Localised and non-localised promotion of fruit development by seeds in Arabidopsis
Catherine M. Cox A B and Stephen M. Swain B CA School of Agricultural Science, La Trobe University, Bundoora, Vic. 3083, Australia.
B CSIRO Plant Industry, 585 River Ave, South Merbein, Vic. 3505, Australia.
C Corresponding author. Email: Steve.Swain@csiro.au
D This paper originates from the Peter Goldacre Award 2004 of the Australian Society of Plant Scientists, received by S. M. Swain.
Functional Plant Biology 33(1) 1-8 https://doi.org/10.1071/FP05136
Submitted: 3 June 2005 Accepted: 29 August 2005 Published: 3 January 2006
Abstract
In Arabidopsis, as in the majority of flowering plants, developing seeds promote fruit growth. One method to investigate this interaction is to use plants with reduced seed set and determine the effect on fruit growth. Plants homozygous for a transgene designed to ectopically express a gene encoding a gibberellin-deactivating enzyme exhibit reduced pollen tube elongation, suggesting that the plant hormone gibberellin is required for this process. Reduced pollen tube growth causes reduced seed set and decreased silique (fruit) size, and this genotype is used to explore the relationship between seed set and fruit elongation. A detailed analysis of seed set in the transgenic line reveals that reduced pollen tube growth decreases the probability of each ovule being fertilised. This effect becomes progressively more severe as the distance between the stigma and the ovule increases, revealing the complex biology underlying seed fertilisation. In terms of seed-promoted fruit growth, major localised and minor non-localised components that contribute to final silique length can be identified. This result demonstrates that despite the relatively small size of the fruit and associated structures, Arabidopsis can be used as a model to investigate fundamental questions in fruit physiology.
Introduction
Flower development and structure is intimately related to the physiological processes of seed set and fruit development (Koltunow et al. 2002). One well studied example is the wild type (WT) Arabidopsis thaliana ovary, which has a pistil composed of two congenitally fused carpels, divided by a postgenitally fused false septum, topped by a single style and stigma (Fig. 1; Bowman et al. 1999; Alvarez and Smyth 2002). Each carpel contains two linear rows of ovules, which under the conditions used here typically contain approximately 12 more-or-less evenly-spaced ovules, all of which are potentially able to be fertilised and develop into seeds (Bowman et al. 1999). Each ovule arises from, and is attached via a funiculus to, a placenta. A total of four placentae are present in each ovary: in each carpel two placentae form along the carpel margin adjacent to the septum (Fig. 1). Thus, each ovary contains a total of approximately 50 ovules in four rows along the length of the pistil. Pollination (Fig. 1A) and pollen grain germination (Fig. 1B) occurs on the stigmatic papillae situated at the distal end of the ovary, farthest from the flower base. Because of the position of the stigma and the orientation of the four ovule rows, a progressive increase in the distance from the stigma, and hence the distance that pollen tubes are required to grow to fertilise each ovule, occurs along the length of each ovule row (Fig. 1C). Consequently, pollen tubes are able, in the absence of any regulation by the pistil tissues, to reach the ovules at the top of the ovary (nearest the stigma) before they reach the more distant ovules at the base of the ovary (farthest from the stigma).
