Leaf water δ18O reflects water vapour exchange and uptake by C3 and CAM epiphytic bromeliads in Panama
Monica Mejia-Chang A , Casandra Reyes-Garcia A B , Ulli Seibt A C , Jessica Royles A , Moritz T. Meyer A , Glyn D. Jones A , Klaus Winter D , Miquel Arnedo E and Howard Griffiths
D , Miquel Arnedo E and Howard Griffiths  A F
A F
A Physiological Ecology Group, Department of Plant Sciences, University of Cambridge, Cambridge, CB2 3EA, UK.
B Unidad de Recursos Naturales, Centro de Investigación Científica de Yucatán, Calle 43 Num. 130 Churburná de Hidalgo, Mérida, 97200, México.
C Department of Atmospheric and Oceanic Sciences, UCLA, Los Angeles, CA, USA.
D Smithsonian Tropical Research Institute, Apartado 0843-03092, Balboa, Republic of Panama.
E Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals, Fac. Biologia, Universitat de Barcelona, Av. Diagonal 645, 08028 Barcelona, Spain.
F Corresponding author. Email: hg230@cam.ac.uk
Functional Plant Biology 48(7) 732-742 https://doi.org/10.1071/FP21087
Submitted: 23 March 2021 Accepted: 20 April 2021 Published: 21 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
The distributions of CAM and C3 epiphytic bromeliads across an altitudinal gradient in western Panama were identified from carbon isotope (δ13C) signals, and epiphyte water balance was investigated via oxygen isotopes (δ18O) across wet and dry seasons. There were significant seasonal differences in leaf water (δ18Olw), precipitation, stored ‘tank’ water and water vapour. Values of δ18Olw were evaporatively enriched at low altitude in the dry season for the C3 epiphytes, associated with low relative humidity (RH) during the day. Crassulacean acid metabolism (CAM) δ18Olw values were relatively depleted, consistent with water vapour uptake during gas exchange under high RH at night. At high altitude, cloudforest locations, C3 δ18Olw also reflected water vapour uptake by day. A mesocosm experiment with Tillandsia fasciculata (CAM) and Werauhia sanguinolenta (C3) was combined with simulations using a non-steady-state oxygen isotope leaf water model. For both C3 and CAM bromeliads, δ18Olw became progressively depleted under saturating water vapour by day and night, although evaporative enrichment was restored in the C3 W. sanguinolenta under low humidity by day. Source water in the overlapping leaf base ‘tank’ was also modified by evaporative δ18O exchanges. The results demonstrate how stable isotopes in leaf water provide insights for atmospheric water vapour exchanges for both C3 and CAM systems.
Keywords: C3, CAM, Crassulacean acid metabolism, Tillandsia fasciculata, Werauhia sanguinolenta, photosynthetic pathway, gas exchange, epiphyte, oxygen isotopes, altitudinal gradient, mesocosm.
Introduction
Epiphytes provide sensitive climatic indicators, with their distribution reflecting both the microclimate within a particular forest canopy, as well as altitudinal zonation between forest formations (Gómez González et al. 2017; Horwath et al. 2019). Vascular epiphytes can comprise a significant component of diversity in lower montane forest canopies, and the distribution of C3 and Crassulacean acid metabolism (CAM) epiphytic bromeliads within neotropical forests has long provided a model system to integrate physiological ecology, life-form and habitat preference (Osmond 1978; Griffiths and Smith 1983; Crayn et al. 2015; Males and Griffiths 2018). Here, we analyse the distribution of C3 and CAM bromeliads along an altitudinal gradient in western Panama using stable isotopes to evaluate contrasting strategies for water use and exchange of water vapour.
Epiphytic bromeliad species exhibit great diversity in functional forms through an array of morphological and physiological traits. The vegetative body of epiphytic bromeliads is mostly comprised of leaves, whereas roots and stems are highly reduced (Males 2016; Leroy et al. 2019). Leaves are displayed in a rosette that often forms a water reservoir or ‘tank’ between overlapping leaf bases. The water-impounding tank constitutes a stable water source between rain events (North et al. 2013; Males 2016) but may be subject to direct evaporation (Zotz and Thomas 1999; Males and Griffiths 2018). With extensive leaf trichome cover (Benzing 1976), leaf wetting characteristics (Pierce et al. 2001; Leroy et al. 2019) also allow bromeliads to inform current studies on direct water uptake by leaves (Dawson and Goldsmith 2018; Berry et al. 2019).
Water availability and storage are a major determinant of bromeliad morphology and physiology (North et al. 2013; Males 2016; Males and Griffiths 2018). The C3 pathway tends to predominate in areas where rainfall is more frequent, whereas nocturnal stomatal opening (when transpiration is reduced) allows CAM bromeliads to predominate more in semiarid tropical forests or exposed portions of the canopy (Griffiths and Smith 1983). Bromeliad stomata are particularly sensitive to ambient vapour pressure (Lange and Medina 1979). The stable isotope signals in leaf carbon (δ13C) distinguish those species with CAM (Osmond 1978), whereas oxygen isotopes (δ18O) reflect water use characteristics (Seibt et al. 2008; Cernusak et al. 2016; Dubbert et al. 2017). Precipitation and source water inputs are usually depleted in 18O by around –5 to –10‰ relative to the Vienna standard mean ocean water (VSMOW) mass spectrometric standard but varies on a seasonal basis (Cernusak et al. 2016). For C3 plants, leaf water is normally evaporatively enriched in 18O during transpiration, and then subject to an additional biochemical enrichment of some +27‰ when transferred into organic material (Sternberg et al. 2006; Cernusak et al. 2016; Lehmann et al. 2020).
The δ18O signal in leaf water and organic material in bromeliads reflects the relative inputs from contrasting water sources such as precipitation, tank water (which may become evaporatively enriched between rain events) and atmospheric water vapour (Farquhar and Cernusak 2005; Seibt et al. 2008; Cernusak et al. 2016; Lehmann et al. 2020; Benettin et al. 2021). The net efflux of water vapour during gas exchange normally leads to evaporative enrichment of 18O in leaf water (δ18Olw) (Harwood et al. 1998), whereas water vapour ingress under high ambient humidity can lead to more depleted δ18Olw (Farquhar and Cernusak 2005), allowing niche specialisation to be defined for C3 and CAM epiphytic bromeliads (Helliker and Griffiths 2007; Reyes-García et al. 2008; Helliker 2011; Lehmann et al. 2020). Provided that the interplay between water sources, water use and degree of steady-state equilibration for isotopic signals in both C3 and CAM tissues can be well defined, 18O signals in both leaf water (δ18Olw) and organic material (δ18OOM) of epiphytes can be used to model climatic conditions along altitudinal and latitudinal gradients for the epiphytic bromeliad Tillandsia usneoides Sw. (Helliker 2014).
