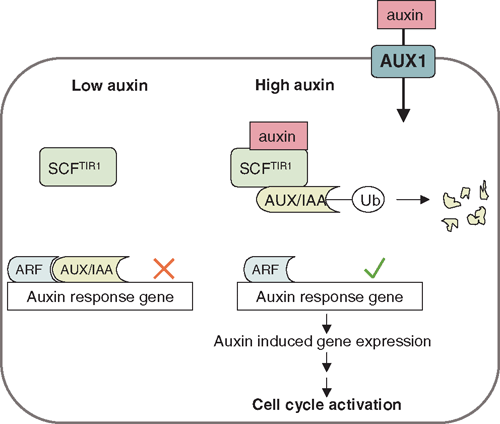
|
Activation of the cell cycle in founder cells by auxin is not sufficient for lateral root initiation, which also requires cell fate re-specification by auxin through the auxin response protein SOLITARY ROOT/IAA14 (Vanneste et al. 2005). NPA application inhibits the induction of lateral roots, and at high (10 μm) concentrations it can block lateral root initiation at the earliest stage of founder pericycle cell division (Casimiro et al. 2001). NPA does not appear to be able to re-specify the identity of the pericycle founder cells as it does not alter the expression of a pericycle marker, nor does it prevent lateral roots from forming when it is applied at the same time as the auxin NAA (Casimiro et al. 2001). Thus, NPA appears to inhibit lateral root initiation by limiting auxin availability in the root. Measurements of IAA levels in roots treated with NPA show that increasing levels of NPA reduce IAA levels, except for the first 3 mm of the root tip where auxin accumulates (Casimiro et al. 2001). This is consistent with the expression pattern of the auxin responsive promoter DR5 in Arabidopsis (Sabatini et al. 1999).
The role of auxin transport in primordium initiation and lateral root emergence
The directional transport of auxin is crucial for lateral root initiation and emergence. Whereas the former requires auxin transport from the root tip into the basal part of the root, the latter is dependent on transport of auxin from the shoot to the root (Reed et al. 1998; Casimiro et al. 2001; Bhalerao et al. 2002). Mutants with reduced auxin transport, for example the pinoid (Benjamin et al. 2001) and tir3 (Ruegger et al. 1997) mutants, are characterised by reduced lateral root numbers. Auxin exporters of the PIN and PGP family are also important for lateral root initiation. Individual PIN genes show overlapping but slightly distinct expression patterns in early lateral root primordia, and altered auxin localisation in lateral root primordia of Arabidopsis pin mutants is correlated with retarded lateral root initiation (Benkovà et al. 2003). GNOM, which is important for correct localisation of PIN, also affects lateral root initiation (Geldner et al. 2004). PIN gene expression is important for the creation of local auxin gradients in the lateral root primordium, and these gradients are likely to regulate cell specification (Vanneste et al. 2005; Vieten et al. 2005). The Arabidopsis mdr1 mutant, which has reduced root acropetal auxin transport, was defective in lateral root elongation but not initiation (Wu et al. 2007). In contrast, decreased auxin uptake in the pgp4 mutant correlated with elevated auxin levels and temporarily increased numbers of lateral roots in young Arabidopsis seedlings (Santelia et al. 2005). Flavonoid-deficient Arabidopsis mutants with increased auxin transport rates have a somewhat increased density of lateral roots (Brown et al. 2001). The auxin importer AUX1 might have a dual role during lateral root development, at least in Arabidopsis. During the initiation phase AUX1 appears to facilitate IAA unloading at the root tip, providing auxin to the initiating lateral roots. During the emergence phase, AUX1 facilitates export of IAA from the shoot and unloading of IAA at the site of a forming lateral root primordium (Marchant et al. 2002).
Auxin response changes during lateral root initiation
In addition to changes in auxin transport direction, the transition from lateral root initiation to lateral root emergence requires an altered auxin response. High auxin levels usually promote the initiation of lateral root primordia, whereas auxin levels need to drop afterwards in the primordium to allow its differentiation and elongation (Wightman et al. 1980; Laskowski et al. 1995). At a later differentiation phase, it gains ‘autonomy’ by synthesising its own auxin (Ljung et al. 2005), and in contrast with that of the primary root, elongation of the lateral root is stimulated by auxin (Muday and Haworth 1994). The changes in auxin response are reflected in the expression patterns of the auxin response gene DR5, which is localised in the earliest dividing pericycle cells and the early lateral root primordium, but disappears from an emerging lateral root, except for expression remaining in the lateral root tip (Benkovà et al. 2003). A microarray analysis confirmed these studies, showing that auxin response genes are activated during the very early cell divisions in a lateral root primordium, and later stages are characterised by downregulation of auxin biosynthesis genes and upregulation of auxin conjugation genes (Vanneste et al. 2005).
Negative regulation of lateral root initiation
Lateral root initiation and development are also under the control of negative regulators. Cytokinins inhibit lateral root initiation at the earliest stage of the asymmetric pericycle cell division, and it has been suggested that cytokinins could interfere with the cell fate re-specification mediated by PIN gene expression, because cytokinins inhibit PIN gene expression during lateral root initiation (Laplaze et al. 2007). In addition, early cell cycle activation in the pericycle is inhibited by cyclin dependent kinase (CDK) inhibitors, i.e. kip-related proteins (KRPs), which are repressed by auxin and are localised in cells not destined for lateral root initiation (Beeckman et al. 2001; Himanen et al. 2002).
How do rhizobia interfere with the root auxin balance?
Similar to lateral root development, the initiation of a nodule requires re-programming of pericycle cells. Unlike lateral root development though, cortical cells inside the root are also re-programmed during nodulation. Both cell types re-activate their cell cycle to form a new meristematic centre of actively dividing cells, although the cortical cells appear to be arrested in the G0 phase of the cell cycle, rather than in G2 or in an active state of cell cycling, as for xylem-pole pericycle cells (Foucher and Kondorosi 2000; Roudier et al. 2003). The group of early dividing cells is called a primordium, which is later invaded by rhizobia. Cells adjacent to the nodule primordium located in the central cortex divide to form the nodule meristem, which gives rise to the nodule parenchyma, vascular traces, vascular endodermis and nodule endodermis. A group of outer cortical cells divides and enlarges to give rise to the nodule cortex, and the nodule base is formed from cell divisions in the pericycle (Hirsch 1992). The centre of the emerging nodule is colonised by rhizobia which differentiate into bacteroids and fix nitrogen. The following section examines the roles of auxin at different stages of nodule development, in the early stage of nodule progenitor cell initiation (founder cell specification), in the stimulation of early cell divisions, in the differentiation of the nodule, and in the systemic regulation of nodule numbers.
Role of auxin in nodule founder cell specification
Nodules are initiated by Nod factors in pericycle and/or cortical cells, usually in front of xylem poles. There is a developmental ‘window’ of susceptibility of root cells to Nod factors that is located in the root elongation and differentiation zone (Bhuvaneswari et al. 1981). In addition, some legumes are susceptible to nodule formation at sites of lateral and adventitious root emergence. It is not exactly known what specifies the nodule founder cells, or what determines the difference in founder cells between legumes that form indeterminate (inner cortex and pericycle) or determinate (initially outer cortex) nodules. Cell division is regulated by two crucial plant hormones that regulate cell cycle progression, auxin and cytokinin (Kondorosi et al. 2005). Both the concentration and the ratio of these two hormones can determine whether and where cells divide in the plant. Experiments with excised root sections have shown that altering the auxin to cytokinin balance specifies whether root cells divide in the pericycle or in the cortex of legumes (Libbenga et al. 1973).
