Exogenous zinc application mitigates negative effects of salinity on barley (Hordeum vulgare) growth by improving root ionic homeostasis
Waleed Amjad Khan A , Beth Penrose A , Ping Yun A , Meixue Zhou A and Sergey Shabala
A and Sergey Shabala  A B C *
A B C *
A
B
C
Abstract
Detrimental effects of salinity could be mitigated by exogenous zinc (Zn) application; however, the mechanisms underlying this amelioration are poorly understood. This study demonstrated the interaction between Zn and salinity by measuring plant biomass, photosynthetic performance, ion concentrations, ROS accumulation, antioxidant activity and electrophysiological parameters in barley (Hordeum vulgare L.). Salinity stress (200 mM NaCl for 3 weeks) resulted in a massive reduction in plant biomass; however, both fresh and dry weight of shoots were increased by ~30% with adequate Zn supply. Zinc supplementation also maintained K+ and Na+ homeostasis and prevented H2O2 toxicity under salinity stress. Furthermore, exposure to 10 mM H2O2 resulted in massive K+ efflux from root epidermal cells in both the elongation and mature root zones, and pre-treating roots with Zn reduced ROS-induced K+ efflux from the roots by 3–4-fold. Similar results were observed for Ca2+. The observed effects may be causally related to more efficient regulation of cation-permeable non-selective channels involved in the transport and sequestration of Na+, K+ and Ca2+ in various cellular compartments and tissues. This study provides valuable insights into Zn protective functions in plants and encourages the use of Zn fertilisers in barley crops grown on salt-affected soils.
Keywords: antioxidant activity, barley, calcium, non-selective cation channels, potassium, reactive oxygen species, root epidermis, sodium.
Introduction
Soil salinity is a major environmental constraint that affects productivity of agricultural soils, costing over AU$30 billion per year in lost production worldwide (Shahid et al. 2018). Salinity affects plant growth and development by imposing osmotic and oxidative stress and causing disturbances to ion homeostasis (e.g. Na+ toxicity and K+ loss) (Roy et al. 2014; Zhao et al. 2020). These constraints have a major negative impact on plant photosynthesis and energy supply, resulting in reduced shoot biomass and grain yield (Hedrich and Shabala 2018; Pan et al. 2021). Salinity tolerance is a very complex trait and relies on the orchestrated operation of several complementary mechanisms such as Na+ exclusion from uptake and sequestration in vacuoles, cytosolic K+ retention, control of xylem ion loading, osmotic adjustment in roots and shoots, efficient stomata operation and maintenance of optimal redox balance (Munns and Tester 2008; van Zelm et al. 2020; Zhao et al. 2020).
Zinc (Zn) is an essential micronutrient for all living organisms (Broadley et al. 2007). It is the second most abundant metal cofactor after iron (Fe) and the only metal that operates in all enzyme classes as a catalytic or structural cofactor (Stanton et al. 2022). Zinc is crucial for regulating numerous physiological and metabolic processes, such as the biosynthesis and degradation of nucleic acids, proteins, carbohydrates, lipids and hormones (Broadley et al. 2007; Hara et al. 2017). Zn2+ is redox-inactive and a stable ion in biological media (Robson 2012). Additionally, Zn is essential for the functioning of >300 enzymes (Ireland and Martin 2019). Of these, some are directly related to plant redox regulation, e.g. as a catalyst for superoxide dismutase (SOD) and catalase (CAT), the two major antioxidant enzymes that detoxify reactive oxygen species (ROS) in stress-exposed plants (Habashy et al. 2019; Liu et al. 2019).
The provision of adequate Zn supply has been shown to improve plant performance under abiotic stress conditions such as drought (Anwar et al. 2021), heat (Agarwal and Khurana 2018), cold (Li et al. 2017) and salinity (Tavallali et al. 2010; Weisany et al. 2014; Iqbal et al. 2018; Tufail et al. 2018; Tolay 2021). The mechanistic basis of this amelioration, however, remains largely unexplored, with most of the papers reporting the beneficial effects of Zn on whole-plant physiological characteristics such as chlorophyll content, stomata operation, or water use efficiency. Some authors linked the observed tolerant phenotype with increased antioxidant activity (Tavallali et al. 2009), while others reported Zn-induced changes in the abundance of proteins involved in photosynthesis, carbon metabolism, amino acids, phenolics, nucleotide metabolism and signal transduction (Tavallali et al. 2010; Li et al. 2017; Wu et al. 2018). The critical role of Zn in plant abiotic stress tolerance may also be related to Zn being an essential structural component of zinc finger proteins (Han et al. 2020, 2022). It is suggested that RING zinc finger proteins on the plasma membrane function as sensors or receptors of abscisic acid (ABA), thus mediating abiotic stress signalling under conditions of salinity or drought (Han et al. 2022). However, the functional evidence is still lacking.
Maintaining optimal cytosolic ion homeostasis is arguably the most critical trait that determines the ability of plant species to grow in saline environments. Plants maintain an optimal cytosolic K/Na ratio through the precise regulation of numerous Na+ and K+ transporters that confer uptake, retention and sequestration of these ions in various cellular compartments (Zhao et al. 2020). One of the key players in this process are non-selective cation channels (NSCCs), which represent a major pathway for Na+ entry into the cell. These NSCCs are also permeable to a wide range of cations, including both macro- (K+; Ca2+; Mg2+; NH4+) and micro- (Zn2+; Fe2+) nutrients (Demidchik and Maathuis 2007). Most of the NSCCs found in plants are ROS-inducible and can be gated by various ROS species such as H2O2 (Pei et al. 2000; Demidchik et al. 2003, 2007) or hydroxyl radicals (Zepeda-Jazo et al. 2011; Velarde-Buendía et al. 2012). Both of these types of ROS are produced in plants under saline conditions (Demidchik 2015), and it has been shown on numerous occasions that superior salinity stress tolerance in halophyte species and salt-tolerant glycophytic crops is conferred by the reduced sensitivity of NSCC to ROS (Bose et al. 2014; Wang et al. 2018). Given the above role of Zn as a constitutive component of several types of antioxidant enzymes, we hypothesised in this work that the beneficial role of Zn in plant adaptive responses to salinity may be related to modulation of the activity of Na+, K+ and Ca2+ permeable ROS-activated NSCC channels and maintenance of more optimal cytosolic K/Na homeostasis.
Materials and methods
Plant materials and growth conditions
Four commercially grown barley (Hordeum vulgare L.) cultivars (Yerong, Sahara, Franklin and Clipper) were used in this study with the purpose of testing the consistency in results of zinc and salinity effects across the different cultivars. The seeds of barley cultivars were obtained from the Australian Winter Cereal Collection. Seeds were surface sterilised with a 5% commercial bleach solution for 10 min, followed by rinsing with running tap water for 15–20 min. Seeds were then sown in pots of 10 L volume filled with sand and perlite mix (70:30) and grown in a glasshouse under controlled temperatures of 26°C day/18°C night; relative humidity 65 ± 5%; day length 14 h. Plants were irrigated with a modified half-strength Hoagland’s solution that had two Zn and two NaCl levels (Table 1). Pots were placed in a large tray in a randomised manner and irrigated with the respective nutrient solutions using a semi-hydroponic system. Each pot contained three plants, and there were three pots per genotype for each treatment as biological replicates. Initially, two sets of plants were grown with each –Zn and +Zn supply. Three weeks after sowing, 200 mM NaCl was added to the nutrient solution of one out of these two sets of salinity treatments, represented as –Zn +NaCl and +Zn +NaCl. All nutrient solutions were renewed weekly and aerated with a pump throughout the duration of the experiment.
| Treatments (abbreviation) | Composition | |
|---|---|---|
| Low zinc (–Zn) | Half-strength Hoagland’s minus ZnSO4 | |
| Adequate zinc (+Zn) | Half-strength Hoagland’s plus 10 μM ZnSO4 | |
| Low zinc + salinity (–Zn + NaCl) | Half-strength Hoagland’s minus ZnSO4 + 200 mM NaCl | |
| Adequate zinc + salinity (+Zn + NaCl) | Half-strength Hoagland’s plus 10 μM ZnSO4 + 200 mM NaCl |
Agronomical and physiological assessment
Non-destructive measurements were conducted on the third leaf of 6-week-old seedlings. Leaf chlorophyll content was quantified by measuring SPAD, using a SPAD metre (SPAD-502; Konica Minolta, Japan). The efficiency of PSII (chlorophyll fluorescence Fv/Fm ratio) was estimated by using a pulse-amplitude modulation (PAM) fluorometer (Junior-PAM, Walz, Germany), where leaves were dark-adapted for 30 min prior to measurements.