The objectives of this study were, first, to define the distribution of C3 and CAM epiphytic bromeliads along an altitudinal gradient in western Panama, using δ13C analyses to identify the metabolic pathway; and second, to use a combination of field sampling and laboratory experimentation to identify the determinants of δ18Olw and δ18OOM signals in the two photosynthetic pathways. We expected that CAM species would be more prevalent in the drier lowlands, with a higher abundance of C3 species in higher elevation forest systems. The contrasting climatic conditions and water source inputs along the altitudinal gradient allowed us to test the hypothesis that net water vapour uptake under high humidity would re-set the δ18Olw for epiphytes (Farquhar and Cernusak 2005; Helliker and Griffiths 2007; Reyes-García et al. 2008; Seibt et al. 2008; Lehmann et al. 2020). For C3 bromeliads, we hypothesised that that δ18Olw would show evaporative enrichment in lowland habitats, but would be more depleted at high altitude cloudforest habitats due to net water vapour inputs. For CAM bromeliads, we predicted that δ18Olw would also be depleted, due to water vapour inputs associated with stomatal opening at night under high atmospheric humidity (Helliker and Griffiths 2007; Reyes-García et al. 2008). Finally, we also set out to test whether water source inputs, from precipitation trapped in the bromeliad tanks, would be affected by growing season and leaf morphology, with higher evaporative 18O enrichment associated with more open leaf rosettes.
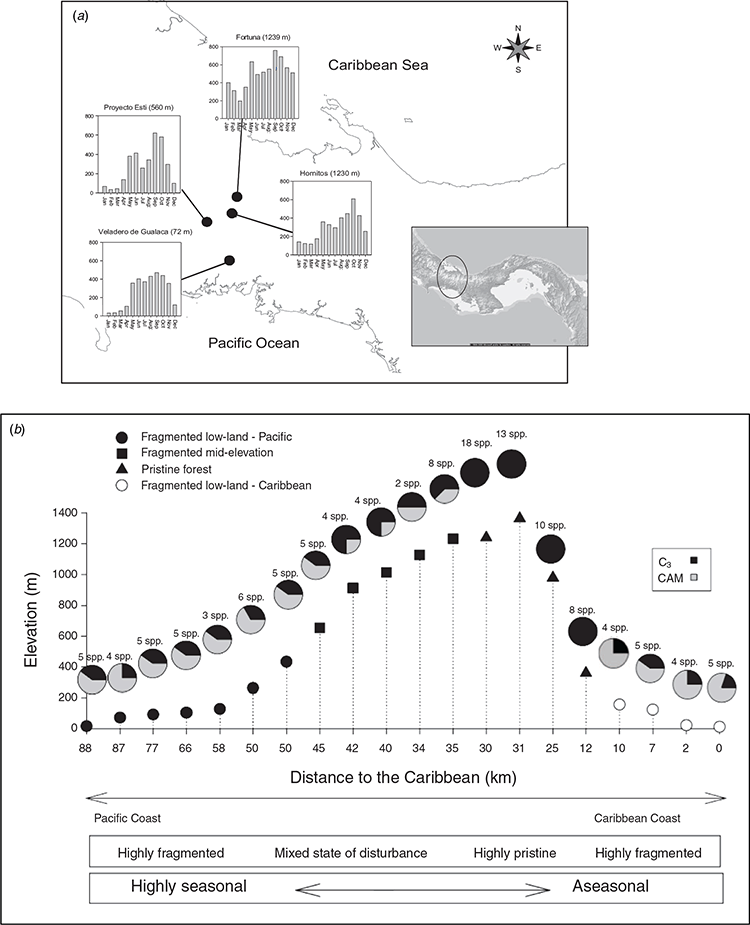
To test these hypotheses, and summarised in Fig. 1, we compared δ18Olw, δ18OOM and source water inputs for epiphytic CAM and C3 bromeliads along a 1300 m gradient over the Central Cordillera in western Panama, from the Pacific Coast in Chiriquí province, north to the Caribbean coast in Bocas del Toro. Measurements were taken in both rainy and dry seasons to capture the variations in source water δ18O inputs and likely evaporative demand. A more detailed analysis was undertaken for two sympatric species, Tillandsia fasciculata (CAM) and Werauhia sanguinolenta (Linden ex Cogniaux & Marchal) J.R.Grant (C3), that dominate the lower part of the altitudinal transect (between sea level and 600 m; Pierce et al. 2002a; Zotz et al. 2005). These species were also investigated experimentally, using an enclosed mesocosm, to measure the direct contribution from water vapour uptake under well-watered and drought-stressed conditions for C3 and CAM systems (Helliker and Griffiths 2007). This study confirmed the field work observations, showing that water vapour uptake at high humidities masked evaporative enrichment during transpiration, and was consistent with a mechanistic model of 18O enrichment, explaining depleted δ18Olw for CAM and upland C3 bromeliad epiphytes.
|
Materials and methods
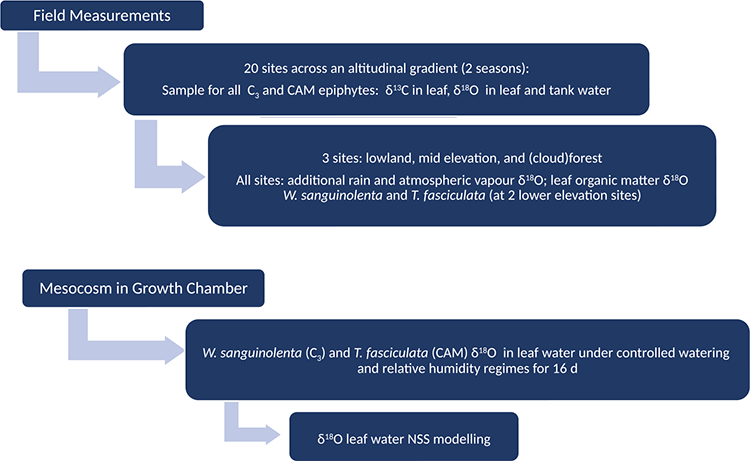
Field measurements
Fieldwork was conducted in 2006 along an altitudinal and precipitation gradient across the Central Cordillera of western Panama, where the dry season extends from January to April (Cavelier et al. 1996). The gradient encompassed 20 sites, incorporating lowland coastal sites located within 1 km of the Pacific Coast, up to pristine montane forest sites at 1300 m above sea level and back down to pastureland within 500 m of the Caribbean Coast (see Supplementary material Table S1). Monthly rainfall during the wet season is over 700 mm in the montane forest, but around 400 mm in the lowlands. During the dry season, the lowlands receive minimal rainfall, with montane sites receiving around 150 mm (data provided by Mr Ambrosio Morales, ETESA (Empresa de Transmisión Eléctrica, Panama)).
In order to determine the natural range of spatial and seasonal variation in δ18Olw and δ18OOM for C3 and CAM species, samples of leaf tissue from 36 bromeliad species (Table S2) were collected during the dry (February) and rainy (June) seasons in 2006 along the altitudinal gradient (Fig. 2). Leaf material was taken from the middle portion of fully expanded leaves to reduce isotopic variation associated to different leaf portions (Helliker and Ehleringer 2000; Ogée et al. 2007). Leaf samples were stored in sealed glass tubes. To avoid differential effects of transpiration all collections were made between 1100 and 1400 hours. If available, samples of tank water and precipitation were also collected with leaf tissue, for subsequent isotopic analysis.
For three of the sites, representing contrasting environmental conditions, an additional water source, atmospheric vapour, was sampled in order to understand the relationship with δ18Olw. The sites represented lowland (90 m elevation, 8°15′839′′N, 81°51′243′′W) and mid elevation (650 m elevation, 8°35′134′′N, 82°14′125′′W) pasture lands with dispersed trees, as well as a cloudforest (978 m elevation, 8°35′125′′N, 82°14′151′′W). Because δ18O of atmospheric water vapour can be very variable across the day due to atmospheric conditions, the samples were taken at five times during the day: 0600, 1000, 1400, 1800, and 2200 hours. Values shown here represent the mean of those measurements. Atmospheric vapour samples were collected using a dry ice-ethanol trap (Helliker et al. 2002); and were processed at the University of Cambridge, UK.
