Comparative transcript abundance of gibberellin oxidases genes in two barley (Hordeum vulgare) genotypes with contrasting lodging resistance under different regimes of water deficit
Shoaib Liaqat A , Zulfiqar Ali A B C * , Muhammad Abu Bakar Saddique A , Rao Muhammad Ikram D and Imtiaz Ali E
A B C * , Muhammad Abu Bakar Saddique A , Rao Muhammad Ikram D and Imtiaz Ali E
A
B
C
D
E
Abstract
Barley (Hordeum vulgare) is the world’s fourth most important cereal crop, and is particularly well adapted to harsh environments. However, lodging is a major productivity constraint causing 13–65% yield losses. Gibberellic acid (GA) homeostatic genes such as HvGA20ox, HvGA3ox and HvGA2ox are responsible for changes in plant phenotype for height and internodal length that contribute towards lodging resistance. This study explored the expression of different HvGAox transcripts in two contrasting barley genotypes (5-GSBON-18, lodging resistant; and 5-GSBON-70, lodging sensitive), which were sown both under controlled (hydroponic, completely randomised factorial design) and field conditions (split-plot, completely randomised block design) with two irrigation treatments (normal with three irrigation events; and water deficit with one irrigation event). In the hydroponic experiment, expression analysis was performed on seedlings at 0, ¾, 1½, 3 and 6 h after application of treatment. In the field experiment, leaf, shoot nodes and internodes were sampled. Downregulation of HvGA20ox.1 transcript and 2-fold upregulation of HvGA2ox.2 transcript were observed in 5-GSBON-18 under water deficit conditions. This genotype also showed a significant reduction in plant height (18–20%), lodging (<10%), and increased grain yield (15–18%) under stress. Utilisation of these transcripts in barley breeding has the potential to reduce plant height, lodging and increased grain yield.
Keywords: barley, GA20ox, GA2ox, GA3ox, gibberellins, internodal length, lodging, plant height, transcript abundance, water stress.
Introduction
Barley (Hordeum vulgare) ranks fourth worldwide in terms of cultivation area and total production among all cereals. It has been widely used as human food, animal feed and as a fermentable grain for the beer industry (Peng et al. 1999). Lodging, defined as the permanent displacement of aboveground parts, is a common problem in barley to cause yield loss up to 80%. However, its severity depends on many plant characteristics and environmental factors.
Plant height is one of the most important morphological traits that play a significant role in determining whether a plant lodges or not (Kim et al. 2009). Taller plants are more prone to lodging risk, while shorter plants have more potential to withstand lodging. It is an important factor that influences lodging and ultimately grain yield. In the past, it has been hypothesised that shorter heights could limit physico-chemical processes that could reduce yield. However, tall varieties generally have a heavier canopy, which limits the ability to resist lateral forces, resulting in a higher lodging index (Okuno et al. 2014). In shorter stature varieties, plant height is directly related to the centre of gravity of the culm, in that a shorter plant reduces the value of the centre of gravity, which provides resistanceagainst lodging (Khobra et al. 2019). Similarly, internodal length, along with plant height, are also important factors that increases lodging resistance. The short length of basal internodes supports the development of lodging-resistant ideotype (Khalid et al. 2023). The shorter the basal internodes, the stronger and more resistant the plant will be against lodging. Strong and positive association of plant height, internodal length and lodging have been observed that reflects the role of these plant traits in the development of lodging-resistant ideotype (Peng et al. 2014). At the grain filling stage, as assimilates accumulation occurs in the spike, it increases the centre of gravity of plant height and reduces the bending strength through translocation of assimilates from stem to spikes (Berry et al. 2015). Therefore, reduced plant height and shorter length of basal internodes contribute towards the development of an ideal culm structure that increases the bending strength of the plant and ultimately enhances lodging resistance. In addition to being crucial for plant vigour and protection from biotic and abiotic stresses, lignin and cellulose are also important components of the plant cell wall. Higher lignin contents in the basal internodes are significantly associated with stem strength lodging resistance in cereals (Zhang et al. 2017).
The root system of the plant also has a major role in its growth and development, anchoring the plant, and for transport water and nutrient from the soil to different parts of the plant. Under harsh environments, roots are considered an important part of signalling against biotic and abiotic stresses including root lodging (Shah et al. 2019). It is quite evident that lodging-resistant cultivars have phenotypic differences between root system from lodging-vulnerable cultivars. For example, when plant standing on flexible and vertical roots do not penetrate deep in the soil thus reducing the anchorage strength. A positive correlation between the spread of seminal and lateral roots and culm lodging resistance in wheat has been observed. The root angle, at which roots penetrate the soil is an important factor to combat lodging resistance in plants (Berry et al. 2000).
The introduction of dwarfing genes in cereal crops was necessary to develop robust and sturdy types of plants that were able to support the heavy spikes with maximum grain numbers and resulted in a significant increase in lodging resistance and productivity (Dockter and Hansson 2015). In cereals, genes of the Green Revolution that control the semi-dwarf growth habit were discovered as reduced height (rht) genes in wheat (Triticum aestivum) (Peng et al. 1999) and semi-dwarf1 (sd1) gene in rice (Oryza sativa) (Kim et al. 2009). In rice, semi-dwarf1 (sd1), was one of the important genes that were used extensively in rice breeding (Spielmeyer et al. 2002). Semi-dwarfing phenotypes with sdw1/denso genes have proved to be one of the best genes that control the gibberellin homeostasis and contributed to various morphological, agronomic and quality parameters (Cuesta-Marcos et al. 2009). These semi-dwarfing genes were known to be highly pleiotropic and their main features include shortening of culm internodal length with enhanced lodging resistance. It reduces stem length by up to 30 cm (Gibson and Weir 2005).