The findings that NPA can induce spontaneous nodules (Hirsch et al. 1989), that rhizobia inhibit GH3 expression within 10 h of inoculation in white clover and that this inhibition is mimicked by Nod factors, NPA and flavonoids (Mathesius et al. 1998b ), suggest that rhizobia inhibit auxin transport in legumes forming indeterminate nodules before the onset of cell divisions (Fig. 4). This is supported by measurements of radio-labelled auxin transport in roots of garden vetch (Vicia sativa L.), which showed that rhizobia, and specifically functional Nod factors, inhibit polar auxin transport within 24 h of inoculation (Boot et al. 1999). Similar inhibition of auxin transport was found in M. truncatula (van Noorden et al. 2006; Wasson et al. 2006). However, no inhibition of auxin transport was detectable in L. japonicus, which forms determinate nodules, before nodule initiation (Pacios-Bras et al. 2003). Known regulators of auxin transport (Fig. 2) are flavonoids (Jacobs and Rubery 1988) and ethylene (Burg and Burg 1966). Both flavonoids (Mathesius et al. 1998a ) and ethylene (Ligero et al. 1987) are induced early during nodulation. Ethylene is a negative regulator of nodulation (Guinel and Geil 2002), and is, thus, not a likely candidate for the early inhibition of auxin transport. Flavonoids are induced specifically in the precursor cells of a nodule after application of nodulating rhizobia or Nod factors (Mathesius et al. 1998a ); they also accumulate after treatment of roots with cytokinin (Mathesius et al. 2000a ). To test if flavonoids are required for auxin transport inhibition by rhizobia, Wasson et al. (2006) silenced the first enzyme of the flavonoid biosynthetic pathway, chalcone synthase, using RNA interference in M. truncatula hairy roots. These flavonoid-deficient roots did not nodulate, and auxin transport inhibition by rhizobia was abolished, confirming that flavonoids are necessary for nodulation and for auxin transport inhibition in M. truncatula (Fig. 4). However, silencing of the isoflavonoid pathway in the legume soybean showed that isoflavonoids are crucial for nodulation as Nod gene inducers, but are unlikely to be required for auxin transport regulation in the development of a determinate nodule (Subramanian et al. 2006). Since no auxin transport inhibition was detectable preceding nodulation in soybean (Subramanian et al. 2006) or in L. japonicus (Pacios-Bras et al. 2003), it is possible that the early auxin transport inhibition is unique to legumes forming indeterminate nodules and required for nodule initiation from the pericycle and inner cortical cells (Wasson et al. 2006; Subramanian et al. 2007).
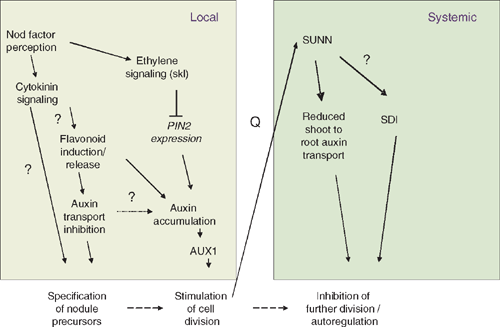
|
What is the effect of inhibiting auxin transport? Most likely, a reduction in auxin transport at the site of nodule initiation would initially reduce the auxin availability and therefore the auxin to cytokinin ratio in the inoculated root zone and root tip. In addition, there is strong evidence that rhizobia induce cytokinin signalling in the root before nodule initiation (Fig. 4) and that this is a required step for cortical cell divisions and for the induction of early nodulins like ENOD40 (Fang and Hirsch 1998; Gonzalez-Rizzo et al. 2006; Murray et al. 2007). The localisation of the cytokinin-inducible ENOD40 expression in pericycle and inner cortical cells several hours before the onset of cell division during nodulation supports a role for cytokinin in specifying the nodule founder cells (Charon et al. 1997; Mathesius et al. 2000a ).
Both inhibition of auxin transport by NPA and the constitutive activity of the cytokinin receptor have been reported to be sufficient to initiate nodule-like structures in the absence of rhizobia (Hirsch et al. 1989; Tirichine et al. 2007). Either of those situations might be extreme when compared with what happens during nodule initiation by rhizobia, and a concomitant inhibition of auxin transport and increased cytokinin synthesis or response might be necessary for determining whether and where a nodule is initiated. So far it is unknown whether cytokinin signalling and auxin transport regulation are functionally linked, but there are indications that cytokinin can alter PIN gene expression (Laplaze et al. 2007) and can cause flavonoid as well as auxin accumulation in dividing cortical cells (Mathesius et al. 2000a ). Future studies are needed to investigate whether the required ratios or sensitivities to auxin and cytokinin differ between legumes forming determinate and indeterminate nodules and specify the site of nodule initiation.
Role of auxin in nodule initiation and differentiation
Auxin transport inhibition in the hours preceding nodule initiation is followed by increased auxin transport and increased GH3::GUS expression in all cell layers at the site of nodule initiation in white clover (Mathesius et al. 1998b ). Increased auxin levels were also found within 24 h of inoculation in bean (Fedorova et al. 2000), and strong induction of two auxin hydrolases, which release active auxin from conjugate forms, was found within 24 h of inoculation in M. truncatula (Campanella et al. 2008). Alternatively, the increase in auxin levels could be the result of the preceding inhibition of auxin export below the site of inoculation, which could cause acropetally-transported auxin to accumulate above that site (Fig. 4). Similar to the case of lateral root initiation, GH3::GUS expression experiments indicate that auxin is localised in the earliest dividing cells of a nodule primordium. In legumes forming indeterminate nodules, including white clover and M. truncatula, expression was localised in the pericycle and inner cortex (Mathesius et al. 1998b ; van Noorden et al. 2007) and in the legume L. japonicus, which forms determinate nodules, expression was localised in the dividing outer cortex (Pacios-Bras et al. 2003). Therefore, in contrast to the requirement for auxin in the specification of founder cells, auxin appears to accumulate similarly in the early dividing cells of legumes forming either determinate or indeterminate nodules, and it is likely that auxin acts to stimulate cell cycle activity (Roudier et al. 2003).
Retention of auxin in dividing cells might be mediated by the spatially overlapping accumulation of flavonoids in the nodule precursor cells and early primordia. Certain flavonoids and other phenolics can inhibit the action of peroxidases and auxin oxidases (Furuya et al. 1962; Grambow and Langenbeck-Schwich 1983), and in white clover, those flavonoids accumulating in the inner cortical cells inhibit auxin breakdown by peroxidase (Mathesius 2001). Consistent with that, flavonoids have been shown to accumulate in outer cortical cells of the legume siratro (Macroptilium purpureum (DC.) Urb.), in which nodules are initiated in the outer cortex (Mathesius et al. 1998a ), although their influence on auxin in legumes forming determinate nodules has not been investigated.
A study whereby the expression of members of the auxin import protein family MtLAX was localised showed that this transporter is strongly expressed in young nodule primordia in M. truncatula (de Billy et al. 2001). Likewise, the expression of the auxin export proteins PIN1 and PIN2 is localised in early nodule primordia in M. truncatula and their silencing by RNAi led to a reduction in nodule numbers (Huo et al. 2006). These studies strongly suggest that auxin transport into the initiating nodule could be responsible for the observed auxin accumulation in the primordium (Fig. 4). Whether or not changes in PIN and LAX gene expression or protein localisation are under the control of the flavonoid changes occurring during nodule initiation is so far unknown.
In a proteomic study comparing root responses to rhizobia with root responses to auxin (IAA) 24 h after each of their application, a high overlap (>80%) of protein changes was found in response to both treatments in M. truncatula, suggesting that increased auxin levels in the root could mediate some or many of the responses of the root to rhizobia (van Noorden et al. 2007). The necessity of auxin action during nodule initiation is supported by the fact that the auxin action inhibitor PCIB (p-chlorophenoxyisobutyric acid) reduces nodule numbers significantly (van Noorden et al. 2006). Auxin action is likely to be optimal only at a certain window of concentration, because increased exogenous auxin levels are known to inhibit nodulation, whereas very low exogenous auxin levels (<10−8 m) stimulate nodulation (van Noorden et al. 2006). Unfortunately, no auxin response mutants have been available yet to test for the role of auxin response during nodulation.
The action of auxin during nodulation is linked with that of other plant hormones, for example cytokinin and gibberellic acid (GA). Cytokinins are likely to be required with auxin to sustain cell divisions in the nodule primordium. The cytokinin sensitive reporter ARR5 (Arabidopsis response regulator 5) was localised to early nodule primordia in L. japonicus (Lohar et al. 2004), and cytokinin-inducible ENOD40 expression was also localised in nodule primordia (Crespi et al. 1994). In addition, cytokinin-insensitive plants are impaired in nodule initiation (Gonzalez-Rizzo et al. 2006; Murray et al. 2007).
The action of auxin during nodule initiation could also be linked to the effects of GA. GA synthesis has been shown to require IAA (Ross et al. 2000), and GA can stimulate IAA synthesis in nodule extracts (Dullaart and Duba 1970). The observations that GA-deficient pea mutants are defective in nodulation (Ferguson et al. 2005) and that GA is required for nodule primordium formation during lateral root-based nodulation in S. rostrata (Lievens et al. 2005) suggest that GA and auxin could act synergistically during primordium formation.