Humidity chamber measurements
Individuals of Werauhia sanguinolenta (Linden ex Cogniaux & Marchal) J.R.Grant (C3) and Tillandsia fasciculata (Sw. var. fasciculata; CAM) adapted to greenhouse conditions in Cambridge, UK were used to investigate the relationship between liquid water and atmospheric water vapour as determinants of C3 and CAM leaf water isotopic composition. Following Helliker and Griffiths (2007), plants were placed inside 50 L sealed mesocosm within a growth chamber with a 12 h photoperiod (300 μmol m–2 s–1 at plant height; day/night temperatures within the mesocosm held at 25 and 20°C respectively, monitored with thermocouple thermometer). The epiphytes rested on a mesh above a water-filled tray, with three 5 cm electric fans facing across the reservoir used to generate a high humidity environment; for the low humidity regime, the mesocosm water reservoir was emptied. Two successive 16 day treatments were undertaken with either ‘fully hydrated’ plants (water in tanks, n = 14) or ‘water-stressed’ treatments (no water in tanks, n = 15). Plants were kept at continuously high RH regime (High/High, day: 90%, night: 90%) for 8 days, and for a further 8 days at diurnally variable RH (with water removed from mesocosm reservoir, leading to a Low/High RH regime, day: 50%, night: 90%). The tanks of fully hydrated plants were emptied and refilled each day with local tap water with a δ18O of –7.0 ‰; for water stressed plants with empty tanks atmospheric vapour was the only water source. The replicate plants (14 or 15 in the two experiments) had rosettes with many leaves, allowing triplicate leaf samples to be collected at random from different plants on days 0, 3, 7, 8, 9, 10, 11, 15, and 16 for isotopic analyses. Leaf relative water content (RWC) was measured at day 0 and at the end of each 8-day period using the relationship RWC = (fresh weigh – dry weight)/(saturated weight – dry weight) × 100. Over the 16-day experimental period, tissue RWC decreased respectively from 91.6 to 86.6 ± 0.8% (CAM) and 68 to 65.4 ± 2.1% (C3) for the well-watered treatments (s.e. ranging from 0.8 to 2.1%), with a further decline to 62.3 ± 2.6% (CAM) and 31.3 ± 1.0% (C3) following the drought treatment. The mean δ18O of the water used in the reservoir to generate water vapour was –6.2‰ (s.e. ± 0.2‰) (n = 14), and the atmospheric water vapour of air passing through the experimental chamber was –19.3‰ (s.e. ± 0.4‰) and –17.8‰ (s.e. ± 0.3‰), for the water-stressed (n = 15) and fully hydrated treatments (n = 14), respectively.
Determination of δ18Olw
Leaf tissues sampled in the field and from the mesocosm experiment were initially stored in 12 mL glass tubes sealed with a screw cap and Viton rubber seal. Bulk leaf water was extracted from leaf tissue using a cryogenic vacuum distillation system and analysed isotopically after equilibration with CO2 on a mass spectrometer (VG SIRA 10, Modified by ProVac Systems, Crewe, UK), as described by Reyes-García et al. (2008). All δ18O values are reported with respect to VSMOW.
Leaf water δ18O values were corrected for atmospheric water vapour adsorbed to trichomes using the method of Helliker and Griffiths (2007). The adsorbed water was estimated to be 4 and 11% of the total collected water volume for 50 and 90% relative humidity, increasing δ18Olw by 0.1 and 0.5‰, respectively.
Organic material isotope composition
A sub-sample of leaves from the most common species collected in the field was oven-dried at 70°C for 3 days and ground to a fine powder and subdivided for isotopic analysis of organic matter. δ18OOM analysis was completed by Dr C. Keitel, Australian National University, Canberra, following (Farquhar et al. 1997). δ13COM was determined at the Godwin Laboratory, University of Cambridge, by an elemental analyser (Thermo Finnigan TC/EA) attached to a Thermo Delta V mass spectrometer (for details, see Horwath et al. 2019). Organic material was not sampled from the mesocosm experiment as insufficient new growth would have occurred during the short experimental period.
Model simulations
Patterns of δ18Olw during the chamber experiments were simulated using a non-steady-state (NSS) model of leaf water enrichment (Seibt et al. 2008), taking into account the isotopic composition of source water and water vapour, the equilibrium liquid-vapour fractionation (9.8‰ at 20°C), the kinetic fractionation during diffusion of vapour (26.5‰, based on the ratio of stomatal and leaf boundary layer conductances; Farquhar et al. 1989; Farquhar and Cernusak 2005; Barbour 2007; Cernusak et al. 2016), RH of air, leaf temperature, stomatal conductance, and changes in leaf water content. Calculations started from observed δ18Olw values. For the well-watered plants, the isotopic composition of tank water was used as the source water term. Stomatal conductance (gs) was parameterised from gas exchange measurements (using a Li-Cor 6400 system, Li-Cor Biosciences) for W. sanguinolenta (C3) during the day (0.016 mol m–2 s–1) and set to a small value at night (0.001 mol m–2 s–1). For T. fasciculata (CAM), gs was set to the opposite diurnal pattern (0.016 mol m–2 s–1 at night, 0.001 mol m–2 s–1 during the day). The difference between evaporating site and bulk leaf water due to the Péclet effect was also taken into account (eqns 17, 21, and 22 in Farquhar and Cernusak 2005). As the bromeliad leaves had a high leaf water content (22–34 mol m–2 for well-watered plants), an effective path length of 30 cm was used to calculate the Péclet number.
For plants under water stressed conditions (no source water input), the model becomes equivalent to that of Helliker and Griffiths (2007). Stomatal conductance was calculated from the observed leaf water loss. Assuming again small gs at night (C3) or day (CAM), gs during the day (C3) or night (CAM) was adjusted so that the cumulative transpiration matched the observed water loss from the leaves. The resulting gs values were 0.005 mol m–2 s–1 and 0.001 mol m–2 s–1 (C3), and 0.004 mol m–2 s–1 and 0.003 mol m–2 s–1 (CAM), for the two experimental periods (High/High, Low/High RH), respectively. In the absence of advection from source water towards the sites of evaporation, there is no Péclet effect. In addition, the Péclet number would be small (0.02) due to the small transpiration rates, so that the difference between evaporating site and bulk leaf water would only be in the order of 1% (Farquhar and Cernusak 2005).
Statistical analyses
Statistical analysis was carried out in StatView version 5.0 (SAS Institute Inc.) and R (ver. 3.6.3; R Core Team 2018). All analyses were considered significant at P < 0.05 and data presented as mean ± s.e. Depending upon the normality of the data distribution, t-test or Wilcoxon analyses were used to compare dry vs rainy season variation in water sources, as well as overall seasonal variation in isotopic composition of leaf water for C3 and CAM species. Where appropriate, ANOVA’s were used and if significant differences were found, Tukey´s method for pair-wise comparison was applied to discriminate differences within variables. Paired t-test analyses were used to compare the isotopic variation in the humidity chamber experiments. Variation in δ18Olw in relation to δ18O tank water along the altitudinal gradient was evaluated with a regression analysis. All analyses were considered significant at P < 0.05 and data presented as mean ± s.e.
