Expressing Arabidopsis thaliana V-ATPase subunit C in barley (Hordeum vulgare) improves plant performance under saline condition by enabling better osmotic adjustment
Getnet D. Adem A , Stuart J. Roy B C , Yuqing Huang D , Zhong-Hua Chen D , Feifei Wang A , Meixue Zhou A , John P. Bowman A , Paul Holford D and Sergey Shabala A EA School of Land and Food, University of Tasmania, Private Bag 54, Hobart, Tas. 7001, Australia.
B Australian Centre for Plant Functional Genomics, Private Mail Bag 1, Glen Osmond, SA 5064, Australia.
C School of Agriculture, Food and Wine, University of Adelaide, Private Mail Bag 1, Glen Osmond, SA 5064, Australia.
D School of Science and Health, Hawkesbury Institute for the Environment, Western Sydney University, Penrith, NSW 2751, Australia.
E Corresponding author. Email: sergey.shabala@utas.edu.au
Functional Plant Biology 44(12) 1147-1159 https://doi.org/10.1071/FP17133
Submitted: 4 May 2017 Accepted: 28 July 2017 Published: 27 September 2017
Abstract
Salinity is a global problem affecting agriculture that results in an estimated US$27 billion loss in revenue per year. Overexpression of vacuolar ATPase subunits has been shown to be beneficial in improving plant performance under saline conditions. Most studies, however, have not shown whether overexpression of genes encoding ATPase subunits results in improvements in grain yield, and have not investigated the physiological mechanisms behind the improvement in plant growth. In this study, we constitutively expressed Arabidopsis Vacuolar ATPase subunit C (AtVHA-C) in barley. Transgenic plants were assessed for agronomical and physiological characteristics, such as fresh and dry biomass, leaf pigment content, stomatal conductance, grain yield, and leaf Na+ and K+ concentration, when grown in either 0 or 300 mM NaCl. When compared with non-transformed barley, AtVHA-C expressing barley lines had a smaller reduction in both biomass and grain yield under salinity stress. The transgenic lines accumulated Na+ and K+ in leaves for osmotic adjustment. This in turn saves energy consumed in the synthesis of organic osmolytes that otherwise would be needed for osmotic adjustment.
Additional keywords: organic osmolytes, osmotic adjustment, potassium, salinity stress tolerance, sodium, vacuolar sequestration.
Introduction
As the world population has been estimated to exceed 9.3 billion people by 2050, there has been a shift towards agricultural production systems on marginal saline lands (Shabala 2013; Panta et al. 2014). The problem of salinity is exacerbated by a reduction in precipitation in subtropical areas, resulting in farmers irrigating with brackish and low-quality water, thereby increasing the salinity of soils (Munns and Tester 2008; Barrett-Lennard and Setter 2010). The estimated cost of salinity-induced loss to world crop production is believed to be in excess of US$27 billion per year (Qadir et al. 2014). Improving performance of crop plants so that they produce a better yield under saline conditions is of paramount importance for using these marginal lands (Munns 2002; Roy et al. 2013).
Excess Na+ in the cytosol, however, is toxic to cellular processes, regardless of whether plants are halophytes or glycophytes (Flowers and Colmer 2008). Therefore, for Na+ to be an effective osmolyte it must be sequestered in the vacuole. Three components are essential for the storage of Na+ in the vacuole: (i) establishment of the electrochemical gradient required to transport Na+ into the vacuole; (ii) Na+ transport across the tonoplast into the vacuole using ion transporters; and (iii) retention of Na+ within the vacuole (Shabala 2013).
Tonoplast Na+/H+ antiporters, such as members of the Na+/H+ exchanger (NHX) family, have been shown to be important in the loading of Na+ into the vacuole (Blumwald and Poole 1985; Barkla et al. 1995; Gaxiola et al. 1999; Apse and Blumwald 2007). NHXs rely on tonoplast localised proton pumps, namely vacuolar ATPases (VHAs) (Vera-Estrella et al. 1999; Wang et al. 2001; Krebs et al. 2010) and vacuolar pyrophosphatases (V-PPases) (Parks et al. 2002; Vera-Estrella et al. 2005; Guo et al. 2006) to generate a proton gradient between the vacuole and the cytoplasm. Once sequestered in to the vacuole, Na+ is prevented from leaking back into the cytosol by the efficient control of and regulation of activity of sodium permeable slow (SV) and fast (FV) vacuolar channels (Pantoja et al. 1989; Shabala and Mackay 2011; Bonales-Alatorre et al. 2013a, 2013b).
There have been many studies in different plant species investigating whether improvements in vacuolar Na+ sequestration through the enhancement of NHX expression can improve plant salinity tolerance. Some studies have found enhanced expression of NHX genes can enhance growth under saline conditions (Apse et al. 1999; Zhang and Blumwald 2001; Zhang et al. 2001; Bayat et al. 2011) whereas others have discerned no yield improvements (Adem et al. 2015). A better approach to enhance growth under salt may be manipulating the expression of genes encoding proteins which establish the proton gradient necessary for effective Na+ sequestration in the vacuole, in particular manipulating of the expression of VHA and V-H+-PPases.
Manipulation of genes encoding H+-PPases has often resulted in enhanced performance under salt, although vacuolar H+-PPases are often considered a supporting mechanism to VHA in the acidification of the vacuole (Maeshima 2000). Hence, investigating whether greater improvements in vacuolar storage of Na+, and thus plant salinity tolerance, can be made by enhancing the abundance of VHA is important.
Unlike vacuolar H+-PPases, vacuolar H+-V-ATPase (VHA) are made of several protein subunits, which are encoded by different genes. Studies have investigated, however, whether manipulation of the expression of genes encoding specific VHA subunits can improve plant growth under saline conditions. Expressing the VHA-c-1 subunit from the halophyte grass Spartina alterniflora Loisel. improved transgenic rice growth under saline conditions (Baisakh et al. 2012). However, the beneficial effect was reported only for vegetative plant growth and not for grain yield. However, VHA-c-1 expressing rice had early closure of the leaf stomata and reduced stomata density (Baisakh et al. 2012) during salt stress. This seems to contradict the finding that transgenic plants had increased biomass, as it is assumed these lines would have a reduced capacity to assimilate CO2.
More recently, wheat genes encoding individual V-ATPase subunits were expressed in Arabidopsis (He et al. 2014). Although improvements in the salt tolerance of seedling of transgenic Arabidopsis plants were observed, no insights into the mechanisms behind the improved tolerance were reported (He et al. 2014). Similar results were reported when wheat V-ATPase subunit B (VHA-B) (Wang et al. 2011) and subunit E (VHA-E) (Zhao et al. 2009) were expressed in Arabidopsis and when the V-ATPase subunit c (VHA-c) from Limonium bicolor was expressed in tobacco (Xu et al. 2011).