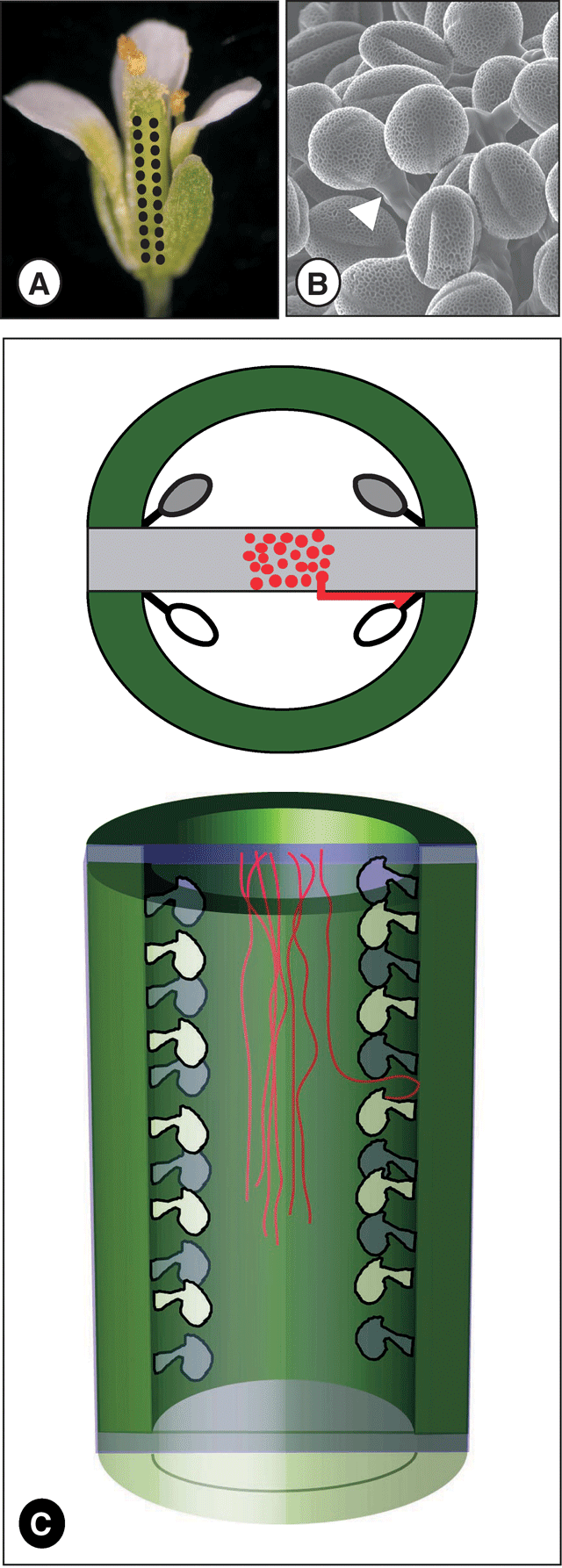
|
In the majority of Angiosperm species, the formation of seeds is intimately linked with fruit growth and development (Koltunow et al. 2002; Ozga et al. 2002). In many plants, ovaries in which no ovules are fertilised fail to develop further and subsequently senesce and then abort (e.g. Garcia-Martinez et al. 1991). In other plants, including Arabidopsis, ovaries without seeds exhibit limited development, but are usually smaller than seeded fruit (e.g. Vivian-Smith and Koltunow 1999; Vivian-Smith et al. 2001). Final fruit size is strongly influenced by seed set, and a positive relationship exists between total seed number and weight, and fruit size (e.g. Groot et al. 1987). Seeds often have both a general effect on fruit development, for example preventing fruit abscission, and a more localised influence on the growth of adjacent pericarp tissue (Nitsch 1952). For example, in tomatoes and pears partial seed set can lead to unevenly shaped fruit, while in citrus fruit shape is not affected by the number or position of seeds. Plant hormones have often been implicated in this process, largely because different hormones, alone or in combination, can substitute for seeds in promoting fruit growth (e.g. Eeuwens and Schwabe 1975; Vivian-Smith and Koltunow 1999). Seeds are also a rich source of plant hormones, including auxin and gibberellins (GAs). The best-studied system in regard to the control of fruit growth by seed-derived signals is the pea fruit, in which fruit (pod) growth is completely dependent on seed set in WT plants. Developing pea seeds contain a modified auxin, 4Cl-IAA, which has been proposed to move from the seed, via the funiculus, to the surrounding pericarp tissue where it promotes GA biosynthesis and hence fruit growth (Ozga et al. 2002, 2003). This model is also supported by studies in elongating internodes of pea and other species in which apically derived auxin promotes the production of active GAs (Ross and O’Neill 2001; O’Neill and Ross 2002).
Genetics is a powerful technique for investigating plant physiology, including reproductive development in general and fruit growth in particular. In model plants with the full range of modern tools and resources this includes both forward and reverse genetics and the ability to introduce transgenes designed to specifically alter gene function in particular ways. One approach that has been used to investigate the role of GAs in seed set is ectopic expression in transgenic Arabidopsis of a cDNA, isolated from Pisum sativum L. (garden pea), encoding a GA 2-oxidase. This 2-oxidase, PsGA2ox2 (also known as 2ox2), can convert the active GAs, GA1 and GA4, into the irreversibly inactivated products, GA8 and GA34, respectively (Lester et al. 1999). In several independent 35S : 2ox2 lines seed development is abnormal and siliques contain seeds that have aborted at various stages after fertilisation (Singh et al. 2002). This observation supports results obtained with GA-deficient mutants of pea that also suggest an essential role for GAs during the early stages of seed development (Swain et al. 1995, 1997; Swain and Singh 2005). An additional phenotype of the 35S : 2ox2 lines is defective pollen tube growth, which in hemizygous plants (i.e. only a single transgenic chromosome) affects pollen tubes carrying the transgene but not non-transgenic pollen tubes. As described in more detail below, this phenotype suggests that GAs are also essential for normal pollen tube growth and hence ovule fertilisation.