Results
Field location and distribution of C3 and CAM bromeliad epiphytes
In Fig. 2a, the seasonality of precipitation is depicted across the continental divide in Panama, running south to north from the Province of Chiriqui (Pacific) to Bocas del Toro (Caribbean). A more distinct seasonal pattern of rainfall is found for the low altitude Pacific Coast, as compared with the high altitude cloudforest site at the Fortuna Nature Reserve (1239 m). Although the focus of this study was primarily on the southern aspect of this altitudinal transect, it is intriguing that the distribution of CAM bromeliads predominates in the lowland habitats on both sides of the continental divide (Fig. 2b), consistent with the higher temperatures and evaporative demand likely to be experienced in these habitats. At the higher altitude sites and those facing the prevailing rainfall inputs on the northern slope, the number of bromeliad epiphytes increases and become dominated by those expressing the C3 pathway (Fig. 2b).
Measurements of epiphytic bromeliad isotope components in the field
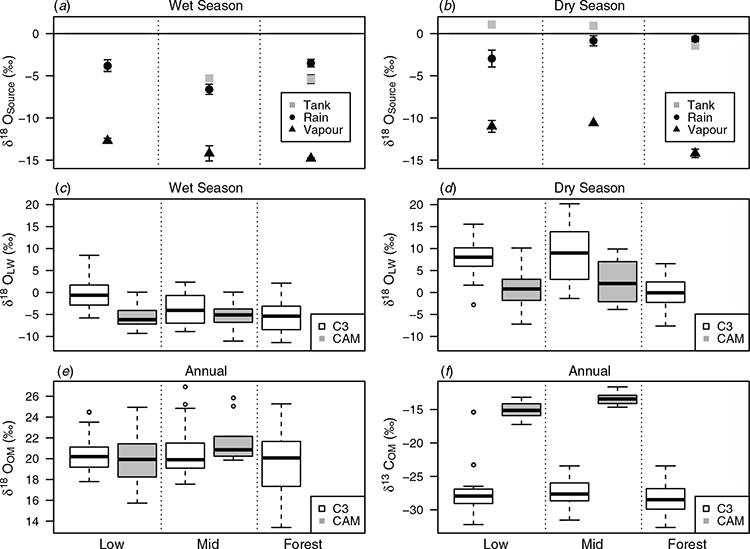
Water vapour was significantly depleted in δ18O, by around 7‰ relative to rainfall (t-test, t = –16.47, P < 0.001, Fig. 3a,b). Seasonal differences in precipitation, tank water and water vapour were significant (factorial ANOVA, seasonal effect, P < 0.01). Dry season water vapour was significantly enriched compared with the wet season (t-test, t = 2.34, P = 0.037). Water vapour from the lowland sites was significantly enriched in δ18O, in relation to the cloudforest site (t-test, t = –4.63, P < 0.001). Rainfall δ18O was also significantly more enriched during the dry season than during the wet season (W = 1262, P < 0.0001). In the wet season there was no significant difference between the tank water and rainfall δ18O (W = 3310, P = 0.9, Fig. 3a). During the dry season, evaporative enrichment increased the tank water δ18O above that of rainfall at low and mid-altitude sites (t = –4.47, P = 0.0005), whereas in the cloudforest sites, the two water sources were not significantly different (W = 258.5, P = 0.06) so tank water represented precipitation inputs (Fig. 3b).
|
Within all CAM and C3 species, δ18Olw values were significantly isotopically depleted during the wet season (C3: –2.6 ± 1.2‰, CAM: –7.1 ± 0.7‰; Fig. 3c) than in the dry season (C3: +3.7 ± 0.9‰, CAM: –0.6 ± 0.8‰), with CAM species having significantly more depleted δ18Olw than C3 species (factorial ANOVA seasonal and photosynthetic pathway effects P < 0.05; Fig. 3d). The high altitude, montane forest species had the most isotopically depleted C3 δ18Olw values in the wet and dry season (factorial ANOVA, significant season, elevation and photosynthetic pathway effects P < 0.05 and Fig. 3c, d). In comparison, for organic material, which integrates wet and dry season precipitation inputs, there was no significant variation in δ18OOM between elevation for either CAM species (W = 223.5, P = 0.06) or C3 species (ANOVA P = 0.1318, F = 2.095, DF = 2). CAM species growing at mid-elevation sites had significantly less negative values of δ13C (–13.2‰ ± 0.2) than plants at the lowlands (–15.0‰ ± 0.2) (Fig. 3f) (W = 6688, P < 0.001), and in contrast, no significant variation was detected for C3 plants across habitats (ANOVA P > 0.05). As would be expected due to the different metabolic pathways, an offset in δ13COM of ~10‰ was measured between CAM and C3 bromeliads.
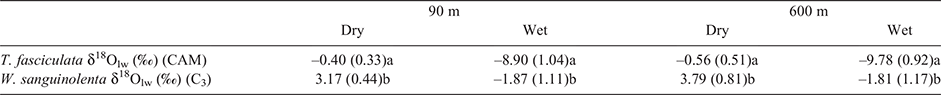
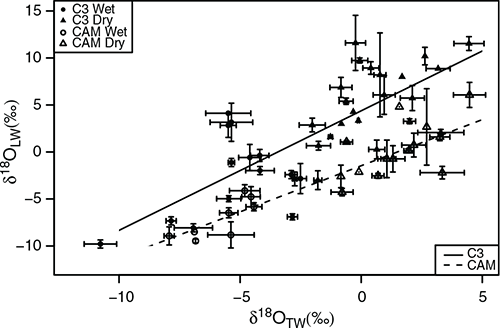
The overlapping leaf bases ‘tanks’ provide a reservoir of water to sustain hydraulic supply between rain events, although tank water also tends to evaporate directly during the dry season. Fig. 4 shows the systematic variations in C3 and CAM δ18Olw in relation to tank water δ18, for all samples across both wet and dry seasons. For both C3 and CAM species, when calculated independently of elevation and season, δ18Olw was positively correlated with the δ18O of source tank water (Fig. 4, P < 0.0001, r2 = 0.57 and P < 0.0001, r2 = 0.75 for C3 and CAM species, respectively). Across the range of tank water δ18Olw values measured, there is a tendency for C3 leaf water δ18Olw to be more enriched than CAM (t-test, t = –3.57, P = 0.0007) with the offset reduced in the more negative values seen for the wet season (Fig. 4). This data is consistent with leaf water values for the two individual C3 (W. sanguinolenta) and CAM (T. fasciculata) species shown in Table 1. The δ18Olw signals of C3 leaves was enriched in 18O relative to CAM, and were consistent with the overall pattern for all other sympatric C3 and CAM species (Fig. 3c, d). There was also a shift within δ18Olw for the CAM T. fasciculata, which was more pronounced from dry to wet season (depleted by around 9‰ at both altitudes) than in the C3 W. sanguinolenta (Table 1).
|
Measurements of isotopic exchange by C3 and CAM tank bromeliads in a humidity chamber
We explored the systematic differences in δ18Olw between two CAM and C3 plants under controlled conditions in a mesocosm experiment, and analysed the role of water sources, photosynthetic pathways and evaporative control using a non-steady-state (NSS) model of leaf water enrichment (Farquhar and Cernusak 2005). For plants without source water input (drought stressed), the model is equivalent to that of Helliker and Griffiths (2007).
Although the tank water was replaced on a daily basis in the well-watered treatment (with local tap water with δ18O –7.0‰), over each 24 h period tank water became enriched for T. fasciculata (–4.8‰ ± 0.1) (n = 32) relative to W. sanguinolenta (–5.4‰ ± 0.1) (n = 40) (t-test, P < 0.0001), probably reflecting the more open leaf structure and shallower tank in T. fasciculata.