As the nodule primordium differentiates, GH3 expression is retained in peripheral cell layers of the primordium but disappears from the central tissue (Fig. 1). In mature nodules, high GH3 expression is found in vascular tissues and the apical meristem and these expression patterns are similar to those in differentiating lateral roots (Mathesius et al. 1998b ; Pacios-Bras et al. 2003). The expression pattern of MtAUX1 is similar, with high expression in peripheral tissues of a nodule and central tissues of lateral roots, indicating that expression overlaps with regions of vascular tissue or endodermal differentiation in both organs (de Billy et al. 2001). These expression patterns support the role of auxin in vascular differentiation (Aloni et al. 2006) and in nodule meristem maintenance, for example through cell cycle activation (Roudier et al. 2003; Kondorosi et al. 2005). Like in lateral root formation, the expression patterns also suggest that auxin levels must drop at the differentiation stage relative to the primordium initiation phase (Laskowski et al. 1995). The loss of auxin in central parts of legume nodules could be regulated by peroxidases that destroy auxin accumulating inside the nodule (Fedorova et al. 2000; Mathesius 2001).
The cochleata mutant of pea forms hybrid structures of nodules and lateral roots, where the nodule meristem appears to be re-specified into a lateral root meristem (Ferguson and Reid 2005). As the cochleata phenotype also includes agravitropism and its nodules resemble auxin-induced nodule-like structures in non-legumes, the authors of this study suggested that an abnormal auxin response in this mutant could be responsible for the altered nodule meristem phenotype.
The localisation and role of auxin in the initiation of legume nodules appears to differ from that in actinorhizal nodules. Examination of the role of AUX1 in Swamp Oak (C. glauca), which forms nodules with actinorhizal bacteria, showed that expression is localised in infected cells, first in the pre-nodule in cortical cells and later in the nodule (Peret et al. 2007). A possible role of the purported high auxin levels in infected cells could be to mediate cell hypertrophy or cell wall remodelling during infection (Peret et al. 2007). However, expression is absent from nodule primordia, even though the same gene is strongly expressed in lateral root primordia in Casuarina. These data suggest that despite the similarities of actinorhizal nodules to lateral roots, their initiation might require distinct auxin responses. In the actinorhizal plant Eleagnus umbellate Thundb., high levels of an auxin-responsive protein have been found in the nodule fixation zone, although it is not clear if this expression pattern reflects auxin levels (Kim et al. 2007). These two reports suggest that high auxin levels in actinorhizal nodules might be derived from auxin synthesis of the symbiont (Peret et al. 2007). Since no Nod factor-related signal molecules have been identified yet from Frankia, it remains unclear whether all the reported changes in AUX1 expression are due to auxin from the symbiont, or to changes in auxin as a result of signal transduction events in the root.
Role of auxin in the regulation of nodule numbers
Nodule numbers are regulated by several mechanisms. If sufficient nitrogen is available in the growth medium, plants prefer nitrogen uptake from nitrate or ammonium over the costly establishment of a nitrogen-fixing symbiosis. Both nitrate and ammonium inhibit nodulation at different stages of infection, nodule development and nitrogen fixation, although the mechanisms are mostly unknown (Streeter 1988). Whether auxin is involved in this inhibition is unclear. Nitrate regulates lateral root initiation and elongation by both local and systemic mechanisms, and this regulation involves auxin signalling, suggesting that similar mechanisms might be involved in nodulation (Walch-Liu et al. 2006).
The plant also has an internal, systemic regulatory mechanism to limit the numbers of nodules on a root system. This mechanism has been termed autoregulation of nodulation (AON) and is dependent on the action of a leucine-rich repeat receptor-like kinase (LRR-RLK), also termed nodulation autoregulation receptor kinase (NARK) acting in the shoot (Kinkema et al. 2006). After the first few nodules are formed on a root system, autoregulation inhibits further formation of nodules, probably to limit the amount of carbon redirected towards nodules. Split-root experiments have shown that an early event during nodule formation sends a signal (termed Q) to the shoot, where it, or a derivative signal, is perceived by NARK and causes another signal (shoot-derived inhibitor, or SDI) to move back to the root system to limit further nodulation (Fig. 4) (Kinkema et al. 2006). These long-distance signals have so far not been identified. As auxin is known to be a long-distance signal from the shoot to the root, which is important for lateral root formation, the role of shoot-to-root transported auxin was investigated for its role in autoregulation. van Noorden et al. (2006) showed that the M. truncatula autoregulation mutant sunn (super numeric nodules) (Schnabel et al. 2005) transports approximately three times as much auxin from the shoot to the root as the wild type. Auxin concentrations in the zone of the root susceptible for nodule initiation were similarly increased in sunn. In addition, the auxin response gene GH3 was expressed at much higher levels in inoculated sunn roots than in wild-type roots (Penmetsa et al. 2003). Within 24 h of inoculating the root tip with compatible rhizobia, long-distance auxin transport from the shoot to the root was reduced in wild-type seedlings, correlating with the onset of autoregulation in M. truncatula (van Noorden et al. 2006). However, no inhibition of long-distance auxin transport occurred in sunn, suggesting that SUNN regulates long-distance auxin transport changes in response to inoculation. Treatment of the shoot–root junction of sunn with NPA caused a reduction in nodule numbers to levels similar to the untreated wild type (van Noorden et al. 2006). In the model suggested by van Noorden et al. (2006), AON-regulated auxin transport positively correlates with nodule numbers, in contrast with the finding that AON induces a shoot-derived inhibitor that negatively correlates with nodule numbers. So far it is not known whether, in addition to the changes in auxin transport, a separate SDI signal is under the control of SUNN (Fig. 4). It is also unknown whether long-distance auxin transport occurs as part of AON in other legumes, in particular in legumes forming determinate nodules.
In soybean, inoculation of wild-type roots led to increased root auxin content after 48 h, whereas this increase was not detected in the nts382 (nitrate tolerant supernodulation) supernodulation mutant (Caba et al. 2000). It was, therefore, suggested that autoregulation is caused by a burst of auxin in soybean (Gresshoff 1993). Although no long distance auxin transport measurements have been made in legumes with determinate nodules, it is likely that legumes forming determinate and indeterminate nodules might differ in their perception or requirement for auxin in the regulation of nodule numbers.
It is important to note that the long-distance regulation of auxin transport during AON in legumes forming indeterminate nodules is regulated independently of local auxin transport inhibition that occurs at the root tip within hours of inoculation and is necessary for the initiation of the first nodules on the root (Fig. 4). The sunn mutant shows local auxin transport inhibition after inoculation with rhizobia similar to the wild type, despite the difference in long distance transport (van Noorden et al. 2006).
Nodule numbers are also regulated by ethylene, which is demonstrated in the hypernodulation phenotype of the ethylene insensitive skl (sickle) mutant (Penmetsa and Cook 1997). The gene mutated in the skl mutant has been shown to encode an orthologue of the Arabidopsis ethylene signalling protein EIN2 (Penmetsa et al. 2008). The effect of ethylene is local, i.e. ethylene acts in the root, as established from grafting experiments in M. truncatula (Prayitno et al. 2006b ). Ethylene might have several roles, one in the regulation of defence responses that could restrict infection (Penmetsa and Cook 1997; Prayitno et al. 2006a ; Penmetsa et al. 2008) and the other in the regulation of auxin transport. After inoculation, auxin transport inhibition at the root tip still occurred in skl (Prayitno et al. 2006b ), consistent with the requirement of auxin transport inhibition for nodule initiation. Within 24 h, the increase in auxin transport observed in the wild type was exaggerated in skl, and this was accompanied by an increased expression of PIN2 and increased numbers of nodules initiated at the site (Prayitno et al. 2006b ). This suggests that ethylene synthesis or perception could downregulate the auxin accumulation at the site of nodule initiation (Fig. 4). This observation is in accordance with the ability of ethylene or its precursors to inhibit auxin transport (Burg and Burg 1966; Prayitno et al. 2006b ). Ethylene is induced during nodule initiation (Ligero et al. 1986), and could be a signal to limit nodule numbers, as it also negatively influences Nod factor signalling (Sun et al. 2006). Ethylene also affects translocation of auxin from the shoot to the root. Although long-distance auxin transport was normal in uninoculated skl plants, the downregulation of auxin transport observed in wild type 24 h after inoculation with rhizobia did not occur in skl (Prayitno et al. 2006b ). The relatively increased long-distance auxin transport in skl correlates with higher numbers of nodules formed in the root, in a manner similar to the higher long-distance auxin transport in the supernodulating mutant sunn.