Because of the defects in seed development and reduced pollen tube elongation, homozygous 35S : 2ox2 plants could only be isolated for a single independent line. This line, 35S : 2ox2 / 28c, exhibits relatively subtle reproductive phenotypes and does not possess a detectable defect in seed development. In this homozygous line, all pollen tubes carry the 35S : 2ox2 / 28c transgene and exhibit reduced growth, although at least some do eventually reach the base of the ovary. The fact that all pollen tubes behave similarly leads to significant experimental advantages and this line has consequently formed the basis of detailed in vivo and in vitro analysis that has confirmed the 35S : 2ox2 transgene causes reduced pollen tube growth. Consistent with an essential role for GAs in pollen tube elongation, this phenotype can be partially or completely suppressed by reducing expression from the 35S : 2ox2 transgene, or by increasing GA levels or response in pollen tubes (Singh et al. 2002; Swain et al. 2004; Swain and Singh 2005). The reduced growth of 35S : 2ox2 / 28c pollen tubes, combined with the structure of the Arabidopsis ovary, has a dramatic effect on seed set and consequently on fruit development. As ovules are only able to be fertilised for a limited period after anthesis, 35S : 2ox2 / 28c pollen tubes have a reduced ability to reach and fertilise ovules, particularly those farthest from the stigma. This leads to a distinctive fruit phenotype: in the majority of siliques seeds are only present near the stigma. The reduced seed set leads to a reduction in final fruit length, to about half the length of self-pollinated WT fruit. Reciprocal crosses between WT and 35S : 2ox2 / 28c plants demonstrate that the 35S : 2ox2 / 28c phenotype is entirely due to reduced pollen tube growth, rather than a direct effect of the 35S : 2ox2 transgene on ovule, seed or fruit development.
In this paper, the 35S : 2ox2 / 28c line is used to explore the relationship between pollen tube growth, fertilisation and seed set, and fruit elongation. The significance of these results to seed set and fruit development in other plants is also discussed.
Materials and methods
Wild type Arabidopsis thaliana plants of ecotype Landsberg erecta (La-er) were grown under typical laboratory conditions: an 18-h photoperiod consisting of 60–70 μmol photons m–2 s–1 of fluorescent light at 22–24°C. The 35S : 2ox2 / 28c homozygous line is in the same genetic background, and has been described previously (Singh et al. 2002).
Seed set was determined on self-pollinated plants by examining the silique at the third reproductive node on the main inflorescence stem. A single node was used as previous analysis demonstrated that the 35S : 2ox2 / 28c phenotype is consistent over at least the first ten reproductive nodes (Swain et al. 2004). Dry, fully mature siliques were removed from the plant, and seed set recorded at each ovule position in each carpel, using a dissecting microscope, after carefully removing each valve in turn. The seeds were then removed and the remaining fruit tissue, consisting of the pedicel, septum, funiculi, style and stigma, placed between two glass slides hinged along one side with tape. Owing to the structure of the Arabidopsis pistil, each carpel could then be viewed through one of the two slides. When six fruit had been placed between the two slides, the slides were taped together for subsequent photography of each carpel with a digital camera attached to a dissecting microscope. The position of the seeds and unfertilised ovules was then digitally marked on each image, and software (ImageJ ver. 1.24, National Institutes of Health, Millersville University, Millersville, PA) used to measure the distance from the style to each ovule / seed in each of the four ovule rows. To make presentation of the data simpler, seed set was averaged across the four ovule rows at each ovule position (see Results). A similar approach was used for interovule distances, except that only the first ten interovule distances (between ovules 1 and 11) were analysed. This approach overcame complications arising from the potential variation in ovule number in each of the four rows within and between each WT and 35S : 2ox2 / 28c silique. All values are shown as mean ± standard error of the mean (s.e.m.).
To estimate interovule distances in WT ovaries at anthesis valve length was determined in 12 flowers and divided by the mean number of ovules (12.17 ± 0.41) + 1 (to account for the spaces between each end of the ovary and the nearest ovule). To determine interovule distances in fully-elongated fruit without seeds, WT flowers were emasculated and valve length determined after elongation ceased. WT flowers were chosen as previous analysis of emasculated WT and 35S : 2ox2 / 28c flowers, at anthesis or when fully elongated, failed to uncover an effect of the transgene on seedless fruit growth (D.P. Singh and S.M. Swain unpubl. data).
For controlled crosses, WT flower buds at stage 12 (Smyth et al. 1990) were emasculated by removing the sepals, petals and anthers. Pollen from either WT or 35S : 2ox2 / 28c flowers at anthesis was placed onto the stigma of these WT flowers, and seed set observed when the fruit was mature.