In the present study, we generated lines of barley expressing the Arabidopsis V-ATPase subunit C (VHA-C) and assessed their physiological and agronomical performance under saline conditions. The V-ATPase subunit C (VHA-C) was chosen as it plays a role in the hydrolysis of ATP in the V1 complex of the vacuolar ATPase structure (Sze et al. 2002; Krebs et al. 2010), and can directly enhance the activity of the V-ATPase. Barley lines expressing the AtVHA-C gene were found to have smaller salinity induced reductions both in biomass and grain yield compared with non-transformed wild-type plants. These beneficial effects may be linked to the improved ability of the transgenic lines to maintain higher stomata conductance, resulting from increased Na+ and K+ accumulation in the leaf which contributed to osmotic adjustment and made plants less reliant on de novo synthesis of organic osmolytes.
Materials and methods
Generating transgenic barley plants expressing VHA-C (Arabidopsis V-ATPase Subunit C)
For the experiments wild-type barley (accession WI4330) was kindly supplied by the University of Adelaide Barley Breeding Program. The coding sequence of AtVHA-C (At1g12840) was amplified from the cDNA of Arabidopsis thaliana (L.) Heynh. (ecotype Col-0) utilising primer pairs, 5ʹ-ATG ACT TCG AGA TAT TGG GTG-3ʹ and 5ʹ-TTA AGC AAG GTT GAT AGT GAA G-3ʹ and high-fidelity DNA polymerase (Elongase, Invitrogen). The amplified sequence was introduced into the Gateway enabled entry vector pCR8/GW/TOPO TA (Invitrogen). AtVHA-C was recombined into the transformation vector pMDC32 vector via an LR recombination reaction (Invitrogen) (Curtis and Grossniklaus 2003), under the control of a CaMV 35S promoter. The AtVHA-C pMDC32 vector was transformed into wild-type barley WI4330 via Agrobacterium tumefaciens-mediated transformation (Tingay et al. 1997; Jacobs et al. 2007). After antibiotic selection, the transformed plantlets were regenerated in soil (Singh et al. 1997; Jacobs et al. 2007) and grown to produce T1 seed. After one round of seed multiplication the T2 plants were grown for experiments at the University of Tasmania.
Plant growth conditions
Four AtVHA-C expressing (35S:AtVHA-C) T2 lines described here as OE1, OE2, OE3 and OE4, and one non-transformed WI4330 were grown in 2 L pots containing potting mix. The four lines were selected as a reasonable compromise between the reliability of the data and the workload. The potting mix was composed of 70% composted pine bark; 20% coarse sand; 10% sphagnum peat; Limil at 1.8 kg m–3, dolomite at 1.8 kg m–3). The plant nutrient balance was maintained by adding the slow release fertiliser Osmocote Plus (Scotts Australia) at 6 kg m–3 and ferrous sulfate (at 500 g m–3) (Bonales-Alatorre et al. 2013a). The plants were grown in a glasshouse with regulated temperature (24−20°C day/15°C night), 15 h day/8 h night period and artificial light 150 µmol m–2s–1 for ~2 weeks after seedling establishment (third leaf stage). Plants were grown in 2 L pots, with three plants per pot and 4–5 pots per treatment/genotype. Salinity treatment was administered by adding 300 mM NaCl irrigation water based on water requirement and uniform to the treatments until maturity. The experiment was conducted between January and April 2014.
DNA extraction and PCR analysis
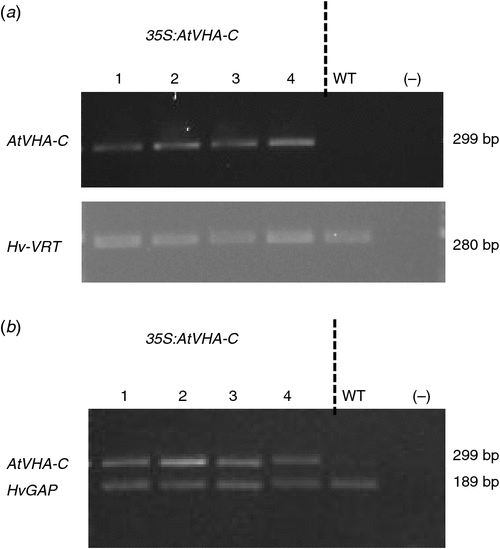
Barley genomic DNA from the above lines was extracted using the method described by Edwards et al. (1991). PCR was performed to confirm the presence of the AtVHA-C transgene in the transformed plants using a forward (5ʹ-AGA GAC TCG TAA ACA AGA G-3ʹ) and reverse (5ʹ-CAG CCA TGG CTC CTG CA-3ʹ) primer, which amplified a fragment of 299 bp in size. The Hv-VRT2 vernalisation gene (GenBank DQ201168) was used as a control gene, and this gene was amplified using Hv-VRT2 specific forward (5ʹ-CCG AAT GTA CTG CCG TCA TCA CAG-3ʹ) and reverse (5ʹ-TGG CAG AGG AAA ATA TGC GCT TGA-3ʹ) primers, which amplifies a fragment of 280 bp in size. PCR reactions included 2 × reaction ImmoMix (Bioline), 10 µM of forward and reverse primers, 1 mg mL–1 BSA, MilliQ water and 0.5 µL of template DNA. The PCR conditions used were similar for both the VRT2 and the VHA-C (95°C for 10 min, followed by 1 min at 94°C, 1 min at 55°C and 1 min at 72°C, repeated for 35 cycles, with a final extension of 72°C for 10 min). PCR products were visualised by gel electrophoresis using a 2% agarose gel containing Gel Red Nucleic acid stain (Biotium) (5 µl per 100 ml of gel) and a photograph of the gel was obtained (Molecular imager, Gel Doc imaging system, Bio-Rad).
RNA extraction and gene expression analysis
Total RNA was extracted from leaf tissue using Bioline plant Isolate II RNA kit (www.bioline.com/isolate, accessed 11 August 2017). The RNA was diluted to 10 ng μL–1 and subjected to RT-PCR with the reaction mix containing 5 × buffer, 10 mM dNTP, water and enzyme mix (Taq polymerase and reverse transcriptase), forward and reverse primers for AtVHA-C and HvGAP (VHA-C gene: forward primer 5ʹ-AGA GAC TCG TAA ACA AGA G-3ʹ and reverse primer 5ʹ-CAG CCA TGG CTC CTG CA-3ʹ, and Hv-GAP: forward primer 5ʹ-GTG AGG CTG GTG CTG ATT ACG-3ʹ and reverse primer 5ʹ-TGG TGC AGC TAG CAT TTG ACA C-3ʹ) and RNA at 10 ng μL–1. Reverse transcription was performed first at 50°C for 30 min, before PCR (initial PCR activation 95°C for 15 min, then 35 cycles of 94°C for 1 min, of 55°C for 1 min and 72°C for 1 min, before a final extension 72°C for 10 min). PCR products were visualised by gel electrophoresis as described above.
Biomass and grain yield
As plants were grown to maturity to collect grain, only the DW of the barley plants (no fresh weights) were recorded at the end of experiment. Plant height was measured at the same period of time as DW. The number of seeds per plant and their total weight were recorded. The sample size for biomass and yield data for the salt treated plants range from eight to 15 plants.