Plant shoots were harvested after 6 weeks of growth and fresh weight was recorded. This was followed by the drying of the harvested plant samples at 60°C for 72 h and the dry weight was recorded. The samples were then ground to powder using a coffee grinder and stored in labelled plastic bags at room temperature until further use. For the ionomic analysis, a 0.05-g subsample of ground homogenised plant material was transferred to a 15-mL centrifuge tube. The extraction of Na and K was performed as described previously (Begum et al. 2015). Briefly, 10 mL of distilled water was initially added to the tubes and then they were placed in a water bath of boiling water for 90 min. The homogenate was centrifuged at 3000g for 15 min, and the supernatant was filtered through Whatman filter paper grade 2. An aliquot of filtrate was used for Na+ and K+ determination using a flame photometer (PFP7, Jenway; Bibby Scientific Ltd, Stone, UK).
Antioxidant enzyme activity
Fresh leaf tissue (0.5 g) was ground to powder with 5 mL extraction buffer (100 mM potassium phosphate buffer, pH 7.8; 0.1 mM ethylenediamine tetra acetic acid and 0.1 g polyvinylpyrrolidone) in a chilled pestle and mortar. The homogenate was centrifuged at 15,000g at 4°C for 20 min and the supernatant was collected to perform antioxidant assays. Superoxide dismutase (SOD) activity was determined by measuring the inhibition in photoreduction of nitroblue tetrazolium (NBT) dye by superoxide radicals, which are generated by the autoxidation of hydroxylamine hydrochloride (Senthilkumar et al. 2021). The colour was developed by adding 1.3 mL of sodium carbonate buffer, 500 μL of NBT and 100 μL of Triton X-100 to the test cuvette. A total of 100 μL of hydroxylamine hydrochloride was added to initiate the reaction. After 2 min of incubation at room temperature, 70 μL of crude enzyme extract was added and the absorbance was measured immediately at 560 nm at every 15 s for 1–2 min using a UV-Vis spectrophotometer (Thermo Scientific, USA). Catalytic activity was assayed by measuring the consumption of H2O2 for 1 min (Aebi 1984). The reaction mixture (4 mL volume) consisted of 50 mM potassium phosphate buffer pH 7; 100 μL 10 mM H2O2 and 100 μL enzyme extract. The change in absorbance was determined at 240 nm to calculate the specific CAT activity using a UV-Vis spectrophotometer (Thermo Scientific, USA).
ROS visualisation
Barley seedlings were grown using the paper roll method (Pandolfi et al. 2010) and kept in an incubator at 25 ± 1°C for 3 days. Seedlings were supplied with basic solution medium (BSM; 0.1 mM CaCl2 and 0.5 mM KCl, pH 5.6) for no Zn treatment (control), with two additional appropriate concentrations (10 and 100 μM) of zinc sulfate (ZnSO4) as Zn treatments. Root tips were visualised for measuring salt-induced ROS accumulation using the fluorescent probe 2′, 7′-dichlorofluorescin diacetate (H2DCFDA; catalogue no. D6883, Sigma-Aldrich). Roots were immersed in 100 μM DCFDA for 25 min in the dark, then washed thoroughly with distilled water prior to examination under a fluorescent microscope (Leica MZ12; Leica Microsystems, Wetzlar, Germany) fitted with an I3-wavelength filter (Leica Microsystems). Images were collected with excitation and emission wavelengths of 488–525 nm and processed by LAS V3.8 software (Leica Microsystems). The green fluorescence signal intensity in the captured images was measured with the Image J software (Schneider et al. 2012).
Non-invasive ion flux measurements
Net fluxes of K+ and Ca2+ across cellular membranes were quantified using non-invasive ion-selective microelectrodes (the MIFE technique; Shabala et al. 1997). Borosilicate glass capillaries (GC150-10, Harvard Apparatus, UK) were used to pull out blank microelectrodes with the help of a vertical puller. Blank electrodes were then dried at 225°C in an oven over night and silanised with chlorotributylsilane (282707-25G, Sigma-Aldrich, Sydney, NSW, Australia) on the following day. The tips of these electrodes were then flattened to a diameter of 2–3 μm and backfilled with respective backfilling solutions (200 mM KCl for K+ and 500 mM CaCl2 for Ca2+). The commercial ionophore cocktails (Cat. 99311 for K+ and 99310 for Ca2+, Sigma-Aldrich, Australia) were then used to fill up the front tips of electrodes. The prepared microelectrodes were then mounted in the electrode holders of the MIFE set-up and calibrated with a set of respective calibration solutions before and after the measurements. Electrodes with a correlation coefficient ≥0.999 and a slope value ≥50 and 25 mV per decade for K+ and Ca2+, respectively, were considered suitable for any flux measurements. Calibrated electrodes were co-focused and positioned 40–50 μm away from the measuring site on the root before starting the experiment. After commencing, a computer-controlled stepper motor (hydraulic micromanipulator) moved microelectrodes 100 μm away from the positioned site and back in a 12 s square-wave cycle to measure electrochemical gradient potential between two positions. The CHART software was used to acquire data and ion fluxes were calculated using the MIFEFLUX program (Shabala et al. 2006). Using this system, the net flux for each ion is calculated by Eqn 1:
where the net ionic flux J is equal to the product of the ion concentration c (mol m−3), the mobility of the ion u (speed per unit force, ms−1 per newton mol−1), the force per mole, which is the electrochemical potential gradient (dV/dx) and z is the constant of the measuring ion.
Two types of MIFE experiments were conducted, studying the impact of Zn availability on ROS-induced ion flux kinetics across the plasma membrane of: (1) root epidermal cells; and (2) leaf mesophyll. For root experiments, plants were grown as described in ‘ROS visualisation’. Roots were immobilised in Petri dishes containing 30 mL of nutrient solution (BSM solution with or without Zn) and left for 60 min for adaptation prior to the measurement. Net fluxes of were measured from roots at elongation (1.5 mm from the root apex) and mature zones (2–3 cm from the root apex). The calibrated Ca2+ and K+ electrodes were co-focused and positioned 40 μm above the measuring site on the root before starting the experiment. During measurements, a computer-controlled stepper motor (hydraulic micromanipulator) moved microelectrodes in a 12-s square-wave cycle between two positions, original (40 μm above the root surface) and 100 μm away, to measure the electrochemical gradient potential between the two positions.
After measuring steady-state fluxes for 5–10 min, 10 mM H2O2 was added to the roots, and transient K+ and Ca2+ flux responses were measured for an additional 40–45 min. Net fluxes of ions were then calculated by using MIFEFLUX software (Shabala et al. 2006) and expressed in nmol m−2 s−1. The first 30–60 s after adding the treatment solution were discarded during data analyses in agreement with the MIFE theory, which requires non-stirred conditions.
For flux measurements in the mesophyll, seeds were sown in pots filled with sand and perlite mix (70:30) and grown in a glasshouse at a controlled temperature as described in ‘Plant materials and growth conditions’. The third leaves were excised, and 5 × 7 mm segments were cut and left floated overnight (10–12 h) in Petri dishes using BSM (as a control) and two appropriate concentrations (10 and 100 μM) of ZnSO4 (as Zn treatments). Leaf segments were then immobilised, as described in Wu et al. (2018). Mounted samples were left to acclimatise to ionic and light conditions for 1 h prior to commencing recordings, and then transient responses from mesophyll cells to H2O2 treatment were measured as described above for root epidermis.