Results
Plants homozygous for the 35S : 2ox2 / 28c transgene (hereafter referred to as 35S : 2ox2) have previously been shown to possess reduced seed set, with the majority of seeds present at the end of the silique nearest the stigmatic papillae, the site of pollen grain germination (Singh et al. 2002; Swain et al. 2004). In these analyses seed set was described by measuring the total number of seeds (i.e. fertilised ovules in all four ovule rows) per silique in addition to the combined numbers of unfertilised ovules farther from the stigma the than the most distant seed in each ovule row (i.e. unfertilised ovules at the silique base; see Swain et al. 2004). The latter value provides an indication of the ovules that cannot be reached by 35S : 2ox2 pollen tubes, but provides only limited information of how many ovules in the region nearest the stigma remain unfertilised. To describe seed set in more detail, each ovule position in each of the four rows was examined from self-pollinated WT and 35S : 2ox2 plants to determine if fertilisation had occurred (Fig. 2). To allow the results to be presented in a simpler format, and because the four ovule rows within the same silique are not statistically independent of each other, the data for each ovule position from the four rows were combined into a single value by averaging the number of fertilised seeds. Thus, for each ovule position the average number of seeds potentially varied between 0 (no ovules fertilised) and 1 (all 4 ovules fertilised). At the base of each silique, where the ovules farthest from the stigma are positioned, a problem arises because not all rows within and between siliques have the same number of ovules (data not shown). To deal with this situation, it was assumed that all rows contain the same number of ovules, the maximum value observed from all siliques examined, and that the ‘missing’ ovules were not fertilised. Consequently, at positions 12 and 13, the apparent low seed set in WT fruit reflects the assumption that all rows contain the same number of ovules, as described above. For the first 11 ovules in WT, seed set was high, at or approaching the maximum possible value of all 4 ovules fertilised. This result is consistent with previous observations that WT plants are very fertile, with almost all ovules fertilised in most fruit, under the typical laboratory conditions used here (see Materials and methods). In contrast to WT, and consistent with previous analysis, seed set was clearly reduced at all ovule positions up to ovule 12 in 35S : 2ox2 siliques (Fig. 2). Furthermore seed set in 35S : 2ox2 siliques decreases with increasing distance of the ovule from the stigma, reflecting a greater distance that the pollen tube fertilising that ovule was required to grow. The differences in seed set also resulted in differences in final silique length: WT and 35S : 2ox2 fruit were 10.61 ± 0.14 mm and 4.83 ± 0.25 mm long, respectively.
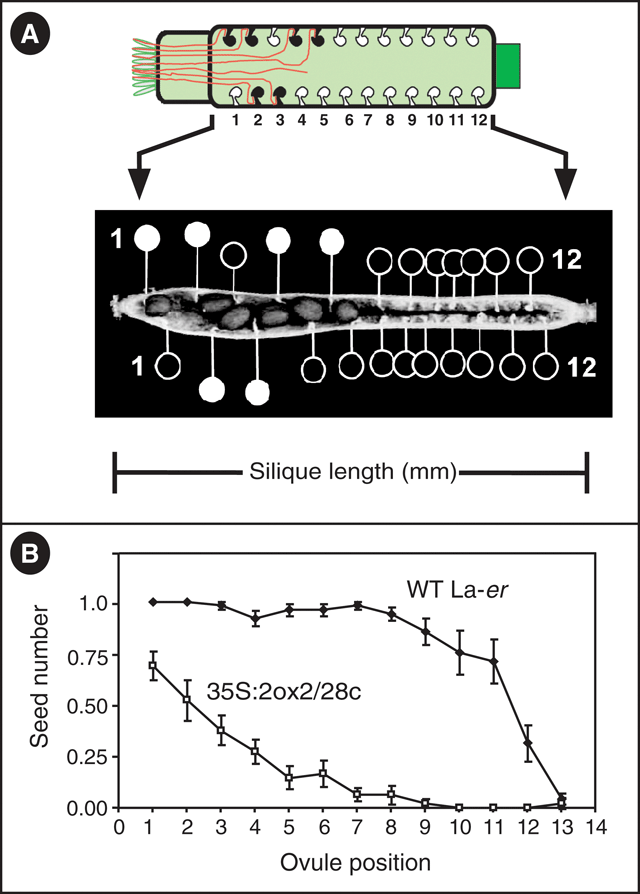
|
An important observation provided by these data relates to ovules that are nearer to the stigma than the most distant fertilised seed in any particular ovule row. For example, if ovule number five is fertilised in one ovule row of a 35S : 2ox2 silique, this does not mean that all ovules closer to the stigma (numbers one to four) are also fertilised. An example of this situation is illustrated by the top row of the 35S : 2ox2 silique shown in Fig. 2A. In this case the pollen tubes that fertilised ovules at positions four and five must have grown past ovule three, despite that fact that ovule three was not ultimately fertilised. To confirm that the reduced seed set in 35S : 2ox2 siliques can be fully explained by impaired pollen tube function (Singh et al. 2002), controlled pollinations were performed. WT flowers were pollinated with either WT or 35S : 2ox2 pollen and seed set observed. Consistent with the results from self-pollinated flowers, many WT ovules remained unfertilised by 35S : 2ox2 pollen between the stigma and the most distant seed. This result is also consistent with the near-WT seed numbers in self-pollinated plants hemizygous for the 35S : 2ox2 / 28c transgene in which 50% of the pollen tubes do not carry the transgene, and the majority of ovules not fertilised by 35S : 2ox2 pollen tubes are instead fertilised by WT pollen tubes (Swain et al. 2004).
