Short-term and long-term fluoride stress induce differential molecular and transcriptional regulation and variable ranges of fluoride tolerance in two indica rice (Oryza sativa) varieties
Ankur Singh A , Aditya Banerjee A and Aryadeep Roychoudhury B *
B *
A
B
Abstract
The aim of this study was to decipher the reprogramming of protective machineries and sulfur metabolism, as responses to time-dependent effect of fluoride stress for 10 and 20 days in two indica rice (Oryza sativa) varieties. Unregulated accumulation of fluoride via chloride channels (CLC1 and CLC2) in 10-day-old (cv. Khitish) and 20-day-old (cv. MTU1010) seedlings caused higher accumulation of H2O2 and superoxide anion that eventually incited chlorophyll loss and electrolyte leakage, along with the formation of malondialdehyde and methylglyoxal. Higher fluoride accumulation also enhanced lipoxygenase and NADPH oxidase activities, which further aggravated the oxidative damages. However, for stressed 20-day-old Khitish and 10 day-old MTU1010 seedlings, plant growth was maintained with lesser oxidative damages due to upregulated expression of H+-ATPase and FEX along with the elevated level of cysteine and H2S, which could be linked with higher activity of ATP-S, OASTL, and DES. The activity of the enzymatic antioxidants (superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidase, and glutathione peroxidase) and level of non-enzymatic antioxidants (anthocyanins and flavonoids) were also enhanced that strengthened the antioxidative potential of the seedlings. Our work demonstrated that differential reprogramming of the protective metabolites and sulfur assimilation pathways is responsible for the differential pattern of adaptive strategies against fluoride stress in the two indica rice varieties, with Khitish exhibiting tolerance against long-term fluoride stress, whilst MTU1010 showing high susceptibility to the same.
Keywords: antioxidants, chloride channels, fluoride, fluoride exporters, isozymes, rice, oxidative damage, sulfur.
Introduction
Anthropogenic activities lead to the gradual build up of toxic elements in the atmosphere. Fluoride (F), being one such toxic element, is continuously being accumulated in the environment due to various human activities such as excess application of fertilizer in the field, and release of untreated water from industries and household activities. In addition, various natural sources like emissions from volcanic mountains, weathering of minerals, and marine aerosols also contribute to the level of F in the surrounding environment (Hong et al. 2016; Singh et al. 2018). When discharged into the environment, F is readily mixed with ground and surface water. Bhattacharya and Samal (2018) reported earlier that the level of F in drinking water in some parts of India such as Newai Tehsil (Rajasthan), and Purulia and Bankura (West Bengal) districts was as high as 0.3–9.8 mg L−1 and 0.8–1.3 mg L−1, respectively. Unplanned utilization of such contaminated water for irrigation purpose further leads to F uptake by the plants. De et al. (2021) reported that the level of F in the soil of Purulia and Bankura districts of West Bengal has risen abruptly to 126 ± 65 mg kg−1 and 114 ± 59 mg kg−1, respectively, affecting pulses, cereals, and leafy and non-leafy vegetables. Excess accumulation of F in crops like spinach (Spinacia oleracea) and fenugreek (Trigonella foenum-graecum) when treated with F salts (7.4–14 mg L−1) was also noted by Gautam et al. (2010). Water-intensive crops like rice (Oryza sativa) take up F salts from the soil through chloride channels (CLC1 and CLC2) that induces oxidative damages. This triggers the formation of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide radicals (Hong et al. 2016; Banerjee et al. 2019). Enhanced ROS accumulation further causes membrane lipid oxidation due to elevated lipoxygenase (LOX) and NADPH oxidase (NOX) activity that eventually causes chlorophyll loss, leakage of cellular electrolytes along with the formation of cytotoxic metabolites such as malondialdehyde (MDA) and methylglyoxal (MG) (Singh and Roychoudhury 2023).
To tolerate adverse conditions, plants have developed a wide range of protective machineries comprising of non-enzymatic metabolites and enzymatic antioxidants that counteract ROS by scavenging them. Superoxide dismutase (SOD) converts superoxide radicals to H2O2, which is converted to non-toxic molecules via enzymatic action of catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPoX), and glutathione peroxidase (GPX) (Yadu et al. 2017; Singh and Roychoudhury 2020). The protective role of these enzymatic antioxidants during F stress has been widely reported in several crops like rice, tea (Camellia sinensis), safflower (Carthamus tinctorius), pigeon pea (Cajanus cajan), and wheat (Triticum aestivum) (Yadu et al. 2017; Ghassemi-Golezani and Farhangi-Abriz 2019; Niu et al. 2020; Pelc et al. 2020; Singh et al. 2020). Apart from enzymatic antioxidants, the non-enzymatic antioxidants such as anthocyanins and flavonoids also detoxify ROS and stabilize the lipid membrane by interacting with the polyunsaturated acyl moiety of the lipids (Waśkiewicz et al. 2014).
Sulfur (S) is one of the most important macronutrients that regulate normal growth and development of plants by controlling various biological functions (Ernst et al. 2008; Kumaran et al. 2008). S is mostly involved in the formation of sulfur-containing amino acids such as cysteine (Cys), proteins, vitamins, chlorophyll and protective metabolites involved in stress tolerance (Rausch and Wachter 2005; Burritt 2008; Spadaro et al. 2010). Sulfur, which is mostly present as sulfate (SO42−) in soil, is taken up by the plants via sulfate transporters (Davidian and Kopriva 2010). Once SO42− is accumulated within the cells, it is converted to adenosine 5′-phosphosulfate (APS) by the enzymatic action of ATP-sulfurylase (ATP-S) (first enzyme of S assimilatory pathway) (Leustek et al. 1994). APS is finally reduced to sulfide (S2−), which reacts with O-acetyl serine to form Cys in presence of O-acetylserine(thiol) lyase (OAS-TL). Cys is the first organic compound of S assimilatory pathway that contains reduced S in plant cells (Gill and Tuteja 2011). Cys can be further catabolized into hydrogen sulfide (H2S) by the catalytic action of L-cysteine desulfhydrase (DES) that helps in maintaining the level of Cys in cells (Vojtovič et al. 2020). H2S also plays a pivotal role in regulating the tolerance capability of plants under stressed conditions by activation of defense mechanisms and alleviating stress-induced injuries in cells (Corpas 2019; Hancock 2019). Various studies have shown that exogenous application of S plays a pivotal role in abrogating the negative effects of environmental stressors by maintaining usual metabolic process (Hasanuzzaman et al. 2018). Sheng et al. (2016) reported that exogenous application of S induces the survival capability of manganese-stressed wheat plants by upregulating the antioxidative defense and sequestering excess manganese into the vacuoles. Similar reports were also published by Lou et al. (2017) and Liang et al. (2016) where it was shown that exogenous application of S promoted cadmium tolerance by upregulating the activity of OAS-TL and ATP-S, respectively, which eventually hindered the translocation of cadmium from root to shoot.
Although there has been research on the effect of F stress in different plant species, detailed study on the time-dependent effect of F toxicity in rice is still relatively unknown. This study aimed to examine the effect of F stress in two indica rice cultivars (Khitish and MTU1010) exposed to short-term (10 days) and long-term (20 days) F stress. After 10 and 20 days, F accumulation in seedlings was measured along with monitoring the expression level of the major transporters (CLC1, CLC2, H+-ATPase, and FEX). Physiological parameters associated with oxidative damages in seedlings, and the fate of protective metabolites (e.g. level of non-enzymatic antioxidants and activity of enzymatic antioxidants) were also analyzed. The regulation of S metabolism in seedlings during F toxicity was studied. The main aim of this work was to investigate the differential regulation of the protective metabolites, sulfur assimilation, and transcriptional regulation of the major transporters to identify their functional role in the survival of the two rice varieties, when exposed to F toxicity for 10 and 20 days. The data obtained here showed that sulfur assimilated in rice seedlings, together with higher expression of H+-ATPase and FEX, played a major role in regulating their tolerance capability. Thus, it can be presumed that by overinduction of enzymes involved in sulfur assimilatory pathways, together with overexpression of transporters such as H+-ATPase and FEX, tolerance capability of crops can be enhanced against F stress.