The interaction of auxin and ethylene during nodulation is supported by the finding that root growth in sunn was less sensitive to ethylene than in the wild type (Penmetsa et al. 2003). Ethylene inhibits root growth via effects on auxin (Stepanova et al. 2007). If auxin is already at super-optimal levels for root growth in sunn, it is possible that ethylene has a relatively reduced effect on inhibition of root growth in sunn.
Differences and similarities between lateral root and nodule development
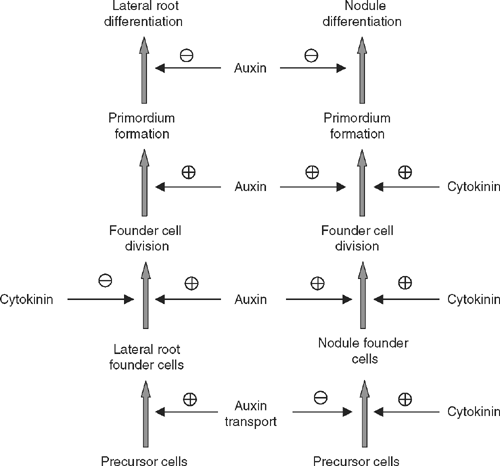
Despite the clear similarities between the development of lateral roots and nodules, in most legumes these organs are distinct. Lateral roots are pre-specified during plant development; they arise from pericycle cells and form a central stele. Nodules in many legumes are initiated de novo at unspecified times during plant development; they arise from both pericycle and cortical cells, and typically have peripheral vascular strands. The data discussed above suggest that the major difference lies in the specification of the founder cells of these organs, whereas their development might be regulated similarly (Fig. 5). The separate specification of the founder cells is supported by different requirements for auxin and cytokinin. Whereas high auxin concentrations increase the numbers of lateral roots formed, high auxin concentrations inhibit the formation of nodules (van Noorden et al. 2006). Cytokinin has the opposite effect, and inhibits lateral root formation but increases nodule numbers (Lohar et al. 2004), and can lead to spontaneous nodule formation (Gonzalez-Rizzo et al. 2006). Lateral roots and nodules also differ in their requirement for flavonoids. Whereas nodule initiation requires the presence of flavonoids, presumably because of the temporary action of flavonoids in inhibiting auxin transport, flavonoid-deficient M. truncatula plants still form lateral roots (Wasson et al. 2006) and lateral root numbers are slightly increased in Arabidopsis, possibly due to higher auxin transport in flavonoid-deficient mutants (Brown et al. 2001). In addition, distinct flavonoids mark the precursor cells of lateral roots and nodules (Mathesius et al. 1998a ; Morris and Djordjevic 2006).
|
It might, therefore, seem surprising that despite the differences in organ initiation, several studies have shown a genetic link between the numbers of nodules and lateral roots formed on a legume root system. This was first studied by Nutman, who observed that different cultivars of red clover showed a positive correlation between the number of lateral roots and nodules (Nutman 1948). One of the determinants of lateral root and nodule numbers could be certain plant hormones. For example, in pea mutants deficient in GA and brassinosteroids, nodule numbers were reduced, in concert with reduced lateral root numbers (Ferguson et al. 2005). Another shared determinant of lateral root and nodule numbers appears to be the autoregulation gene. The autoregulation mutants nts of soybean (Searle et al. 2003), har1 (hypernodulation aberrant root) of L. japonicus (Wopereis et al. 2000) and sunn of M. truncatula (van Noorden 2006) form more lateral roots than the wild type in the absence of rhizobia. However, in these studies inoculation led to a significant reduction of lateral root numbers of the root system, although this can be transient and total numbers of lateral roots can be increased in nodulated mature root systems. Therefore, it is possible that the plant regulates the total number of lateral root organs on a root system systemically, perhaps through an autoregulatory system related to the AON mechanisms. The systemic regulation might be acting independently of the local specification of organ founder cells. This could simply reflect a system to balance resource availability in the whole root system, and it would be interesting to test whether the long-distance transport of auxin could determine resource allocation from the shoot to the root.
A model for the role of auxin in nodulation
A hypothesis suggested by the sum of the data discussed above is that the requirement of lowering the auxin to cytokinin ratio is a specific step in the initiation of nodules. This is opposite to the initiation of lateral roots, which is promoted by high auxin to cytokinin ratios. Both the reduction of auxin transport by Nod factors and the induction of cytokinin signalling, which precede nodule initiation, could be crucial steps in the specification of nodule precursor cells in legumes forming indeterminate nodules (Fig. 5).
Once the organ is specified, it is likely that auxin plays similar roles in activating cell cycle activity in both types of primordia. A drop in auxin levels or response might be a shared requirement for the subsequent organ differentiation, although auxin remains a positive regulator for vascular differentiation and ongoing meristem activity (Fig. 5). This is supported by the similar patterns of auxin accumulation during lateral root and nodule formation after founder cell specification (Fig. 1). Independent of the local role of auxin in nodule initiation, long distance auxin transport is a mechanism that has been shown to control both lateral root and nodule numbers. Another shared aspect of lateral root and nodule differentiation is that the emergence of the organs, i.e. the activation of the meristem, requires the action of the same gene, LATD, which regulates ABA response in the root (Bright et al. 2005; Liang et al. 2007).
Future directions
Key questions about the role of auxin in nodulation remain; specifically, the differences in auxin requirements in founder cell specification in determinate and indeterminate nodule types, the mechanism of auxin transport regulation by the autoregulation of nodulation process in indeterminate nodulation, as well as the interaction between auxin and cytokinin signalling during nodule initiation. The biggest impact in our knowledge of lateral root regulation by auxin has been through the analysis of various auxin mutants of Arabidopsis. Such mutants are currently lacking in legumes, but would be very useful for future research. For example, if autoregulation mutants could be rescued by mutations in the auxin transport machinery, this would support a link between auxin transport and nodule number regulation. Auxin response and auxin transport mutants of legumes forming determinate and indeterminate nodules could be used to test whether different auxin responsiveness is required for these two nodule types. Alternatively, cell-specific silencing of auxin response genes in inner and outer cortical cells could be expected to selectively inhibit the initiation of indeterminate or determinate nodule types. Advances in our understanding of auxin and cytokinin interactions could be made by studying auxin responses in cytokinin-insensitive mutants during nodulation. These and other genetic studies are likely to happen in some of the legume model species (Smit and Bisseling 2008). However, a wealth of information could be gained from the analysis of non-model legumes with a variety of nodule organogenesis programs that range from modified lateral roots to de novo formed nodules (Hirsch et al. 2001; Sprent and James 2008).
Acknowledgements
I am grateful for the encouragement, discussion and collaboration of past and present laboratory members and colleagues involved in the presented research, in particular Giel van Noorden, Anton Wasson, Flavia Pellerone, Karsten Oelkers, Joko Prayitno, Tursun Kerim, Melinda Aprelia, Robert Wiblin, Alexander Ivakov, Julia Frugoli, Brett Ferguson, John Ross, Jim Reid, Peter Gresshoff, Christine Beveridge, Michael Djordjevic and Barry Rolfe. I would also like to thank the reviewers for many constructive comments and the Australian Research Council for funding through an Australian Research Fellowship (DP0557692) and through the ARC Centre of Excellence for Integrative Legume Research (CE0348212). A special ‘thank you’ to the Australian Society of Plant Scientists and Functional Plant Biology for their encouragement through the Goldacre award.
Allen EK,
Allen ON, Newman AS
(1953) Pseudonodulation of leguminous plants induced by 2-bromo-3,5-dichlorobenzoic acid. American Journal of Botany 40, 429–435.
| Crossref | GoogleScholarGoogle Scholar |