The reduced seed set in 35S : 2ox2 siliques provides an opportunity to investigate the relationship between seed set and fruit growth in Arabidopsis that is not usually available in WT plants. Because seed set is not reduced uniformly for all ovule positions, the distance between each adjacent ovule pair in each ovule row in self-pollinated WT and 35S : 2ox2 was determined (Fig. 3). As for seed set, the values for each interovule distance were averaged for the four rows in each silique. In WT siliques, the interovule distance was similar along the length of the silique, averaging ∼0.7 mm, suggesting that seed-promoted fruit growth is similar throughout the ovary. By contrast, interovule distances varied with distance from the stigma in 35S : 2ox2 siliques. Distances were similar to, but slightly lower than, those in WT siliques at the stigmatic end where seed set in 35S : 2ox2 was highest, but interovule distances decreased as 35S : 2ox2 seed set declined at more distant ovules (Fig. 3C).
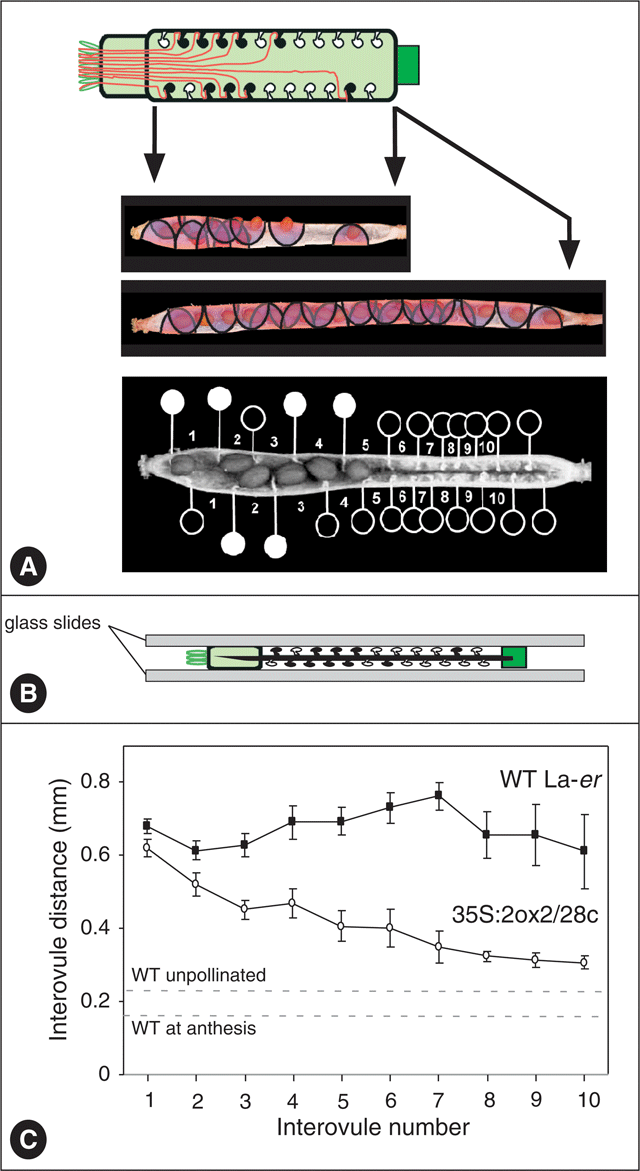
|
Based on the average length of WT ovaries (2.06 ± 0.04 mm) and the average number of ovules in one row (12.17 ± 0.41), unfertilised ovules are calculated to be approximately 0.16 mm apart at anthesis. Unlike many plants, if WT Arabidopsis ovaries are left unpollinated the ovary does not abscise and limited elongation growth occurs. For example, unfertilised (emasculated) WT ovaries reached a final length of 3.06 ± 0.10 mm, indicating that these ovules were eventually approximately 0.23 mm apart on average. Thus, seed-independent interovule growth of ∼0.07 mm (an increase in distance of ∼45%) occurs even in the absence of fertilisation and seed set for an individual fruit. The remaining potential growth, ∼0.47 mm for each interovule region, is therefore seed-dependent. Significantly, the extent of the fertilisation-independent elongation in WT siliques is only slightly less than the growth that occurs between ovules at the base of 35S : 2ox2 siliques where seed set is essentially absent. Thus, the presence of seeds in the upper part of the silique has only a small effect on promotion of the elongation growth of the region without seeds. This observation suggests the majority of the growth-promoting effects of seeds act locally and seeds have only a very limited effect on growth of more distant regions of the ovary.