Pigment content and stomatal conductance
The number of chlorotic and necrotic leaves per plant was recorded after 5 weeks of salt treatment. Leave which were partially yellow leaves were deemed as chlorotic leaves. Completely dead and dried leaves were deemed as necrotic. Relative leaf chlorophyll content was measured using a SPAD-502 Chlorophyll Meter (Konica Minolta). Measurements were taken on the third true leaf, at a position about one-quarter of the length of the leaf from the leaf tip. The same leaf/position was used to measure stomatal conductance (gs) from control and salt treated plants using a Decagon leaf porometer (Decagon Devices Inc.), under constant light conditions (artificial light of 150 μmol m−2 s–1) between 1100 and 1500 hours.
Na+ and K+ concentration
The third leaf from both salt treated and control plants were excised after 5 weeks of salt treatment for Na+ and K+ ion concentration measurement. Leaves were dried in the oven at 65°C for 72 h. A subsample of a known weight (~0.1 g) was digested in a solution consisting of 5 mL of 65–70% HNO3 and 2 mL of 30% H2O2 using a microwave digester (MDS-2000, CEM Corp.). The digested samples were diluted 1 : 5 using distilled water (Skoog et al. 2000). The Na+ and K+ ion concentration was measured using a flame photometer (Model PFP7-flame photometer, Jenway).
Sap osmolality measurements and calculation of contribution of inorganic ions towards leaf osmotic adjustment
After 5 weeks of treatment with 300 mM NaCl, the third leaf of the barley transgenic and WT plants harvested and stored at −20°C. Leaf sap was extracted using a freeze–thaw method (Tomos et al. 1984) and osmolality was measured using the vapour pressure osmometer (Vapro, Wescor Inc.). The sample size for both control and salt treated plants was n = 6. The measured Na+ and K+ concentrations in the tissue were used to calculate the relative contribution of these ions towards overall sap osmotic potential. Contribution of Cl− was estimated as 1.2 of that for Na+, as previously described (James et al. 2006; Puniran-Hartley et al. 2014).
Estimation of vacuolar and cytosolic pH using fluorescence dyes
In this study, the gradient of the pH between the cytosol and the vacuole was estimated with a qualitative method using one wavelength (488 nm). The gradient of the pH in different cellular compartments in mesophyll cells from AtVHA-C expressing and non-transformed WT barley plants were measured using pH sensitive dye (2ʹ,7ʹ-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)-AM after 2 weeks of 300 mM NaCl treatment (see Figs S1 and S2, available as Supplementary Material to this paper). Dye loading was performed essentially according to Krebs et al. (2010). The choice of optimal BCECF-AM dye concentration was based on the published work on Arabidopsis (Scheuring et al. 2015). False colour images were obtained using a confocal laser-scanning microscope (SP5, Leica) with ×20 and ×40 objectives after excitation with 488 nm and recording emissions between 520 nm and 550 nm. A further validation of the above protocol was conducted using mesophyll samples co-stained with BCECF-AM and N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide (FM4–64), a dye that stains both plasma and vacuole membranes (Wu et al. 2015) and allow a better resolution between intra-cellular compartments. After 1 h incubation with 20 μM BCECF-AM, the same mesophyll samples were then incubated together with 20 μM FM4–64 for 30 min to visualise tonoplast. Mesophyll samples were then rinsed with buffer solution (10 mM KCl, 5 mM Ca2+-MES, pH 6.1) for 3 min. For FM4–64 fluorescence, the 488-nm excitation was used and collected with a 615 nm long-pass filter. Experiments were conducted with WT and three OE lines (OE1, OE2 and OE3) in both control conditions and 2 weeks of 300 mM NaCl exposure. Chloroplast fluorescence was detected between 680 and 700 nm in order to separate the autofluorescence of chlorophyll in chloroplasts from the BCECF fluorescence. Images were analysed with LAS AF software (Leica Microsystems), and ImageJ software (National Institutes of Health) which was also used to calculate the corrected total cell fluorescence and estimated fluorescence for cytosol, vacuole and chloroplast after background subtraction (Bonales-Alatorre et al. 2013b).
Statistical analysis
The data were analysed using Student’s t-tests. Significant differences were determined and indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001 using a statistical software SPSS ver. 22 (IBM SPSS).
Results
Generation of barley plants expressing V-ATPase Subunit C
Four AtVHA-C expressing lines, designated as OE1 to OE4 (35S:AtVHA-C-1 to 35S:AtVHA-C-4), and one non-transformed WI4330 line (WT) were included in this study. The OE4 line did not show a consistent phenotype throughout all experiments, most likely due to a detrimental effect from the transformation process, and was therefore omitted from analysis. The presence of the VHA-C gene in the genome of transgenic lines and its absence in the WT were confirmed using transgene specific PCR on genomic DNA extracted from the leaf (Fig. 1a). The expression of the VHA-C gene in transgenic lines and the absence of its expression in the WT were confirmed by RT-PCR (Fig. 1b).
|
Transgenic barley expressing V-ATPase subunit C has improved overall salinity stress tolerance and grain yield
VHA-C expressing barley produced more tillers and maintained better shoot health when grown on 300 mM NaCl for 5 weeks compared with WT plants, which showed signs of severe senescence and wilting (Fig. 2). The DW of OE lines and WT under control conditions at 0 mM NaCl was not affected (Fig. 3a), and an increase in the DW of transgenic plants under salt stressed conditions for OE3 line was observed at 300 mM NaCl (Fig. 3b), and resulted in transgenic lines exhibiting an improvement in salt tolerance (as measured by biomass in 300 mM NaCl divided by the biomass in 0 mM NaCl) when compared with WT plants (Fig. 3c), 43 to 47% in AtVHA-C lines vs 35% in WT.