Materials and methods
Plant growth and stress treatment
Seeds of rice varieties Khitish and MTU1010 were obtained from Rice Research Station located in Chinsurah, Hooghly, West Bengal, India. The seeds were surface sterilised by rinsing with mercuric chloride (HgCl2) followed by thorough washing with distilled water. Seeds were then soaked over sterile gauge and placed in Petri dishes containing NaF solution (25 mg L−1). They were germinated for 3 days at 32°C in the dark. After 3 days, the germinated seeds were divided into two sets and exposed to F stress for two time duration treatments: (1) 7 days, so that upon completion of F exposure, seedlings were 10 days old; and (2) 17 days, so that upon completion of F exposure, seedlings were 20 days old. Plants for both time duration treatments were maintained at 32°C under 16/8 light/dark photoperiod, following the work of Singh et al. (2020). After 10 and 20 days, seedlings were collected and stored at −20°C for experimental analysis. The concentration of NaF (25 mg L−1) was chosen based on the work of Singh et al. (2020) and Banerjee et al. (2019) where it was reported that the treatment of rice varieties Khitish and IR-64 with 25 mg L−1 NaF caused significant damage to both the varieties.
Estimation of F accumulation
A total of 200 mg of seedlings from both time duration treatments was homogenised in total ionic strength adjustment buffer (TISAB, pH 5.2) followed by estimation of F in the homogenate using F-sensitive electrode (Singh et al. 2020). The level of F accumulated in the seedlings was expressed as mg kg−1 FW.
Parameters related to oxidative damages
Chlorophyll loss in the seedlings was estimated following Paul and Roychoudhury (2017). Formation of H2O2 and superoxide anion in the tissues was estimated following Yadu et al. (2017). Electrolyte leakage and MDA formed in the tissues was determined following Singh et al. (2020). The level of MG formed in the tissues was estimated following Singh and Roychoudhury (2020). The work of Basu et al. (2012) was followed for the determination of LOX activity. NOX activity was measured at 470 nm, following the work of Majumdar and Kar (2018).
Estimation of sulfur-containing compounds and related enzymes
The level of Cys present in the seedlings was estimated using the procedure described by Gaitonde (1967). Concentration of H2S was obtained following Li (2015). The activity of ATP-S and OAS-TL was determined following Santiago et al. (2020). Activity of DES was determined by obtaining the absorbance of samples at 667 nm, following Li et al. (2012).
Activity of enzymatic antioxidants
Activity of SOD was estimated by recording the absorbance of sample at 560 nm, following Singh et al. (2020). For estimation of APX and CAT activities, the protocols of Campos et al. (2019) was followed. GPoX activity was determined at 470 nm, as described by Singh et al. (2020). GPX activity was measured at 340 nm, following the work of Banerjee and Roychoudhury (2019).
Estimation of non-enzymatic antioxidants
The level of anthocyanins was measured at 525 nm, following the protocol of Singh and Roychoudhury (2020). Flavonoids formed in the seedlings were estimated at 415 nm (Singh et al. 2020). 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) scavenging activity and ferric reducing antioxidant power (FRAP) of the seedlings were estimated, following the work of Lalhminghlui and Jagetia (2018).
In-gel isozyme detection
Isozyme analyses of enzymatic antioxidants (SOD, APX, CAT, GPoX, and GPX) were carried out by electrophoresis at 4°C in 10% (w/v) native polyacrylamide gel. To analyze band of SOD isozymes, the gel was incubated in dark (Beauchamp and Fridovich 1973). For APX detection, the gel was treated according to the protocol of Yoshimura et al. (2000). For CAT detection, the protocol developed by Woodbury et al. (1971) was used. Activity of GPoX was examined by equilibrating the gel according to the protocol described by Banerjee and Roychoudhury (2019). For visualization of GPX isozymes, we followed the protocol described by Lin et al. (2002).
Immunoblot analysis
For analysis of accumulation of FEX and H+-ATPase, 100 μg of protein from seedlings was loaded in sodium dodecyl sulfate (SDS)-polyacrylamide gel followed by transfer to nitrocellulose membrane (GE Healthcare, USA), using Mini Trans-Blot Cell (Bio-Rad, USA) (Banerjee et al. 2019). The membrane was incubated with specific antibodies and the protein bands were visualized after staining the membrane in substrate solution containing nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP). The anti-histone H3 antibody was used as the control.
Expression analysis of transporter genes
A total of 100 mg of the seedlings was used for RNA extraction following the protocol described by Paul and Roychoudhury (2018). Isolated RNA (5 μg) was reverse-transcribed to monitor gene expression, using primers specific for the genes. β-actin was used as internal control for all the genes.
Protein estimation
Protein isolated from the seedlings was estimated using Bradford reagent (Bradford 1976). Equal amount of protein was used for the determination of enzyme activity or for immunoblot analysis.
Results and discussion
Soil pollution due to irrigation with F-contaminated water is relatively less explored. In recent times, several investigations are being carried out to elucidate the effects of F pollution on surrounding environment and on plants. Prolonged exposure of plants to F leads to its uptake in tissues via chloride channels that eventually lead to the generation of ROS and other cytotoxic metabolites like MDA and MG (Singh and Roychoudhury 2020). To ensure protection from environmental stress, plants regulate the level of endogenous defensive metabolites such as enzymatic and non-enzymatic antioxidants. Antioxidants scavenge excess ROS formed in plants and also aid in maintaining the integrity of lipid membranes (Singh et al. 2020). Macro-elements such as S also regulate the normal growth and development of plants by controlling various biological functions under stressed environment (Ernst et al. 2008; Kumaran et al. 2008). Previously, we showed the toxic effect of F (25 mg L−1 NaF) treatment on various rice varieties (Banerjee et al. 2019; Singh and Roychoudhury 2023). However, to the best of our knowledge, there is currently no adequate study showing varietal response of rice plants to F, applied for varying time periods. Therefore, our study was designed to explore the physiological and molecular responses of two rice varieties (Khitish and MTU1010), when exposed to F for 10 and 20 days. We also attempted to identify the role of protective metabolites and enzymes involved in S cycle in curbing the effects of F toxicity.
Uptake and accumulation of F− ions
When treated with NaF solution for 10 days, the growth of Khitish seedlings was stunted; in contrast, MTU1010 seedlings showed no stunting in growth. When compared to control seedlings, stunting was not observed in 20-day-old Khitish seedlings, whereas the growth of MTU1010 seedlings (20-day-old) was significantly reduced (Fig. 1).
Effect of application of 25 mg L−1 NaF on growth of Khitish and MTU1010 rice seedlings after 10 and 20 days. Controls were seedlings grown in water.