Since ovules are essentially uniformly distributed along the ovary at anthesis, the data in Figs 2 and 3 can be used to determine the relationship between seed numbers and interovule distance along the length of the silique (Fig. 4). To analyse this relationship, interovule distance was plotted as a function of the combined number of seeds at both adjacent ovule positions, assuming that seed-derived, growth-promoting signals would move away from the funiculus in both directions along the length of the ovary. This analysis reveals that, for the seed-dependent component of silique elongation, a simple positive relationship exists between combined seed numbers on each side of an interovule region (between 0 and 2), and the interovule distance. Both linear and quadratic functions can be used to create a line of best fit with similar R2 values (Fig. 4).
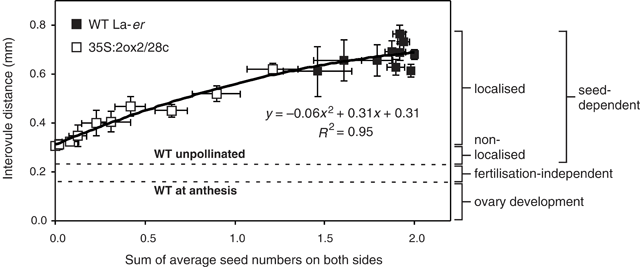
|
Discussion
Fertilisation and seed set
A detailed analysis of seed set in self-pollinated 35S : 2ox2 siliques reveals that, compared with WT fruit, seed set is reduced at all ovule positions, even those ovules nearest to the site of pollen grain germination, the stigma. Seed set progressively decreases as the distance between the stigma and ovule increases, so that the majority of more distant ovules are not fertilised by 35S : 2ox2 pollen. This results in the typical 35S : 2ox2 phenotype with seeds only present in the ovary region nearest the stigma (Singh et al. 2002).
An important aspect of the reduced seed set in 35S : 2ox2 is that unfertilised ovules are also present nearer to the stigma than the most distant fertilised ovule in that ovule row. In other words, some ovules are missed by 35S : 2ox2 pollen, but not simply because the 35S : 2ox2 pollen tubes cannot grow that far in the period that ovules remain receptive. Controlled crosses with WT or 35S : 2ox2 pollen onto WT pistils confirms that this is not due to defects in 35S : 2ox2 female tissues (data not shown). Based on the assumption that ovules nearest the stigma can be fertilised before more distant ones, the following model of Arabidopsis fertilisation can be proposed. Pollen tubes grow down the transmitting tract towards the base of the ovary, and only a small proportion of the total is potentially attracted to any one ovule during a limited period in which each ovule is receptive. This model is consistent with the results obtained when controlled pollinations with limited numbers (approximately five) of pollen grains are performed (Hulskamp et al. 1995). Although pollination with a single pollen grain produces a pollen tube that tends to emerge at the ovule closest to the stigma, additional pollen tubes tend to target more distant ovules. Thus, even when the total number of pollen tubes is small, it appears that only one is usually influenced by a single ovule to leave the transmitting tract (Shimizu and Okada 2000). Observations of septum and inner carpel tissues during and after fertilisation are also consistent with this model as ‘lost’ pollen tubes that left the transmitting tract but failed to find an ovule are not observed in great numbers (Hulskamp et al. 1995). The mechanism of pollen tube attraction by ovules is not completely understood, but in part it involves gamma-amino butyric acid (GABA) gradients (Palanivelu et al. 2003). It is possible that only pollen tubes in a very limited region of the transmitting tract closest to a particular ovule are able to perceive the GABA gradient, and that once a pollen tube grows towards that ovule other pollen tubes in the transmitting tract are unable to respond to the same gradient.
A key assumption of this model is that the timing of ovule receptivity is optimised for normal anthesis and self-pollination and for the typical WT pollen tube growth rate. The slow growth of 35S : 2ox2 pollen tubes would mean that the number of pollen tubes near each ovule during the receptive period, and potentially able to be attracted by the ovule, would be greatly reduced in self-pollinated 35S : 2ox2 ovaries. The absolute difference in distance from the stigma for typical WT and 35S : 2ox2 pollen tubes would become larger as the total distance grown increases so that 35S : 2ox2 pollen tubes would increasingly ‘fall behind’ WT pollen tubes, consistent with previous analysis of WT and 35S : 2ox2 pollen tube growth (Singh et al. 2002). Thus, the probability of no fertilisation would increase as the distance between the ovule and the stigma increases, and this is in fact what is observed with 35S : 2ox2 pollen. Towards the base of the ovary almost no 35S : 2ox2 pollen tubes would arrive in time to fertilise the ovules, explaining the almost complete lack of seeds in this region.