Gibberellins (GAs) are the plant hormone that regulate various growth and plant developmental processes (Hedden and Phillips 2000), especially stem elongation (Zhu et al. 2006). Therefore, it is necessary to find the relationship of plant height with GAs. The GA 20oxidases (GA20ox), GA 3oxidases (GA3ox) and GA 2oxidases (GA2ox) are the essential enzymes that play a significant role in GA homeostasis (Olszewski et al. 2002). These genes have significant effects on plant height and many other morphological, physiological and anatomical traits depending on the plant’s genetic background and environmental conditions. Under drought conditions, activation of GA2ox gene has been observed in guard cell and leaf tissues due to inhibited expression of the GA20ox gene (Shohat et al. 2021). The concentration of GA influences the regulation of oxidase gene expression. Any change in essential enzymes of the GA biosynthesis seems to affect the phenotype of plants, especially plant height. If loss of function occurs in GA20ox and GA3ox, the GA concentration will be low and give rise to a short phenotype but their overexpression will enhance the GA concentration and result in a tall plant. In contrast, for the catalytic gene GA2ox, the loss of function produces a tall phenotype due to an increase in GA concentration but its overexpression reduces the GA level and develops a short phenotype (He et al. 2019). This study investigated the spatial and temporal expression profiling of different HvGA20ox, HvGA3ox and HvGA2ox transcripts based on the hypothesis that HvGA20ox and HvGA3ox transcripts are downregulated and HvGA2ox transcript is upregulated in the lodging-resistant genotypes. Most tolerant and sensitive genotypes against lodging were characterised based on morphological, yield and lodging traits and exposed to different water stress both in growth chamber and field conditions. This will help the plant breeders improve the phenotypic and physiological features for the improvement of lodging resistance.
Materials and methods
Plant material and growing conditions
Two contrasting genotypes of barley (Hordeum vulgare L.): (1) 5-GSBON-18 (lodging resistant); and (2) 5-GSBON-70 (lodging sensitive) were selected from previously characterised 251 barley genotypes (S. Liaqat, unpubl. data). For the hydroponic experiment, genotypes were grown with two treatments: (1) normal, Hoagland solution; and (2) osmotic stress, Hoagland solution + 20% polyethylene glycol (PEG-6000) under completely randomised design with factorial arrangements in three replications. The Hoagland solution was prepared following the protocol of Hoagland and Arnon (1950). Treatments were applied 1 week after germination. For the field experiment, the genotypes were grown at the research area of MNS University of Agriculture, Multan, Pakistan (30°08′50″N, 71°26′50″E), with two different treatments: (1) normal, with three irrigation events applied at 25 days after sowing (DAS), 70 DAS, 110 DAS; (2) water deficit with one irrigation event applied at 25 DAS. The field experiment was a randomised complete block design (RCBD) with split-plots during the growing season 2019–2020. Plot size was maintained at 1 m2 and seed rate at 10 g m−2. Diammonium phosphate and sulfate of potash were applied as a basal dose at the rate of 50 kg ha−1.
Climatic parameters and soil condition
The climatic parameters at the study site were recorded using the weather station with data logger CR1000X (Campbell Scientific Inc., Logan, UT, USA) that was installed at the experimental site. It is also equipped with sensors for wind (03002 wind sentry set, measured both wind speed and direction, rain (gauge, CS120A and CS125 visibility sensor), temperature (probe, CS215), relative humidity (probe, CS300), total sun and solar radiation (pyranometers, CS301), and Dielectric Leaf Wetness Sensor (LWS). The soil texture was loam with pH (1:1) was 7.98 ± 0.05 with organic matter 0.55 ± 0.05%. Phosphorous content was 8.48 ± 0.70 mg kg−1, and potassium content was 202.50 ± 957 mg kg−1.
Morphological, physiological and biochemical traits
At 2 weeks after treatment, five plants per treatment per replication were measured for coleoptile length (cm), number of seminal roots, root length (cm), shoot length (cm), root angle (°), stomatal conductance (mmol H2O m−2 s−1), photosynthesis (μmol CO2 m−2 s−1), leaf temperature (°C) and transpiration (mmol H2O m−2 s−1), water use efficiency (kg ha−1 mm−1), chlorophyll contents (%), cellulose (%) and lignin (%) contents from the shoot sample. To record root angle, the genotypes were photographed at the seedling stage. Root angle (°) was analysed by using ImageJ 1.53e (Java 1.8.0_172 software). The physiological parameters were measured from flag leaf using the 80018-3 CIRAS-3 portable photosynthesis system (CIRAS-3, PP Systems, Amesbury, MA, USA). The values of chlorophyll contents were recorded from the base, mid and upper part of the flag leaf using SPAD (SPAD-502, Minolta Corp.). Cellulose and lignin contents were measured by isolation of the cell wall, following the method described by Foster et al. (2010). For the field experiment, data of five randomly selected plants of both genotypes from each treatment per replication were recorded for physiological and biochemical traits as described above as well as morphological traits such as plant height (cm), internodal length (cm), nodal width (mm), stem diameter (mm), stem wall width (mm), number of nodes, lodging (%), 1000-grain weight (g), grain yield/plant (g) were recorded. Lodging was scored by using the following formula described by Fischer and Stapper (1987):
Bioinformatics analysis of HvGA20ox, HvGA3ox and HvGA2ox transcripts
The protein sequences for the different HvGA20ox, HvGA3ox and HvGA2ox transcripts were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov/). Phylogenetic analysis was performed using MEGA X. For phylogenetic analysis, full-length protein sequences of HvGA20ox, HvGA3ox and HvGA2ox transcripts of barley, Arabidopsis thaliana L., wheat (Triticum aestivum L.), rice and Micromonas pusilla (unicellular) were aligned. Multiple sequence alignment was done using the ClustalW program. For the exon and introns prediction and organisation, Gene Structure Display Server was used (http://gsds.cbi.pku.edu.cn/).