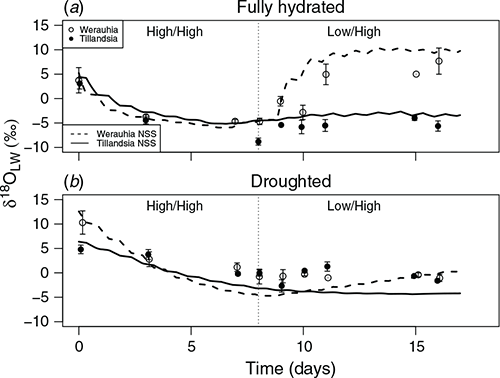
For well-watered plants, δ18Olw decreased over the first 8 days of high RH (12‰ for T. fasciculata, 8‰ for W. sanguinolenta, P < 0.003) (Fig. 5a). After the switch to low daytime RH, isotopic enrichment occurred progressively for W. sanguinolenta, and after 8 days the δ18Olw was enriched more in the C3 (12‰, P = 0.0007) than the CAM plant (3‰, P = 0.04), relative to the respective values following the high humidity regime (Fig. 5a). The model simulations compare well to the observed δ18Olw values (Fig. 5a). The model reproduces the gradual decrease of δ18Olw in both species during the initial period of constant high RH (High/High), and the subsequent evaporative increase of δ18Olw for the C3 W. sanguinolenta but constant with depleted δ18Olw for the CAM T. fasciculata during the final period of low daytime RH (Low/High).
|
Water-stressed plants also experienced a depletion in δ18Olw over the first 8 days at high RH (4‰ for T. fasciculata, 10‰ for W. sanguinolenta, P < 0.008) (Fig. 5b). No changes in isotopic values were found in either species during the low daytime RH treatment (Fig. 5b). For hydrated plants the model simulations reproduce the general patterns in δ18Olw, but under droughted conditions the model under-predicted δ18Olw for both species towards the end of the high-RH period and for the first few days of the low-RH period (Fig. 5b). The model captures reduced level of enrichment seen under droughted conditions in the C3 W. sanguinolenta, with minimal gas exchange is occurring under low daytime humidity (Fig. 5b) consistent with directly measured stomatal conductance (data not shown).
Discussion
The distribution of CAM and C3 epiphytic bromeliads across the continental divide in western Panama was used as the basis for a comparison of the coupling in water budgets for the two photosynthetic pathways. The study set out to define whether leaf water oxygen stable isotopes (δ18Olw) reflect the seasonal variability in precipitation, changes in δ18O of tank water reserves, and contrasting daily gas exchange patterns, along an altitudinal transect running north from the Pacific to montane cloud forest at Fortuna (Cavelier et al. 1996; Zotz et al. 2005; Gómez González et al. 2017; Benettin et al. 2021). The overall aim was to investigate whether water vapour exchange across leaf surfaces, when atmospheric water vapour concentrations are close to saturation, leads to significant inward fluxes of water which ‘reset’ the δ18Olw under natural conditions in the field (Farquhar and Cernusak 2005; Seibt et al. 2006; Helliker and Griffiths 2007; Helliker 2011; Lehmann et al. 2020) in addition to potential liquid water uptake (Pierce et al. 2001; Dawson and Goldsmith 2018; Berry et al. 2019).
The distribution of C3 and CAM bromeliad epiphytes did conform to the hypothesis that C3 species would dominate in higher, cloudforest habitats (Fig. 2), and we explore the integration of bromeliad hydraulic properties and δ18O signals in ecological terms in the final section below. We also set out to test the hypothesis that the gas exchange of C3 and CAM bromeliads would result in contrasting temporal and spatial drivers for δ18Olw, when compared with source water δ18O inputs measured in terms of precipitation, tank water and water vapour (Dubbert et al. 2017; Benettin et al. 2021). Depending on seasonal conditions (dry or wet season), we thought that daytime gas exchange associated with C3 species should be subject to evaporative enrichment in δ18Olw at lower altitudes in the dry season. The impact of net water vapour uptake under high humidities was predicted to result in lower δ18Olw at high altitude cloudforest sites in the dry season, and for low altitude sites in the wet season, as seen in experimental studies (Helliker and Griffiths 2007; Reyes-García et al. 2008; Lehmann et al. 2020).
The data shown in Fig. 3 confirm these predictions, where the significant shifts in δ18O source water inputs as enriched precipitation, tank water and water vapour in the dry season, relative to the wet season (Fig. 3a, b). The associated δ18Olw signals in C3 bromeliads were evaporatively enriched at lower altitudes, and showed a progressive depletion with altitude in the rainy season, consistent with an increasing role for water vapour uptake, particularly in humid cloudforest habitats (Fig. 3b, c). These shifts are consistent with those seen experimentally with a range of C3 species (Lehmann et al. 2020), as well as cloudforest bryophyte communities, where water vapour was also shown to dominate hydrological budgets (Horwath et al. 2019).
The night-time stomatal opening associated with the CAM pathway should allow for net water vapour uptake to dominate δ18Olw signals, although we expected to see contrasting responses between rainy and dry seasons. The data for the range of CAM species found at lower altitude sites were again consistent with these predictions, with significantly lower δ18Olw values when compared with sympatric C3 species, and the general predictions of water vapour uptake seen previously for CAM plants (Helliker and Griffiths 2007; Reyes-Garcia et al. 2008; Helliker 2011, 2014; Lehmann et al. 2020).
The δ18Olw signal was highly correlated with the tank water δ18O (Fig. 4) and isotopic signatures of tank water reflected evaporative enrichment relative to precipitation under more arid, lowland conditions (Fig. 3a–d). At high altitude, the δ18O of precipitation inputs tended to be similar to regularly replenished tank water 18O in both wet and dry seasons (Fig. 3a, b). Integration of leaf water signal into organic matter occurs over time (Barbour 2007; Cernusak et al. 2016), and thus δ18Olw represents a more instantaneous marker for coupling of water budgets relative to the organic matter 18O signal (Fig. 3e), although the sampling in this study did not show systematic shifts in the δ18OOM for bromeliad tissues, relative to those seen in other studies (Cernusak et al. 2016; Horwath et al. 2019).
The study then focussed on two sympatric species, T. fasciculata (CAM) and W. sanguinolenta (C3), which are widely distributed across the lower altitudes of the transect (from sea level to 600 m) (Pierce et al. 2002a; Zotz et al. 2005). Overall, the variation in δ18Olw was highly responsive to seasonality, precipitation inputs and ambient humidity (Table 1; Figs 3a–d, 4) and, by inference, stomatal sensitivity during gas exchange (Lange and Medina 1979; Griffiths et al. 1986). The experimental manipulation of both species in a humidity chamber mesocosm provided additional evidence for the interplay between water vapour inputs, recharge by precipitation, the holding capacity of the bromeliad ‘tank’, and the regulation of net leaf water use.