Allen ON, Allen EK
(1940) Response of the peanut plant to inoculation with rhizobia, with special reference to morphological development of the nodules. Botanical Gazette 102, 121–142.
| Crossref | GoogleScholarGoogle Scholar |

Aloni R,
Aloni E,
Langhans M, Ullrich CI
(2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany 97, 883–893.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Badescu GO, Napier RM
(2006) Receptors for auxin: will it all end in TIRs? Trends in Plant Science 11, 217–223.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Beeckman T,
Burssens S, Inzé D
(2001) The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany 52, 403–411.
| PubMed |

Benjamins R,
Quint A,
Weijers D,
Hooykaas P, Offringa R
(2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128, 4057–4067.
| PubMed |

Benkovà E,
Michniewicz M,
Sauer M,
Teichmann T,
Seifertova D,
Jürgens G, Friml J
(2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bernasconi P
(1996) Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiologia Plantarum 96, 205–210.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Bhalerao RP,
Eklof J,
Ljung K,
Marchant A,
Bennett M, Sandberg G
(2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal 29, 325–332.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bhuvaneswari TV,
Bhagwat AA, Bauer WD
(1981) Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiology 68, 1144–1149.
| PubMed |

Blakeslee JJ,
Bandyopadhyay A,
Lee OR,
Mravec J, Titapiwatanakun B ,
et al
.
(2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. The Plant Cell 19, 131–147.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boerjan W,
Cervera MT,
Delarue M,
Beeckman T,
Dewitte W,
Bellini C,
Caboche M,
Van Onckelen H,
van Montagu M, Inzé D
(1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. The Plant Cell 7, 1405–1419.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boot KJM,
van Brussel AAN,
Tak T,
Spaink HP, Kijne JW
(1999) Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Molecular Plant-Microbe Interactions 12, 839–844.
| Crossref | GoogleScholarGoogle Scholar |

Bright LJ,
Liang Y,
Mitchell DM, Harris JM
(2005) The LATD gene of Medicago truncatula is required for both nodule and root development. Molecular Plant-Microbe Interactions 18, 521–532.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brown DE,
Rashotte AM,
Murphy AS,
Normanly J,
Tague BW,
Peer WA,
Taiz L, Muday GK
(2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiology 126, 524–535.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Buer CS, Muday GK
(2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. The Plant Cell 16, 1191–1205.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Buer CS,
Sukumar P, Muday GK
(2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiology 140, 1384–1396.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Burg SP, Burg EA
(1966) The interaction between auxin and ethylene and its role in plant growth. Proceedings of the National Academy of Sciences of the United States of America 55, 262–269.
|
CAS |
Crossref |
PubMed |

Caba JM,
Centeno ML,
Fernandez B,
Gresshoff PM, Ligero F
(2000) Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta 211, 98–104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Campanella JJ,
Smith SM,
Leibu D,
Wexler S, Ludwig-Müller J
(2008) The auxin conjugate hydrolase family of Medicago truncatula and their expression during the interaction with two symbionts. Journal of Plant Growth Regulation 27, 26–38.
| Crossref | GoogleScholarGoogle Scholar |

Casimiro I,
Marchant A,
Bhalerao RP,
Beeckman T, Dhooge S ,
et al
.
(2001) Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell 13, 843–852.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Casimiro I,
Beeckman T,
Graham N,
Bhalerao R,
Zhang HM,
Casero P,
Sandberg G, Bennett MJ
(2003) Dissecting Arabidopsis lateral root development. Trends in Plant Science 8, 165–171.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Charon C,
Johansson C,
Kondorosi E,
Kondorosi A, Crespi M
(1997)
enod40 induces dedifferentiation and division of root cortical cells in legumes. Proceedings of the National Academy of Sciences of the United States of America 94, 8901–8906.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Christiansen-Weniger C
(1998) Endophytic establishment of diazotrophic bacteria in auxin-induced tumors of cereal crops. Critical Reviews in Plant Sciences 17, 55–76.
| Crossref | GoogleScholarGoogle Scholar |

Clark E,
Manulis S,
Ophir Y,
Barash I, Gafni Y
(1993) Cloning and characterization of IAAM and IAAH from Erwinia herbicola pathovar gypsophilae. Phytopathology 83, 234–240.
| Crossref | GoogleScholarGoogle Scholar |

Crespi MD,
Jurkevitch E,
Poiret M,
d’Aubenton-Carafa Y,
Petrovics G,
Kondorosi E, Kondorosi A
(1994)
ENOD40, a gene expressed during nodule organogenesis, codes for a non-translatable RNA involved in plant growth. EMBO Journal 13, 5099–5122.
| PubMed |

de Billy F,
Grosjean C,
May S,
Bennett M, Cullimore JV
(2001) Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Molecular Plant-Microbe Interactions 14, 267–277.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

De Smet I,
Vanneste S,
Inzé D, Beeckman T
(2006) Lateral root initiation or the birth of a new meristem. Plant Molecular Biology 60, 871–887.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

De Smet I,
Tetsumura T,
De Rybel B,
Frey NFD, Laplaze L ,
et al
.
(2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134, 681–690.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dharmasiri S,
Swarup R,
Mockaitis K,
Dharmasiri N, Singh SK ,
et al
.
(2006) AXR4 is required for localization of the auxin influx facilitator AUX1. Science 312, 1218–1220.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dhonukshe P,
Grigoriev I,
Fischere R,
Tominaga M, Robinson DG ,
et al
.
(2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proceedings of the National Academy of Sciences of the United States of America 105, 4489–4494.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dobbelaere S,
Croonenborghs A,
Thys A,
Vande Broek A, Vanderleyden J
(1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant and Soil 212, 153–162.
| Crossref | GoogleScholarGoogle Scholar |

Dubrovsky JG,
Doerner PW,
Colon-Carmona A, Rost TL
(2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology 124, 1648–1657.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dullaart J, Duba LI
(1970) Presence of gibberellin-like substances and their possible role in auxin bioproduction in root nodules and roots of Lupinus luteus L. Acta Botanica Neerlandica 19, 877–883.

