Truncation of grain filling in wheat (Triticum aestivum) triggered by brief heat stress during early grain filling: association with senescence responses and reductions in stem reserves
Hamid Shirdelmoghanloo A , Daniel Cozzolino B C , Iman Lohraseb A and Nicholas C. Collins A DA The Australian Centre for Plant Functional Genomics, School of Agriculture Food and Wine, The University of Adelaide, PMB1 Glen Osmond, SA 5064, Australia.
B School of Agriculture Food and Wine, the University of Adelaide, PMB1 Glen Osmond, SA 5064, Australia.
C Present address: School of Medical and Applied Sciences, Central Queensland University, Rockhampton North Campus, Bruce Highway, Qld 4701, Australia.
D Corresponding author. Email: nick.collins@acpfg.com.au
Functional Plant Biology 43(10) 919-930 https://doi.org/10.1071/FP15384
Submitted: 22 December 2015 Accepted: 5 May 2016 Published: 1 August 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Short heat waves during grain filling can reduce grain size and consequently yield in wheat (Triticum aestivum L.). Grain weight responses to heat represent the net outcome of reduced photosynthesis, increased mobilisation of stem reserves (water-soluble carbohydrates, WSC) and accelerated senescence in the grain. To compare their relative roles in grain weight responses under heat, these characteristics were monitored in nine wheat genotypes subjected to a brief heat stress at early grain filling (37°C maximum for 3 days at 10 days after anthesis). Compared with the five tolerant varieties, the four susceptible varieties showed greater heat-triggered reductions in final grain weight, grain filling duration, flag leaf chla and chlb content, stem WSC and PSII functionality (Fv/Fm). Despite the potential for reductions in sugar supply to the developing grains, there was little effect of heat on grain filling rate, suggesting that grain size effects of heat may have instead been driven by premature senescence in the grain. Extreme senescence responses potentially masked stem WSC contributions to grain weight stability. Based on these findings, limiting heat-triggered senescence in the grain may provide an appropriate focus for improving heat tolerance in wheat.
Additional keywords: grain weight, photosynthesis, stay-green, temperature, water-soluble carbohydrate.
Introduction
Temperatures increase during the growing period in most wheat (Triticum aestivum L.) growing regions of the world and brief heat waves of +34°C are not uncommon during the sensitive reproductive stages of development (Wardlaw and Wrigley 1994; Asseng et al. 2011). Such heat events can significantly reduce both grain number and individual size, and hence yield. For example, in a correlation study using data from over 600 field trials in southern Australia, yield losses of 15% were attributed to every day above 30°C at or around flowering (Telfer et al. 2013). Similarly, Talukder et al. (2013) reported that a single day of heat stress (maximum temperature, 30°C), applied to field plots at reproductive growth stages using drop-on heated chambers, caused a ~11–23% reduction in grain number and a ~10–26% reduction in individual grain size, relative to the nonstressed controls. Needless to say, with climate change, this situation is predicted to worsen (Asseng et al. 2011). Hence further improvements to heat tolerance in wheat should help ensure global food security.
An understanding of the physiological processes governing genetic variation for heat tolerance in wheat may lead to the identification of traits that are likely to be useful for indirect selection of heat tolerance in breeders’ trials or novel strategies for engineering heat tolerance. In addition to its effect on grain number when it occurs before anthesis (Saini and Aspinall 1982), heat can affect grain size when it occurs around anthesis and early grain filling. Heat reduces grain size mainly by affecting the deposition of starch, which normally makes up ~70% of the grain mass (Jenner 1994). Heat may affect grain size by reducing sugar supply to the developing grain or by impacting processes within the grain that convert the delivered sugars into starch.