Analysing the controls on leaf water enrichment with a NSS model
The experimental component of our study validated the notion that under high humidity, the gross diffusive exchanges of water vapour can dominate leaf water isotopic signatures (Farquhar and Cernusak 2005; Seibt et al. 2006; Helliker and Griffiths 2007; Seibt et al. 2007; Reyes-García et al. 2008; Helliker 2011, 2014; Lehmann et al. 2020). Understanding NSS controls on the isotopic signature of plant water and tissue organic material is important during gas exchange by certain species (Farquhar and Cernusak 2005; Cernusak et al. 2008; Lai et al. 2008; Cernusak et al. 2016; Benettin et al. 2021). Our simulations with a NSS leaf water model illustrate how the contrasting diurnal patterns of gas exchange affect δ18Olw for the two species during the humidity chamber experiments.
For the initial experimental period, despite the distinct stomatal opening patterns of C3 and CAM, the δ18Olw became progressively depleted under saturating atmospheric water vapour by day and night. In contrast, for the final period of low RH during the day, which more closely mimics field conditions at lower altitudes, the daytime rates of C3 gas exchange coincided with low RH, resulting in evaporative leaf water enrichment for the well-watered plants (Fig. 5a). As a consequence of the high leaf water content, low stomatal conductance and low leaf hydraulic conductance representative of tank bromeliads (North et al. 2013; Males 2016; Males and Griffiths 2018), both species in our study had very low rates of leaf water turnover and thus their δ18Olw values changed relatively slowly over a time course of days (Helliker and Griffiths 2007; Cernusak et al. 2008; Lehmann et al. 2020).
Alternatively, under droughted conditions, the sensitivity of bromeliad stomata to humidity (Lange and Medina 1979; Griffiths et al. 1986) resulted in reduced water vapour exchange and reduced evaporative enrichment in the C3 δ18Olw measured empirically and modelled (Fig. 5b). The NSS model also utilised bromeliad tank water as an external water source for leaves similar to soil water in rooted plants. Thus, the gradual evaporative enrichment of tank water over time, seen in both the laboratory mesocosm on a daily basis, and under seasonal conditions in the field (Figs 3, 4) represents an important control on δ18Olw and organic material in tank bromeliads.
Implications of water vapour for leaf water signatures: from mesocosm to the field
The manipulative component of our study showed that at high RH, leaf water δ18O signals of both species were dominated by water vapour exchange, even as the water status of the plants, measured as relative water content (RWC), declined (Fig. 4; see ‘Materials and methods’ for experimental details). Our result is consistent with previous observations demonstrating that δ18Olw signals in atmospheric CAM bromeliads were dominated by water vapour when exposed to high RH (Helliker and Griffiths 2007; Reyes-García et al. 2008; Helliker 2011, 2014; Lehmann et al. 2020).
The control of water vapour on δ18Olw under high humidity conditions did not lead to significant shifts in organic material isotopic signatures in the samples analysed under field conditions. The lower δ18Olw values for C3 bromeliads in the high altitude cloudforest sites, relative to lowland sites, were consistent with the more negative δ13C values found in organic material of those species (Fig. 3f), also consistent with higher stomatal conductances (Seibt et al. 2008). The comparison of sympatric C3 and CAM species across the lower altitudinal gradient (Table 1) showed that T. fasciculata (CAM) performing gas exchange under high RH at night consistently showed depleted δ18Olw. In contrast, when W. sanguinolenta (C3) was exposed to lower RH during gas exchange and δ18Olw was evaporatively enriched. The mesocosm manipulations showed that C3 species are as susceptible to the influx of water vapour as CAM species, which was supported by the field data for bromeliads from the high elevation cloud forest sites, which has been confirmed by the recent manipulations of C3 and CAM species under saturating water vapour (Lehmann et al. 2020). Evidence of water vapour as a main control on δ18Olw for Inga sp., a C3 understory tree, has been reported for the Amazon rainforest (Lai et al. 2008). Furthermore, demonstration of nocturnal transpiration and stomatal conductance across different biomes (Dawson et al. 2007; Seibt et al. 2007) indicate that the contribution of water vapour might be significant to leaf water at a global scale, particularly when RH is high, in addition to liquid water uptake by wet leaves (Pierce et al. 2001; Dawson and Goldsmith 2018; Berry et al. 2019; Leroy et al. 2019).
Conversely, the relatively small fluctuations observed in δ18Olw during water stress and low RH in the mesocosm (Fig. 4) reflects the overriding control of stomatal conductance, as stomatal sensitivity to low RH and drought has been consistently shown for bromeliads (Fig. 5, see also Lange and Medina 1979; Griffiths et al. 1986; Zotz and Andrade 1998). The temporal separation of gas exchange for C3 and CAM species is also reflected in the differential signatures for the bromeliads growing along the altitudinal gradient (Fig. 3), where C3 species consistently showed higher δ18Olw in comparison with sympatric CAM species. Our findings suggest that δ18Olw in C3 bromeliads provide clear markers of local climatic conditions of humidity and water sources.
Ecological implications
The results of this study highlight the sensitivity of epiphytic bromeliads to drought, water stress and changes in local relative humidity, and offers information on the life form and metabolic pathway variation in δ18Olw for plants exposed to common environmental conditions. The δ18Olw signal in bromeliad epiphytes can be used as a proxy to understand shifts in the water use of the species which may result from deforestation and its effect on microclimate, as it diminishes rain and fog inputs (Gómez González et al. 2017; Horwath et al. 2019) and increases canopy openness that in turn increases light incidence and evaporative demand (Williams et al. 2020). This type of monitoring is also relevant as temperatures increase and precipitation patterns are affected by global warming (Horwath et al. 2019). Shifts in δ18Olw and δ18OOM were found to reflect water vapour inputs for the atmospheric CAM T. usneoides, as precipitation δ18O varied along a latitudinal gradient (Helliker 2014).
We explored an altitudinal gradient and did not find a clear trend of higher enrichment organic material at the lower, more open, drier forests, compared with the more moist, upper montane forests. This lack of a correlation may be explained because most of the sites were secondary forests and pastures, with reduced canopy cover below 1000 m (Fig. 2) or differing growing seasons for C3 and CAM bromeliads. Significant within-site variation was possibly introduced by the different species sampled, which have different tank capacities, plant size, succulence, trichome cover and other ecophysiological traits that influence water use (Pierce et al. 2002a, 2002b; North et al. 2013; Males 2016; Males and Griffiths 2018).
Finally, we also note that the overall distribution of CAM bromeliads across the altitudinal transect is consistent with the predicted capacity to cope with semiarid habitats (Osmond 1978) as seen in lowland habitats on both sides of the continental divide in Panama (Fig. 2) and other tropical coastal locations (Griffiths and Smith 1983; Griffiths et al. 1986). Although sadly there were no CAM bromeliads found in the cloud forest habitat at Fortuna (in contrast to that seen in eastern Panama, Pierce et al. 2002b), the combined use of stable isotopes of oxygen and carbon have helped to de-mistify bromeliads, which continue to set CAM as a curiosity in context (Osmond 1978).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Monica Mejía-Chang was a recipient of a British Council Chevening Fellowship, a Short-term Fellowship from the Smithsonian Tropical Research Institute and a PhD scholarship from the government of Panama through the Senacyt-IFARHU 2005–2010 program. Casandra Reyes-García received a traveling grant from Fondo Sectorial SEP-CONACYT 221490. Jessica Royles was supported by NERC NE/M001946/1.
Acknowledgements
We would like to thank A. Virgo for laboratory assistance in Panama and M. García for construction of the water vapour trap used in the field collections. Valuable help was provided by the staff of the Fortuna Biological Station, and by N. González in the field. We are grateful for the generous advice and design of the growth chamber mesocosm by Dr Brent Helliker. All of us wish to acknowledge the career trajectories which, directly or indirectly, have been influenced by the insights, guidance and support of Professor C. Barry Osmond FAA FRS.