Total RNA extraction, primer design and semi-qPCR
For the hydroponic experiment, RNA samples of each genotype were taken from root, coleoptile, leaf and shoot tissues (GS12: Zadoks et al. 1974) both under normal and osmotic stress conditions. For the field experiment, RNA samples were taken from leaf and shoot at the tillering stage (GS22: Zadoks et al. 1974), and from leaf, nodes (5) and internodes (5) at the grain filling stage (GS75: Zadoks et al. 1974). RNA was extracted from the samples using the TRIzol method (Liu et al. 2018) following the manufacturer’s instructions and quantified by spectrophotometer (Colibri Microvolume Spectrometer, Globalsave, Harrogate, UK). The integrity of isolated RNA was checked on the gel electrophoresis apparatus. The first strand cDNA was synthesised through reverse transcription PCR (RT-PCR) using RevertAid First Strand cDNA Synthesis Kit by Thermo Scientific from USA. The synthesised cDNA was stored at −80°C. Primers were synthesised using the Amplifix software ver. 1.7.0 to check the expression of HvGA20ox, HvGA3ox and HvGA2ox transcripts along with the one housekeeping gene (ubiquitin) in the synthesised cDNA samples. For the sequences of primers, see Supplementary Table S1. An optimisation of UBQ gene (internal control) for semi-qPCR cycles was done at 30, 32, 34, 35, 36, 39 and 40 cycles to obtain the product to reach their plateau stage. The reaction mixture and PCR (BioRad T100 Thermal Cycler, Sharjah, UAE) conditions are in Tables S2 and S3, respectively. The PCR product was stained with ethidium bromide, run on 1% of agarose gel and observed under UV light using gel documentation apparatus (Omega Fluor Plus Gel Documentation System). The gel image was used to quantify the relative expression level by using ImageJ 1.53e (Java 1.8.0_172 software). The change in transcript expression level was measured through numerical values that represented band strength (Saddique et al. 2020).
Results
Weather conditions
The data of average air temperature (°C), rainfall (mm), wind speed (m/s), solar radiation (MJ/m2) and relative humidity (%) were logged daily during the entire crop growing period from November 2019 to April 2020 are in Fig. S1. Rainfall during the barley growing season 2019–2020 was recorded 6.08 mm and maximum wind speed 4.63 m/s was observed. Maximum rainfall and higher wind speed were observed during the end of February and the first week of March.
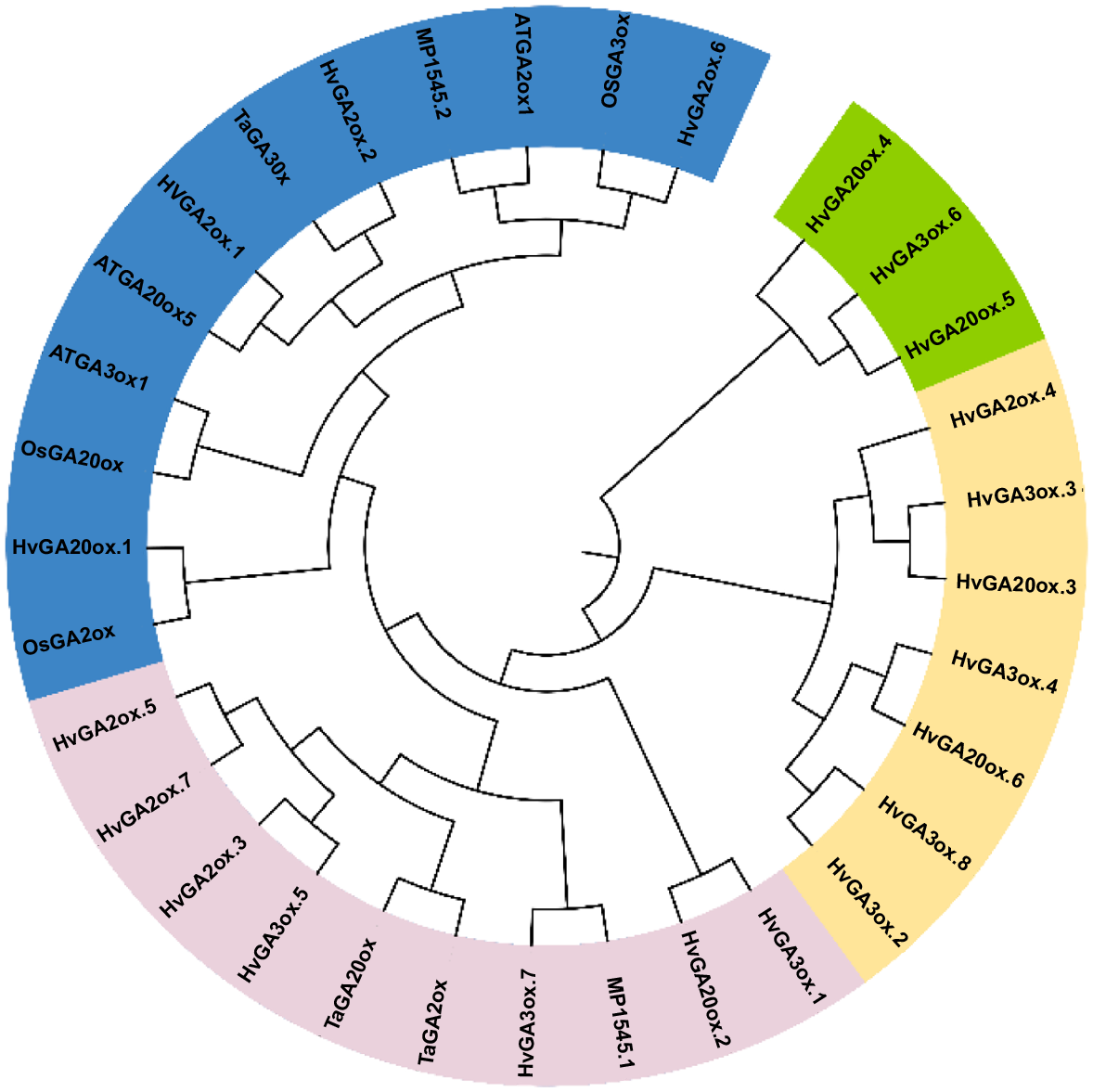
Phylogenetic and gene structure analysis of HvGA20ox, HvGA3ox and HvGA2ox transcripts
The phylogenetic tree was generated in MEGA X and different groups were observed based on sequence evolutionary relationship (Fig. 1). Two transcripts of HvGA20ox were observed in the same group in which Arabidopsis, wheat and unicellular were clustered, which indicates that these transcripts originated from early land plants’ phylostratum and must have gone through maximum selection pressure. Further, gene structure analysis (Fig. 2) revealed the intron and exon pattern of HvGA20ox, HvGA3ox and HvGA2ox transcripts. The HvGA3ox transcripts have fewer introns and more exons while HvGA2ox transcripts have more introns and less exons. The number of introns ranged from 1 to 2 while exons ranged from 2 to 4. Most of them showed both upstream and downstream regions. Closely related transcripts have shown a similar structure.