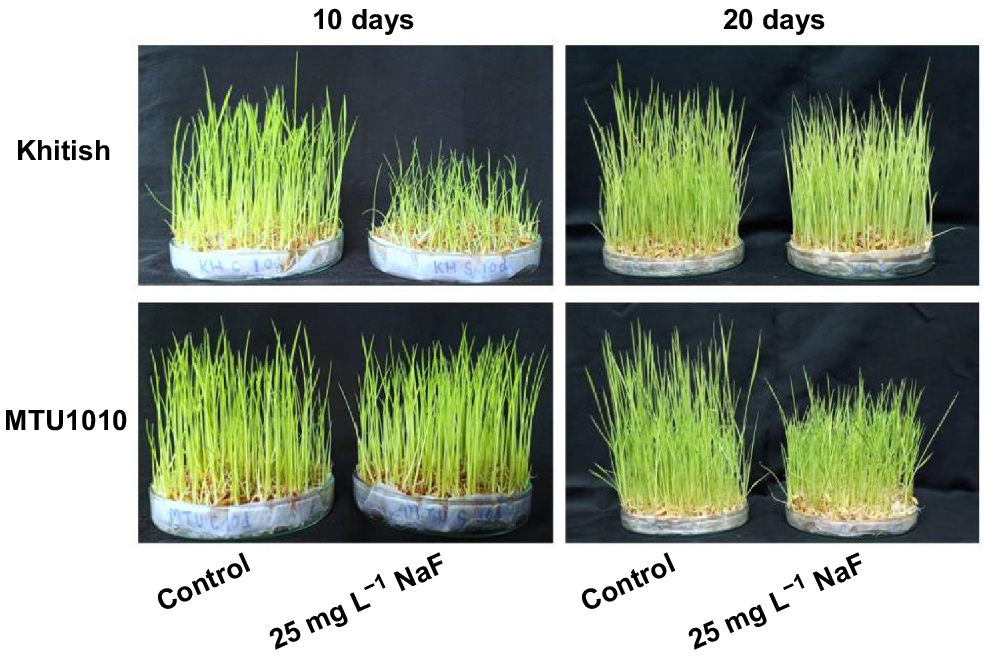
Reduced growth of 10-day-old Khitish and 20-day-old MTU1010 seedlings could be attributed to the 27.7-fold and 40.1-fold higher accumulation of F− ions, respectively (Fig. 2a). Higher uptake of F− ions by seedlings could be linked with the higher expression of CLC1 and CLC2 genes, which facilitated the uptake of F− ions via roots (Fig. 2b, c). Our results were in line with the previous work of Banerjee et al. (2019) where they showed that higher accumulation of F− ions in two different rice varieties (IR-64 and Gobindobhog) was facilitated by these chloride channels. They further supported their observation with the fact that chloride channels have larger passage diameter when compared to the size of F− ions and thus can facilitate the uptake of F− ions. In contrast, regulated uptake of F− ions, as seen in 20-day-old Khitish and 10 day-old MTU1010 seedlings may be due to down regulation of both the chloride channels in the two varieties. In addition to chloride channel, analysis of the expression pattern (at both gene and protein level) of H+-ATPase (involved in maintaining the proton gradient across the cellular membrane) and FEX (selectively exporting F− ions) revealed that both the transporters were induced in expression in the stressed 20-day-old Khitish and 10-day-old MTU1010 seedlings when compared to the corresponding same varieties at 10 days and 20 days, respectively (Fig. 2d–g). Higher expression of H+-ATPase and FEX plays a pivotal role in stressed seedlings in regulating the cellular homeostasis. Earlier, Baunthiyal and Sharma (2014) reported that higher expression of H+-ATPase was important in regulating the tolerance potential of three semi-arid F− hyperaccumulator plants (Cassia fistula, Acacia tortilis, and Prosopis juliflora) when exposed to F stress. Similarly, in another study, Banerjee et al. (2019) observed higher expression of H+-ATPase in the stressed seedlings of the tolerant variety when compared to stressed seedlings of a sensitive variety. Recently, Agarwal et al. (2021) reported that the expression of FEX was highly induced in Vigna radiata that assisted in the exclusion of the accumulated F- ions from the plasma membrane of cells, imparting higher survival rate to the seedlings. Banerjee and Roychoudhury (2021) also demonstrated that overexpression of FEX in Nicotiana benthamiana was efficient to promote fluoride tolerance, which is in agreement with our observation. Thus, unregulated uptake of F− ions in 10-day-old Khitish seedlings via CLC1 and CLC2 was responsible for stunted growth of the seedlings; however, the seedlings were less affected during long term (20 days) exposure to F because of the enhanced expression of H+-ATPase and FEX that maintained cellular homeostasis. Moreover, downregulated expression of CLC1 and CLC2 also controlled the uptake of F− ions. However, contrasting behavior was noted in MTU1010 seedlings where the entry of F− ions was initially regulated due to reduced expression of chloride channels. However, with longer exposure (20 days), this regulation proved ineffective, which resulted in higher accumulation of F− ions in seedlings that eventually led to significant stunting in seedlings of this variety.
Fluoride accumulation (a), expression pattern of genes CLC1 (b), CLC2 (c), H+-ATPase (d), and FEX (e), and the accumulation of H+-ATPase (f) and FEX (g) proteins in Khitish and MTU1010 seedlings grown in 25 mg L−1 NaF at 10 days and 20 days. *P ≤ 0.05 between control and stressed seedlings; #P ≤ 0.05 between the two varieties; +P ≤ 0.05 between the seedlings grown for 10 and 20 days. Histone H3 was used as a control in immunoblot analysis to check equal loading of protein (h); KT represents Khitish and M1010 represents MTU1010 (applicable for f, g, and h).

Higher F accumulation leads to higher oxidative damages
Higher accumulation of F− ions in 10-day-old Khitish and 20-day-old MTU1010 seedlings led to the enhanced formation of ROS such as H2O2 (2.7-fold and 2.4-fold, respectively) and superoxide radicals (2.0-fold and 2.4-fold, respectively), which caused peroxidation of lipid membrane, leading to 2.5 and 2.6 times higher leakage of electrolytes from the respective cells. This also induced the formation of cytotoxic metabolites like MG and MDA in the stressed seedlings when compared to the control (non-stressed) seedlings. Higher accumulation of ROS also led to 1.3 and 1.6 times higher chlorophyll loss in 10-day-old Khitish and 20-day-old MTU1010 seedlings under F stress. A similar trend was also noted in case of LOX and NOX activity where exposure to F stress enhanced their activity by 2.2 and 2.0 fold, respectively, in 10-day-old Khitish, and by 2.8 and 2.0 fold, respectively, in 20-day-old MTU1010 seedlings (Table 1). Higher activity of LOX and NOX induced the accumulation of ROS in seedlings, which further affected plant growth. A similar response was reported by Singh and Roychoudhury (2020) where it was shown that higher F accumulation in NaF-treated rice variety resulted in higher formation of H2O2 in seedlings, which hampered the overall integrity of cell membrane, leading to higher electrolyte leakage along with induced formation of MDA and MG, higher chlorophyll loss, and LOX activity. An opposite trend was noted for 20-day-old Khitish and 10-day-old MTU1010 seedlings, which may be due to controlled uptake of F− ions, restricting the formation of ROS with lower extent of peroxidation of lipid membrane and reduced formation of cytotoxic metabolites (Table 1). Our observation supports the findings of Banerjee and Roychoudhury (2019) where it was reported that F− accumulation in the tolerant rice variety was significantly lower than the susceptible variety when treated with 15 and 25 mg L−1 NaF solution, causing lower oxidative damage and maintaining the growth of the seedlings.