Heat can accelerate senescence in photosynthetic organs, causing reductions in chlorophyll content and functionality. There is evidence that chla and chlb pigments can be reduced differentially by heat (Al‐Khatib and Paulsen 1984), which has implications for their respective functions. PSII and the thylakoid membranes on which it resides are among the most heat-sensitive components of plant cells (Ristic et al. 2007 and references therein). Heat typically causes a (reversible) loss in PSII functionality, as reflected by changes in the chlorophyll fluorescence parameter Fv/Fm (Haque et al. 2014), indicating a further way in which heat reduces photosynthetic capacity. Reductions in Fv/Fm under heat can show a strong positive correlation with chlorophyll loss, suggesting that thylakoid membrane damage and mechanisms of chlorophyll loss are linked (Ristic et al. 2007). The ability of some wheat genotypes to maintain a high chlorophyll content (‘stay-green’) and chlorophyll functionality under heat is a trait that could help maintain sugar supply and hence high rates of grain filling during and after heat stress. Indeed, the ability to maintain grain weight under heat stress conditions in the field has been found to correlate with the stay-green trait (Kumari et al. 2007; Lopes and Reynolds 2012; Talukder et al. 2014). High temperatures can also affect photosynthetic activity (and other plant processes) indirectly by compromising plant water status as a consequence of increased evaporative demand.
Mobilisation of water soluble carbohydrate (WSC) reserves from the wheat stem to the developing grains also makes significant contributions to grain filling. The relative contribution of WSCs to grain filling can increase under conditions of stress such as drought (e.g. from 13% to 27%; Bidinger et al. 1977), which reduces current photosynthesis capacity. Talukder et al. (2013) reported a positive correlation between stem WSC mobilisation and heat stability of grain filling in a study of six wheat genotypes. Otherwise, evidence that WSCs contribute to variability in the ability of wheat genotypes to maintain grain size under heat seems scarce. The WSC content levels in wheat stems are dynamic and are the net consequence of deposition, remobilisation and losses due to other processes such as respiration. However, an estimate of mobilised WSC can be obtained from the difference between peak and minimum WSC content during grain filling (expressed as an absolute or a percentage of peak content). Methods using infrared reflectance spectra and modelling for determination of WSC content have been adapted to high-throughput nondestructive applications in the field (Dreccer et al. 2014).
Several processes within or near the developing grain have been linked to the heat responsiveness of starch deposition. Soluble starch synthase has been identified as both the rate-limiting and most heat-sensitive component of the starch biosynthetic machinery of the developing wheat grain, although the effect of heat on soluble starch synthase appears to be reversible (Jenner 1994). Elevated temperatures raise respiration rates in the grains and spike, thereby limiting the sugar pools available for starch deposition (Wardlaw et al. 1980). Heat stress during early grain filling results in earlier but lower peaks of expression of starch biosynthesis genes in the developing grain (Hurkman et al. 2003). Increasing temperatures, at least within the ~15−30°C range, can accelerate the rate of grain filling and there is genetic variation for this response in wheat (e.g. Sofield et al. 1977; Wardlaw and Moncur 1995). Short or long heat treatments during grain filling invariably truncate grain filling. This response (and genetic variation for it) has been linked to signalling by the senescence hormone ethylene within the grain (Hays et al. 2007). Rates of grain filling are responsive to changes in sugar supply to the developing grains (source strength), as demonstrated using experimental manipulations other than temperature (Sofield et al. 1977). Therefore, measuring the components of grain filling dynamics can provide clues about the drivers of grain weight reductions under heat.
In this study, aspects of the aforementioned heat responses were concurrently studied in a set of nine wheat genotypes to gain insights into their relative contributions to grain weight loss (and its genetic variability) as well as interactions. Heat was applied for a short time (3 days) during early grain filling using a growth chamber, in order to mimic the heat waves that can be most damaging to wheat crops.
Materials and methods
Two experiments were conducted in parallel in the same greenhouse to study effects of a brief heat stress at grain filling stage on grain growth, chlorophyll loss, chlorophyll fluorescence and stem WSC. In Experiment 1, chlorophyll fluorescence was measured nondestructively during and shortly after the heat treatment; at maturity, plants were harvested to measure yield components. In Experiment 2, grain growth, chla and chlb content and stem WSC were measured using destructive methods starting 10 days after anthesis (DAA) and concluding at 58 DAA, as described in the following sections.