Fang YW, Hirsch AM
(1998) Studying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa. Plant Physiology 116, 53–68.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fedorova EE,
Zhiznevskaya GY,
Kalibernaya ZV,
Artemenko EN,
Izmailov SF, Gus’kov AV
(2000) IAA metabolism during development of symbiosis between Phaseolus vulgaris and Rhizobium phaseoli.
Russian Journal of Plant Physiology: a Comprehensive Russian Journal on Modern Phytophysiology 47, 203–206.

Ferguson BJ, Reid JB
(2005) Cochleata: getting to the root of legume nodules. Plant & Cell Physiology 46, 1583–1589.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ferguson BJ,
Ross JJ, Reid JB
(2005) Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiology 138, 2396–2405.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Foucher F, Kondorosi E
(2000) Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Molecular Biology 43, 773–786.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friml J
(2003) Auxin transport – shaping the plant. Current Opinion in Plant Biology 6, 7–12.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Friml J,
Yang X,
Michniewicz M,
Weijers D, Quint A ,
et al
.
(2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306, 862–865.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fukaki H,
Okushima Y, Tasaka M
(2007) Auxin-mediated lateral root formation in higher plants. International Review of Cytology – a Survey of Cell Biology 256, 111–137.

Furuya M,
Garlston AW, Stowe BB
(1962) Isolation from peas of co-factors and inhibitors of indolyl-3-acetic acid oxidase. Nature 193, 456–457.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Geisler M,
Blakeslee JJ,
Bouchard R,
Lee OR, Vincenzetti V ,
et al
.
(2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. The Plant Journal 44, 179–194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Geldner N,
Anders N,
Wolters H,
Keicher J,
Kornberger W,
Müller P,
Delbarre A,
Ueda T,
Nakano A, Jürgens G
(2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112, 219–230.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Geldner N,
Richter S,
Vieten A,
Marquardt S,
Torres-Ruiz RA,
Mayer U, Jürgens G
(2004) Partial loss-of-function alleles reveal a role for GNOM in auxin trans port-related, post-embryonic development of Arabidopsis. Development 131, 389–400.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gherbi H,
Markmann K,
Svistoonoff S,
Estevan J, Autran D ,
et al
.
(2008) SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proceedings of the National Academy of Sciences of the United States of America 105, 4928–4932.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gil P,
Dewey E,
Friml J,
Zhao Y,
Snowden KC,
Putterill J,
Palme K,
Estelle M, Chory J
(2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes & Development 15, 1985–1997.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Giraud E,
Moulin L,
Vallenet D,
Barbe V, Cytryn E ,
et al
.
(2007) Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316, 1307–1312.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gonzalez-Rizzo S,
Crespi M, Frugier F
(2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti.
The Plant Cell 18, 2680–2693.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Goormachtig S,
Capoen W, Holsters M
(2004) Rhizobium infection: lessons from the versatile nodulation behaviour of water-tolerant legumes. Trends in Plant Science 9, 518–522.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Grambow HJ, Langenbeck-Schwich B
(1983) The relationship between oxidase activity, peroxidase activity, hydrogen peroxide, and phenolic compounds in the degradation of indole-3-acetic acid in vitro. Planta 157, 132–137.
| Crossref | GoogleScholarGoogle Scholar |

Gualtieri G, Bisseling T
(2000) The evolution of nodulation. Plant Molecular Biology 42, 181–194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guinel FC, Geil RD
(2002) A model for the development of the rhizobial and arbuscular mycorrhizal symbioses in legumes and its use to understand the roles of ethylene in the establishment of these two symbioses. Canadian Journal of Botany – Revue Canadienne de Botanique 80, 695–720.
| Crossref | GoogleScholarGoogle Scholar |

Heisler MG,
Ohno C,
Das P,
Sieber P,
Reddy GV,
Long JA, Meyerowitz EM
(2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current Biology 15, 1899–1911.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Himanen K,
Boucheron E,
Vanneste S,
Engler JD,
Inzé D, Beeckman T
(2002) Auxin-mediated cell cycle activation during early lateral root initiation. The Plant Cell 14, 2339–2351.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Himanen K,
Vuylsteke M,
Vanneste S,
Vercruysse S,
Boucheron E,
Alard P,
Chriqui D,
van Montagu M,
Inzé D, Beeckman T
(2004) Transcript profiling of early lateral root initiation. Proceedings of the National Academy of Sciences of the United States of America 101, 5146–5151.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirsch AM
(1992) Developmental biology of legume nodulation. New Phytologist 122, 211–237.
| Crossref | GoogleScholarGoogle Scholar |

Hirsch AM, Fang YW
(1994) Plant hormones and nodulation – what’s the connection. Plant Molecular Biology 26, 5–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirsch AM, LaRue T
(1997) Is the legume nodule a modified root, stem or an organ sui generis? Critical Reviews in Plant Sciences 16, 361–392.
| Crossref | GoogleScholarGoogle Scholar |

Hirsch AM,
Bhuvaneswari TV,
Torrey JG, Bisseling T
(1989) Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proceedings of the National Academy of Sciences of the United States of America 86, 1244–1248.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirsch AM,
Lum MR, Downie JA
(2001) What makes the rhizobia–legume symbiosis so special? Plant Physiology 127, 1484–1492.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huo XY,
Schnabel E,
Hughes K, Frugoli J
(2006) RNAi phenotypes and the localization of a protein: GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. Journal of Plant Growth Regulation 25, 156–165.
| Crossref | GoogleScholarGoogle Scholar |

Jacobs M, Rubery PH
(1988) Naturally occurring auxin transport regulators. Science 241, 346–349.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jones AM
(1998) Auxin transport: down and out and up again. Science 282, 2201–2202.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kefford NP,
Brockwell J, Zwar JA
(1960) The symbiotic synthesis of auxin by legumes and nodule bacteria and its role in nodule development. Australian Journal of Biological Sciences 13, 456–467.

Kim HB,
Lee H,
Oh CJ,
Lee NH, An CS
(2007) Expression of EuNOD-ARP1 encoding auxin-repressed protein homolog is upregulated by auxin and localized to the fixation zone in root nodules of Elaeagnus umbellata.
Molecules and Cells 23, 115–121.
| PubMed |

Kinkema M,
Scott PT, Gresshoff PM
(2006) Legume nodulation: successful symbiosis through short- and long-distance signalling. Functional Plant Biology 33, 707–721.
| Crossref | GoogleScholarGoogle Scholar |

Kondorosi E,
Redondo-Nieto M, Kondorosi A
(2005) Ubiquitin-mediated proteolysis. To be in the right place at the right moment during nodule development. Plant Physiology 137, 1197–1204.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Laplaze L,
Benkovà E,
Casimiro I,
Maes L, Vanneste S ,
et al
.
(2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell 19, 3889–3900.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Laskowski MJ,
Williams ME,
Nusbaum HC, Sussex IM
(1995) Formation of lateral root meristems is a two-stage process. Development 121, 3303–3310.
| PubMed |

Liang Y, Harris JM
(2005) Response of root branching to abscisic acid is correlated with nodule formation both in legumes and nonlegumes. American Journal of Botany 92, 1675–1683.
| Crossref | GoogleScholarGoogle Scholar |

Liang Y,
Mitchell DM, Harris JM
(2007) Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Developmental Biology 304, 297–307.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Libbenga KR,
van Iren F,
Bogers RJ, Schraag-Lamers MF
(1973) The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 114, 29–39.
| Crossref | GoogleScholarGoogle Scholar |

Lievens S,
Goormachtig S,
Den Herder J,
Capoen W,
Mathis R,
Hedden P, Holsters M
(2005) Gibberellins are involved in nodulation of Sesbania rostrata.
Plant Physiology 139, 1366–1379.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ligero F,
Lluch C, Olivares J
(1986) Evolution of ethylene from roots of Medicago sativa plants inoculated with Rhizobium meliloti.
Journal of Plant Physiology 125, 361–365.