| Parameters | 10 days | 20 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | ||
| Chlorophyll (μg g−1 FW) | 100.67 ± 10.98 | 78.98 ± 9.87* | 110.87 ± 8.87 | 89.98 ± 6.87 | 150.87 ± 11.87+ | 134.89 ± 12.36*+ | 165.98 ± 9.87+ | 100.87 ± 12.98*#+ | |
| H2O2 (μM g−1 FW) | 0.13 ± 0.04 | 0.35 ± 0.01* | 0.15 ± 0.02 | 0.21 ± 0.03# | 0.19 ± 0.04 | 0.29 ± 0.06 | 0.17 ± 0.05 | 0.40 ± 0.08*#+ | |
| Superoxide anion (μM g−1 FW) | 2.12 ± 0.12 | 4.19 ± 0.67* | 2.09 ± 0.98 | 3.16 ± 0.87 | 2.68 ± 0.57 | 4.26 ± 0.46* | 2.87 ± 0.33 | 6.78 ± 0.82*#+ | |
| Electrolyte leakage (%) | 15.31 ± 1.21 | 39.12 ± 1.56* | 14.98 ± 2.12 | 21.87 ± 1.67# | 15.98 ± 1.45 | 35.65 ± 2.87* | 16.90 ± 1.20 | 43.61 ± 1.43*+ | |
| MDA (μM g−1 FW) | 2.11 ± 0.98 | 4.89 ± 0.87* | 1.97 ± 0.43 | 2.98 ± 0.57# | 2.56 ± 0.52 | 4.15 ± 0.87* | 2.19 ± 0.45 | 6.86 ± 3.38*#+ | |
| MG (mg g−1 FW) | 24.89 ± 1.56 | 35.18 ± 1.17* | 21.87 ± 0.98 | 28.19 ± 1.56# | 26.92 ± 1.19 | 39.87 ± 0.59* | 24.87 ± 1.67 | 40.97 ± 1.49*#+ | |
| LOX activity (unit mg−1 leaf protein) | 5.87 ± 0.45 | 12.87 ± 0.98* | 4.98 ± 0.87 | 8.98 ± 0.34# | 6.89 ± 1.11 | 15.98 ± 0.76* | 6.98 ± 0.15 | 19.98 ± 1.78*+ | |
| NOX activity (unit mg−1 leaf protein) | 22.27 ± 1.19 | 43.87 ± 0.95* | 24.87 ± 1.67 | 34.98 ± 0.56# | 27.98 ± 0.81 | 37.98 ± 1.39 | 29.76 ± 2.67 | 59.67 ± 1.33*#+ | |
Data represented are the mean value (n = 3) ± s.e.
*P ≤ 0.05 between control and stressed seedlings; #P ≤ 0.05 between the two varieties; +P ≤ 0.05 between seedlings grown for 10 and 20 days.
Role of S in regulating the tolerance mechanism of seedlings
Plants absorb a wide range of macro and micro nutrients from the soil that enable them to survive under harsh environmental conditions by enhancing the functioning of protective machineries. Sulfur, being one such essential macronutrient, serves numerous plant functions and is vital for the metabolic processes. When exposed to F toxicity, there was lowering in the activity of the major S assimilatory enzymes; i.e. ATP-S (1.4 and 1.2 times, respectively) and OASTL (1.6 and 1.3 times, respectively) that led to decrease in the formation of Cys by 1.6 and 1.3 folds, respectively in 10-day-old Khitish and 20-day-old MTU1010 seedlings, thus reducing their tolerance level under F stress (Table 2). Our results support the findings of Santiago et al. (2020) where it was shown that higher ATP-S and OASTL activity was observed in two varieties of arugula (Eruca sativa) compared to two varieties of lettuce (Lactuca sativa), which maintained the tolerance level of the seedlings when treated with various concentration of selenate (Na2SeO4). Contrasting results were noted in case of 20-day-old Khitish and 10-day-old MTU1010 seedlings, explaining the role of S as an important protective element against F-toxicity. Another important S-containing metabolite that also acts as a major signalling molecule is H2S. In presence of F stress, the level of H2S was decreased by 1.5 and 1.3 times in 10-day-old Khitish and 20-day-old MTU1010 seedlings, respectively, which may be due to reduced formation of Cys that acts as a precursor of H2S or it can also be attributed to the reduced activity of DES (the sole enzyme responsible for the generation of H2S in plant cytosol) by 1.8 and 1.7 folds, respectively. On the contrary, higher H2S level was noted in 20-day-old stressed Khitish seedlings, which might be due to enhanced DES activity, when compared to 10-day-old stressed seedlings, that eventually contributed towards higher tolerance level in the former. Reduced DES activity and H2S level in 20-day-old MTU1010 seedlings, with respect to 10-day-old seedlings, heightened the F-induced damage in the older seedlings (Table 2). Kushwaha and Singh (2020) also observed that the treatment of tomato (Solanum lycopersicum), pea (Pisum sativum), and brinjal (Solanum melongena) seedlings with 25 μM potassium dichromate inhibited the formation of Cys and H2S in leaves and roots. The activity of DES was restored in the stressed seedlings when treated with potassium sulfate (additional source of S), which ultimately resulted in higher formation of H2S, thereby maintaining the tolerance capability of seedlings.
| Parameters | 10 days | 20 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Khitsh control | Khitish stress | MTU1010 control | MTU1010 stress | Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | ||
| Cys (μg g−1 FW) | 1.54 ± 0.12 | 0.98 ± 0.09* | 1.67 ± 0.28 | 1.78 ± 0.12# | 2.78 ± 0.09 | 4.77 ± 0.15*+ | 2.56 ± 0.20 | 2.01 ± 0.21# | |
| H2S (μM g−1 FW) | 8.97 ± 0.54 | 5.78 ± 0.65* | 7.98 ± 0.76 | 10.98 ± 1.12# | 11.94 ± 1.29 | 17.89 ± 0.98*+ | 12.76 ± 0.67+ | 9.67 ± 0.32# | |
| ATP-S activity (μM PPi min−1 mg−1 leaf protein) | 6.78 ± 0.38 | 4.76 ± 0.87* | 7.98 ± 0.37 | 10.67 ± 0.76# | 7.98 ± 1.10 | 12.87 ± 0.98*+ | 9.67 ± 0.99 | 8.14 ± 1.09 | |
| OAS-TL activity (μM Cys min−1 mg−1 leaf protein) | 3.12 ± 0.76 | 1.98 ± 0.16* | 4.67 ± 0.18 | 5.45 ± 0.38# | 3.98 ± 0.18 | 5.76 ± 0.67*+ | 5.12 ± 0.17 | 3.98 ± 0.87*+ | |
| DES activity (μM H2S min−1 mg−1 leaf protein) | 0.98 ± 0.01 | 0.54 ± 0.07 | 1.12 ± 0.12 | 1.08 ± 0.06*# | 1.54 ± 0.09 | 1.98 ± 0.06*+ | 1.43 ± 0.03 | 0.86 ± 0.01*# | |
Data represented are the mean value (n = 3) ± s.e.
*P ≤ 0.05 between control and stressed seedlings; #P ≤ 0.05 between the two varieties; +P ≤ 0.05 between seedlings grown for 10 and 20 days.