Plant material
Nine bread wheat varieties (Triticum aestivum L. cvv. Drysdale, Frame, Gladius, Lyallpur-73, Millewa, Reeves, Sunco, Waagan and Young) were used. These were chosen because they varied in their heat responses of chlorophyll content and single-grain weight (SGW) at maturity in our previous studies (H. Shirdelmoghanloo, unpubl. data); Gladius, Millewa, Sunco, Waagan and Young were relatively heat-tolerant, but Drysdale, Frame, Lyallpur-73 and Reeves were intolerant. Some were also parents of available mapping populations.
Experimental design, plant growth and heat stress conditions
The experiments were set up in a split-plot (Experiment 1, six blocks or replicates) and a split-split-plot (Experiment 2, four blocks or replicates) design. In Experiment 1, each block was split into nine main plots (genotypes) and two subplots (control v. heat); in Experiment 2, each block was split into seven main plots (time of sampling), nine subplots (genotypes) and two sub-subplots (control v. heat). Both experiments were sown at the same time in late winter (early August 2013) and grown in the same naturally lit greenhouse compartment (The Plant Accelerator, the University of Adelaide, Waite Campus, Adelaide). Plant growth and heat stress conditions were similar to those of Maphosa et al. (2014). Plants were pruned back to the single main culm by removing tillers as they appeared. We previously established that under these conditions, pruning has little if any effect on grain weight under heat (I. Lohraseb, unpubl. data). Plants were kept well watered. Measured greenhouse conditions were ~20°C ; 17°C, 14 h : 10 h day : night (Table S1, available as Supplementary Material to this paper). Each plant’s anthesis date was recorded. For heat treatment, plants at 10 DAA were individually moved to a growth chamber (BDW120, Conviron) set at 37°C : 27°C day : night for 3 days, before being returned to the greenhouse. The temperature of 37°C was held for 8 h each day, with 3-h periods used either side to ramp down from and up to the night temperature. Under similar treatment conditions, Tashiro and Wardlaw (1990) have reported that a treatment at 10 DAA was late enough to avoid producing any ‘sterile’, ‘parthenocarpic’, ‘abortive’ or ‘shrunken’(wrinkly) wheat grains, but was not late enough to make some grains appear abnormal in other ways (notched, split or opaque). Such a treatment is still early enough to substantially reduce the final grain dry mass of susceptible varieties (Stone and Nicolas 1995). Pots were placed in trays of water to ~2-cm depth while in the chamber to minimise drought stress. The chamber had the same block layout as the greenhouse and although pots were moved to the corresponding chamber block, they were otherwise located within these chamber blocks at random. Average day : night relative humidity in the chamber was measured at 60% : 80%.
Data collection
Chlorophyll fluorescence was investigated in Experiment 1. The maximum quantum efficiency of PSII (Fv/Fm) was monitored using a portable chlorophyll fluorometer (MINI-PAM, Walz). Measurements were taken on the left-hand side of the flag leaf between the midrib and leaf margin, halfway between the base and the tip, after dark-adapting the leaf segment for 30 min. Measurements were taken at around midday on a daily basis from 10 to 15 DAA. This trait was not measured in the late-flowering variety Frame due to a lack of equipment availability.
Grain number per spike (GNS), grain weight per spike and SGW at maturity were also evaluated in Experiment 1. The collected spikes were oven-dried for 3 days at 85°C. Whole spikes were then threshed and grains of all sizes were manually counted and weighed. SGW was calculated as GWS divided by grain number per spike.