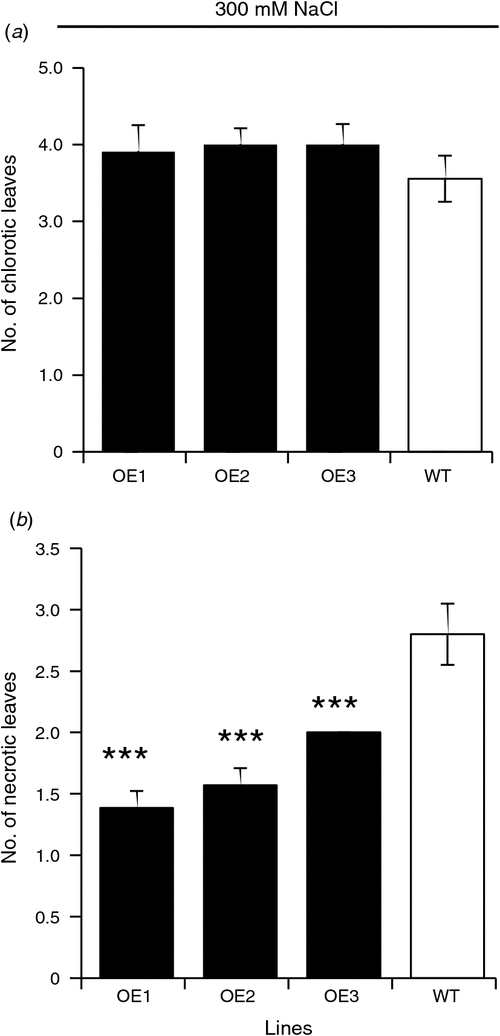
The number of chlorotic leaves in salt-grown plants did not differ significantly between WT and OE lines (Fig. 4a). At the same time, the WT had a significantly (P ≤ 0.001) higher number of necrotic leaves compared with AtVHA-C expressing lines when grown for 5 weeks on 300 mM NaCl (Fig. 4b). We noted that there were large differences in stomatal conductance observed between both WT and OE lines under control and salt stressed conditions. While the stomatal conductance of the OE lines was significantly lower than the wild type in 0 mM NaCl (Fig. 5a), all three OE lines had significantly higher (P ≤ 0.001) stomatal conductance than WT when the plants treated with 300 mM NaCl for 5 weeks (Fig. 5b).
|
As expected, the chlorophyll content was not significantly different between OE lines and WT under control conditions (Fig. 5d) but WT plants had a significant reduction in chlorophyll content under saline conditions when compared with AtVHA-C expressing barley (significant at P ≤ 0.05; Fig. 5d, e). Also significantly higher was relative chlorophyll content in transgenic lines compared with WT barley (Fig. 5f, significant at P ≤ 0.001). At harvest, AtVHA-C expressing lines (OE) had larger seed size and were significantly (P ≤ 0.001) heavier in seed weight compared with WT barley plants after growing under 300 mM NaCl stress (Fig. 6; Table 1). Under 0 mM NaCl there was no significant difference (P ≤ 0.05) between the numbers of seeds per plant between OE lines and WT neither (Fig. 7a). However, an increase in the number of seeds per plant in the transgenic lines in 300 mM NaCl, when compared with WT, (Fig. 7b) resulted in an increase in salinity tolerance (as measured by yield under 300 mM NaCl divided by the yield under 0 mM NaCl) (Fig. 7c). Although the grain yield per plant (g plant–1) was not significantly (at P ≤ 0.05) different between the OE lines and WT under 0 mM NaCl conditions (Fig. 7d), there was significantly higher grain yield in two of three AtVHA-C expressing lines under 300 mM (OE-1 and OE-2; significant at P ≤ 0.001 and P ≤ 0.01 respectively; Fig. 7e). This resulted in a greater maintenance of grain yield in the transgenic lines compared with the control (Fig. 7f).

|
Transgenic barley expressing V-ATPase subunit C has increased leaf Na+ and K+ and these ions contributed towards osmotic adjustment
The OE lines had significantly higher leaf Na+ concentration than WT plants, when grown in both 0 mM NaCl (Fig. 8a) and 300 mM NaCl (Fig. 8b) (P ≤ 0.001). However, the relative increase in leaf Na+ under salt stress was less in OE lines compared with WT (6- to 7-fold vs 9.2-fold in WT; significant at P ≤ 0.001; Fig. 8c). The leaf K+ concentration for all the OE lines and WT was not significantly different (P ≤ 0.05) under control conditions (Fig. 8d) but all the OE lines showed significantly higher leaf K+ concentration compared with WT under saline conditions (significant at P ≤ 0.001; Fig. 8e). Two out of three OE lines (OE-1 and OE-2) have significantly increased the overall leaf K+ concentration (1.5- and 1.4-fold respectively) whereas WT-plants were not able to do this when grown under saline conditions (Fig. 8f).
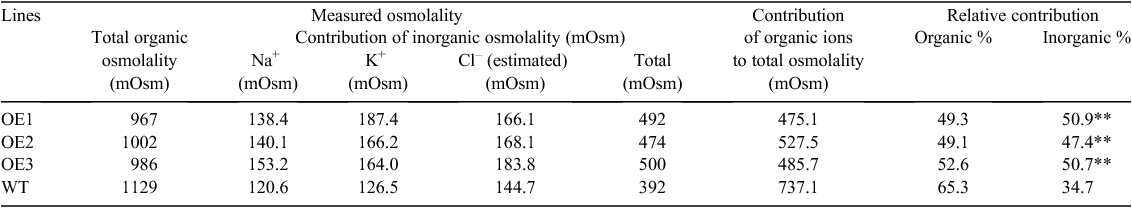
The osmolality of leaf sap showed no differences between OE lines and WT (Table 2) but the measured contribution of inorganic ions in WT plants was lower than in OE lines. We estimated the relative contribution of inorganic and organic osmolytes towards osmotic adjustment in barley leaves. In WT, the overall contribution of three major inorganic osmolytes (Na+, Cl–, and K+) was only ~34%, implying that the major bulk of osmotic adjustment under saline conditions (~66%) was achieved by other means, such as de novo synthesis of organic osmolytes (Table 2). In a stark contrast, contribution of organic osmolytes towards osmotic adjustment in OE lines varied between 49 and 52% (Table 1; significant compared with WT at P ≤ 0.01), e.g. was by ~15% less than in WT.
Enhancing Na+ accumulation transgenic barley may be associated with cellular pH adjustment
We further estimated the relative changes in cytosolic and vacuolar pH in leaf mesophyll cells of WT and OEs for control and 300 mM NaCl treated plants (Fig. 9). As confocal microscopy was not suitable for ratiometric pH quantification, BCECF-AM was used to estimate changes in the relative fluorescent intensity in both the cytosol and vacuole in order to evaluate the H+ gradients across the tonoplast. We used corrected cell fluorescence by subtracting the background fluorescence similar to our work in Na+ measurements (Bonales-Alatorre et al. 2013a, 2013b; Wu et al. 2015). We also sampled a large number of cells (>200) in order to reduce systematic errors. We first conducted experiments to separate BCECF fluorescence signals between cytosol and vacuole using FM4-64 and auto fluorescence of chloroplasts (Fig. 9), to show intracellular pH distribution in the mesophyll of WT after 2 weeks of 300 mM NaCl. This was achieved by selecting one typical cell possessing a large central vacuole for the region of interest (ROI) analysis (Fig. 9a–e). ROI lines were then drawn crossing the cytosol twice and the large vacuole in the cells (Fig. 9f–i), and intracellular pH distribution was quantified as relative intensity of BCECF fluorescence in mesophyll cells in the control and salt treatment (Fig. 9j, k).