Ligero F,
Lluch C, Olivares J
(1987) Evolution of ethylene from roots and nodulation rate of alfalfa (Medicago sativa L.) plants inoculated with Rhizobium meliloti as affected by the presence of nitrate. Journal of Plant Physiology 129, 461–467.

Ljung K,
Hull AK,
Kowalczyk M,
Marchant A,
Celenza J,
Cohen JD, Sandberg G
(2002) Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana.
Plant Molecular Biology 49, 249–272.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ljung K,
Hull AK,
Celenza J,
Yamada M,
Estelle M,
Nonmanly J, Sandberg G
(2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. The Plant Cell 17, 1090–1104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lohar DP,
Schaff JE,
Laskey JG,
Kieber JJ,
Bilyeu KD, Bird DM
(2004) Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. The Plant Journal 38, 203–214.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Malamy JE, Benfey PN
(1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana.
Development 124, 33–44.
| PubMed |

Marchant A,
Bhalerao R,
Casimiro I,
Eklof J,
Casero PJ,
Bennett M, Sandberg G
(2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell 14, 589–597.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Markmann K,
Giczey G, Parniske M
(2008) Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biology 6, e68.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mathesius U
(2001) Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. Journal of Experimental Botany 52, 419–426.
|
CAS |
PubMed |

Mathesius U,
Bayliss C,
Weinman JJ,
Schlaman HRM,
Spaink HP,
Rolfe BG,
McCully ME, Djordjevic MA
(1998a) Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Molecular Plant-Microbe Interactions 11, 1223–1232.
| Crossref | GoogleScholarGoogle Scholar |

Mathesius U,
Schlaman HRM,
Spaink HP,
Sautter C,
Rolfe BG, Djordjevic MA
(1998b) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. The Plant Journal 14, 23–34.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mathesius U,
Charon C,
Rolfe BG,
Kondorosi A, Crespi M
(2000a) Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Molecular Plant-Microbe Interactions 13, 617–628.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mathesius U,
Weinman JJ,
Rolfe BG, Djordjevic MA
(2000b) Rhizobia can induce nodules in white clover by ‘hijacking’ mature cortical cells activated during lateral root development. Molecular Plant-Microbe Interactions 13, 170–182.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mitchell EK, Davies PJ
(1975) Evidence for three different systems of movement of indoleacetic-acid in intact roots of Phaseolus coccineus.
Physiologia Plantarum 33, 290–294.
| Crossref | GoogleScholarGoogle Scholar |

Morris AC, Djordjevic MA
(2006) The Rhizobium leguminosarum biovar trifolii ANU794 induces novel developmental responses on the subterranean clover cultivar Woogenellup. Molecular Plant-Microbe Interactions 19, 471–479.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Morris ME, Zhang SZ
(2006) Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sciences 78, 2116–2130.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Muday GK, DeLong A
(2001) Polar auxin transport: controlling where and how much. Trends in Plant Science 6, 535–542.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Muday GK, Haworth P
(1994) Tomato root-growth, gravitropism, and lateral development – correlation with auxin transport. Plant Physiology and Biochemistry 32, 193–203.
| PubMed |

Murphy A, Taiz L
(1999) Naphthylphthalamic acid is enzymatically hydrolyzed at the hypocotyl-root transition zone and other tissues of Arabidopsis thaliana seedlings. Plant Physiology and Biochemistry 37, 413–430.
| Crossref | GoogleScholarGoogle Scholar |

Murphy A,
Peer WA, Taiz L
(2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211, 315–324.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Murphy AS,
Hoogner KR,
Peer WA, Taiz L
(2002) Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiology 128, 935–950.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Murray JD,
Karas BJ,
Sato S,
Tabata S,
Amyot L, Szczyglowski K
(2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Noh B,
Murphy AS, Spalding EP
(2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. The Plant Cell 13, 2441–2454.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

van Noorden GE,
Kerim T,
Goffard N,
Wiblin R,
Pellerone FI,
Rolfe BG, Mathesius U
(2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti.
Plant Physiology 144, 1115–1131.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

van Noorden GE,
Ross JJ,
Reid JB,
Rolfe BG, Mathesius U
(2006) Defective long distance auxin transport regulation in the Medicago truncatula super numerary nodules mutant. Plant Physiology 140, 1494–1506.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nutman PS
(1948) Physiological studies on nodule formation. I. The relation between nodulation and lateral root formation in red clover. Annals of Botany 12, 81–96.

Paciorek T,
Zazimalova E,
Ruthardt N,
Petrasek J, Stierhof YD ,
et al
.
(2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pacios-Bras C,
Schlaman HRM,
Boot K,
Admiraal P,
Langerak JM,
Stougaard J, Spaink HP
(2003) Auxin distribution in Lotus japonicus during root nodule development. Plant Molecular Biology 52, 1169–1180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parry G, Estelle M
(2006) Auxin receptors: a new role for F-box proteins. Current Opinion in Cell Biology 18, 152–156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pawlowski K, Bisseling T
(1996) Rhizobial and actinorhizal symbioses: what are the shared features? The Plant Cell 8, 1899–1913.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peer WA, Murphy AS
(2007) Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science 12, 556–563.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peer WA,
Brown DE,
Tague BW,
Muday GK,
Taiz L, Murphy AS
(2001) Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiology 126, 536–548.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peer WA,
Bandyopadhyay A,
Blakeslee JJ,
Makam SI,
Chen RJ,
Masson PH, Murphy AS
(2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana.
The Plant Cell 16, 1898–1911.
| Crossref | GoogleScholarGoogle Scholar |

Penmetsa RV, Cook DR
(1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Penmetsa RV,
Frugoli JA,
Smith LS,
Long SR, Cook DR
(2003) Dual genetic pathways controlling nodule number in Medicago truncatula.
Plant Physiology 131, 998–1008.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Penmetsa RV,
Uribe P,
Anderson J,
Lichtenzveig J, Gish J-C ,
et al
.
(2008) The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. The Plant Journal 55, 580–595.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peret B,
Swarup R,
Jansen L,
Devos G, Auguy F ,
et al
.
(2007) Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca.
Plant Physiology 144, 1852–1862.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Petrasek J,
Mravec J,
Bouchard R,
Blakeslee JJ, Abas M ,
et al
.
(2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pii Y,
Crimi M,
Cremonese G,
Spena A, Pandolfini T
(2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biology 7, 21.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Prayitno J,
Imin N,
Rolfe BG, Mathesius U
(2006a) Identification of ethylene-mediated protein changes during nodulation in Medicago truncatula using proteome analysis. Journal of Proteome Research 5, 3084–3095.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Prayitno J,
Rolfe BG, Mathesius U
(2006b) The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiology 142, 168–180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Raven JA, Edwards D
(2001) Roots: evolutionary origins and biogeochemical significance. Journal of Experimental Botany 52, 381–401.
| PubMed |

Reed RC,
Brady SR, Muday GK
(1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiology 118, 1369–1378.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rolfe BG, Gresshoff PM
(1988) Genetic analysis of legume nodule initiation. Annual Review of Plant Physiology and Plant Molecular Biology 39, 297–319.