Another important conclusion from the results presented here applies to seed set in plants that, unlike Arabidopsis, do not have placentae that generate rows of ovules at increasing distance from the stigma. The data in Fig. 2 suggest that even in fruit in which all of the ovules are an equal distance from the stigma, a reduction in pollen tube growth will reduce seed set, depending on the distance from the stigma to the ovules, the timing of ovule receptivity, and the relative change in pollen tube growth rate (Fig. 5). Thus, reduced pollen tube growth, due to GA-deficiency or other causes, would be expected to decrease seed set in crops in which all ovules are approximately the same distance from the stigma, such as grapes, and in monocotyledonous crops with a single ovule per ovary, such as rice and wheat. Furthermore, as recently demonstrated in tobacco, plants in which all pollen tubes are severely defective in growth will be functionally male sterile regardless of ovule number and positioning (de Groot et al. 2004).
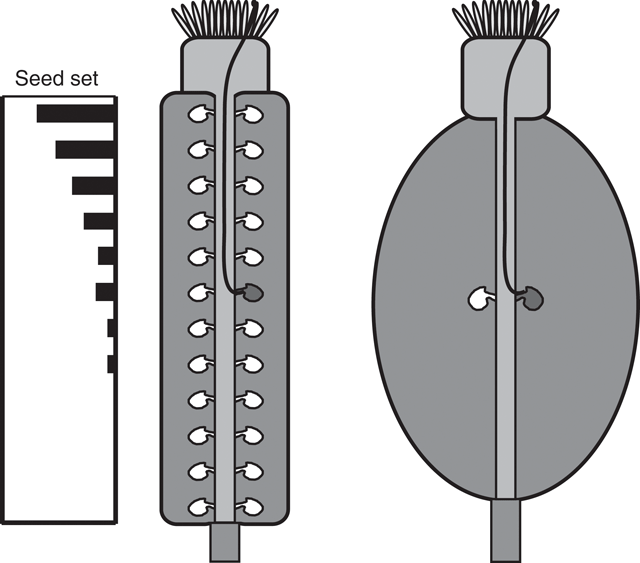
|
Localised and non-localised effects of seeds on fruit development
In fertilised WT flowers, silique length is mainly determined by the seed-dependent component of growth. Limited fertilisation-independent ovary growth also occurs before and after anthesis (Fig. 4). The observation that a simple near-linear relationship exists between seed numbers and the length of neighbouring interovule regions suggests that seeds promote silique elongation in an essentially local fashion. Thus, the presence of seeds near the stigma of 35S : 2ox2 siliques has only a minor effect on the elongation growth of the basal region where seeds are absent. The relationship shown in Fig. 4 also reveals that individual seeds contribute to final fruit length in a close to additive manner; each seed contributes to growth largely independently of the number of neighbouring seeds. This means that ten seeds spread throughout the silique will result in similar overall fruit length as the same number clustered near the stigma.
Clearly, changes in any of the growth components described above have the potential to alter final fruit size. For example, the strongest manifestation of true parthenocarpy involves a complete loss of the requirement for seeds so that the relationship between seed numbers and growth is a horizontal line and maximal fruit growth occurs independently of seed set. Increased carpel growth before anthesis can also result in partially seed-independent fruit growth, as described for Arabidopsis overexpressing a gene encoding a cytochrome P450 enzyme, CYP78A9 (Ito and Meyerowitz 2000).
While Arabidopsis seeds promote fruit elongation in a mostly localised manner, fertilisation affects other aspects of fruit development in a more general way. For example, the dehiscence zone, along which the valves will detach at fruit maturity to release seeds, does not complete development in unfertilised ovaries. By contrast, the presence of only a single seed allows the dehiscence zone in both carpels to form along the length of the silique, and not only in the region where the seed is present. Similarly, dehiscence always occurs along the entire valve in 35S : 2ox2 plants, even at the silique base.
Conclusion
The results presented here confirm that Arabidopsis, despite the relatively small size of its fruit and associated structures, can be used to investigate fundamental questions concerning the relationship between pollen tube growth, seed set, and fruit elongation. This approach, and the knowledge gained, can be used to improve our understanding of these fundamental processes in the wide range of crops in which seed and fruit development are of critical importance.