References
Barbour MM (2007) Stable oxygen isotope composition of plant tissue: a review. Functional Plant Biology 34, 83–94.| Stable oxygen isotope composition of plant tissue: a review.Crossref | GoogleScholarGoogle Scholar | 32689335PubMed |
Benettin P, Nehemy MF, Cernusak LA, Kahmen A, McDonnell JJ (2021) On the use of leaf water to determine plant water source: A proof of concept. Hydrological Processes 35, e14073
| On the use of leaf water to determine plant water source: A proof of concept.Crossref | GoogleScholarGoogle Scholar |
Benzing DH (1976) Bromeliad trichomes: structure, function, and ecological significance. Selbyana 1, 330–348.
Berry ZC, Emery NC, Gotsch SG, Goldsmith GR (2019) Foliar water uptake: Processes, pathways, and integration into plant water budgets. Plant, Cell & Environment 42, 410–423.
| Foliar water uptake: Processes, pathways, and integration into plant water budgets.Crossref | GoogleScholarGoogle Scholar |
Cavelier J, Solis D, Jaramillo MA (1996) Fog interception in montane forests across the Central Cordillera of Panamá. Journal of Tropical Ecology 12, 357–369.
| Fog interception in montane forests across the Central Cordillera of Panamá.Crossref | GoogleScholarGoogle Scholar |
Cernusak LA, Mejia-Chang M, Winter K, Griffiths H (2008) Oxygen isotope composition of CAM and C3 Clusia species: non-steady-state dynamics control leaf water 18O enrichment in succulent leaves. Plant, Cell & Environment 31, 1644–1662.
| Oxygen isotope composition of CAM and C3 Clusia species: non-steady-state dynamics control leaf water 18O enrichment in succulent leaves.Crossref | GoogleScholarGoogle Scholar |
Cernusak LA, Barbour MM, Arndt SK, Cheesman AW, English NB, Feild TS, Helliker BR, Holloway-Phillips MM, Holtum JAM, Kahmen A, McInerney FA, Munksgaard NC, Simonin KA, Song X, Stuart-Williams H, West JB, Farquhar GD (2016) Stable isotopes in leaf water of terrestrial plants. Plant, Cell & Environment 39, 1087–1102.
| Stable isotopes in leaf water of terrestrial plants.Crossref | GoogleScholarGoogle Scholar |
Crayn DM, Winter K, Schulte K, Smith JAC (2015) Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species. Botanical Journal of the Linnean Society 178, 169–221.
| Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species.Crossref | GoogleScholarGoogle Scholar |
Dawson TE, Goldsmith GR (2018) The value of wet leaves. New Phytologist 219, 1156–1169.
| The value of wet leaves.Crossref | GoogleScholarGoogle Scholar |
Dawson TE, Burgess SSO, Tu KP, Oliveira RS, Santiago LS, Fisher JB, Simonin KA, Ambrose AR (2007) Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiology 27, 561–575.
| Nighttime transpiration in woody plants from contrasting ecosystems.Crossref | GoogleScholarGoogle Scholar | 17241998PubMed |
Dubbert M, Kübert A, Werner C (2017) Impact of leaf traits on temporal dynamics of transpired oxygen isotope signatures and its impact on atmospheric vapor. Frontiers in Plant Science 8, 5
| Impact of leaf traits on temporal dynamics of transpired oxygen isotope signatures and its impact on atmospheric vapor.Crossref | GoogleScholarGoogle Scholar | 28149303PubMed |
Farquhar GD, Cernusak LA (2005) On the isotopic composition of leaf water in the non-steady state. Functional Plant Biology 32, 293–303.
| On the isotopic composition of leaf water in the non-steady state.Crossref | GoogleScholarGoogle Scholar | 32689132PubMed |
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537.
| Carbon isotope discrimination and photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Farquhar GD, Henry BK, Styles JM (1997) A rapid on-line technique for determination of oxygen isotope composition of nitrogen-containing organic matter and water. Rapid Communications in Mass Spectrometry 11, 1554–1560.
| A rapid on-line technique for determination of oxygen isotope composition of nitrogen-containing organic matter and water.Crossref | GoogleScholarGoogle Scholar |
Gómez González DC, Quiel CR, Zotz G, Bader MY (2017) Species richness and biomass of epiphytic vegetation in a tropical montane forest in western Panama. Tropical Conservation Science 10, 1–17.
| Species richness and biomass of epiphytic vegetation in a tropical montane forest in western Panama.Crossref | GoogleScholarGoogle Scholar |
Griffiths H, Smith JAC (1983) Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 60, 176–184.
| Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM.Crossref | GoogleScholarGoogle Scholar | 28310484PubMed |
Griffiths H, Lüttge U, Stimmel KH, Crook CE, Griffiths NM, Smith JAC (1986) Comparative ecophysiology of CAM and C3 bromeliads. III: Environmental influences on CO2 assimilation and transpiration. Plant, Cell & Environment 9, 385–393.
| Comparative ecophysiology of CAM and C3 bromeliads. III: Environmental influences on CO2 assimilation and transpiration.Crossref | GoogleScholarGoogle Scholar |
Harwood KG, Gillon JS, Griffiths H, Broadmeadow MSJ (1998) Diurnal variation of Δ13CO2, ΔC18O16O and evaporative site enrichment of δH218O in Piper aduncum under field conditions in Trinidad. Plant, Cell & Environment 21, 269–283.
| Diurnal variation of Δ13CO2, ΔC18O16O and evaporative site enrichment of δH218O in Piper aduncum under field conditions in Trinidad.Crossref | GoogleScholarGoogle Scholar |
Helliker BR (2011) On the controls of leaf-water oxygen isotope ratios in the atmospheric Crassulacean acid metabolism epiphyte Tillandsia usneoides. Plant Physiology 155, 2096–2107.
| On the controls of leaf-water oxygen isotope ratios in the atmospheric Crassulacean acid metabolism epiphyte Tillandsia usneoides.Crossref | GoogleScholarGoogle Scholar | 21300917PubMed |
Helliker BR (2014) Reconstructing the δ18O of atmospheric water vapour via the CAM epiphyte Tillandsia usneoides: seasonal controls on δ18O in the field and large-scale reconstruction of δ18Oa. Plant, Cell & Environment 37, 541–556.
| Reconstructing the δ18O of atmospheric water vapour via the CAM epiphyte Tillandsia usneoides: seasonal controls on δ18O in the field and large-scale reconstruction of δ18Oa.Crossref | GoogleScholarGoogle Scholar |
Helliker BR, Ehleringer JR (2000) Establishing a grassland signature in veins: O18 in the leaf water of C3 and C4 grasses. Proceedings of the National Academy of Sciences of the United States of America 97, 7894–7898.
| Establishing a grassland signature in veins: O18 in the leaf water of C3 and C4 grasses.Crossref | GoogleScholarGoogle Scholar | 10884421PubMed |
Helliker BR, Griffiths H (2007) Towards a plant-based proxy for the isotope ratio of atmospheric water vapor. Global Change Biology 13, 723–733.
| Towards a plant-based proxy for the isotope ratio of atmospheric water vapor.Crossref | GoogleScholarGoogle Scholar |
Helliker BR, Roden JS, Cook C, Ehleringer JR (2002) A rapid and precise method for sampling and determining the oxygen isotope ratio of atmospheric water vapor. Rapid Communications in Mass Spectrometry 16, 929–932.