Phenotyping at seedling stage
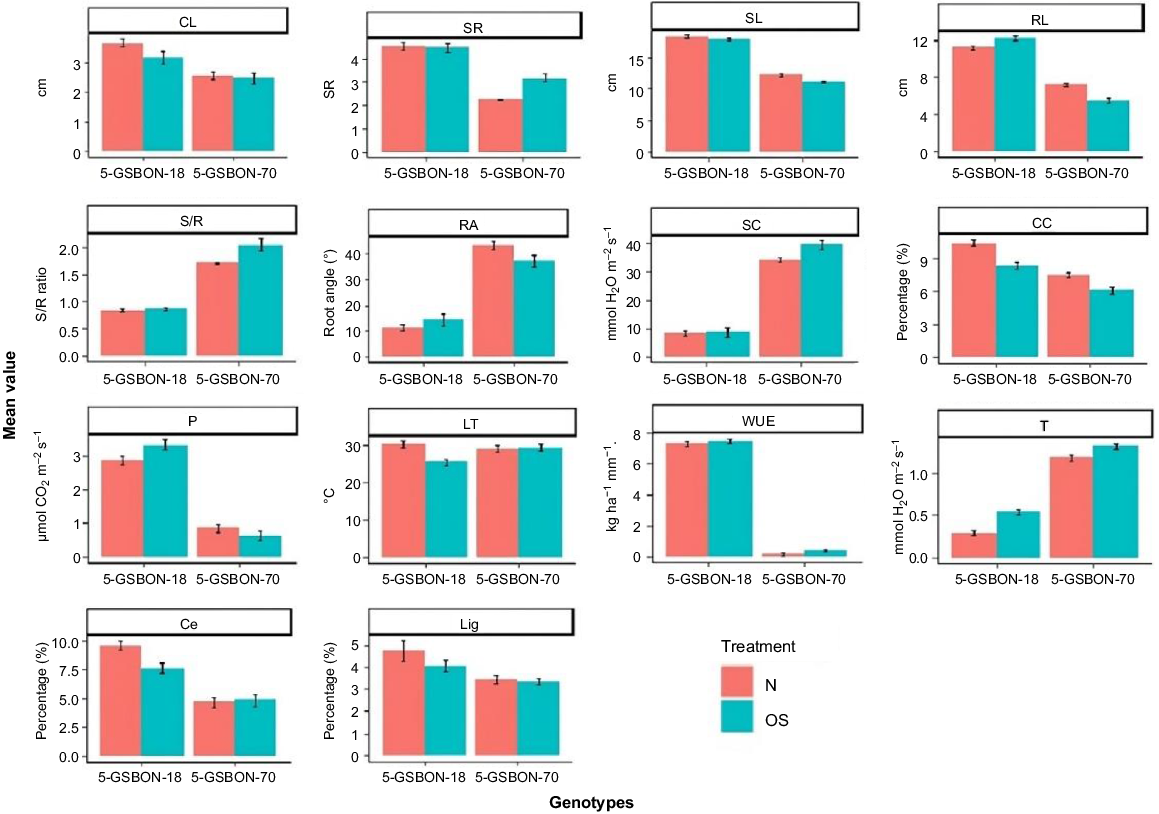
Mean data of both genotypes for different morphological, physiological and biochemical traits under normal and osmotic stress are in Fig. 3. Results indicate that genotype 5-GSBON-18 had the greater coleoptile length (3.4 cm) and root length (10.2 cm) under normal conditions, and 3.1 cm and 11.2 cm under water deficit conditions, respectively, in comparison with 5-GSBON-70. Under water deficit conditions, the number of seminal roots were higher in 5-GSBON-18 (4.4) than 5-GSBON-70 (3.1). Shoot/root ratio was lower (0.9) in lodging-resistant genotype than the lodging-sensitive genotype (2.0). The values for chlorophyll contents, photosynthesis and water use efficiency were observed higher in 5-GSBON-18 in comparison with 5-GSBON-70. Under water deficit conditions, cellulose (7.7%) and lignin (3.9%) contents were also higher for lodging-resistant 5-GSBON-18 than the lodging-sensitive genotype 5-GSBON-70.
Mean data of morphological and physiological traits of genotypes 5-GSBON-18 (lodging resistant) and 5-GSBON-70 (lodging sensitive) grown under normal (N) and osmotic stress (OS) conditions. CL, coleoptile length; SR, seminal roots numbers; SL shoot length; RL, root length; S/R, shoot/root ratio; RA, root angle; SC, stomatal conductance; CC, chlorophyll contents, P, photosynthesis; LT, leaf temperature; WUE, water use efficiency; T, transpiration; Ce, cellulose; and Lig, lignin. Bars indicate s.d.
Transcript profiling
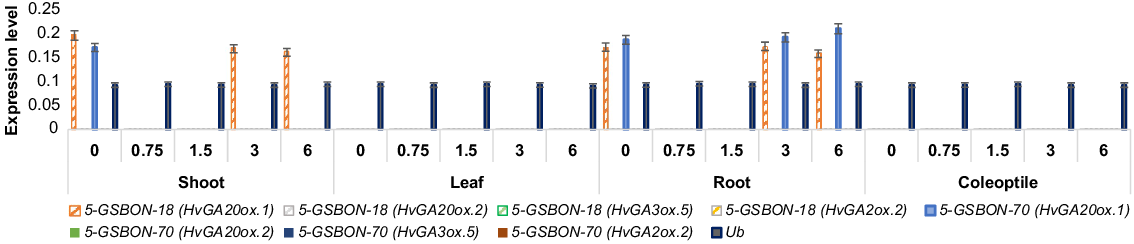
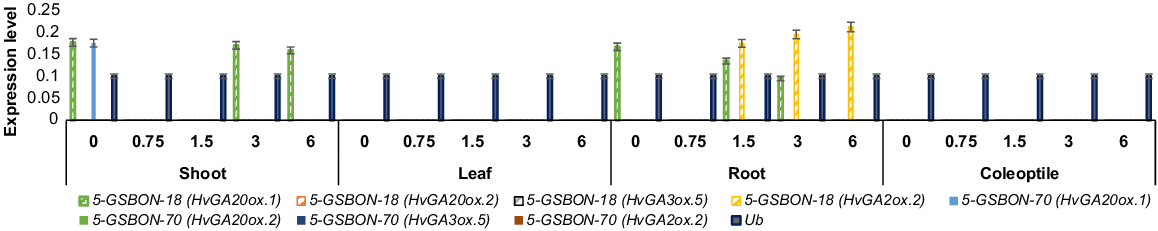
The quantified expression level of HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.2 transcripts from shoot, leaf, coleoptile and root after 0, 0.75, 1.5, 3 and 6 h of treatment under normal and osmotic stress, respectively, are in Figs 4 and 5. Under normal conditions, genotype 5-GSBON-70, showed 2-fold and 2.1-fold upregulation of HvGA20ox.1 transcript from the shoot at 3 and 6 h, respectively. Lower expression of HvGA20ox.1 transcript was observed for genotype 5-GSBON-18, at 0, 3 and 6 h from root and shoot tissues. Under osmotic stress, 2-fold upregulation of HvGA2ox.2 transcript from shoot was observed for genotype 5-GSBON-18 after 3 and 6 h of treatment. While the same genotype showed downregulation of HvGA20ox.1 at 3 h of treatment. No expression was observed from the leaf and coleoptile under both treatments.