Results
Whole-plant responses
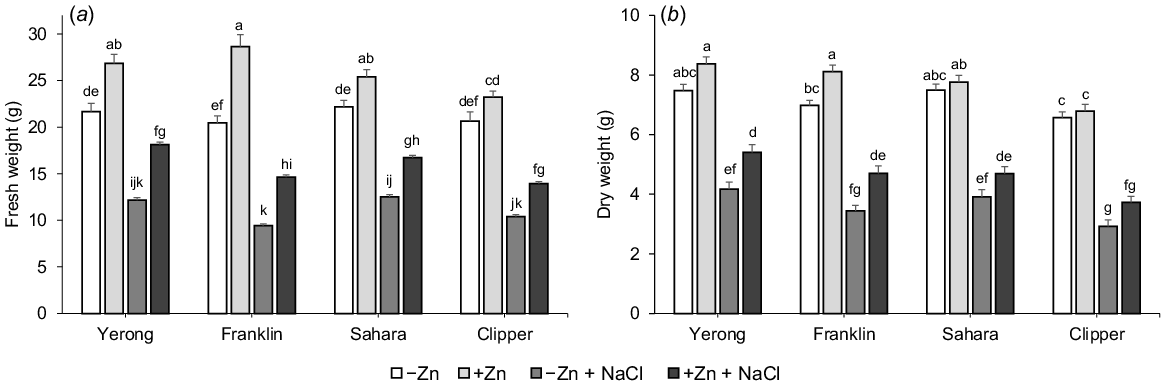
In this study, zinc and salinity applications strongly influenced plant agronomical characteristics. Both fresh (FW) and dry (DW) weights of plants grown under saline conditions were significantly reduced (by 52.4% and 50.6%, respectively) compared to those grown under non-saline conditions in all cultivars (Fig. 1). The plants supplied with adequate Zn performed much better compared to plants with depleted Zn supply under saline conditions, with ~30% greater FW and DW when averaged for all cultivars (Fig. 1). This indicates that Zn application has mitigating effects on salinity stress in barley. The lowest difference in biomass between plants treated with or without salt was found in Yerong, whereas the highest difference was observed in Franklin.
The influence of supplemental Zn (10 μM ZnSO4) and salinity (200 mM NaCl) on shoot fresh (a) and dry (b) weights of barley. Mean ± s.e. (n = 9). Data labelled with different lowercase letters are significantly different at P < 0.05 level.
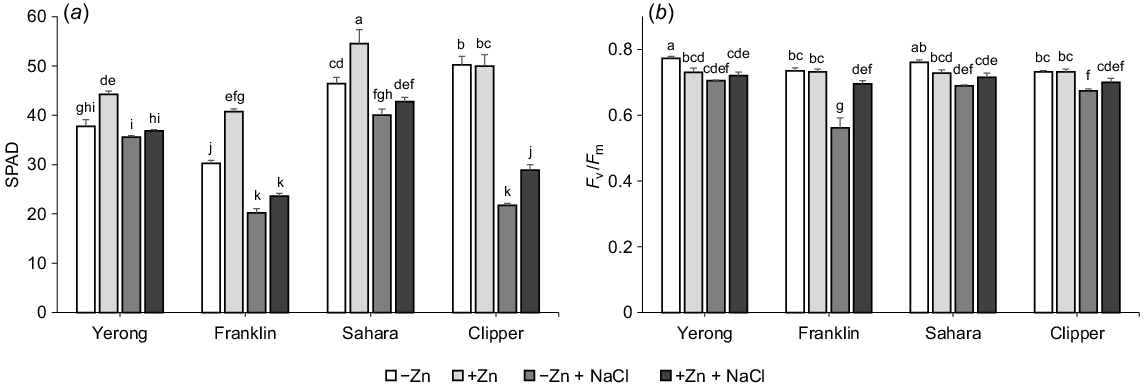
The above phenotyping data was further confirmed by observations of the changes in leaf chlorophyll content. Under low Zn + salinity conditions (–Zn + NaCl), plants on average had 29% lower SPAD readings compared with +Zn + NaCl treatment (Fig. 2a). Ameliorative effects of Zn were also evident for the operation of photosynthetic machinery in leaf mesophyll, with chlorophyll fluorescence Fv/Fm values (a measure of the maximum photochemical activity of PSII) being higher in Zn treated plants compared with low Zn treatment under saline conditions (Fig. 2b). The most pronounced effect was reported for Franklin, which was deemed most salt sensitive (Fig. 1), consistent with previous reports (Chen et al. 2007).
The influence of supplemental Zn (10 μM ZnSO4) and salinity (200 mM NaCl) on (a) chlorophyll content (SPAD values), and (b) maximum photochemical efficiency of PSII (Fv/Fm chlorophyll fluorescence ration of barley. Mean ± s.e. (n = 9). Data labelled with different lowercase letters are significantly different at P < 0.05 level.
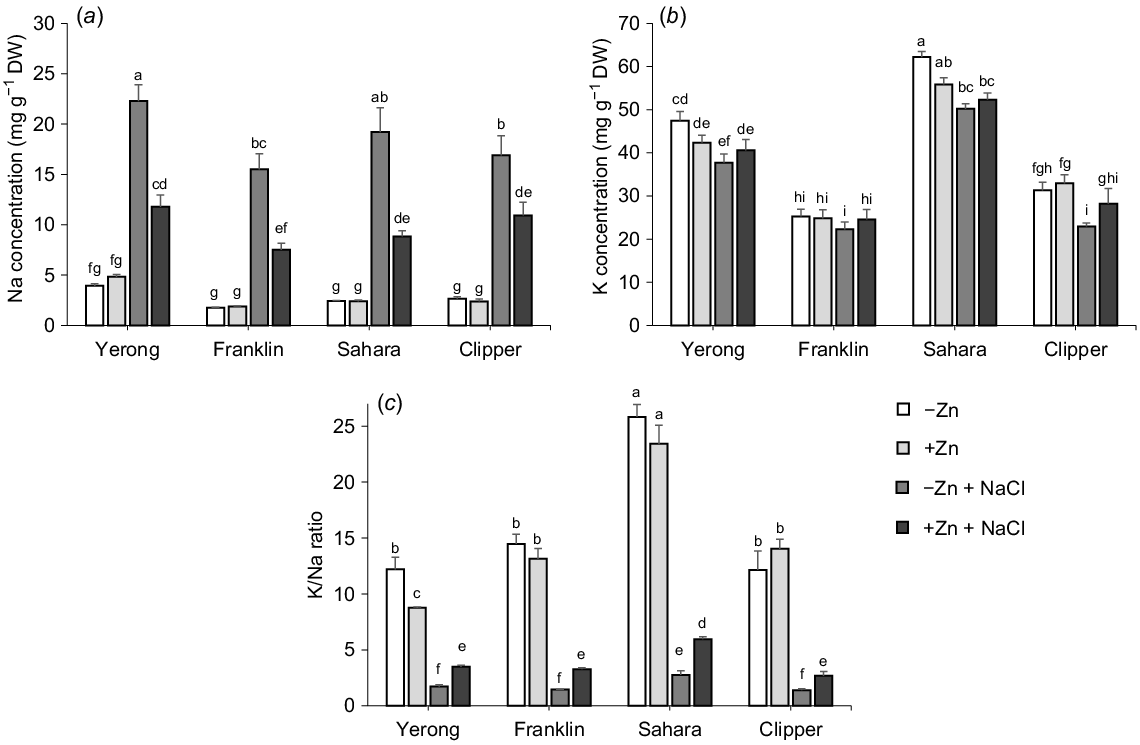
Na+ and K+ concentration in the shoot
Salinity treatment resulted in a massive Na+ accumulation in barley shoots (a 4-fold increase; Fig. 3a); at the same time, shoot K content was reduced on average by 15% in Zn-deprived plants (Fig. 3b). Consequently, the K/Na ratio in shoots was substantially greater (15.5) in non-salt stressed plants (e.g. −Zn and +Zn) than in salt stressed plants (2.8) (e.g. −Zn+ NaCl and +Zn+ NaCl) (Fig. 3c). Plants supplied with adequate Zn had an average 50% less Na+ accumulated in the shoot under saline conditions, implying a major impact of Zn on root Na+ acquisition and/or translocation to the shoot. No obvious trends were found for the Zn effect on shoot K content. Overall, plants supplemented with adequate Zn showed a 5.5-fold greater K/Na ratio in shoots than those depleted in Zn when exposed to salinity stress for 3 weeks.