Enzymatic antioxidants regulating the level of ROS in seedlings
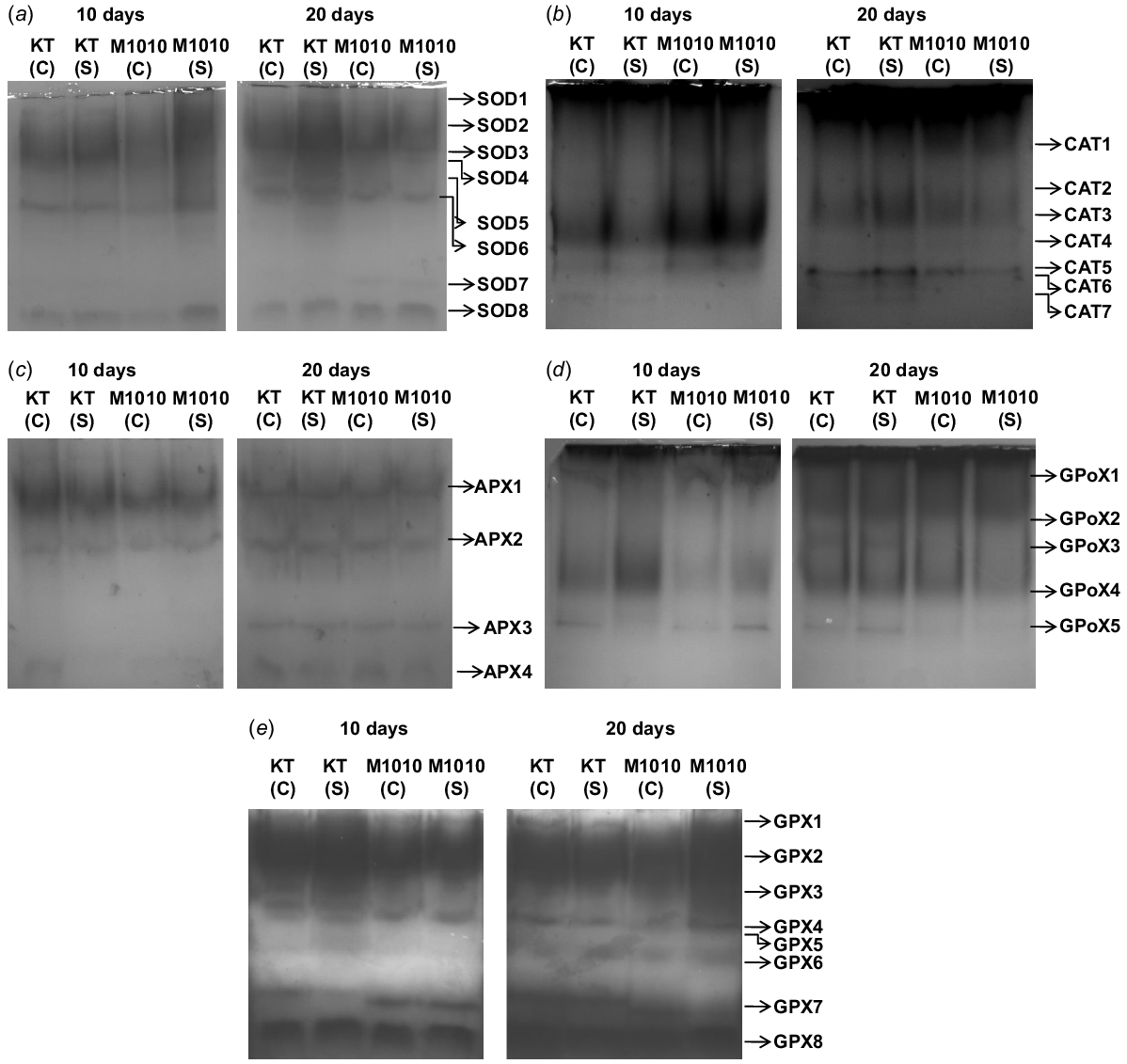
Enzymatic antioxidants play a pivotal role in regulating the stress tolerance capability of plants by scavenging the free radicals formed, thus maintaining cellular homeostasis and redox status. In F-stressed Khitish and MTU1010 seedlings, the activity of SOD was enhanced by 1.8 and 2.4 folds, respectively, in 10-day-old seedlings, and by 2.2 and 1.4 folds, respectively, in 20-day-old seedlings, which enabled detoxification of superoxide anions. The tolerance in 20-day-old Khitish and 10-day-old MTU1010 seedlings was governed by the rise in the activity of the enzyme (Table 3). Similar trend was noted with isozyme profiling, where eight different isoforms of SOD were observed. Of all the isoforms, SOD4 and SOD5 were prominently enhanced in 20-day-old Khitish and 10-day-old MTU1010 seedlings, respectively (Fig. 3a). SOD converts superoxide anions into H2O2, which is detoxified by CAT, APX, GPoX, and GPX into non toxic metabolites. Almost 1.3, 1.4, and 1.5 times higher activity of APX, GPoX, and GPX, respectively, was noted in 10-day-old Khitish seedlings, whereas the activity of these enzymes was enhanced by 1.4, 1.6, and 1.6 times in 20-day-old Khitish seedlings, when compared to the control seedlings. This suggested that the enhanced activity of enzymatic antioxidants helped in the adaptation of seedlings to F stress. Similarly, the activity of these protective enzymes was increased by 1.7, 2.2, and 1.4 folds, respectively, in 10-day-old MTU1010 seedlings, whereas in 20-day-old stressed seedlings, the respective rise in the activity of the enzymes was only 1.1, 1.6 and 1.3 times, when compared to water-treated seedlings. In case of MTU1010, the activity of enzymatic antioxidants did not rise significantly, leading to the higher accumulation of ROS, thereby increasing the susceptibility of the seedlings (Table 3). The enhanced activity of enzymatic antioxidants is corroborated by the findings of Banerjee and Roychoudhury (2019) who showed that F stress significantly enhanced the activity of the antioxidative enzymes in the tolerant cultivar Gobindobhog; whereas in case of the susceptible variety (IR-64), such induction in the activity of the enzymatic antioxidants was insignificant, leading to higher damage in the seedlings. Contrasting results were noted with regard to CAT activity where F stress inhibited its activity by 1.5 and 1.3 folds in 10-day-old Khitish and 20-day-old MTU1010 seedlings, respectively, whereas no such inhibition was noted in 20-day-old Khitish and 10-day-old MTU1010 seedlings, respectively (Table 3). Our observation of reduced CAT activity is supported by the previous observation of Kumar et al. (2009) where it was shown that F− ions replaced the hydroxyl moiety present in the ferric group, residing at the enzyme active site, which was responsible for maintaining the active conformation of this enzyme. Similar results were also demonstrated by Singh and Roychoudhury (2023) where higher accumulation of F in rice seedlings restricted the activity of CAT, whereas the activity of APX, SOD, GPX, and GPoX was induced in seedlings. They further demonstrated that in presence of a protective agent (salicylic acid), the activity of these enzymatic antioxidants along with CAT was further enhanced that abrogated the F-induced damage in the seedlings. Isozyme analysis of APX revealed four isoforms in seedlings, where APX3 isoform was only detected in 20-day-old seedlings of both the varieties. In case of GPoX, five isoforms were noted. GPoX3 was observed in only 20-day-old Khitish seedlings along with higher induction of GPoX5, which was absent in 10-day-old Khitish seedlings. This suggested that GPoX3 and GPoX5 might contribute to tolerance of the seedlings. In case of GPX, eight isoforms were observed and activity of GPX7 was reduced in 10-day-old Khitish and 20-day-old MTU1010 seedlings, respectively, whereas contrasting effect was noted in case of 20-day-old Khitish and 10-day-old MTU1010 seedlings. This suggested the involvement of GPX7 isoform in F tolerance. Analysis of CAT isozyme showed seven different isoforms, of which CAT4 was reduced in 10-day-old Khitish seedlings. Two new isoforms (CAT6 and CAT7) were observed in 20-day-old Khitish seedlings, which suggested the importance of these two isoforms in ameliorating the adverse effects of F stress (Fig. 3).
| Parameters | 10 days | 20 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | ||
| SOD activity (unit mg−1 protein) | 2.12 ± 0.12 | 3.78 ± 0.34 | 1.98 ± 0.14 | 4.67 ± 0.19* | 2.67 ± 0.78 | 5.98 ± 0.98*+ | 2.76 ± 0.37 | 3.97 ± 0.67# | |
| CAT activity (μM H2O2 min−1 mg−1 protein) | 20.89 ± 1.98 | 14.09 ± 0.87* | 22.98 ± 1.92 | 30.17 ± 2.87*# | 28.17 ± 2.87 | 35.38 ± 1.54*+ | 27.89 ± 0.46 | 21.08 ± 1.74*+ | |
| APX activity (μM ascorbate min−1 mg−1 protein) | 168.98 ± 10.27 | 220.72 ± 12.82 | 146.83 ± 11.87 | 250.98 ± 9.76* | 219.67 ± 8.8 | 298.67 ± 12.29*+ | 200.98 ± 9.46 | 230.56 ± 12.78# | |
| GPoX activity (μM tetraguaiacolmin−1 mg−1 protein) | 15.87 ± 1.53 | 22.63 ± 1.89 | 12.37 ± 0.78 | 26.82 ± 1.01* | 19.65 ± 0.67 | 31.67 ± 1.17*+ | 13.87 ± 1.98 | 22.29 ± 0.83*# | |
| GPX activity (μM NADPH min−1 mg−1 protein) | 45.98 ± 2.76 | 69.18 ± 3.82* | 51.72 ± 3.16 | 72.82 ± 3.91* | 61.98 ± 1.87 | 98.37 ± 2.82*+ | 57.19 ± 3.28 | 75.38 ± 2.72*# | |
Data represented are the mean value (n = 3) ± s.e.