Chlorophyll a and b, and total chlorophyll content were measured in Experiment 2. Total chlorophyll pigments were extracted using the DMSO method described by Hiscox and Israelstam (1979). This method was chosen because it requires no grinding or centrifugation steps and chlorophyll is more stable in DMSO than in other solvents such as acetone and ethanol (Richardson et al. 2002). Leaf samples (~100 mg FW) were collected from the flag leaves at 10, 13, 23, 33, 43 and 53 DAA, from the same plants that were used in the grain growth study. Samples were transferred to glass centrifuge vials containing 7 mL DMSO, heated at 65°C for ~1 h in a water bath to extract the chlorophyll pigments and the volume increased to 10 mL with DMSO. After being allowed to cool to room temperature, 1 mL of each extract was transferred to a disposable polystyrene cuvette and the absorbance was measured at 645 and 663 nm using a UV–visible light spectrophotometer (model UV-160A, Shimadzu). The concentrations of chla, chlb and total chlorophyll were estimated according to Arnon’s equations (Arnon 1949). Chla, chlb and total chlorophyll content were presented over time and also as an average across all harvest times (averaged total chlorophyll content, TotChlav).
Experiment 2 also investigated grain growth. Grain samples were collected at 10 DAA (directly before treatment), 13 DAA (directly after treatment) and at 5-day intervals thereafter up to 58 DAA. On each date, 10 grains per spike were collected from the two basal floret positions of spikelets from around the middle of the spike from a single plant from each of the four blocks or replicates. For 10, 13 and 58 DAA, each plant was sampled at only one time point, taking five grains from each side of the spike from the central spikelets. For 18 to 53 DAA, individual plants were used for two consecutive samplings due to limitations in growth space (i.e. samplings at 18 and 23 DAA, 28 and 33 DAA, 38 and 43 DAA, and 48 and 53 DAA), taking 10 grains from one and then the other side of the spike at the first and the second time point, respectively. The anthesis date of each plant was defined as the day that extruded anthers first became visible. Anthesis normally begins in the middle of the wheat spike and at the most basal floret positions in each spikelet (McMaster 1997), which were the florets that were sampled. The collected grains were oven-dried for 3 days at 85°C before being weighed.
Grain weight measurements were fitted to the following logistic equation (Eqn. 1) to estimate grain growth characteristics, where W(t) is SGW (mg) at time t (day) after anthesis; c predicts the final SGW at maturity (SGWpred; mg); b is the slope parameter that controls the steepness of the curve; m (day) is the time from anthesis to the inflection point, the inflection point being defined as the point of maximum grain growth rate (MGR), and e is Napier’s number (a mathematical constant of ~2.718281828).

MGR (mg day–1) and grain filling duration (GFD, in days) were obtained using the following equations, as described by Zahedi and Jenner (2003).


To estimate sustained grain growth rate (SGR), a linear regression was applied to data from the linear phase of grain growth as described previously (Loss et al. 1989).
WSC were measured in Experiment 2. Stem samples (including leaf sheaths) were collected at 10, 13, 23, 33, 43 and 53 DAA from the same plants as were used in the grain growth study. At each date, stems were cut at the soil surface, the leaf blades and the spike were removed, and the stem was divided into three segments: peduncle, penultimate internode (the internode below the peduncle) and the lower internodes (the remaining portion). Samples were snap-frozen in liquid N and freeze-dried for ~20 h. Each segment was then weighed, chopped into ~5 mm segments, placed in a 10-mL Falcon tube containing two 5-mm ball bearings and reduced to a fine powder using a Geno/Grinder high-throughput plant and tissue homogeniser (SPEX SamplePrep). Samples were then scanned using a platinum diamond attenuated total reflectance single reflection sampling module cell mounted in a Bruker Alpha instrument (Bruker Optics GmbH), using air for normalisation. The attenuated total reflection mid infrared (ATR-MIR) reflectance spectra were recorded on OPUS software (ver. 7.0) provided by Bruker Optics and then exported to Unscrambler X software (ver. 10.1, CAMO ASA) for analysis.
Spectra were recorded for all harvested stem samples. WSC was measured directly in a subset of 125 samples using the anthrone method (see below), and the spectra and WSC values from this subset were used to derive a calibration model for predicting WSC quantities in all of the samples. The 125 samples were chosen for developing the model because they showed the maximum spectral variability in a principal component analysis, with the Mahalanobis distance applied as a measure of variability. WSC were measured in the 125 samples by extraction in 80% ethanol solution and then 100% water (van Herwaarden et al. 1998), followed by quantification using the anthrone method (Yemm and Willis 1954), using absorbance at 620 nm on a UV–visible light spectrophotometer (model UV-160A, Shimadzu) and fructose as the standard. Samples with absorbance values <0.2 or >1.83 were in the nonlinear range for absorbance v. WSC concentration and were remeasured after being extracted in a smaller volume or diluted 3×, respectively.