A higher H+ pump activity by the VATPase should cause an acidification in the vacuole and alkalinisation of the cytosol. The proton increase in the vacuole can then be used for Na+/H+ antiportation, accumulating Na+ into the vacuole. Cytosolic pH is usually maintained at around 7.5 (low H+ concentration) in plant cells, which was indicated by a low BCECF fluorescence intensity (Fig. 9e, j). In contrast, vacuolar pH is normally between 4.5 and 5.5 (high H+ concentration), which was clearly shown as much higher by BCECF fluorescence (Scheuring et al. 2015) compared with the cytosolic pH (Fig. 9e, k). Moreover, salt treatment led to no significant (at P < 0.05) changes in cytosolic pH changes in mesophyll cells of WT and three OE barley lines (Fig. 9j), suggesting cytosolic pH was tightly regulated even under high salinity stress. However, overexpressing AtVHA-C resulted in significant acidification of vacuolar pH especially in mesophyll cells of line OE1 (Fig. 9k). The BCECF fluorescence intensity in mesophyll cells of WT and three barley lines overexpressing AtVHA-C was significantly reduced by salt stress (P < 0.001). In addition, vacuolar pH in mesophyll cells of OE2 showed significantly high BCECF fluorescence intensity after salt tress as compared with WT and other two OE lines (Fig. 9k). Again, the overexpressing AtVHA-C-induced vacuolar pH changes matched well with the overall high salt tolerance in OE1 and OE2 in contrast to WT and OE3 (Figs 2–8). Therefore, the results demonstrate that the overexpression of V-ATPase subunit C in barley may have resulted in a vacuolar high H+ pump activity for more NHX-mediated Na+ sequestration in vacuole, potentially leading to an alkalinisation of the vacuole (e.g. from pH 5 to 6) and slight acidification of cytosol (e.g. from pH 7.5 to 7.2).
Discussion
Transgenic VHA-C barley improved growth and grain yield under saline growth condition
The activity of VHA has been shown to increase under salt stress (Silva et al. 2010), accompanied by an upregulation of both VHA and NHX transcripts under salt stress (Qiu et al. 2007). Transgenic plants expressing subunits of VHA have shown improvement in growth and performance under saline condition in several species, such as the expression of the VHA-c-1 subunit in rice (Baisakh et al. 2012) and tobacco (Xu et al. 2011), as well as the expression of several VHA subunits in Arabidopsis (Zhao et al. 2009; Wang et al. 2011; He et al. 2014). The possible mechanisms for increased pump activity by overexpressing a certain subunit (subunit C in this work) may be due to the fact that this subunit is functioning in the active centre of the pump that hydrolyses ATP. Hence, having a higher number of subunits during the protein assembly of the pump will increase the probability of having more pumps with a higher catalytic activity. This is especially true for subunit C that is a catalytic subunit and it can be limiting as compared with the other subunits.
To date, the expression of VHA has been enhanced in plants which are considered to exclude Na+ from the shoot and do not normally rely on vacuolar Na+ sequestration mechanisms (Shabala 2013). To understand the role of VHA in a crop plant, which can accumulate a substantial amount of shoot Na+ in the shoot, the VHA-C gene was successfully cloned from Arabidopsis and transformed into barley under the control of the CaMV 35S promoter. In this study we show, for the first time, that expressing VHA-C from Arabidopsis also enhances salinity stress tolerance in a crop plant (barley) which has the ability to accumulate significant levels of shoot Na+.
The transgenic lines expressing VHA-C not only showed significant (P < 0.05 and higher) dry weight and plant height when grown under saline conditions (Fig. 3) but also higher grain yield (Fig. 7c, e). As far as we are aware, this is the first work showing transgenic plants expressing VHA subunits have improvements in this important agronomical trait.
Increased stomatal conductance contributes to enhanced salinity tolerance in transgenic plants
Previous reports suggest the beneficial effects of overexpressing VHA transporter subunits were related to either improved plant water status, resulting from reduced stomatal density and early stomatal closure (Baisakh et al. 2012), or from increased activity of enzymatic antioxidants (AO) in transgenic lines (He et al. 2014). However, plant biomass (DW) is directly proportional to the ability of the plant to assimilate CO2 (which relies on gas exchange through open stomates). Therefore reductions in stomatal density and early stomatal closure, as suggested by Baisakh et al. (2012), cannot be the sole reason VHA expressing plants to have greater salinity tolerance, as it would be expected that CO2 assimilation would be lower in these plants. In addition, there are many reports showing there is no direct correlation between AO activity and salinity stress tolerance in crops (Maksimović et al. 2013). Many salt tolerant plants (such as halophytes) do not require high AO activity as they prevent formation of high levels of reactive oxygen species in the first place (Bose et al. 2014). Therefore, there must be other mechanisms by which VHA can improve salt tolerance in transgenic plants. From the data reported here, two physiological mechanisms were identified which would lead to improved performance of overexpression lines. The first one was reduced number of necrotic leaves (Fig. 4) and the second one – higher relative stomatal conductance (Fig. 5). The association between expressing VHA subunits and stomatal opening and closing has been reported by (Allen et al. 2000) using VHA-C mutant (det3), and also by RNAi knockdown of OsVHA-A (Zhang et al. 2013). Two to 3-fold higher gs values in transgenic lines compared with WT (Fig. 5b) might account for better CO2 assimilation, and fewer necrotic leaves increased the overall photosynthetic leaf surface in transgenic place. Both these factors could contribute to increase plant biomass and, ultimately, higher grain yield in OE lines.
Transgenic lines expressing VHA-C have better Na+ sequestration
All the transgenic plants expressing VHA-C showed higher leaf Na+ concentration than the WT when grown under saline condition (Fig. 8b). At the same time, the chlorophyll content was not affected (Fig. 4), and the number of necrotic leaves was reduced in OE lines (Fig. 4). Taken together, these facts point out at highly efficient vacuolar Na+ sequestration in transgenic plants. All OE lines also showed higher Na+ concentration under control conditions, that can be interpreted as plants using freely available Na+ (always present in the soil) as a cheap osmoticum to generate turgor and enable shoot growth.
Na+/H+ antiporters, such as members of the NHX family (Blumwald and Poole 1985; Barkla et al. 1995; Gaxiola et al. 1999; Apse and Blumwald 2007) energised by the proton motive force generated by proton pumps run the Na+ sequestration process. The improvement in salinity tolerance in the VHA-C expressing barley may be due to an improvement in the H+ gradient between the vacuole and the cytosol, so that NHX transporters could compartmentalise more Na+. Expression of the subunit C of the VHA, therefore, could be instrumental to increase vacuolar H+-ATPase phosphorylation (Armbrüster et al. 2004) or stability of its operation (Sze et al. 2002), therefore better energising the NHX activity and thus assisting Na+ sequestration process. This is further confirmed in experiments using fluorescent pH dye (Fig. 9). Consistent with this model, transgenic Arabidopsis expressing wheat VHA subunit E showed increased Na+ accumulation after 10 days of 120 mM NaCl but with a lower Na+ accumulation in the cytosol (Zhao et al. 2009). It remains to be seen whether the improved sequestration in the vacuole was due solely to an increase in the activity of Na+/H+ antiporter which were already present in the cell or if there is also an upregulation of genes encoding Na+/H+ antiporters, resulting in a greater concentration (and more activity) of the transporters.