*P ≤ 0.05 between control and stressed seedlings; #P ≤ 0.05 between the two varieties; +P ≤ 0.05 between seedlings grown for 10 and 20 days.
F stress regulates the level of non-enzymatic antioxidants in seedlings
Along with enzymatic antioxidants, plants also harbor several endogenous protective metabolites that help in ameliorating the damaging effects of stress. One such group of protective metabolite constitutes the non-enzymatic antioxidants that regulate the expression of genes associated with stress tolerance in plants (Waśkiewicz et al. 2014). Almost 1.3 and 1.2 times enhanced level of anthocyanins and flavonoids, respectively, was noted in 10-day-old stressed seedlings, when compared to water-treated seedlings of Khitish. Exposure of seedlings to F for 20 days further enhanced the level of these metabolites by 1.7 and 1.4 folds, respectively, in Khitish. This suggested that the F-induced damages were mitigated by magnifying the amount of such protective metabolites (Table 4). In case of MTU1010 seedlings, the level of anthocyanins and flavonoids was enhanced by 1.5 and 1.5 folds, respectively, in 10-day-old stressed seedlings, whereas lower increments (1.4 and 1.2 folds) were observed in 20-day-old seedlings, which suggested that with the progression in duration of stress, there was reduced accumulation of metabolites, when compared to early response (10 days) that ultimately compromised the tolerance level of this variety (Table 4). Higher content of anthocyanins and flavonoids in 20-day-old Khitish and 10-day-old MTU1010 seedlings could be further linked with higher assimilation of S-containing compounds. Lian et al. (2023) also showed that application of H2S enhanced the level of anthocyanins and flavonoids, which could be correlated with the higher activity of phenylalanine ammonia lyase (an essential enzyme involved in the synthesis of secondary metabolites). Similarly, Zahid et al. (2024) showed that the application of S-rich thiourea enhanced the level of non-enzymatic antioxidants that lowered the damaging effects of cobalt stress in wheat seedlings. Our observation agrees with that of Singh and Roychoudhury (2020) where it was shown that in F-stressed seedlings, the level of anthocyanins and flavonoids was enhanced in Khitish and their levels could be elevated in presence of some exogenous protective agents like silicon, leading to higher tolerance level. The enhancements in the level of these antioxidants also led to the increment in ABTS scavenging activity and FRAP in 10-day-old Khitish seedlings by 1.1 and 1.3 folds, respectively, and by 1.7 and 1.3 folds, respectively, in 20-day-old Khitish seedlings, which indicated higher antioxidative potential as adaptive strategy against F stress. In the case of MTU1010 seedlings, ABTS scavenging activity and FRAP were enhanced by 1.7 and 1.3 folds, respectively, in 10-day-old stressed seedlings, and by 1.5 and 1.4 folds, respectively, in 20-day-old stressed seedlings, showing that the antioxidative potential was more compromised during long-term exposure of MTU1010 seedlings to F stress, with pronounced susceptibility (Table 4). A similar trend was also earlier reported by Banerjee and Roychoudhury (2019) where they demonstrated that the radical scavenging potential was reduced in the susceptible variety, as compared to that of the tolerant variety, where significant enhancement in antioxidative potential enhanced the tolerance capability.
| Parameters | 10 days | 20 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | Khitish control | Khitish stress | MTU1010 control | MTU1010 stress | ||
| Anthocyanins (μM g−1 FW) | 20.92 ± 1.28 | 27.19 ± 2.28 | 19.65 ± 1.63 | 30.38 ± 1.83*# | 25.28 ± 2.29 | 42.29 ± 2.98*#+ | 27.29 ± 1.19 | 38.29 ± 1.80 | |
| Flavonoids (μg g−1 FW) | 182.28 ± 7.38 | 220.28 ± 9.82* | 167.38 ± 8.34 | 250.38 ± 9.27* | 200.28 ± 10.28 | 289.29 ± 12.29*+ | 187.29 ± 12.10 | 229.29 ± 10.18# | |
| ABTS scavenging activity (mg ascorbic acid equivalents 100 g−1 FW) | 6.98 ± 0.91 | 7.78 ± 1.01 | 5.87 ± 0.87 | 10.27 ± 1.12*# | 7.98 ± 1.01 | 13.58 ± 0.98*+ | 8.89 ± 0.67+ | 11.38 ± 0.76 | |
| FRAP (mg ferrous sulfate equivalents 100 g−1 FW) | 10.23 ± 0.78 | 12.98 ± 0.67 | 9.38 ± 0.78 | 14.29 ± 1.00* | 11.92 ± 0.67 | 15.38 ± 1.21*+ | 11.27 ± 0.87 | 15.98 ± 0.71* | |
Data represented are the mean value (n = 3) ± s.e.
*P ≤ 0.05 between control and stressed seedlings; #P ≤ 0.05 between the two varieties; +P ≤ 0.05 between seedlings grown for 10 and 20 days.
A model representing the effect of F toxicity in 10-day-old and 20-day-old Khitish and MTU1010 seedlings, grown in 25 mg L−1 NaF is represented in Fig. 4.