Impact of Zn on ionic relations and ROS accumulation in roots
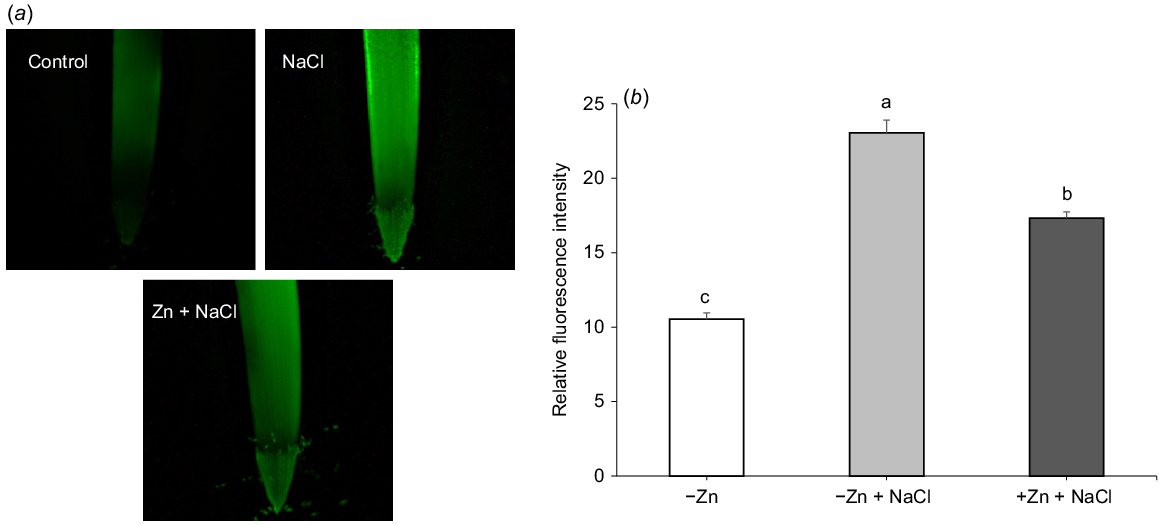
According to the classical view (Munns 2002), Na+ toxicity in the shoot becomes a limiting factor after several weeks of salinity exposure. The processes in the root operate on a much faster timescale, with the loss of cell viability and salinity-induced programmed cell death (PCD) occurring within hours of exposure to salt. Keeping this in mind, we looked at the effect of Zn on ionic relations and oxidative stress markers in roots. The root treatment with 200 mM NaCl for 4 days resulted in a 2-fold increase in H2O2 accumulation in the root apex (Fig. 4); the presence of Zn reduced this increase by 50%.
Visualisation of H2O2 accumulation at root apex of barley (cv. Franklin) during salt stress by 2′,7′-dichlorofluorescin diacetate fluorescent dye. Plants were treated with 200 mM NaCl for salt stress and 10 μM ZnSO4 for Zn treatment for the duration of 4 days: (a) one (of six) typical image is shown for each treatment taken by using fluorescence microscope; (b) relative fluorescence intensity measured in the roots when supplied with different treatments. Data labelled with different lowercase letters are significantly different at P < 0.05 level.
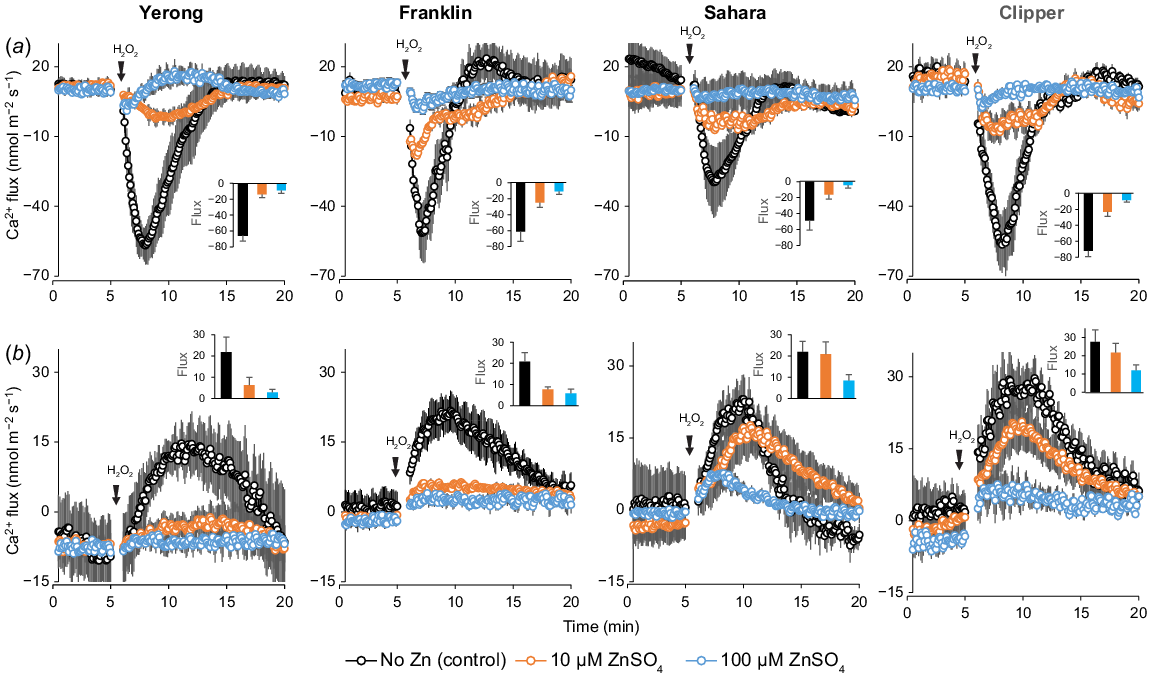
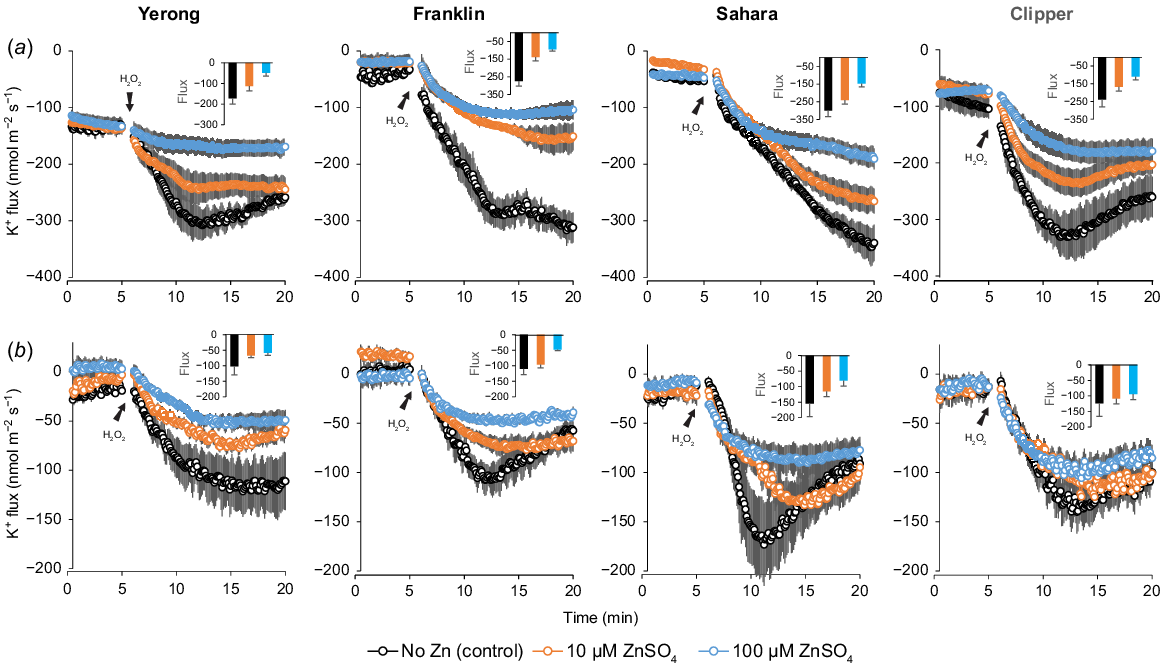
We next looked at the effects of Zn on the sensitivity of membrane transporters to ROS, exposing roots to acute H2O2 treatment and measuring the kinetics of H2O2-induced K+ and Ca2+ fluxes from the root epidermis using the MIFE technique. The addition of 10 mM H2O2 resulted in a massive K+ efflux from root epidermal cells at both the elongation (Fig. 5a) and mature (Fig. 5b) root zones. The seedlings pre-treated with ZnSO4 had a reduced ROS-induced K+ efflux from the roots. The observed effect was dose-dependent, with a 3–4-fold reduction in peak K+ loss observed in plants treated with 100 μM Zn compared with depleted-Zn plants. The ameliorating effects of Zn were more pronounced at the root apex (Fig. 5a), which is a zone that has been shown to be most sensitive to salt stress (Bose et al. 2014; Shabala et al. 2016; Wang et al. 2018). Early pharmacological experiments showed that such H2O2-induced K+ fluxes from roots may be efficiently blocked by both Gd3+ and La3+, known blockers of non-selective cation channels (NSCC; e.g. Shahzad et al. 2022).