Ross JJ,
O’Neill DP,
Smith JJ,
Kerckhoffs LHJ, Elliott RC
(2000) Evidence that auxin promotes gibberellin A(1) biosynthesis in pea. The Plant Journal 21, 547–552.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Roudier F,
Fedorova E,
Lebris M,
Lecomte P,
Gyorgyey J,
Vaubert D,
Horvath G,
Abad P,
Kondorosi A, Kondorosi E
(2003) The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiology 131, 1091–1103.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ruegger M,
Dewey E,
Hobbie L,
Brown D,
Bernasconi P,
Turner J,
Muday G, Estelle M
(1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. The Plant Cell 9, 745–757.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sabatini S,
Beis D,
Wolkenfelt H,
Murfett J, Guilfoyle T ,
et al
.
(1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sachs T
(1981) The control of the patterned differentiation of vascular tissues. Advances in Botanical Research 9, 151–262.
| Crossref |

Santelia D,
Vincenzetti V,
Azzarello E,
Bovet L,
Fukao Y,
Duchtig P,
Mancuso S,
Martinoia E, Geisler M
(2005) MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Letters 579, 5399–5406.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schnabel E,
Journet EP,
de Carvalho-Niebel F,
Duc G, Frugoli J
(2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Molecular Biology 58, 809–822.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Searle IR,
Men AE,
Laniya TS,
Buzas DM,
Iturbe-Ormaetxe I,
Carroll BJ, Gresshoff PM
(2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299, 109–112.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sergeeva E,
Liaimer A, Bergman B
(2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215, 229–238.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Soltis DE,
Soltis PS,
Morgan DR,
Swensen SM,
Mullin BC,
Dowd JM, Martin PG
(1995) Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen-fixation in angiosperms. Proceedings of the National Academy of Sciences of the United States of America 92, 2647–2651.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Spaepen S,
Vanderleyden J, Remans R
(2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiology Reviews 31, 425–448.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Spaink HP
(2004) Specific recognition of bacteria by plant LysM domain receptor kinases. Trends in Microbiology 12, 201–204.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sprent JI
(1989) Which steps are essential for the formation of functional legume nodules? New Phytologist 111, 129–153.
| Crossref | GoogleScholarGoogle Scholar |

Sprent JI
(2007) Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytologist 174, 11–25.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sprent JI, James EK
(2008) Legume–rhizobial symbiosis: an anorexic model? New Phytologist 179, 3–5.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Stenlid G
(1976) Effects of flavonoids on the polar transport of auxins. Physiologia Plantarum 38, 262–266.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Stepanova AN,
Yun J,
Likhacheva AV, Alonso JM
(2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell 19, 2169–2185.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Streeter J
(1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Critical Reviews in Plant Sciences 7, 1–23.
|
CAS |

Subramanian S,
Stacey G, Yu O
(2006) Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum.
The Plant Journal 48, 261–273.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Subramanian S,
Stacey G, Yu O
(2007) Distinct, crucial roles of flavonoids during legume nodulation. Trends in Plant Science 12, 282–285.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sun JH,
Cardoza V,
Mitchell DM,
Bright L,
Oldroyd G, Harris JM
(2006) Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. The Plant Journal 46, 961–970.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Swarup R,
Friml J,
Marchant A,
Ljung K,
Sandberg G,
Palme K, Bennett M
(2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes & Development 15, 2648–2653.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Taylor LP, Grotewold E
(2005) Flavonoids as developmental regulators. Current Opinion in Plant Biology 8, 317–323.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Teale WD,
Paponov IA, Palme K
(2006) Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews. Molecular Cell Biology 7, 847–859.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Terasaka K,
Blakeslee JJ,
Titapiwatanakun B,
Peer WA, Bandyopadhyay A ,
et al
.
(2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. The Plant Cell 17, 2922–2939.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Theunis M,
Kobayashi H,
Broughton WJ, Prinsen E
(2004) Flavonoids, NodD1, NodD2, and nod-box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Molecular Plant-Microbe Interactions 17, 1153–1161.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thimann KV
(1936) On the physiology of the formation of nodules on legume roots. Proceedings of the National Academy of Sciences of the United States of America 22, 511–514.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Timmers ACJ,
Auriac MC, Truchet G
(1999) Refined analysis of early symbiotic steps of the Rhizobium–Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126, 3617–3628.
| PubMed |

Tirichine L,
Sandal N,
Madsen LH,
Radutoiu S,
Albrektsen AS,
Sato S,
Asamizu E,
Tabata S, Stougaard J
(2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trinick MJ
(1979) Structure of nitrogen-fixing nodules formed by Rhizobium on roots of Parasponia andersonii plants. Canadian Journal of Microbiology 25, 565–578.
|
CAS |
PubMed |

Tsurumi S, Ohwaki Y
(1978) Transport of C-14-labeled indoleacetic acid in Vicia root segments. Plant & Cell Physiology 19, 1195–1206.

Vanneste S,
De Rybel B,
Beemster GTS,
Ljung K, De Smet I ,
et al
.
(2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana.
The Plant Cell 17, 3035–3050.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vieten A,
Vanneste S,
Wisniewska J,
Benkovà E,
Benjamins R,
Beeckman T,
Luschnig C, Friml J
(2005) Functional redundancy of PIN proteins is accompanied by auxin dependent cross-regulation of PIN expression. Development 132, 4521–4531.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vieten A,
Sauer M,
Brewer PB, Friml J
(2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science 12, 160–168.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Walch-Liu P,
Ivanov II,
Filleur S,
Gan YB,
Remans T, Forde BG
(2006) Nitrogen regulation of root branching. Annals of Botany 97, 875–881.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wasson AP,
Pellerone FI, Mathesius U
(2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. The Plant Cell 18, 1617–1629.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wheeler CT,
Crozier A, Sandberg G
(1984) The biosynthesis of indole-3-acetic acid by Frankia. Plant and Soil 78, 99–104.
| Crossref | GoogleScholarGoogle Scholar |

Wightman F,
Schneider EA, Thimann KV
(1980) Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiologia Plantarum 49, 304–314.
| Crossref | GoogleScholarGoogle Scholar |

Winkel-Shirley B
(2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology 126, 485–493.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wisniewska J,
Xu J,
Seifertova D,
Brewer PB,
Ruzicka K,
Blilou I,
Rouquie D,
Scheres B, Friml J
(2006) Polar PIN localization directs auxin flow in plants. Science 312, 883.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Woodward AW, Bartel B
(2005) Auxin: regulation, action, and interaction. Annals of Botany 95, 707–735.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wopereis J,
Pajuelo E,
Dazzo FB,
Jiang QY,
Gresshoff PM,
de Bruijn FJ,
Stougaard J, Szczyglowski K
(2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. The Plant Journal 23, 97–114.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wu CW,
Dickstein R,
Cary AJ, Norris JH
(1996) The auxin transport inhibitor N-(1-naphthyl)phthalamic acid elicits pseudonodules on non-nodulating mutants of white sweetclover. Plant Physiology 110, 501–510.
| PubMed |

Wu GS,
Lewis DR, Spalding EP
(2007) Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. The Plant Cell 19, 1826–1837.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Xie H,
Pasternak JJ, Glick BR
(1996) Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida CR12–2 that overproduce indoleacetic acid. Current Microbiology 32, 67–71.
| Crossref | GoogleScholarGoogle Scholar |

Yang Y,
Hammes UZ,
Taylor CG,
Schachtman DP, Nielsen E
(2006) High-affinity auxin transport by the AUX1 influx carrier protein. Current Biology 16, 1123–1127.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang XC,
Wu XL,
Findley S,
Wan JR,
Libault M,
Nguyen HT,
Cannon SB, Stacey G
(2007) Molecular evolution of lysin motif-type receptor-like kinases in plants. Plant Physiology 144, 623–636.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