Pattern of expression level of HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.2 transcripts under normal (N) condition. Numeric values on the x-axis show time interval (h). Genotype 5-GSBON-70 showed 2-fold upregulation of HvGA20ox.1 transcript from shoot tissue at 3 h. Bars indicate s.d. All samples were analysed in three technical replications. Ub, ubiquitin was used as an internal control. Solid colour, genotype 5-GSBON-70; pattern fill colour, genotype 5-GSBON-18.
Pattern of expression level of HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.2 transcripts under osmotic stress (OS). Numeric values on x-axis shows time interval (h). Genotype 5-GSBON-18, showed pattern of 2-fold upregulation for HvGA2ox.2 transcript at shoot tissue after 3 and 6 h of 20% PEG treatment. Bars indicate s.d. All samples were analysed in three technical replications. Ub, ubiquitin was used as an internal control. Solid colour, genotype, 5-GSBON-70; pattern fill colour, genotype 5-GSBON-18.
Phenotyping under field condition
Data for different morphological and physiological traits of both genotypes under normal and water deficit conditions were recorded in the field and mean data of both genotypes are in Fig. 6. Lodging percentage for the genotype 5-GSBON-70 was higher (55%) than 5-GSBON-18 (no lodging). The plant height of lodging-resistant genotype 5-GSBON-18 was 85.4 cm, and 109.5 cm for 5-GSBON-70. Similarly, internodal length was 10.5 cm for 5-GSBON-18, and 16.5 cm for 5-GSBON-70. Under water deficit condition, stem diameter (3.1 mm) and stem wall (0.31 mm) for the lodging-resistant genotype (5-GSBON-18), and 2.4 mm and 0.15 mm, respectively, for lodging-sensitive genotype (5-GSBON-70). Similarly, 1000-grain weight and grain yield were higher for 5-GSBON-18 than 5-GSBON-70 under both normal and water deficit conditions. Lodging-resistant genotype 5-GSBON-18 had chlorophyll (16.4%), photosynthesis (32.2%), cellulose (14.5%) and lignin (8.5%).
Mean data of morphological, physiological and biochemical traits of genotypes 5-GSBON-18 (lodging resistant) and 5-GSBON-70 (lodging sensitive) grown under normal (N) and water deficit (WD) conditions. PH, plant height; INTL, internodal length; NW, nodal width; SD, stem diameter; SWW, stem wall width; NN, number of nodes; LOD, lodging (LOD); TGW, 1000-grain weight; GY, grain yield; SC, stomatal conductance; CC, chlorophyll contents; P, photosynthesis; LT, leaf temperature; WUE, water use efficiency; T, transpiration, Ce, cellulose; Lig, lignin. Bars indicate s.d.

Transcript profiling
The quantified expression level of both genotypes for HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.5 transcripts from leaf, shoot, nodes and internodes were observed both under normal and drought stress (Figs 7 and 8). The genotype 5-GSBON-70 showed 2-fold upregulated expression for the HvGA20ox.1 transcript at node 3 and internode 2 and 3 at grain filling stage, under normal conditions (N) compared to the genotype 5-GSBON-18. While under water deficit conditions (WD), 2-fold upregulated expression was observed in genotype 5-GSBON-70 for HvGA20ox.1 transcript in shoot tissue at tillering. Expression of HvGA2ox.2 transcript for the genotype 5-GSBON-18 was 2.2-fold upregulated at node 3 and node 4 and 2.6-fold upregulated at internode 2 and 3. Downregulation of HvGA20ox.1 was observed at the third node and internode for genotype 5-GSBON-18, while genotype 5-GSBON-70 showed 2-fold upregulation of HvGA20ox.2 transcript at node 3, 4 and internode 2.
Expression level of HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.2 transcripts under normal (N) condition. Genotype 5-GSBON-70 showed 2-fold upregulation of HvGA20ox.1 transcript from second node and second and third internodes. Bars indicate s.d. All samples were analysed in three technical replications. Ub, ubiquitin was used as an internal control. Numeric values on x-axis shows the node and internode number. Solid colour, genotype 5-GSBON-7; pattern fill colour, genotype 5-GSBON-18. L, leaf; S, shoot; N, node; INT, internode; T, tillering stage; GF, grain filling stage.

Relative expression level of HvGA20ox.1, HvGA20ox.2, HvGA3ox.5 and HvGA2ox.2 transcripts under water deficit (WD) condition. Genotype 5-GSBON-18 had 2.2-fold upregulation at third and fourth node and 2.6 upregulation at second and third internode for the transcript HvGA2ox.2. Bars, s.d. All samples were analysed in three technical replications. Ub, ubiquitin was used as an internal control. Numeric values on x-axis shows the node and internode number. Solid colour, genotype, 5-GSBON-70; pattern fill colour, genotype 5-GSBON-18. L, leaf; S, shoot; N, node; INT, internode; T, tillering stage; GF, grain filling stage.

Discussion
The effect of GAs on plant height is well documented, and it plays an influential role in plant phenotype and final yield performance. Changes in GA metabolism or signalling contributed to the increased grain yield during the 1960’s Green Revolution (Wang and Wang 2022). The Hv20ox, HvGA3ox and HvGA2ox genes proved to be the most effective semi-dwarfing genes that were globally used in barley breeding programmes and contributed largely towards the agronomic and yield factors (Wu et al. 2010).