Net K+ fluxes from root epidermis measured from 4-days-old barley seedlings at two different root zones: (a) elongation, and (b) mature in response to oxidative stress (10 mM H2O2). Seedlings were supplied with basic nutrient solution [0.1 mM CaCl2 and 0.5 mM KCl, pH 5.6] as control with two additional concentrations of ZnSO4 as treatments. Mean ± s.e. (n = 6–8).
Application of H2O2 also triggered a massive transient response in the net Ca2+ flux in barley roots (Fig. 6). In the mature zone of the root (Fig. 6b), exposure to acute H2O2 resulted in transient Ca2+ uptake that peaked 8–10 min after stress onset. Plant supplementation with Zn reduced the magnitude of this H2O2-induced peak Ca2+ influx by 5-fold to 10-fold (Fig. 6b), with the strongest effect reported for salt-tolerant Yerong. In the elongation zone, H2O2 treatment led to massive net Ca2+ loss (Fig. 6a). Application of Zn reduced this net Ca2+ efflux in a dose-dependent manner, with ~10-fold difference reported for the highest Zn concentration.
Effect of Zn on ion transport in leaf mesophyll
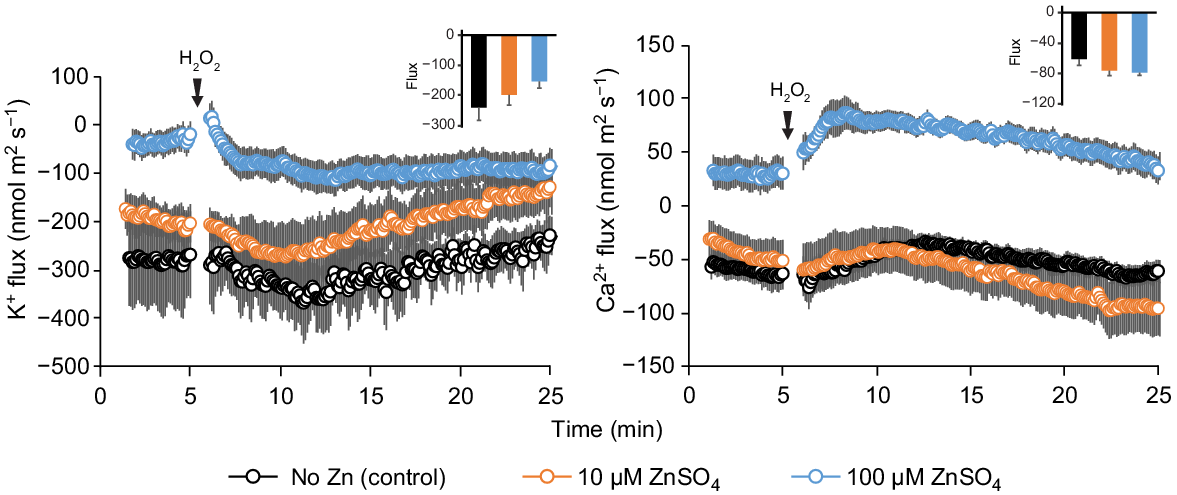
We then looked at the impact of Zn availability on ionic relations in leaf mesophyll. When the mesophyll tissue was incubated for 24 h in a low-K solution (0.5 mM KCl), a significant net K+ loss was measured (0–5 min interval, Fig. 7 using Yerong as a representative example). The addition of Zn to the bath solution reduced this K+ loss, in a dose-dependent manner (Fig. 7a), as well as reducing the magnitude of K+ flux response to H2O2 (an insert in Fig. 7a). The addition of Zn also shifted steady Ca2+ fluxes towards net influx (Fig. 7b), also in a dose-dependent manner. At the same time, the presence of Zn increased the sensitivity of Ca2+-permeable ion channels in leaf mesophyll to H2O2, as evident from higher peak Ca2+ fluxes (an insert in Fig. 7b). Thus, it appears that the effects of Zn on Ca2+ transport and signalling differ between root and leaf tissues.
Net K+ and Ca2+ fluxes measured from mesophyll tissue of the 4–5-week-old barley seedlings (cv. Yerong) in response to oxidative stress (10 mM H2O2). Leaf samples were floated overnight in Petri-dishes containing basic nutrient solution (as control) with two additional concentrations of ZnSO4 as treatments. Mean ± s.e. (n = 6–8).
Antioxidant enzymatic activity
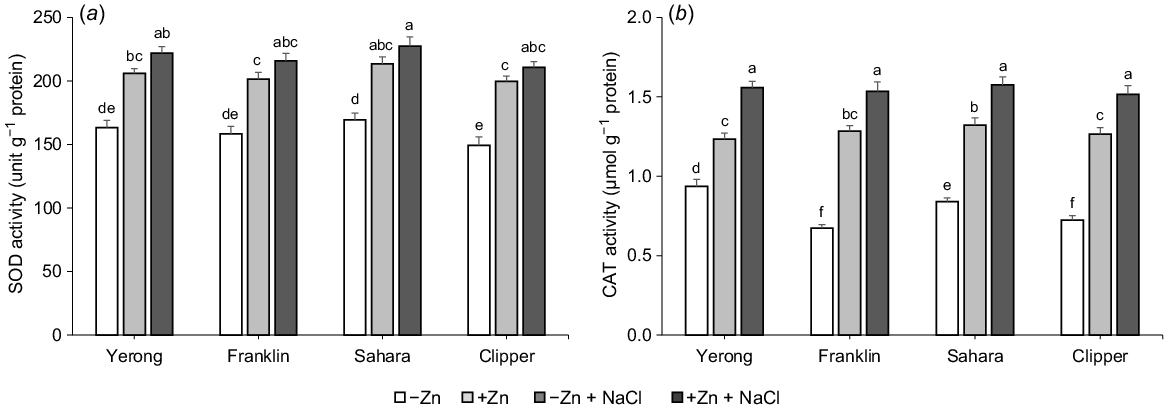
As expected, antioxidant enzymatic activity in the shoots of plants grown under salt stress was higher (on average by 28% for SOD and by 61% for CAT; Fig. 8) as compared to non-stressed plants. The presence of Zn had no statistically significant impact on SOD activity (a 7% increase; not significant at P < 0.05) while a significant 21% increase in CAT activity was reported (Fig. 8b).