The spectra and direct WSC measurements were used to develop the model by partial least-squares regression with full cross-validation. The optimum number of terms in the partial least-squares calibration models was defined as the lowest number of factors that gave the minimum value of the prediction residual error sum of squares in cross-validation in order to avoid overfitting. The resulting calibration equations were evaluated using the coefficient of determination in calibration (R2) and the s.e. in cross-validation. The ratio of s.d. to s.e. in cross-validation, called the residual predictive deviation, was used to test the accuracy of the calibration models. The residual predictive deviation demonstrates how well the calibration models perform in predicting the reference data, with values >3 considered adequate for most ATR-MIR applications (D. Cozzolino, unpubl. data). The residual predictive deviation value for the calibration model in this study was 4. The developed calibration model was then used to predict WSC values for all of the samples. A comparison of the WSC concentration (mg g–1 DW) determined by the anthrone method and data predicted via ATR-MIR analysis in the subset of 125 samples is shown in Fig. S1.
WSC content and average WSC content across all harvest times were also calculated. Maximum WSC content (WSCmax) was defined as the highest WSC content obtained over time for each type of sample. The amount of mobilised WSC (MWSC) in each type of stem segment was calculated as the difference between the minimum WSC content (WSCmin) and WSCmax of the segment. WSC mobilisation efficiency (WSCME) for each stem segment was calculated as the fraction of the maximum WSC content of the segment that was mobilised (Eqn. 4).

The DW of each stem segment over time and on average across all of the harvest times (DWav) were also presented.
Data analysis
ANOVA was carried out for each trait using Genstat ver. 16 (http://www.vsni.co.uk/genstat, accessed 16 May 2016). LSD tests (α = 0.05) were used for mean comparisons. R language (R Development Core Team 2012) was used for model regressions and Pearson correlation tests, and to prepare figures.
Results
Grain number and final size
Means for GNS, grain number per spikelet and final SGW from Experiment 1 are plotted in Fig. S2. Although there was a significant overall genotype effect on GNS and grain number per spikelet (P < 0.001), there was no significant effect of the heat treatment on these traits (P = 0.054 and 0.556, respectively).
For SGW measured at maturity, there were significant effects (P < 0.001) for genotype, treatment and genotype × treatment (G × T). Gladius, Millewa, Sunco, Waagan and Young showed the smallest heat responses (≤8%) and were therefore relatively tolerant, whereas Drysdale, Frame, Lyallpur-73 and Reeves showed the greatest responses (–14 to –28%; Fig. S2) and were therefore relatively intolerant. These results were consistent with tolerance rankings of these genotypes obtained in previous experiments (H. Shirdelmoghanloo, unpubl. data).