Seminal roots and coleoptile length play an important role in stand establishment (Ceccarelli et al. 2004). Long coleoptiles may be indicative of root lodging resistance (Stamp and Kiel 1992). In barley, significant differences were also found among the genotypes for seminal root numbers and root length (Tyagi et al. 2014). The mean data showed a significant difference among lodging-resistance and lodging-susceptible genotypes for coleoptile length, seminal root numbers, shoot/root ratio, water use efficiency, cellulose and lignin contents at the early stage (Fig. 3). Root traits that maintain the structure and functions contribute to minimise the risks of abiotic stresses and should be considered when in breeding programmes. Under field condition, significant differences in lodging, plant height, internodal length, grain yield, photosynthesis, water use efficiency, cellulose and lignin contents were observed among both genotypes (Fig. 6). Our results showed that the dwarf phenotype showed a significant effect on plant growth, increased tillers/plant, grains/spike, reduced lodging and increased grain yield. Reduction in plant height usually leads to strong straw and high resistance to lodging as happened in semi-dwarf rice and wheat cultivars (Jia et al. 2015). Lodging resistance is also associated with stem diameter and stem wall thickness of the basal internodes (Upadhya et al. 2016). Therefore, the basal three internodes and the thickness of the stem wall determine the stem resistance against lodging. Lignin and cellulose are the part of stem wall structure, and provides stability to the stem to resist lodging (Hyles et al. 2017). The association of higher concentration of cellulose and lignin with lodging resistance has been reported in several other investigations (Kong et al. 2013).
HvGA20ox.1 and HvGA20ox.2 were the most expressed transcripts in the vegetative tissues, especially at nodes and internodes (Figs 7 and 8). Several transcripts have very low or undetectable expression such as HvGA3ox transcripts mostly showed no expression from root tissues. Our findings indicated that genotype 5-GSBON-18 showed low expression of HvGA20ox.1 transcript specifically at node 3 and internode 3 and 2-fold upregulation of HvGA2ox.2 transcript in the same tissues under field condition, which suggests that this may affect the internodal length and resulted in a phenotypic change of that genotype. Significant association was found between Hv20ox.2 transcript abundance and plant height, which provide evidence that this semi-dwarfing gene (sdw1/denso) is a candidate gene that can produce significant phenotypic variations (Kuczyńska et al. 2013). Genotype 5-GSBON-18 had reduced plant height and internodal length compared with 5-GSBON-70 (Fig. 6). Similarly, in the case of expression profiling at the seedling stage, in the lodging-sensitive genotype, the abundance of HvGA20ox.1 was observed in the root and shoot both under normal and osmotic stress condition, while upregulation of HvGA2ox.2 was observed in the most lodging-resistant genotype from shoot tissue after application of treatment, which indicated that under water stress, shoot growth was low while roots grew faster comparatively. It has been reported that one member of GA3ox was expressed only in young seedlings and involved in germination only (Coles et al. 1999).
Changes in the expression level of transcripts confirmed that HvGA20ox is involved in the regulation of GA production, and it was also reported that GA promoted stem elongation and was found in higher concentrations in young tissue (Teshome and Kebede 2021). Genotype 5-GSBON-70 showed high abundance of GA20ox transcripts and very low or no abundance of HvGA2ox transcripts. It suggests that this genotype has faster stem elongation and resulted in tall plants and more prone to lodging. Similar observations has been reported in Arabidopsis where high expression of GA20ox.1 and GA3ox.1 genes was observed in newly developed vegetative tissues (Yamaguchi et al. 1998). In comparison, genotype 5-GSBON-18 had reduced expression of HvGA20ox.1 at nodes 3 and 4, while enhanced abundance at node 3, node 4 and internode 3 under water deficit condition. In a previous study, it was found that GA biosynthesis genes (GA20ox and HvGA3ox) were upregulated, and the GA catabolic gene (HvGA2ox) was downregulated in a barley mutant and resulted in dwarf phenotype (Jia et al. 2015). It is also reported that GA20ox and GA3ox gene family members are the major regulator of GA production in different parts of the plant (Wang and Wang 2022). Loss-of-function mutations in the key GA biosynthesis gene, OsGA20ox.2, have been linked to similar height phenotypes in rice (Spielmeyer et al. 2002), and evidence exists that semi-dwarfing of barley by sdw1/denso gene is associated with lower expression of orthologous HvGA20ox2 genes (Jia et al. 2009). Under water deficit conditions, guard cells and leaf tissue exhibited reduced levels of bioactive GAs resulting from reduced expression of GA20ox and high expression of GA2ox (Shohat et al. 2021). Similar results have been observed in our findings in the genotype 5-GSBON-18 (Fig. 8), which ultimately resulted in reduced internodal distance and plant height with increased lodging resistance. It has also been revealed that stem bending strength and internodal length influence the degree of lodging resistance in plants (Wu et al. 2022).
Our results showed that the genotype with high expression of HvGA2ox.2, was also associated with increased grain number, grain yield, photosynthesis and water use efficiency. The chlorophyll contents and stomatal conductance were also higher in crosses with high expression of HvGA2ox.2 transcript. Therefore, these genes significantly increased the lodging resistance and grain yield, specifically under water stress conditions. Several examples of plants with low GA concentration have been reported with a higher number of transcripts of GA20ox and GA3ox genes. Feedback regulation of GA20ox and GA3ox and feed-forward regulation of GA2ox gene expression have also been shown in GA-deficient Arabidopsis mutants (Rieu et al. 2008). Transcript abundance of TaGA20ox.3 and TaGA3ox.3 was very high in the grain, especially at the grain filling stage but the expression was much lower in the other tissues. This is like the expression patterns observed for OsGA20ox.3 and OsGA3ox.1 in rice (Sakamoto et al. 2004). Lignin and cellulose contents were found to be higher in 5-GSBON-18 under both normal and water deficit condition. As expected, no negative effects due to downregulation of HvGA20ox and upregulation of HvGA2ox transcripts were observed on lignin and cellulose contents. Several studies have reported an association between lodging resistance and higher concentrations of lignin and cellulose in the plant stem (Kong et al. 2013). Similarly, chlorophyll contents and photosynthesis were observed in sensitive genotype under water stress condition along with lignin and cellulose contents. It has also been reported that decline in physiological activities has been observed under drought stress condition (Nadeem et al. 2023; Zafar et al. 2023). It was also found that the expression of HvGA20ox in the denso mutant was downregulated by 4-fold, but almost 60-fold upregulated in the sdw1 mutant compared to the control (Jia et al. 2011).