Acknowledgments
Thanks to Judy Tisdall (La Trobe University) for cosupervising CMC, Davinder Pal Singh and Richard Storey for the scanning electron microscope image in Fig. 1, and the Cooperative Research Centre for Viticulture and the Grape and Wine Research and Development Corporation (GWRDC) for financial support to CMC. This work was performed in conjunction with CSIRO Plant Industry and La Trobe University as part of Riverlink.
Alvarez J, Smyth DR
(2002) CRABS CLAW and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. International Journal of Plant Sciences 163, 17–41.
| Crossref | GoogleScholarGoogle Scholar |

Bowman JL,
Baum SF,
Eshed Y,
Putterill J, Alvarez J
(1999) Molecular genetics of gynoecium development in Arabidopsis. Current Topics in Developmental Biology 45, 155–205.
| PubMed |

de Groot P,
Weterings K,
de Been M,
Wittink F,
Hulzink R,
Custers J,
van Herpen M, Wullems G
(2004) Silencing of the pollen-specific gene NTP303 and its family members in tobacco affects in vivo pollen tube growth and results in male sterile plants. Plant Molecular Biology 55, 715–726.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Eeuwens CJ, Schwabe WW
(1975) Seed and pod wall development in Pisum sativum L. in relation to extracted and applied hormones. Journal of Experimental Botany 26, 1–14.

Garcia-Martinez JL,
Marti M,
Sabater T,
Maldonado A, Vercher Y
(1991) Development of fertilized ovules and their role in the growth of the pea pod. Physiologia Plantarum 83, 411–416.
| Crossref | GoogleScholarGoogle Scholar |

Groot SPC,
Bruinsma J, Karssen CM
(1987) The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiologia Plantarum 71, 184–190.

Hulskamp M,
Schneitz K, Pruitt RE
(1995) Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. The Plant Cell 7, 57–64.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ito T, Meyerowitz EM
(2000) Overexpression of a gene encoding a cytochrome P450, CYP78A9, induces large and seedless fruit in Arabidopsis. The Plant Cell 12, 1541–1550.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koltunow, AM ,
Vivian-Smith, A ,
Tucker, MR ,
and
Paech, N (2002). The central role of the ovule in apomixis and parthenocarpy. In ‘Plant reproduction. Vol 6.’ pp. 221–256. (Sheffield Academic Press: Sheffield, UK)
Lester DR,
Ross JJ,
Smith JJ,
Elliott RC, Reid JB
(1999) Gibberellin 2-oxidation and the SLN gene of Pisum sativum. The Plant Journal 19, 65–73.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nitsch JP
(1952) Plant hormones in the development of fruits. The Quarterly Review of Biology 27, 33–57.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

O’Neill DP, Ross JJ
(2002) Auxin regulation of the gibberellin pathway in pea. Plant Physiology 130, 1974–1982.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ozga JA,
van Huizen R, Reinecke DM
(2002) Hormone and seed-specific regulation of pea fruit growth. Plant Physiology 128, 1379–1389.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ozga JA,
Yu J, Reinecke DM
(2003) Pollination-, development-, and auxin-specific regulation of gibberellin 3β-hydroxylase gene expression in pea fruit and seeds. Plant Physiology 131, 1137–1146.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Palanivelu R,
Brass L,
Edlund AF, Preuss D
(2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114, 47–59.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ross J, O’Neill D
(2001) New interactions between classical plant hormones. Trends in Plant Science 6, 2–4.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shimizu KK, Okada K
(2000) Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127, 4511–4518.
| PubMed |

Singh DP,
Jermakow AM, Swain SM
(2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. The Plant Cell 14, 3133–3147.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Smyth DR,
Bowman JL, Meyerowitz EM
(1990) Early flower development in Arabidopsis. The Plant Cell 2, 755–767.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Swain SM, Singh DP
(2005) Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends in Plant Science 10, 123–129.
| PubMed |

Swain SM,
Ross JJ,
Reid JB, Kamiya Y
(1995) Gibberellins and pea seed development. Expression of the lhi, ls and le5839
mutations. Planta 195, 426–433.
| Crossref | GoogleScholarGoogle Scholar |

Swain SM,
Reid JB, Kamiya Y
(1997) Gibberellins are required for embryo growth and seed development in pea. The Plant Journal 12, 1329–1338.
| Crossref | GoogleScholarGoogle Scholar |

Swain SM,
Muller AJ, Singh DP
(2004) The gar2 and rga alleles increase the growth of gibberellin-deficient pollen tubes in Arabidopsis. Plant Physiology 134, 694–705.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vivian-Smith A, Koltunow AM
(1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiology 121, 437–451.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vivian-Smith A,
Luo M,
Chaudhury A, Koltunow A
(2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128, 2321–2331.
| PubMed |