Grain growth
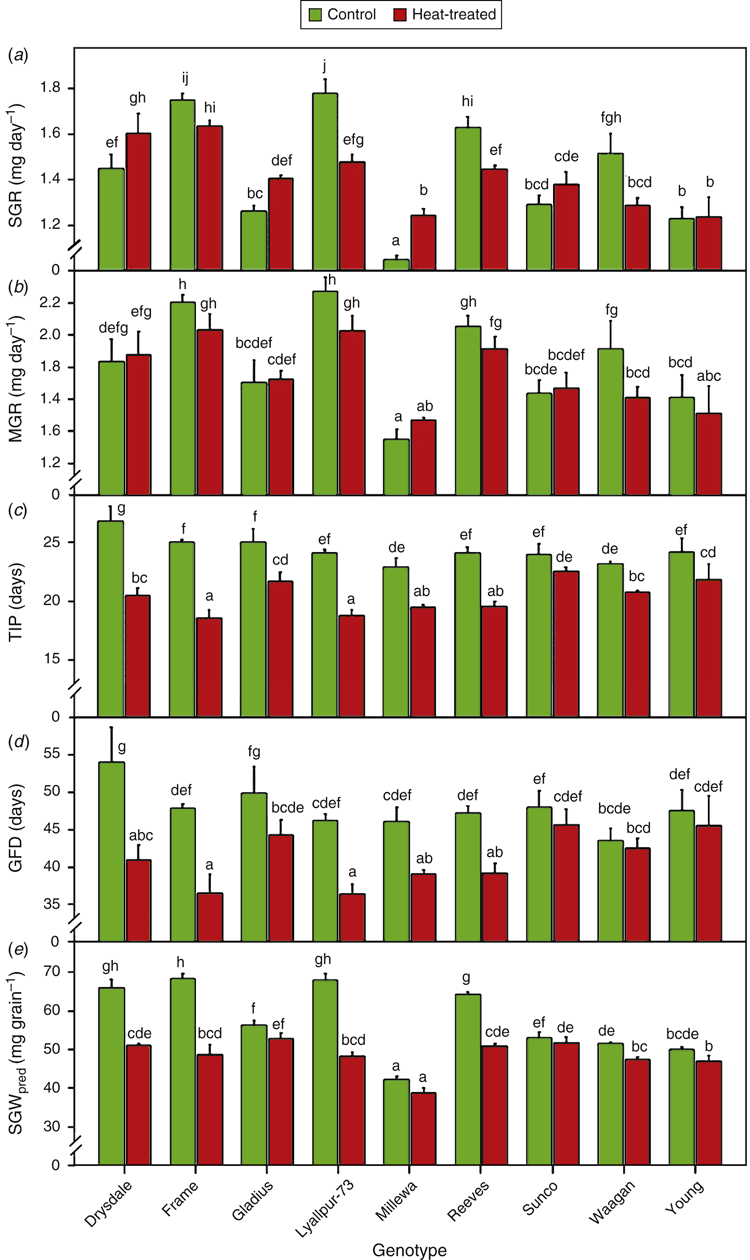
Grain filling dynamics in control and heat-treated plants of each genotype, fitted to logistic models, are illustrated in Fig. 1. There was a consistent trend (albeit nonsignificant) for heat treatment to temporarily increase the rate of grain filling during the 3-day heat treatment (Fig. 1). However, the most prominent effect of the heat treatment was a levelling off of grain filling rate in the heat-treated plants from about 2 weeks after the heat treatment. The grain filling profiles were in line with the tolerance classifications of the varieties based on final SGW, with the five most tolerant genotypes (Gladius, Millewa, Sunco, Waagan and Young) showing few or no time points with significant differences between the grain weight of the control and heat-treated plants (Fig. 1).
Grain filling parameters estimated from the logistic models (or linear models for SGR) were subjected to ANOVA. The output, together with the means and ranges of heat treatment effects from Experiment 2, are presented in Table S2. All grain filling parameters showed significant genotype effects (P < 0.001). There was no significant heat treatment effect detected for sustained growth rate (SGR). The treatment effect was borderline nonsignificant for MGR (P = 0.057) but it was highly significant (P < 0.001) for the remaining grain traits. Except for MGR, G × T effects were significant (P < 0.05 for GFD, P < 0.001 for the rest). Heat reduced the predicted grain weight (final SGW), time to grain growth inflection point and GFD, all by between 13% and 16% on average, and from <5% to >20% across genotypes.
Theoretical final SGW estimated from the logistic models in Experiment 2 showed a strong positive genotypic correlation (r = 0.84; P < 0.01) with SGW measured at maturity in Experiment 1, indicating a good estimation of the grain weight at maturity by the models.
The means of grain filling attributes in the individual genotypes in Experiment 2 are summarised in Fig. 2. The four intolerant genotypes Drysdale, Frame, Lyallpur-73 and Reeves were mainly distinguished from the five tolerant varieties by having large and significant reductions in GFD due to heat, whereas they showed no significant effects of heat on MGR. However Lyallpur-73 and Reeves did show significant reductions in SGR under heat (reductions of 16.8% and 11.2%, respectively). The tolerant varieties Gladius and Millewa showed smaller but significant reduction in GFD due to heat, but had compensatory increases in SGR of 11.3% and 18.3%, respectively.