Plants expressing VHA-C showed higher K+ retention
The barley plants expressing VHA-C showed significantly higher leaf K+ concentration compared with WT (Fig. 8). This feature correlated with the fewer number of necrotic leaves in salt stressed plants (Fig. 4). This suggests that K+ retention in the shoot was essential for preventing salt stress-induced senescence, a programmed cell death process (PCD). The loss of cytosolic K+ in cells under stress induces the activation of caspase-like proteases and endonucleases and, hence causes PCD in plants (Shabala et al. 2007; Demidchik et al. 2010) and mammalian cells (Hughes and Cidlowski 1998, 1999). Potassium retention in both shoots and roots is an important mechanism in salt tolerance in barley, which was corroborated by K+ kinetics study on shoots and roots of barley and on roots using electrophysiological studies (Adem et al. 2014). Recently, Wu et al. (2013) and Wu et al. (2014) demonstrated that K+ retention in the leaf mesophyll is a contributing factor towards salt tolerance in barley, as measured by non-invasive ion flux. Notably, several NHX transporters have been shown to have a higher affinity for K+ than Na+ (Rodríguez-Rosales et al. 2008; Leidi et al. 2010; Bassil et al. 2011; Barragan et al. 2012) – enhanced proton pumping in VHA-C expressing barley may therefore may enable higher accumulation of K+ in cell vacuoles. This may contribute to increased cell turgor and better osmotic adjustment under saline conditions (as discussed below). Higher K+ concentration in leaves may be also essential for more efficient stomatal control, given the critical role of this nutrient in stomata movements (Anschütz et al. 2014).
Relative contribution of organic and inorganic osmolytes
Synthesis of organic osmolytes is energy expensive, particularly in stressful environments (Raven 1985), and plants with enhanced salt tolerance tend to use Na+ as a cheap osmoticum for osmotic adjustment (Chen et al. 2007). As shown in Table 2, WT plants relied predominantly (65.3% of the cell’s osmotic potential) on organic osmolytes for their osmotic adjustment. AtVHA-C expressing lines, however, had a large proportion of inorganic osmolytes (specifically, Na+ and K+) to achieve ~50% of cell osmotic potential (Table 2). The transgenic lines were therefore likely to expend less energy generating organic osmolytes and make better use of photosynthetic assimilate. Osmotic adjustment using inorganic ions is the predominant mechanism in halophytes (Inan et al. 2004), and it appears here that some ‘halophytism’ was highly beneficial for the transgenic barley plants.
Conclusion
Expressing Arabidopsis VHA-C in barley plants has shown better Na+ and K+ sequestration, which led to the utilisation of these ions as a cheap osmoticum to improve gas exchange characteristics. This result indicates that the presence of vacuolar H+-ATPase has a crucial role in enhancing Na+ and K+ sequestration in the presence of NHX and thereby, improved seed yield. It is therefore strongly advocated that the concurrent expression of the vacuolar H+-ATPase and NHX together in one genotype should be done to enhance plant performance under salt stress conditions.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This paper has been included as Chapter 5 in a PhD thesis of Getnet Adem. This work was supported by the Australian Research Council and Grain research and Development Corporation grants to Sergey Shabala. Zhong-Hua Chen was funded by an ARC DECRA project (DE140101143). Thanks to Dr Anya Salih and the Confocal Bio-imaging Facility at Western Sydney University.
References
Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biology 14, 113| Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley.Crossref | GoogleScholarGoogle Scholar |
Adem G, Roy S, Plett D, Zhou M, Bowman J, Shabala S (2015) Expressing AtNHX1 in barley (Hordium vulgare L.) does not improve plant performance under saline conditions. Plant Growth Regulation 77, 289–297.
| Expressing AtNHX1 in barley (Hordium vulgare L.) does not improve plant performance under saline conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmt12itL0%3D&md5=53c7cc50cb599b931056fc50fd0b85baCAS |
Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schroeder JI (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342.
| Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmvF2ru7o%3D&md5=6a59040df5a0ecd3455da99eb31064d8CAS |
Anschütz U, Beckera D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology 171, 670–687.
| Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment.Crossref | GoogleScholarGoogle Scholar |
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Letters 581, 2247–2254.
| Na+ transport in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1aiurY%3D&md5=bb91df374c86d7235a69927048acb968CAS |
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285, 1256–1258.
| Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXls1Sju7s%3D&md5=5d3bfb350e4abb4f0fcc3a9277402b32CAS |
Armbrüster A, Svergun DI, Coskun U, Juliano S, Bailer SM, Grüber G (2004) Structural analysis of the stalk subunit Vma5p of the yeast V-ATPase in solution. FEBS Letters 570, 119–125.
| Structural analysis of the stalk subunit Vma5p of the yeast V-ATPase in solution.Crossref | GoogleScholarGoogle Scholar |
Baisakh N, RamanaRao MV, Rajasekaran K, Subudhi P, Janda J, Galbraith D, Vanier C, Pereira A (2012) Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Löisel. Plant Biotechnology Journal 10, 453–464.
| Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Löisel.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xot1ygs7s%3D&md5=4cfb5a71753170fd16ffc9095eb5563bCAS |
Barkla BJ, Zingarelli L, Blumwald E, Smith JAC (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiology 109, 549–556.
| Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXoslOqtrc%3D&md5=15bf65222374334bd711ea54925fdb4aCAS |
Barragan V, Leidi EO, Andres Z, Rubio L, De Luca A, Fernandez JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. The Plant Cell 24, 1127–1142.
| Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmsl2jtL4%3D&md5=85113ace15c3f714e56907ed2dc9ef89CAS |
Barrett-Lennard E, Setter T (2010) Developing saline agriculture: from traits and genes to systems. Functional Plant Biology 37, iii–iv.
| Developing saline agriculture: from traits and genes to systems.Crossref | GoogleScholarGoogle Scholar |
Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction The Plant Cell 23, 3482–3497.
| The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproductionCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVGqtrvP&md5=b06a8dd0c7d589e23369e331df749dbdCAS |
Bayat F, Shiran B, Belyaev DV (2011) Overexpression of HvNHX2, a vacuolar Na+/H+ antiporter gene from barley, improves salt tolerance in Arabidopsis thaliana. Australian Journal of Crop Science 5, 428–432.