Conclusion
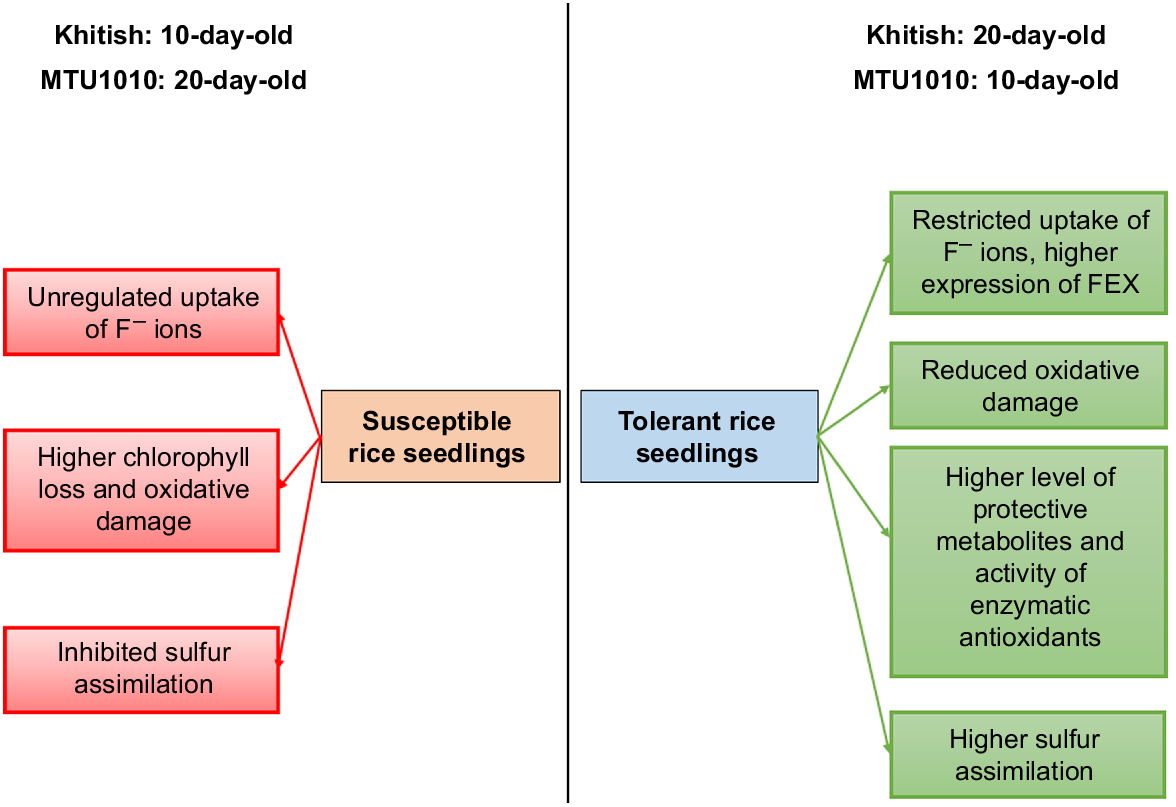
Our study aimed to investigate the genetic and metabolic responses occurring in two different indica rice varieties (Khitish and MTU1010), imposed to F toxicity for 10 and 20 days. We showed that Khitish exhibited tolerance to long-term F stress than MTU1010, which was the more susceptible variety. Initially, Khitish showed higher oxidative damages due to unregulated uptake of F− ions; however, with continued exposure to F for 20 days, this variety showed metabolic adjustments and maintained cellular homeostasis as well as better regulation of F accumulation due to the higher expression of H+-ATPase and FEX. These features characterised Khitish as the more tolerant variety. In contrast, short-term exposure of MTU1010 to F incited less oxidative damages, concomitant with lower F accumulation, which allowed this variety to maintain growth. However, such regulated uptake of F− ions was compromised during prolonged exposure to 20 days when accumulation of toxic metabolites along with higher oxidative damages, reduced the overall growth of the seedlings. These characteristics made MTU1010 to behave more as a susceptible variety. When exposed to 20 days of F stress, the activity of the enzymatic antioxidants was significantly increased in Khitish, compared to MTU1010, enabling Khitish to maintain normal physiology. Similar trend was also noted in non-enzymatic antioxidants. S-containing metabolites such as Cys and H2S played a pivotal role in the survival of the seedlings. In 20-day-old Khitish seedlings, the level of Cys and H2S was enhanced, which was attributed to the higher activity of ATP-S, OASTL, and DES, allowing the seedlings to overcome the detrimental effects of F stress (Fig. 4). Overall, we identified varietal differences between Khitish and MTU1010 in our study. Differential reprogramming of the metabolites and enzymes of S-assimilation pathways, along with antioxidative machineries, were synergistically responsible for the differential pattern of adaptive strategies against F stress in Khitish and MTU1010; Khitish showed resilience against long-term stress, whilst MTU1010 showed high susceptibility. Thus, we conclude that in F-contaminated areas, cultivation of Khitish will be more beneficial for the farmers as it possesses the inherent potential to tolerate F stress for prolonged duration, as compared to that of MTU1010. The genes that control the S metabolic pathway and formation of protective metabolites in Khitish and were upregulated during F stress, may potentally be overexpressed in the susceptible rice varieties to enhance F tolerance.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that there is no conflict of interest in publishing this manuscript.
Declaration of funding
Financial assistance from Science and Engineering Research Board (SERB), Government of India through the grant EMR/2016/004799 and Department of Higher Education, Science and Technology and Biotechnology, Government of West Bengal (DHESTBT) through the grant 264(Sanc.)/ST/P/S&T/1G-80/2017 is gratefully acknowledged. The authors thank Dr. Marc Boutry, Unité de Biochimie Physiologique, Université Catholique de Louvain, Belgium, for providing the anti-P-H+/ATPase primary antibody as a gift.
Author contributions
Ankur Singh performed all the experiments, generated the data, designed the figures and drafted the manuscript. Aditya Banerjee assisted in the generation of anti-FEX antibody and immunoblot analysis. Prof. Aryadeep Roychoudhury supervised the entire work, arranged all resources and fundings, and made necessary corrections within the manuscript.
Reference
Agarwal S, Regon P, Rehman M, Tanti B, Panda SK (2021) Genome-wide analysis of fluoride exporter genes in plants. 3 Biotech 11(3), 124.
| Crossref | Google Scholar | PubMed |
Banerjee A, Roychoudhury A (2019) Differential regulation of defence pathways in aromatic and non-aromatic indica rice cultivars towards fluoride toxicity. Plant Cell Reports 38(10), 1217-1233.
| Crossref | Google Scholar | PubMed |
Banerjee A, Roychoudhury A (2021) Functional and molecular characterization of fluoride exporter (FEX) from rice and its constitutive overexpression in Nicotiana benthamiana to promote fluoride tolerance. Plant Cell Reports 40(9), 1751-1772.
| Crossref | Google Scholar | PubMed |
Banerjee A, Roychoudhury A, Ghosh P (2019) Differential fluoride uptake induces variable physiological damage in a non-aromatic and an aromatic indica rice cultivar. Plant Physiology and Biochemistry 142, 143-150.
| Crossref | Google Scholar | PubMed |
Basu S, Roychoudhury A, Sanyal S, Sengupta DN (2012) Carbohydrate content and antioxidative potential of the seed of three edible indica rice (Oryza sativa L.) cultivars. Indian Journal of Biochemistry & Biophysics 49(2), 115-123.
| Google Scholar |
Baunthiyal M, Sharma V (2014) Response of fluoride stress on plasma membrane H+-ATPase and vacuolar H+-ATPase activity in semi-arid plants. Indian Journal of Plant Physiology 19, 210-214.
| Crossref | Google Scholar |
Beauchamp CO, Fridovich I (1973) Isozymes of superoxide dismutase from wheat germ. Biochimica et Biophysica Acta (BBA) - Protein Structure 317(1), 50-64.
| Crossref | Google Scholar | PubMed |
Bhattacharya P, Samal AC (2018) Fluoride contamination in groundwater, soil and cultivated foodstuffs of India and its associated health risks: a review. Research Journal of Recent Sciences 7(4), 36-47.
| Google Scholar |
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1–2), 248-254.
| Crossref | Google Scholar | PubMed |
Campos FV, Oliveira JA, Pereira MG, Farnese FS (2019) Nitric oxide and phytohormone interactions in the response of Lactuca sativa to salinity stress. Planta 250(5), 1475-1489.
| Crossref | Google Scholar | PubMed |
Corpas FJ (2019) Hydrogen sulfide: a new warrior against abiotic stress. Trends in Plant Science 24(11), 983-988.
| Crossref | Google Scholar | PubMed |
Davidian J-C, Kopriva S (2010) Regulation of sulfate uptake and assimilation—the same or not the same? Molecular Plant 3(2), 314-325.
| Google Scholar |
De A, Mridha D, Ray I, Joardar M, Das A, Chowdhury NR, Roychowdhury T (2021) Fluoride exposure and probabilistic health risk assessment through different agricultural food crops from fluoride endemic Bankura and Purulia districts of West Bengal, India. Frontiers in Environmental Science 9, 713148.
| Crossref | Google Scholar |
Ernst WHO, Krauss G-J, Verkleij JAC, Wesenberg D (2008) Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant, Cell & Environment 31(1), 123-143.
| Google Scholar |
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochemical Journal 104(2), 627-633.
| Crossref | Google Scholar | PubMed |
Gautam R, Bhardwaj N, Saini Y (2010) Fluoride accumulation by vegetables and crops grown in Nawa Tehsil of Nagaur District (Rajasthan, India). J Phytol 2(2), 80-85.
| Google Scholar |
Ghassemi-Golezani K, Farhangi-Abriz S (2019) Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus tinctorius L.) seedlings. Chemosphere 223, 406-415.
| Crossref | Google Scholar | PubMed |
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signaling & Behavior 6(2), 215-222.
| Crossref | Google Scholar | PubMed |
Hancock JT (2019) Hydrogen sulfide and environmental stresses. Environmental and Experimental Botany 161, 50-56.