| A rapid and precise method for sampling and determining the oxygen isotope ratio of atmospheric water vapor.Crossref | GoogleScholarGoogle Scholar | 11968123PubMed |
Horwath AB, Royles J, Tito R, Gudiño JA, Salazar Allen N, Farfan-Rios W, Rapp JM, Silman MR, Malhi Y, Swamy V, Latorre Farfan JP, Griffiths H (2019) Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent. Proceedings of the Royal Society B: Biological Sciences 286, 20182284
| Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent.Crossref | GoogleScholarGoogle Scholar | 30963945PubMed |
Lai CT, Ometto JPHB, Berry JA, Martinelli LA, Domingues TF, Ehleringer JR (2008) Life form-specific variations in leaf water oxygen-18 enrichment in Amazonia vegetation. Oecologia 157, 197–210.
| Life form-specific variations in leaf water oxygen-18 enrichment in Amazonia vegetation.Crossref | GoogleScholarGoogle Scholar | 18543002PubMed |
Lange OL, Medina E (1979) Stomata of the CAM plant Tillandsia recurvata respond directly to humidity. Oecologia 40, 357–363.
| Stomata of the CAM plant Tillandsia recurvata respond directly to humidity.Crossref | GoogleScholarGoogle Scholar | 28309618PubMed |
Lehmann MM, Goldsmith GR, Mirande-Ney C, Weigt RB, Schönbeck L, Kahmen A, Gessler A, Siegwolf RTW, Saurer M (2020) The 18O-signal transfer from water vapour to leaf water and assimilates varies among plant species and growth forms. Plant, Cell & Environment 43, 510–523.
| The 18O-signal transfer from water vapour to leaf water and assimilates varies among plant species and growth forms.Crossref | GoogleScholarGoogle Scholar |
Leroy C, Gril E, Ouali LS, Coste S, Gérard B, Maillard P, Mercier H, Stahl C (2019) Water and nutrient uptake capacity of leaf-absorbing trichomes vs. roots in epiphytic tank bromeliads. Environmental and Experimental Botany 163, 112–123.
| Water and nutrient uptake capacity of leaf-absorbing trichomes vs. roots in epiphytic tank bromeliads.Crossref | GoogleScholarGoogle Scholar |
Males J (2016) Think tank: water relations of Bromeliaceae in their evolutionary context. Botanical Journal of the Linnaean Society 181, 415–440.
| Think tank: water relations of Bromeliaceae in their evolutionary context.Crossref | GoogleScholarGoogle Scholar |
Males J, Griffiths H (2018) Economic and hydraulic divergences underpin ecological differentiation in the Bromeliaceae. Plant, Cell & Environment 41, 64–78.
| Economic and hydraulic divergences underpin ecological differentiation in the Bromeliaceae.Crossref | GoogleScholarGoogle Scholar |
North GB, Lynch FH, Maharaj FDR, Phillips CA, Woodside WT (2013) Leaf hydraulic conductance for a tank bromeliad: axial and radial pathways for moving and conserving water. Frontiers in Plant Science 4, 78
| Leaf hydraulic conductance for a tank bromeliad: axial and radial pathways for moving and conserving water.Crossref | GoogleScholarGoogle Scholar | 23596446PubMed |
Ogée J, Cuntz M, Peylin P, Bariac T (2007) Non-steady-state, non-uniform transpiration rate and leaf anatomy effects on the progressive stable isotope enrichment of leaf water along monocot leaves. Plant, Cell & Environment 30, 367–387.
| Non-steady-state, non-uniform transpiration rate and leaf anatomy effects on the progressive stable isotope enrichment of leaf water along monocot leaves.Crossref | GoogleScholarGoogle Scholar |
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29, 379–414.
| Crassulacean acid metabolism: a curiosity in context.Crossref | GoogleScholarGoogle Scholar |
Pierce S, Maxwell K, Griffiths H, Winter K (2001) Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. American Journal of Botany 88, 1371–1389.
| Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae.Crossref | GoogleScholarGoogle Scholar | 21669669PubMed |
Pierce S, Winter K, Griffiths H (2002a) Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytologist 156, 75–83.
| Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae.Crossref | GoogleScholarGoogle Scholar |
Pierce S, Winter K, Griffiths H (2002b) The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant, Cell & Environment 25, 1181–1189.
| The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2018) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing, Vienna, Austria). Available at https://www.R-project.org/
Reyes-García C, Mejia-Chang M, Jones G, Griffiths H (2008) Water vapour isotopic exchange by epiphytic bromeliads in tropical dry forests reflects niche differentiation and climatic signals. Plant, Cell & Environment 31, 828–841.
| Water vapour isotopic exchange by epiphytic bromeliads in tropical dry forests reflects niche differentiation and climatic signals.Crossref | GoogleScholarGoogle Scholar |
Seibt U, Wingate L, Berry JA, Lloyd J (2006) Non-steady state effects in diurnal 18O discrimination by Picea sitchensis branches in the field. Plant, Cell & Environment 29, 928–939.
| Non-steady state effects in diurnal 18O discrimination by Picea sitchensis branches in the field.Crossref | GoogleScholarGoogle Scholar |
Seibt U, Wingate L, Berry JA (2007) Nocturnal stomatal conductance effects on the δ18O signatures of foliage gas exchange observed in two forest ecosystems. Tree Physiology 27, 585–595.
| Nocturnal stomatal conductance effects on the δ18O signatures of foliage gas exchange observed in two forest ecosystems.Crossref | GoogleScholarGoogle Scholar | 17242000PubMed |
Seibt U, Rajabi A, Griffiths H, Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155, 441–454.
| Carbon isotopes and water use efficiency: sense and sensitivity.Crossref | GoogleScholarGoogle Scholar | 18224341PubMed |
Sternberg L, Pinzon MC, Anderson WT, Jahren AH (2006) Variation in oxygen isotope fractionation during cellulose synthesis: intramolecular and biosynthetic effects. Plant, Cell & Environment 29, 1881–1889.
| Variation in oxygen isotope fractionation during cellulose synthesis: intramolecular and biosynthetic effects.Crossref | GoogleScholarGoogle Scholar |
Williams CB, Murray JG, Glunk A, Dawson TE, Nadkarni NM, Gotsch SG (2020) Vascular epiphytes show low physiological resistance and high recovery capacity to episodic, short-term drought in Monteverde, Costa Rica. Functional Ecology 34, 1537–1550.
| Vascular epiphytes show low physiological resistance and high recovery capacity to episodic, short-term drought in Monteverde, Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Zotz G, Andrade JL (1998) Water relations of two co-occurring epiphytic bromeliads. Journal of Plant Physiology 152, 545–554.
| Water relations of two co-occurring epiphytic bromeliads.Crossref | GoogleScholarGoogle Scholar |
Zotz G, Thomas V (1999) How much water is in the tank? Model calculations for two epiphytic bromeliads. Annals of Botany 83, 183–192.
| How much water is in the tank? Model calculations for two epiphytic bromeliads.Crossref | GoogleScholarGoogle Scholar |
Zotz G, Laube S, Schmidt G (2005) Long-term population dynamics of the epiphytic bromeliad, Werauhia sanguinolenta. Ecography 28, 806–814.
| Long-term population dynamics of the epiphytic bromeliad, Werauhia sanguinolenta.Crossref | GoogleScholarGoogle Scholar |