Blumwald E, Poole RJ (1985) Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiology 78, 163–167.
| Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXktVags74%3D&md5=8e75e24ad9cda9c0ef2f5254d8190eacCAS |
Bonales-Alatorre E, Pottosin I, Shabala L, Chen Z-H, Zeng F, Jacobsen S-E, Shabala S (2013a) Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in halophyte species, Chenopodium quinoa. International Journal of Molecular Sciences 14, 9267–9285.
| Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in halophyte species, Chenopodium quinoa.Crossref | GoogleScholarGoogle Scholar |
Bonales-Alatorre E, Shabala S, Chen Z-H, Pottosin I (2013b) Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa. Plant Physiology 162, 940–952.
| Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Oqs7g%3D&md5=a8869980568d3ab1547f591d4b93c3c5CAS |
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context salinity stress tolerance. Journal of Experimental Botany 65, 1241–1257.
| ROS homeostasis in halophytes in the context salinity stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks12htbY%3D&md5=0e9bfe786dfba2f3a3bf74a46da82d1aCAS |
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shiabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany 58, 4245–4255.
| Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlymurg%3D&md5=eb90624fe98b2124e320a0b4d6be5732CAS |
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469.
| A gateway cloning vector set for high-throughput functional analysis of genes in planta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXosVaqtrw%3D&md5=175088d0c2056c01e842de3c0a1065d0CAS |
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479.
| Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1KmtL0%3D&md5=1ea5171aa64a36e614598240e5e6d344CAS |
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Research 19, 1349
| A simple and rapid method for the preparation of plant genomic DNA for PCR analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitVWju74%3D&md5=5341044301d35e7e48cf4bf5e0c8d213CAS |
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqur%2FE&md5=a36e6fda1d4e8df4e7a168c06e633bccCAS |
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proceedings of the National Academy of Sciences of the United States of America 96, 1480–1485.
| The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhsFSru7o%3D&md5=18e2dea9a24d684103d05f67bb400147CAS |
Guo SL, Yin HB, Zhang X, Zhao FY, Li PH, Chen SH, Zhao YX, Zhang H (2006) Molecular cloning and characterization of a vacuolar H+-pyrophosphatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis. Plant Molecular Biology 60, 41–50.
| Molecular cloning and characterization of a vacuolar H+-pyrophosphatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFynsbc%3D&md5=0032a13ac1029c349d0e4460daa90c97CAS |
He XL, Huang X, Shen YZ, Huang ZJ (2014) Wheat V-H+-ATPase subunit genes significantly affect salt tolerance in Arabidopsis thaliana. PLoS One 9, e86982
| Wheat V-H+-ATPase subunit genes significantly affect salt tolerance in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Hughes FM, Cidlowski JA (1998) Glucocorticoid-induced thymocyte apoptosis: Protease-dependent activation of cell shrinkage and DNA degradation. The Journal of Steroid Biochemistry and Molecular Biology 65, 207–217.
| Glucocorticoid-induced thymocyte apoptosis: Protease-dependent activation of cell shrinkage and DNA degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvVKqsrs%3D&md5=ee0915a34b0aa15c5c3ffe302cc04cedCAS |
Hughes FM, Cidlowski JA (1999) Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Advances in Enzyme Regulation 39, 157–171.
Inan G, Zhang Q, Li PH, Wang ZL, Cao ZY, Zhang H, Zhang CQ, Quist TM, Goodwin SM, Zhu JH, Shi HH, Damsz B, Charbaji T, Gong QQ, Ma SS, Fredricksen M, Galbraith DW, Jenks MA, Rhodes D, Hasegawa PM, Bohnert HJ, Joly RJ, Bressan RA, Zhu JK (2004) Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiology 135, 1718–1737.
| Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtVOqsbg%3D&md5=b4c21c0614c0a20a1b9b71b51e9dc47cCAS |
Jacobs A, Lunde C, Bacic A, Tester M, Roessner U (2007) The impact of constitutive heterologous expression of a moss Na+ transporter on the metabolomes of rice and barley. Metabolomics 3, 307–317.
| The impact of constitutive heterologous expression of a moss Na+ transporter on the metabolomes of rice and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFOntbjL&md5=41c67873af60757f80d4252c9bde30a0CAS |
James RA, Munns R, Von Caemmerer S, Trejo C, Miller C, Condon T (2006) Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– in salt-affected barley and durum wheat. Plant, Cell & Environment 29, 2185–2197.
| Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– in salt-affected barley and durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVaisA%3D%3D&md5=bbe95fed983e525b452e3074e400da38CAS |
Krebs M, Beyhl D, Gorlich E, Al-Rasheid KAS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proceedings of the National Academy of Sciences of the United States of America 107, 3251–3256.
| Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Cgurw%3D&md5=45dfe7dcf8e8c2d0d33d26a9eb475ac7CAS |
Leidi OE, Barragan V, Rubio L, El-Hamdaoui A, Ruiz TM, Cubero B, Fernandez AJ, Bressan AR, Hasagawa MP, Quintero JF, Pardo MJ (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. The Plant Journal 61, 495–506.
| The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitFGqtL0%3D&md5=85de7b47efcd7b4303bf1372421da28aCAS |
Maeshima M (2000) Vacuolar H+-pyrophosphatase. Biochimica et Biophysica Acta (BBA) Biomembranes 1465, 37–51.
| Vacuolar H+-pyrophosphatase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXit1Wgtr4%3D&md5=c1c787a2ab4d887782dabf9e19d91861CAS |
Maksimović JD, Zhang JY, Zeng FR, Živanović BD, Shabala L, Zhou MX, Shabala S (2013) Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant and Soil 365, 141–155.
| Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance?Crossref | GoogleScholarGoogle Scholar |
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell & Environment 25, 239–250.
| Comparative physiology of salt and water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhslakurw%3D&md5=2f4c6c7e2f9463e9f6a3ba186035af1dCAS |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=b30f07d810b3303a6dcacfac2b459d6cCAS |
Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S (2014) Halophyte agriculture: success stories. Environmental and Experimental Botany 107, 71–83.
| Halophyte agriculture: success stories.Crossref | GoogleScholarGoogle Scholar |
Pantoja O, Dainty J, Blumwald E (1989) Ion channels in vacuoles from halophytes and glycophytes. FEBS Letters 255, 92–96.
| Ion channels in vacuoles from halophytes and glycophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXmt1Ogsb8%3D&md5=fc0a61d6bf45c60c7817491c398a2de0CAS |
Parks GE, Dietrich MA, Schumaker KS (2002) Increased vacuolar Na+/H+ exchange activity in Salicornia bigelovii Torr. in response to NaCl. Journal of Experimental Botany 53, 1055–1065.
| Increased vacuolar Na+/H+ exchange activity in Salicornia bigelovii Torr. in response to NaCl.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsFagu7k%3D&md5=f490d70efaa256004b76686ec12ae877CAS |
Puniran-Hartley N, Hartley J, Shabala L, Shabala S (2014) Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance. Plant Physiology and Biochemistry 83, 32–39.
| Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1KlsL7N&md5=139f1b25c74fc2c17a5d19ea17b1a494CAS |
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD (2014) Economics of salt-induced land degradation and restoration. Natural Resources Forum 38, 282–295.
| Economics of salt-induced land degradation and restoration.Crossref | GoogleScholarGoogle Scholar |
Qiu NW, Chen M, Guo JR, Bao HY, Ma XL, Wang BS (2007) Co-ordinate up-regulation of V-H+-ATPase and vacuolar Na+/H+ antiporter as a response to NaCl treatment in a C3 halophyte Suaeda salsa. Plant Science 172, 1218–1225.