Discussion
It has been demonstrated before that plants supplied with adequate Zn are comparatively more salt stress tolerant as compared to plants with low Zn (Mori et al. 2016; Jan et al. 2017; Tolay 2021), although the mechanistic basis behind this phenomenon has remained largely unexplored. This gap in knowledge was partially filled by our work.
In this study, plants supplied with adequate Zn had greater biomass (Fig. 1) as compared to those grown with low Zn supply under both non-saline and salt-stressed conditions. Thus, one could potentially claim that higher shoot FW and DW may be simply a result of the ‘carry-over effect’ caused by the initially higher biomass of Zn-treated plants prior to salinity exposure. However, analysis of relative changes in plant biomass suggests that this was not the case (Fig. S1), with each genotype showing 6–12% higher relative DW in the presence of Zn.
Zn-sufficient plants also had higher SPAD readings (Fig. 2), both under normal and saline conditions. The effect of salinity on chlorophyll content is complicated. On the one hand, high amounts of Na+ in the cytosol of mesophyll cells may impact chlorophyll synthesis (Turan and Tripathy 2015) and a stress-induced increase in ROS content may lead to chlorophyll degradation. On the other hand, the size of the mesophyll cells in salt-grown plants is much smaller (Zhao et al. 2020), so they can ‘condense’ chlorophyll and result in even higher SPAD readings compared with non-stressed plants, where chlorophyll is diluted by the large cell volume. Thus, this parameter is not suitable as a proxy for plant diagnostics for salinity tolerance. As for the beneficial effects of Zn on chlorophyll content in plants grown under normal conditions, this could be due to the expression of genes associated with catalysing chlorophyll synthesis, such as the magnesium-chelatase subunit ChlH chloroplastic, magnesium protoporphyrin IX chelatase and magnesium protoporphyrin IX methyltransferase chloroplastic, which increase in the presence of adequate Zn supply during plant growth (Zhang et al. 2019). Sufficient availability of Zn may also trigger plant hormonal biosynthesis of auxins, which leads to greater production of plant cells and thus, the shoot biomass (Hussein and Alva 2014).
Zinc supplementation led to a reduction in the shoot Na+ load by 2-fold in plants grown under salinity stress conditions (Fig. 3). Control of shoot Na+ content is a complicated process that includes control of Na+ uptake by the root epidermis, its radial transport into the stele, xylem Na+ loading and delivery to the shoot; and retrieval of Na+ from the xylem (Munns and Tester 2008; Shabala 2013; Zhao et al. 2020). Each of these processes is mediated by specific Na+ transporting proteins (e.g. SOS1 Na+/H+ antiporters operating in Na+ exclusion from root uptake (Shi et al. 2000); CCC transporters mediating xylem Na+ loading (Colmenero-Flores et al. 2007; Zhu et al. 2017); HKT1 transporters operating in retrieval of Na+ from the xylem (Byrt et al. 2007). However, to the best of our knowledge, no direct impact of Zn on any of these transporters has been reported in the literature. However, being a divalent cation, Zn2+ has been reported to be an efficient blocker of non-selective Na+ permeable cation channels, with 0.3 mM Zn2+ providing a complete block to Na+ currents through NSCC in patch-clamp experiments (Demidchik and Tester 2002). These findings were then also confirmed by in situ experiments using non-invasive MIFE measuring techniques (Shabala et al. 2005). Given that NSCCs represent a major pathway for Na+ entry into the plant (Zhao et al. 2020), its blockage by Zn2+ may explain the reported 2-fold decline in shoot Na+ content (Fig. 3a). Also, NSCC are involved in Na+ loading into the xylem (at least at initial stages; see Shabala (2013) for thermodynamical analysis), so it is plausible to suggest that the rate of Na+ loading into the xylem (and its subsequent delivery to the shoot) will be reduced in Zn-sufficient plants.
The detrimental effects of salinity on the growth of plants are strongly associated with the oxidative stress induced by ROS (AbdElgawad et al. 2016; Chen et al. 2017; Luo et al. 2021). Indeed, ROS imaging displayed greater H2O2 accumulation in the roots of those barley plants treated with only NaCl as compared to Zn + NaCl, indicating beneficial effects of Zn supplementation on this process (Fig. 4). Previous studies have reported a Zn-related increase in enzymatic antioxidant (AO) activity in salt-treated plants, and our results also showed higher SOD and CAT activity in the shoots of plants supplemented with Zn (Fig. 8). This may be because Zn acts as a cofactor for both CAT and SOD (Natasha et al. 2022) and the antioxidant activity of both these enzymes may increase with Zn supplementation. This may play a dual-beneficial role. Higher AO activity may provide greater protection to the cell structures against the ROS generated by stress (Zafar et al. 2021). Another possibility is that Zn addition activates the antioxidant activity of CAT at the transcript level, as found in Arabidopsis thaliana (Khan et al. 2019). Thus, Zn may help the plants cope with ROS production at both transcriptional and post-translational levels. However, the reported difference in AO activity was statistically significant only for CAT but not for SOD, so the physiological implications of such an increase are rather marginal. Also, the concept ‘the higher antioxidant activity the better salt tolerance’ often does not hold (Maksimović et al. 2013), as plants require stress-induced ROS elevation for signalling purposes. It should be also noted that in this study we analysed activity of all SOD isoforms but not ones specific for Cu/Zn (e.g. Dreyer and Schippers 2019). Given the important role of such isoforms in oxidative stress tolerance (Li et al. 2017) such possibility could not be completely ruled out.
The most drastic effects of Zn supplementation were on ionic relations and transport of K+ and Ca2+ across the plasma membrane in both root (Figs 5 and 6) and leaf mesophyll (Fig. 7) cells. The reported effects were dose-dependent and operated on a minute timescale, indicating direct involvement of Zn in the (de)sensitisation of H2O2-induced K+- and Ca2+-permeable ion channels. These channels are almost equally permeable for K+, Ca2+ and Na+. Some of these NSCC are ROS-activated and may also interact with NADPH oxidase, forming a so-called ‘ROS-Ca2+ Hub’ that amplifies stress-induced ROS and Ca2+ signalling and helps the plant adapt in a saline environment (Demidchik et al. 2018). However, such amplification comes with the danger that, while amplifying Ca2+ signals, plants may also increase Na+ uptake via these NSCCs. Here, reduced Na+ accumulation in Zn sufficient plants (Fig. 4) is in full agreement with the reduced magnitude of H2O2-induced Ca2+ uptake in roots (Fig. 6) supporting this model. In shoots, however, Zn supply has increased sensitivity of Ca2+-permeable channels to ROS, implying a likely role of stress-induced elevation in the cytosolic Ca2+ in triggering a cascade of adaptive responses in the shoot.
Zinc application also led to a significant improvement in K+ retention both in roots (Fig. 5) and in leaves (Fig. 7). Over the past decade, the ability of plant tissues to retain K+ under stress conditions has emerged as a key component of the tissue tolerance mechanism, especially in plants exposed to saline conditions (Shabala and Pottosin 2014; Shabala et al. 2016), with a positive correlation between the overall salinity stress tolerance and the ability of a root tissue to retain K+ reported for barley (Chen et al. 2005; Chen et al. 2007), wheat (Triticum aestivum) (Cuin et al. 2008; Cuin et al. 2009), lucerne (Medicago sativa) (Smethurst et al. 2008; Guo et al. 2016), Capsicum chinense (Bojórquez-Quintal et al. 2014), cotton (Gossypium hirsutum) (Wang et al. 2016), cucumber (Cucumis sativus) (Redwan et al. 2016) and Arabidopsis (Sun et al. 2015). Differential K+ retention ability also confers differential salinity stress tolerance between halophytes and glycophytes (Percey et al. 2016). One of the possible pathways of root K+ loss is via ROS-inducible NSCC channels (Shabala and Pottosin 2014; Wu et al. 2018), and H2O2-induced K+ flux from mature root zone correlate with salt tolerance in a broad range of wheat and barley genotypes (Wang et al. 2018). As for mechanisms underlying this process, a direct blockage of K+-permeable NSCC by Zn2+ is the currently preferred scenario, although other possibilities (including transcriptional regulation) cannot be completely ruled out.