Several morphological, anatomical, physiological and agronomical traits of plants may be affected by the sdw1/denso genes depending on genetic background and/or growth conditions. Additionally, barley genome sequencing will be helpful to understand the impact of linkage and/or pleiotropy in the traits that are genetically controlled with reduced height and semi-dwarfism. From our findings, we can conclude that downregulation of HvGA20ox.1, and 2-fold upregulation of HvGA2ox.2 transcripts results in the change of phenotypic expression through a decrease in internodal length and plant height under both normal and water deficit conditions. The introduction of dwarfing and semi-dwarfing genes exploited in cereal crops has resulted in a significant increase in grain yield, harvest index and lodging resistance (Khalid et al. 2023). As such, these transcripts can be further exploited in the barley breeding program for the development of semi-dwarf phenotypes, which will possess lodging resistance and improvement in yield and quality.
Conclusion
Semi-dwarfing genes still play a major role in barley breeding. Plant height and several morphological, anatomical and biochemical attributes are influenced by the semi-dwarfing genes depending on growth conditions and genetic background. This study reveals that under stress condition, 2-fold upregulation of HvGA2ox.2 transcript at the third and fourth node and second and third internode have significantly played role in reducing the plant height in the lodging resistant genotype 5-GSBON-18 with no negative effects on anatomical, biochemical and yield traits. High expression of HvGA2ox.2 transcript at the prescribed nodes and internodes reduces the plant height and internodal length and mitigates the lodging. Genotype 5-GSBON-18 with high expression of HvGA2ox.2 transcript under water deficit condition can be further utilised in barley breeding programme to develop semi-dwarf lodging resistant barley genotypes with better adaptation to marginal lands.
Author contributions
SL: conducted the experiments; ZA: conceptualisation of the study; ZA and MABS: supervised lab work and data analysis; SL, ZA and IA: manuscript preparation; RMI: supervised field work.
Acknowledgements
The authors thank the Australian Centre for International Agricultural Research (ACIAR) and MNS University of Agriculture, Multan, for their valuable help.
References
Berry PM, Griffin JM, Sylvester-Bradley R, Scott RK, Spink JH, Baker CJ, Clare RW (2000) Controlling plant form through husbandry to minimise lodging in wheat. Field Crops Research 67(1), 59-81.
| Crossref | Google Scholar |
Berry PM, Kendall S, Rutterford Z, Orford S, Griffiths S (2015) Historical analysis of the effects of breeding on the height of winter wheat (Triticum aestivum) and consequences for lodging. Euphytica 203(2), 375-383.
| Crossref | Google Scholar |
Coles JP, Phillips AL, Croker SJ, García-Lepe R, Lewis MJ, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. The Plant Journal 17(5), 547-556.
| Crossref | Google Scholar | PubMed |
Cuesta-Marcos A, Casas AM, Hayes PM, Gracia MP, Lasa JM, Ciudad F, Codesal P, Molina-Cano JL, Igartua E (2009) Yield QTL affected by heading date in Mediterranean grown barley. Plant Breeding 128(1), 46-53.
| Crossref | Google Scholar |
Dockter C, Hansson M (2015) Improving barley culm robustness for secured crop yield in a changing climate. Journal of Experimental Botany 66(12), 3499-3509.
| Crossref | Google Scholar | PubMed |
Fischer RA, Stapper M (1987) Lodging effects on high-yielding crops of irrigated semidwarf wheat. Field Crops Research 17, 245-258.
| Crossref | Google Scholar |
Foster CE, Martin TM, Pauly M (2010) Comprehensive compositional analysis of plant cell walls (Lignocellulosic biomass) part I: lignin. Journal of Visualized Experiments e1745.
| Crossref | Google Scholar |
Gibson G, Weir B (2005) The quantitative genetics of transcription. Trends in Genetics 21(11), 616-623.
| Crossref | Google Scholar | PubMed |
He H, Liang G, Lu S, Wang P, Liu T, Ma Z, Zuo C, Sun X, Chen B, Mao J (2019) Genome-wide identification and expression analysis of GA2ox, GA3ox, and GA20ox are related to gibberellin oxidase genes in grape (Vitis Vinifera L.). Genes 10(9), 680.
| Crossref | Google Scholar | PubMed |
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends in Plant Science 5(12), 523-530.
| Crossref | Google Scholar | PubMed |
Hyles J, Vautrin S, Pettolino F, MacMillan C, Stachurski Z, Breen J, Berges H, Wicker T, Spielmeyer W (2017) Repeat-length variation in a wheat cellulose synthase-like gene is associated with altered tiller number and stem cell wall composition. Journal of Experimental Botany 68(7), 1519-1529.
| Crossref | Google Scholar | PubMed |
Jia Q, Zhang J, Westcott S, Zhang X-Q, Bellgard M, Lance R, Li C (2009) GA-20 oxidase as a candidate for the semidwarf gene sdw1/denso in barley. Functional & Integrative Genomics 9(2), 255-262.
| Crossref | Google Scholar | PubMed |
Jia Q, Zhang X-Q, Westcott S, Broughton S, Cakir M, Yang J, Lance R, Li C (2011) Expression level of a gibberellin 20-oxidase gene is associated with multiple agronomic and quality traits in barley. Theoretical and Applied Genetics 122(8), 1451-1460.
| Crossref | Google Scholar | PubMed |
Jia Q, Li C, Shang Y, Zhu J, Hua W, Wang J, Yang J, Zhang G (2015) Molecular characterization and functional analysis of barley semi-dwarf mutant Riso no. 9265. BMC Genomics 16(1), 927.
| Crossref | Google Scholar |
Khalid MA, Ali Z, Tahir MHN, Ghaffar A, Ahmad J (2023) Genetic effects of GA-responsive dwarfing gene Rht13 on plant height, peduncle length, internodal length and grain yield of wheat under drought stress. Genes 14(3), 699.