| Co-ordinate up-regulation of V-H+-ATPase and vacuolar Na+/H+ antiporter as a response to NaCl treatment in a C3 halophyte Suaeda salsa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFGitbo%3D&md5=4aafc44170ddec3f33d73171b904f0e3CAS |
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular plants – a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytologist 101, 25–77.
| Regulation of pH and generation of osmolarity in vascular plants – a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXmtFWjt7g%3D&md5=2e1b69b49e6dafb61f02bde3d53d4cc7CAS |
Rodríguez-Rosales MP, Jiang XY, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytologist 179, 366–377.
| Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization.Crossref | GoogleScholarGoogle Scholar |
Roy SJ, Huang W, Wang XJ, Evrard A, Schmockel SM, Zafar ZU, Tester M (2013) A novel protein kinase involved in Na+ exclusion revealed from positional cloning. Plant, Cell & Environment 36, 553–568.
| A novel protein kinase involved in Na+ exclusion revealed from positional cloning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1ais7c%3D&md5=e7560c1fa7da78ae87b067326faa0805CAS |
Scheuring D, Schöller M, Kleine-Vehn J, Löfke C (2015) Vacuolar staining methods in plant cells. In ‘Plant cell expansion: methods and Protocols. Methods in molecular biology. Vol. 1242’. (Ed. J Estevez) pp. 83–92. (Humana Press: Clifton, NJ, USA).
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany 112, 1209–1221.
| Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops.Crossref | GoogleScholarGoogle Scholar |
Shabala S, Mackay A (2011) Ion transport in halophytes. Advances in Botanical Research 57, 151–199.
| Ion transport in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1Sit74%3D&md5=2d715f8077e1330e11408ba9452e7346CAS |
Shabala S, Cuin TA, Prismall L, Nemchinov LG (2007) Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress. Planta 227, 189–197.
| Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlyku7zF&md5=4d1e8fec9d499fa7c9c94fa479736102CAS |
Silva P, Facanha AR, Tavares RM, Geros H (2010) Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica Oliv. Journal of Plant Growth Regulation 29, 23–34.
| Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica Oliv.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFWlt7c%3D&md5=0959e88755d3b46bba81722ad3275089CAS |
Singh RR, Kemp JA, Kollmorgen JF, Qureshi JA, Fincher GB (1997) Fertile plant regeneration from cell suspension and protoplast cultures of barley (Hordeum vulgare cv. Schooner). Plant Cell, Tissue and Organ Culture 49, 121–127.
| Fertile plant regeneration from cell suspension and protoplast cultures of barley (Hordeum vulgare cv. Schooner).Crossref | GoogleScholarGoogle Scholar |
Skoog D, West D, Holler F, Crouch S (2000) ‘Analytical chemistry: an introduction.’ (Saunders College Publishing: Philadelphia, PA, USA).
Sze H, Schumacher K, Muller M, Padmanaban S, Taiz L (2002) A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends in Plant Science 7, 157–161.
| A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtVKnsbg%3D&md5=59d689c8ada417c3c37b557fd32eb984CAS |
Tingay S, McElroy D, Kalla R, Fieg S, Wang MB, Thornton S, Brettell R (1997) Agrobacterium tumefaciens-mediated barley transformation. The Plant Journal 11, 1369–1376.
| Agrobacterium tumefaciens-mediated barley transformation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkslajurw%3D&md5=1379b25997fb3cefae0173a5f1f7fad9CAS |
Tomos AD, Leigh RA, Shaw CA, Jones RGW (1984) A comparison of methods for measuring turgor pressures and osmotic pressures of cells of red beet storage tissue. Journal of Experimental Botany 35, 1675–1683.
| A comparison of methods for measuring turgor pressures and osmotic pressures of cells of red beet storage tissue.Crossref | GoogleScholarGoogle Scholar |
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (1999) Salt stress in Mesembryanthemum crystallinum L cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta 207, 426–435.
| Salt stress in Mesembryanthemum crystallinum L cell suspensions activates adaptive mechanisms similar to those observed in the whole plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtlOgtro%3D&md5=44e4c6a5f08bf03eb9a46fda9de0ed02CAS |
Vera-Estrella R, Barkla BJ, Garcia-Ramirez L, Pantoja O (2005) Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiology 139, 1507–1517.
| Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Ogu73F&md5=d8b8d45f96f506507c155087b2bedb00CAS |
Wang BS, Luttge U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. Journal of Experimental Botany 52, 2355–2365.
| Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXptVeisL0%3D&md5=ab2a637d7ccb22816ce1ac4d895f4bddCAS |
Wang L, He XL, Zhao YJ, Shen YZ, Huang ZJ (2011) Wheat vacuolar H+-ATPase subunit B cloning and its involvement in salt tolerance. Planta 234, 1–7.
| Wheat vacuolar H+-ATPase subunit B cloning and its involvement in salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotVeltr8%3D&md5=1a9d3e31fd2c9b84ec7d21ccd47f9fb1CAS |
Wu HH, Shabala L, Barry K, Zhou MX, Shabala S (2013) Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiologia Plantarum 149, 515–527.
| Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslyhtLnL&md5=8aab4fbea16efe03468b821dc99f2ea5CAS |
Wu H, Zhu M, Shabala L, Zhou M, Shabala S (2014) K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: a case study for barley. Journal of Integrative Plant Biology 57, 1–15.
Wu H, Shabala L, Liu X, Azzarello E, Zhou M, Pandolfi C, Chen ZH, Bose J, Mancuso S, Shabala S (2015) Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Frontiers in Plant Science 6, 1–13.
| Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots.Crossref | GoogleScholarGoogle Scholar |
Xu CX, Zheng L, Gao CQ, Wang C, Liu GF, Jiang J, Wang YC (2011) Ovexpression of a Vacuolar H+-ATPase c subunit gene mediates physiological changes leading to enhanced salt tolerance in transgenic tobacco. Plant Molecular Biology Reporter 29, 424–430.
| Ovexpression of a Vacuolar H+-ATPase c subunit gene mediates physiological changes leading to enhanced salt tolerance in transgenic tobacco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltFaqsL8%3D&md5=18f9545df186bac16b0eaf95f0a9ec15CAS |
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology 19, 765–768.
| Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlslektLw%3D&md5=50ce757907071620eade0fd76db6f532CAS |
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proceedings of the National Academy of Sciences of the United States of America 98, 12832–12836.
| Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotFahsLs%3D&md5=1b88ad09ca0c797ef1c04770a0fa5f1fCAS |
Zhang HY, Niu XL, Liu J, Xiao FM, Cao SQ, Liu YS (2013) RNAi-directed downregulation of vacuolar H+-ATPase subunit A results in enhanced stomatal aperture and density in rice. PLoS One 8, 1–18.
Zhao Q, Zhao YJ, Zhao BC, Ge RC, Li M, Shen YZ, Huang ZJ (2009) Cloning and functional analysis of wheat V-H+-ATPase subunit genes. Plant Molecular Biology 69, 33–46.
| Cloning and functional analysis of wheat V-H+-ATPase subunit genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVagsbzO&md5=aa293bdb360414de509ab947bf174a97CAS |