Conclusions
This study demonstrated that Zn supplementation improved the salt tolerance in barley by reducing shoot Na+ load and improving K+ retention in root and mesophyll cells. The observed effects may be causally related to better regulation of cation-permeable NSCC involved in transport and sequestration of Na+, K+ and Ca2+ in various cellular compartments and tissues. The Zn-facilitated increase in antioxidants was relatively minor and unlikely to play a major role in conferring a salt-tolerant phenotype.
Conflicts of interest
Sergey Shabala is the Editor-in-Chief of Functional Plant Biology. To mitigate this potential conflict of interest they had no editor-level access to this manuscript during peer review. The authors declare no other conflicts of interest.
Author contributions
Waleed Amjad Khan: Methodology, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review and Editing, Visualisation. Beth Penrose: Conceptualisation, Writing – Review and Editing, Supervision. Ping Yun: Investigation, Visualisation. Meixue Zhou: Resources, Writing – Review and Editing. Sergey Shabala: Conceptualisation, Methodology, Formal analysis, Writing, Supervision, Project administration.
References
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Frontiers in Plant Science 7, 276.
| Crossref | Google Scholar |
Aebi H (1984) Catalase in vitro. Methods in Enzymology 105, 121-126.
| Google Scholar | PubMed |
Agarwal P, Khurana P (2018) Characterization of a novel zinc finger transcription factor (TaZnF) from wheat conferring heat stress tolerance in Arabidopsis. Cell Stress and Chaperones 23(2), 253-267.
| Crossref | Google Scholar |
Anwar S, Khalilzadeh R, Khan S, Zaib un N, Bashir R, Pirzad A, Malik A (2021) Mitigation of drought stress and yield improvement in wheat by zinc foliar spray relates to enhanced water use efficiency and zinc contents. International Journal of Plant Production 15(3), 377-389.
| Crossref | Google Scholar |
Begum F, Haque MA, Alam MS, Mohanta HC (2015) Evaluation of sweet potato genotypes against salinity. Bangladesh Journal of Agricultural Research 40, 249-257.
| Crossref | Google Scholar |
Bojórquez-Quintal E, Velarde-Buendía A, Ku-González Á, Carillo-Pech M, Ortega-Camacho D, Echevarría-Machado I, Pottosin I, Martínez-Estévez M (2014) Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Frontiers in Plant Science 5, 605.
| Crossref | Google Scholar |
Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, Shabala S (2014) Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Annals of Botany 115(3), 481-494.
| Crossref | Google Scholar |
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytologist 173, 677-702.
| Crossref | Google Scholar | PubMed |
Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiology 143(4), 1918-1928.
| Crossref | Google Scholar |
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell & Environment 28, 1230-1246.
| Crossref | Google Scholar |
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany 58, 4245-4255.
| Crossref | Google Scholar | PubMed |
Chen Z, Xie Y, Gu Q, Zhao G, Zhang Y, Cui W, Xu S, Wang R, Shen W (2017) The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical Biology and Medicine 108, 465-477.
| Crossref | Google Scholar | PubMed |
Colmenero-Flores JM, Martínez G, Gamba G, Vázquez N, Iglesias DJ, Brumós J, Talón M (2007) Identification and functional characterization of cation–chloride cotransporters in plants. The Plant Journal 50(2), 278-292.
| Crossref | Google Scholar |
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany 59, 2697-2706.
| Crossref | Google Scholar | PubMed |
Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S (2009) Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology 36, 1110-1119.
| Crossref | Google Scholar |
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environmental and Experimental Botany 109, 212-228.
| Crossref | Google Scholar |
Demidchik V, Maathuis FJM (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175(3), 387-404.
| Crossref | Google Scholar |
Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology 128, 379-387.
| Crossref | Google Scholar |
Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. Journal of Cell Science 116(1), 81-88.
| Crossref | Google Scholar |
Demidchik V, Shabala SN, Davies JM (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. The Plant Journal 49(3), 377-386.
| Crossref | Google Scholar |
Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytologist 220, 49-69.
| Crossref | Google Scholar | PubMed |
Dreyer BH, Schippers JHM (2019) Copper-zinc superoxide dismutases in plants: evolution, enzymatic properties, and beyond. In ‘Annual plant reviews online. Vol. 2(3)’. (Ed. JA Roberts) pp. 1–36. (American Cancer Society) doi:10.1002/9781119312994.apr0705
Guo P, Wei H, Zhang W, Bao Y (2016) Physiological responses of alfalfa to high-level salt stress: root ion flux and stomatal characteristics. International Journal of Agriculture and Biology 18, 125-133.
| Google Scholar |
Habashy WS, Milfort MC, Rekaya R, Aggrey SE (2019) Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. International Journal of Biometeorology 63, 1569-1584.
| Crossref | Google Scholar | PubMed |
Han G, Lu C, Guo J, Qiao Z, Sui N, Qiu N, Wang B (2020) C2H2 zinc finger proteins: master regulators of abiotic stress responses in plants. Frontiers in Plant Science 11, 115.
| Crossref | Google Scholar |
Han G, Qiao Z, Li Y, Yang Z, Wang C, Zhang Y, Liu L, Wang B (2022) RING zinc finger proteins in plant abiotic stress tolerance. Frontiers in Plant Science 13, 877011.
| Crossref | Google Scholar |
Hara T, Takeda T-A, Takagishi T, Fukue K, Kambe T, Fukada T (2017) Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. The Journal of Physiological Sciences 67, 283-301.
| Crossref | Google Scholar |
Hedrich R, Shabala S (2018) Stomata in a saline world. Current Opinion in Plant Biology 46, 87-95.
| Crossref | Google Scholar |
Hussein MM, Alva AK (2014) Effects of zinc and ascorbic acid application on the growth and photosynthetic pigments of millet plants grown under different salinity. Agricultural Sciences 5, 1253-1260.
| Crossref | Google Scholar |
Iqbal MN, Rasheed R, Ashraf MY, Ashraf MA, Hussain I (2018) Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environmental Science and Pollution Research 25(24), 23883-23896.
| Crossref | Google Scholar |
Ireland SM, Martin ACR (2019) ZincBind—the database of zinc binding sites. Database 2019 baz006.
| Crossref | Google Scholar |
Jan AU, Hadi F, Midrarullah , Nawaz MA, Rahman K (2017) Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiology and Biochemistry 116, 139-149.
| Crossref | Google Scholar | PubMed |
Khan AR, Wakeel A, Muhammad N, Liu B, Wu M, Liu Y, Ali I, Zaidi SHR, Azhar W, Song G (2019) Involvement of ethylene signaling in zinc oxide nanoparticle-mediated biochemical changes in Arabidopsis thaliana leaves. Environmental Science: Nano 6, 341-355.
| Google Scholar |
Li Z, Han X, Song X, Zhang Y, Jiang J, Han Q, Liu M, Qiao G, Zhuo R (2017) Overexpressing the Sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Frontiers in Plant Science 8, 1010.
| Crossref | Google Scholar |
Liu X, Li R, Dai Y, Yuan L, Sun Q, Zhang S, Wang X (2019) A B-box zinc finger protein, MdBBX10, enhanced salt and drought stresses tolerance in Arabidopsis. Plant Molecular Biology 99, 437-447.
| Crossref | Google Scholar | PubMed |
Luo X, Dai Y, Zheng C, Yang Y, Chen W, Wang Q, Chandrasekaran U, Du J, Liu W, Shu K (2021) The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytologist 229, 950-962.