| Crossref | Google Scholar |
Khobra R, Sareen S, Meena BK, Kumar A, Tiwari V, Singh GP (2019) Exploring the traits for lodging tolerance in wheat genotypes: a review. Physiology and Molecular Biology of Plants 25, 589-600.
| Crossref | Google Scholar | PubMed |
Kim SI, McKenzie KS, Tai TH (2009) A molecular survey of SD1 alleles used in U.S. rice cultivars. SABRAO Journal of Breeding and Genetics 41(1), 25-40.
| Google Scholar |
Kong E, Liu D, Guo X, Yang W, Sun J, Li X, Zhan K, Cui D, Lin J, Zhang A (2013) Anatomical and chemical characteristics associated with lodging resistance in wheat. The Crop Journal 1(1), 43-49.
| Crossref | Google Scholar |
Kuczyńska A, Surma M, Adamski T, Mikołajczak K, Krystkowiak K, Ogrodowicz P (2013) Effects of the semi-dwarfing sdw1/denso gene in barley. Journal of Applied Genetics 54, 381-390.
| Google Scholar | PubMed |
Liu L, Han R, Yu N, Zhang W, Xing L, Xie D, Peng D (2018) A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE 13(5), e0196592.
| Crossref | Google Scholar | PubMed |
Nadeem M, Khan AA, Khan AA, Nadeem J, Fatima U (2023) Cloning and characterization of Trichoderma glucanase gene for plant transformation. International Journal of Agriculture and Biosciences 12(1), 31-46.
| Crossref | Google Scholar |
Okuno A, Hirano K, Asano K, Takase W, Masuda R, Morinaka Y, Ueguchi-Tanaka M, Kitano H, Matsuoka M (2014) New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE 9(2), e86870.
| Crossref | Google Scholar | PubMed |
Olszewski N, Sun T-P, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell 14, S61-S80.
| Crossref | Google Scholar | PubMed |
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400(6741), 256-261.
| Crossref | Google Scholar | PubMed |
Peng D, Chen X, Yin Y, Lu K, Yang W, Tang Y, Wang Z (2014) Lodging resistance of winter wheat (Triticum aestivum L.): lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Research 157, 1-7.
| Crossref | Google Scholar |
Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P, Phillips AL (2008) Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. The Plant Cell 20(9), 2420-2436.
| Crossref | Google Scholar | PubMed |
Saddique MAB, Ali Z, Sher MA, Farid B, Ikram RM, Ahmad MS (2020) Proline, total antioxidant capacity, and OsP5CS gene activity in radical and plumule of rice are efficient drought tolerance indicator traits. International Journal of Agronomy 2020, 8862792.
| Crossref | Google Scholar |
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiology 134(4), 1642-1653.
| Crossref | Google Scholar | PubMed |
Shah L, Yahya M, Shah SMA, Nadeem M, Ali A, Ali A, Wang J, Riaz MW, Rehman S, Wu W, Khan RM, Abbas A, Riaz A, Anis GB, Si H, Jiang H, Ma C (2019) Improving lodging resistance: using wheat and rice as classical examples. International Journal of Molecular Sciences 20(17), 4211.
| Crossref | Google Scholar |
Shohat H, Cheriker H, Kilambi HV, Illouz Eliaz N, Blum S, Amsellem Z, Tarkowská D, Aharoni A, Eshed Y, Weiss D (2021) Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytologist 232(5), 1985-1998.
| Crossref | Google Scholar | PubMed |
Spielmeyer W, Ellis MH, Chandler PM (2002) Semidwarf (sd-1),“green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proceedings of the National Academy of Sciences 99(13), 9043-9048.
| Crossref | Google Scholar |
Stamp P, Kiel C (1992) Root morphology of maize and its relationship to root lodging. Journal of Agronomy and Crop Science 168(2), 113-118.
| Crossref | Google Scholar |
Teshome S, Kebede M (2021) Analysis of regulatory elements in GA2ox, GA3ox and GA20ox gene families in Arabidopsis thaliana: an important trait. Biotechnology & Biotechnological Equipment 35(1), 1603-1612.
| Crossref | Google Scholar |
Tyagi K, Lee HJ, Lee CA, Steffenson BJ, Kim YJ, Yun SJ (2014) Variation in seedling root traits in wild barley (Hordeum vulgare L. ssp. spontaneum) germplasm. Plant Genetic Resources 12(S1), S79-S82.
| Crossref | Google Scholar |
Upadhya D, Dhakal R, Khadka K, Rana S, Acharya P, Rana R, Chaudhary P (2016) Local knowledge on climate-induced traits in rice for improving crop yield, food security and climate resilience. International Journal of Agriculture Innovations and Research 5, 385-396.
| Google Scholar |
Wang S, Wang Y (2022) Harnessing hormone gibberellin knowledge for plant height regulation. Plant Cell Reports 41(10), 1945-1953.
| Crossref | Google Scholar | PubMed |
Wu X, Wang Z, Chang X, Jing R (2010) Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. Journal of Experimental Botany 61, 2923-2937.
| Crossref | Google Scholar | PubMed |
Wu W, Shah F, Ma B-L (2022) Understanding of crop lodging and agronomic strategies to improve the resilience of rapeseed production to climate change. Crop and Environment 1, 133-144.
| Crossref | Google Scholar |
Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun T-P (1998) Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. The Plant Cell 10(12), 2115-2126.
| Crossref | Google Scholar | PubMed |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14(6), 415-421.
| Crossref | Google Scholar |
Zafar MM, Chattha WS, Khan AI, Zafar S, Subhan M, Saleem H, Ali A, Ijaz A, Anwar Z, Qiao F, Shakeel A, Seleiman MF, Wasonga DO, Parvaiz A, Razzaq A, Xuefei J (2023) Drought and heat stress on cotton genotypes suggested agro-physiological and biochemical features for climate resilience. Frontiers in Plant Science 14, 1265700.
| Crossref | Google Scholar | PubMed |
Zhang W, Wu L, Ding Y (2017) Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). Journal of Plant Research 130(5), 859-871.
| Crossref | Google Scholar | PubMed |
Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, Mao B, Hanada A, Zhou H, Wang R, Li P, Zhu X, Mander LN, Kamiya Y, Yamaguchi S, He Z (2006) ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. The Plant Cell 18(2), 442-456.
| Crossref | Google Scholar | PubMed |