| Crossref | Google Scholar |
Hasanuzzaman M, Hossain M, Bhuyan M, Al Mahmud J, Nahar K, Fujita M (2018) The role of sulfur in plant abiotic stress tolerance: molecular interactions and defense mechanisms. In ‘Plant nutrients and abiotic stress tolerance’. (Eds M Hasanuzzaman, M Fujita, H Oku, K Nahar, B Hawrylak-Nowak) pp. 221–252. (Springer: Singapore)
Hong B-D, Joo R-N, Lee K-S, Lee D-S, Rhie J-H, Min S-W, Song S-G, Chung D-Y (2016) Fluoride in soil and plant. Korean Journal of Agricultural Science 43, 522-536.
| Crossref | Google Scholar |
Kumar KA, Varaprasad P, Rao AVB (2009) Effect of fluoride on catalase, guaiacol peroxidase and ascorbate oxidase activities in two varieties of mulberry leaves (Morus alba L.). Research Journal of Earth Sciences 1(2), 69-73.
| Google Scholar |
Kushwaha BK, Singh VP (2020) Glutathione and hydrogen sulfide are required for sulfur-mediated mitigation of Cr(VI) toxicity in tomato, pea and brinjal seedlings. Physiologia Plantarum 168(2), 406-421.
| Crossref | Google Scholar | PubMed |
Lalhminghlui K, Jagetia GC (2018) Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Science OA 4(2), FSO272.
| Google Scholar |
Leustek T, Murillo M, Cervantes M (1994) Cloning of a cDNA encoding ATP sulfurylase from Arabidopsis thaliana by functional expression in Saccharomyces cerevisiae. Plant Physiology 105(3), 897-902.
| Google Scholar |
Li Z-G (2015) Quantification of hydrogen sulfide concentration using methylene blue and 5,5′-dithiobis (2-Nitrobenzoic Acid) methods in plants. Methods in Enzymology 554, 101-110.
| Crossref | Google Scholar | PubMed |
Li Z-G, Gong M, Liu P (2012) Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha curcas. Acta Physiologiae Plantarum 34(6), 2207-2213.
| Crossref | Google Scholar |
Lian H, Qin C, Shen J, Ahanger MA (2023) Alleviation of adverse effects of drought stress on growth and nitrogen metabolism in mungbean (Vigna radiata) by sulphur and nitric oxide involves up-regulation of antioxidant and osmolyte metabolism and gene expression. Plants 12(17), 3082.
| Crossref | Google Scholar | PubMed |
Liang T, Ding H, Wang G, Kang J, Pang H, Lv J (2016) Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicology and Environmental Safety 124, 129-137.
| Crossref | Google Scholar | PubMed |
Lin C-L, Chen H-J, Hou W-C (2002) Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecyl sulfate polyacrylamide gels. Electrophoresis 23(4), 513-516.
| Crossref | Google Scholar | PubMed |
Lou L, Kang J, Pang H, Li Q, Du X, Wu W, Chen J, Lv J (2017) Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. International Journal of Molecular Sciences 18(8), 1628.
| Crossref | Google Scholar | PubMed |
Majumdar A, Kar RK (2018) Congruence between PM H+-ATPase and NADPH oxidase during root growth: a necessary probability. Protoplasma 255, 1129-1137.
| Crossref | Google Scholar | PubMed |
Niu H, Zhan K, Xu W, Peng C, Hou C, Li Y, Hou R, Wan X, Cai H (2020) Selenium treatment modulates fluoride distribution and mitigates fluoride stress in tea plant (Camellia sinensis (L.) O. Kuntze). Environmental Pollution 267, 115603.
| Crossref | Google Scholar | PubMed |
Paul S, Roychoudhury A (2017) Seed priming with spermine and spermidine regulates the expression of diverse groups of abiotic stress-responsive genes during salinity stress in the seedlings of indica rice varieties. Plant Gene 11, 124-132.
| Crossref | Google Scholar |
Paul S, Roychoudhury A (2018) Transcriptome profiling of abiotic stress-responsive genes during cadmium chloride-mediated stress in two indica rice varieties. Journal of Plant Growth Regulation 37, 657-667.
| Crossref | Google Scholar |
Pelc J, Śnioszek M, Wróbel J, Telesiński A (2020) Effect of fluoride on germination, early growth and antioxidant enzymes activity of three winter wheat (Triticum aestivum L.) cultivars. Applied Sciences 10(19), 6971.
| Crossref | Google Scholar |
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends in Plant Science 10(10), 503-509.
| Crossref | Google Scholar |
Santiago FEM, Silva MLS, Cardoso AAS, Duan Y, Guilherme LRG, Liu J, Li L (2020) Biochemical basis of differential selenium tolerance in arugula (Eruca sativa Mill.) and lettuce (Lactuca sativa L.). Plant Physiology and Biochemistry 157, 328-338.
| Crossref | Google Scholar | PubMed |
Sheng H, Zeng J, Liu Y, Wang X, Wang Y, Kang H, Fan X, Sha L, Zhang H, Zhou Y (2016) Sulfur mediated alleviation of Mn toxicity in polish wheat relates to regulating Mn allocation and improving antioxidant system. Frontiers in Plant Science 7, 1382.
| Crossref | Google Scholar | PubMed |
Singh A, Roychoudhury A (2020) Silicon-regulated antioxidant and osmolyte defense and methylglyoxal detoxification functions co-ordinately in attenuating fluoride toxicity and conferring protection to rice seedlings. Plant Physiology and Biochemistry 154, 758-769.
| Crossref | Google Scholar | PubMed |
Singh A, Roychoudhury A (2023) Salicylic acid–mediated alleviation of fluoride toxicity in rice by restricting fluoride bioaccumulation and strengthening the osmolyte, antioxidant and glyoxalase systems. Environmental Science and Pollution Research 30, 25024-25036.
| Crossref | Google Scholar |
Singh G, Kumari B, Sinam G, Kriti, Kumar N, Mallick S (2018) Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective– a review. Environmental Pollution 239, 95-108.
| Crossref | Google Scholar | PubMed |
Singh A, Banerjee A, Roychoudhury A (2020) Seed priming with calcium compounds abrogate fluoride-induced oxidative stress by upregulating defence pathways in an indica rice variety. Protoplasma 257, 767-782.
| Crossref | Google Scholar | PubMed |
Spadaro D, Yun B-W, Spoel SH, Chu C, Wang YQ, Loake GJ (2010) The redox switch: dynamic regulation of protein function by cysteine modifications. Physiologia Plantarum 138(4), 360-371.
| Crossref | Google Scholar |
Vojtovič D, Luhová L, Petřivalský M (2020) Something smells bad to plant pathogens: Production of hydrogen sulfide in plants and its role in plant defence responses. Journal of Advanced Research 27, 199-209.
| Crossref | Google Scholar | PubMed |
Woodbury W, Spencer AK, Stahman MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Analytical Biochemistry 44(1), 301-305.
| Crossref | Google Scholar | PubMed |
Yadu B, Chandrakar V, Meena R, Sahu K (2017) Glycinebetaine reduces oxidative injury and enhances fluoride stress tolerance via improving antioxidant enzymes, proline and genomic template stability in Cajanus cajan L. South African Journal of Botany 111, 68-75.
| Google Scholar |
Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2000) Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiology 123(1), 223-234.
| Crossref | Google Scholar | PubMed |
Zahid A, Ul Din K, Ahmad M, Hayat U, Zulfiqar U, Askri SMH, Anjum MZ, Maqsood MF, Aijaz N, Chaudhary T, Ali HM (2024) Exogenous application of sulfur-rich thiourea (STU) to alleviate the adverse effects of cobalt stress in wheat. BMC Plant Biology 24, 126.
| Crossref | Google Scholar | PubMed |