| Crossref | Google Scholar | PubMed |
Maksimović JD, Zhang J, Zeng F, Živanović BD, Shabala L, Zhou M, Shabala S (2013) Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant and Soil 365, 141-155.
| Crossref | Google Scholar |
Mori A, Kirk GJD, Lee J-S, Morete MJ, Nanda AK, Johnson-Beebout SE, Wissuwa M (2016) Rice genotype differences in tolerance of zinc-deficient soils: evidence for the importance of root-induced changes in the rhizosphere. Frontiers in Plant Science 6, 1160.
| Crossref | Google Scholar |
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell & Environment 25(2), 239-250.
| Crossref | Google Scholar |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651-681 [In eng].
| Crossref | Google Scholar |
Natasha N, Shahid M, Bibi I, Iqbal J, Khalid S, Murtaza B, Bakhat HF, Farooq ABU, Amjad M, Hammad HM, Niazi NK, Arshad M (2022) Zinc in soil-plant-human system: a data-analysis review. Science of The Total Environment 808, 152024.
| Crossref | Google Scholar |
Pan T, Liu M, Kreslavski VD, Zharmukhamedov SK, Nie C, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S (2021) Non-stomatal limitation of photosynthesis by soil salinity. Critical Reviews in Environmental Science and Technology 51(8), 791-825.
| Crossref | Google Scholar |
Pandolfi C, Pottosin I, Cuin T, Mancuso S, Shabala S (2010) Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiology 51, 422-434.
| Crossref | Google Scholar | PubMed |
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731-734.
| Google Scholar |
Percey WJ, McMinn A, Bose J, Breadmore MC, Guijt RM, Shabala S (2016) Salinity effects on chloroplast PSII performance in glycophytes and halophytes. Functional Plant Biology 43, 1003-1015.
| Crossref | Google Scholar | PubMed |
Redwan M, Spinelli F, Marti L, Weiland M, Palm E, Azzarello E, Mancuso S (2016) Potassium fluxes and reactive oxygen species production as potential indicators of salt tolerance in Cucumis sativus. Functional Plant Biology 43, 1016-1027.
| Crossref | Google Scholar | PubMed |
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Current Opinion in Biotechnology 26, 115-124.
| Crossref | Google Scholar | PubMed |
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nature Methods 9, 671-675.
| Crossref | Google Scholar | PubMed |
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany 112(7), 1209-1221.
| Crossref | Google Scholar |
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257-279.
| Crossref | Google Scholar | PubMed |
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology 113, 111-118.
| Crossref | Google Scholar | PubMed |
Shabala L, Cuin TA, Newman IA, Shabala S (2005) Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222(6), 1041-1050.
| Crossref | Google Scholar |
Shabala L, Ross T, McMeekin T, Shabala S (2006) Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiology Reviews 30, 472-486.
| Crossref | Google Scholar | PubMed |
Shabala S, Bose J, Fuglsang AT, Pottosin I (2016) On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. Journal of Experimental Botany 67, 1015-1031.
| Crossref | Google Scholar | PubMed |
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. In ‘Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques’. (Eds M Zaman, SA Shahid, L Heng) pp. 43–53. (Springer International Publishing: Cham, Switzerland)
Shahzad B, Yun P, Rasouli F, Shabala L, Zhou M, Venkataraman G, Chen Z-H, Shabala S (2022) Root K+ homeostasis and signalling as a determinant of salinity stress tolerance in cultivated and wild rice species. Environmental and Experimental Botany 201, 104944.
| Crossref | Google Scholar |
Shi H, Ishitani M, Kim C, Zhu J-K (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences 97(12), 6896-6901.
| Crossref | Google Scholar |
Smethurst CF, Rix K, Garnett T, Auricht G, Bayart A, Lane P, Wilson SJ, Shabala S (2008) Multiple traits associated with salt tolerance in lucerne: revealing the underlying cellular mechanisms. Functional Plant Biology 35, 640-650.
| Crossref | Google Scholar | PubMed |
Stanton C, Sanders D, Krämer U, Podar D (2022) Zinc in plants: integrating homeostasis and biofortification. Molecular Plant 15, 65-85.
| Crossref | Google Scholar | PubMed |
Sun Y, Kong X, Li C, Liu Y, Ding Z (2015) Potassium retention under salt stress is associated with natural variation in salinity tolerance among Arabidopsis accessions. PLoS ONE 10, e0124032.
| Crossref | Google Scholar | PubMed |
Tavallali V, Rahemi M, Maftoun M, Panahi B, Karimi S, Ramezanian A, Vaezpour M (2009) Zinc influence and salt stress on photosynthesis, water relations, and carbonic anhydrase activity in pistachio. Scientia Horticulturae 123, 272-279.
| Google Scholar |
Tavallali V, Rahemi M, Eshghi SRT, Kholdebarin B, Ramezanian A (2010) Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turkish Journal of Agriculture and Forestry 34, 349-359.
| Google Scholar |
Tolay I (2021) The impact of different zinc (Zn) levels on growth and nutrient uptake of Basil (Ocimum basilicum L.) grown under salinity stress. PLoS ONE 16, e0246493.
| Crossref | Google Scholar | PubMed |
Tufail A, Li H, Naeem A, Li TX (2018) Leaf cell membrane stability-based mechanisms of zinc nutrition in mitigating salinity stress in rice. Plant Biology 20(2), 338-345.
| Crossref | Google Scholar |
Turan S, Tripathy BC (2015) Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiologia Plantarum 153(3), 477-491.
| Crossref | Google Scholar |
van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annual Review of Plant Biology 71, 403-433.
| Crossref | Google Scholar |
Velarde-Buendía AM, Shabala S, Cvikrova M, Dobrovinskaya O, Pottosin I (2012) Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiology and Biochemistry 61, 18-23.
| Crossref | Google Scholar |
Wang N, Qi H, Su G, Yang J, Zhou H, Xu Q, Huang Q, Yan G (2016) Genotypic variations in ion homeostasis, photochemical efficiency and antioxidant capacity adjustment to salinity in cotton (Gossypium hirsutum L.). Soil Science and Plant Nutrition 62, 240-246.
| Crossref | Google Scholar |
Wang H, Shabala L, Zhou M, Shabala S (2018) Hydrogen peroxide-induced root Ca2+ and K+ fluxes correlate with salt tolerance in cereals: towards the cell-based phenotyping. International Journal of Molecular Sciences 19, 702.
| Crossref | Google Scholar | PubMed |
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Badakhshan H (2014) Effects of zinc application on growth, absorption and distribution of mineral nutrients under salinity stress in soybean (Glycine Max L.). Journal of Plant Nutrition 37(14), 2255-2269.
| Crossref | Google Scholar |
Wu H, Shabala L, Shabala S, Giraldo JP (2018) Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environmental Science: Nano 5, 1567-1583.
| Crossref | Google Scholar |
Zafar Z, Rasheed F, Haq AU, Ibrahim FH, Afzal S, Nazre M, Akram S, Hussain Z, Kudus KA, Mohsin M, Qadeer A, Raza Z, Khan WR (2021) Interspecific differences in physiological and biochemical traits drive the water stress tolerance in young Morus alba L. and Conocarpus erectus L. saplings. Plants 10, 1615 10.3390/plants10081615.
| Google Scholar |
Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiology 157(4), 2167-2180.
| Crossref | Google Scholar |
Zhang J, Wang S, Song S, Xu F, Pan Y, Wang H (2019) Transcriptomic and proteomic analyses reveal new insight into chlorophyll synthesis and chloroplast structure of maize leaves under zinc deficiency stress. Journal of Proteomics 199, 123-134.
| Crossref | Google Scholar | PubMed |
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The Innovation 1, 100017.
| Crossref | Google Scholar | PubMed |
Zhu M, Zhou M, Shabala L, Shabala S (2017) Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context of salinity stress tolerance. Plant, Cell & Environment 40(7), 1009-1020.
| Crossref | Google Scholar |