Chlorophyll fluorescence
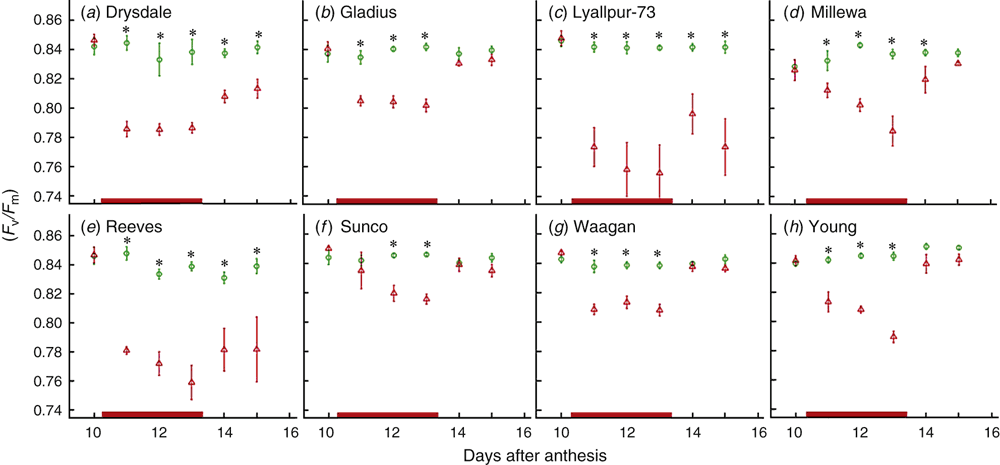
In an ANOVA on the average chlorophyll fluorescence (Fv/Fm) across all time points (10–15 DAA), significant genotype, treatment, and G × T effects (P < 0.001) were found. Fv/Fm before the heat treatment was similar across the varieties except in Millewa, where it was noticeably lower (Fig. 3). The Fv/Fm ratio decreased rapidly and significantly in the first day of treatment in all varieties except Sunco. The intolerant varieties Drysdale, Lyallpur-73 and Reeves showed the largest reductions due to heat (the other intolerant variety, Frame, was not tested for this trait). Fv/Fm decreased further as the heat treatment continued, except in Drysdale, Gladius and Waagan. By the first or second day after the heat treatment, Fv/Fm recovered almost completely to control levels in the tolerant varieties, whereas it only partially recovered in the intolerant varieties Drysdale, Lyallpur-73 and Reeves (Fig. 3).
Chlorophyll content
The heat treatment also decreased the chla and chlb content of flag leaves (averages across all genotypes are shown in Fig. 4). ANOVA on the values averaged across all time points revealed significant genotype and treatment effects (P < 0.001), but no significant G × T effects (P > 0.05). In absolute terms, chla and chlb reductions due to heat peaked at around 30 DAA and were greater for chla than chlb (0.58 v. 0.30 mg g–1 FW, respectively), although in percentage terms, losses in chla were only marginally greater (47.8% v. 41.2%).

|
Time courses of the flag leaf total chla and chlb contents of individual varieties are shown in Fig. S3 and Fig. S4. Similar to Fv/Fm, chla and chlb contents showed the largest overall decreases due to heat in the four intolerant varieties Drysdale, Frame, Lyallpur-73 and Reeves. In these genotypes, decreases were rapid during the heat treatment, then upon relief from heat stress, the rates of loss recovered to be similar to those of control plants, either immediately (Drysdale and Reeves) or after a delay (Frame and Lyallpur-73). In both the tolerant and intolerant genotypes, chlb tended to show greater immediate heat-induced losses than chla (i.e. during the heat treatment), whereas chla tended to show proportionately more loss after a delay (Fig. S3 and Fig. S4). Consequently, the chl a/b ratio was initially higher in heat-treated plants and then became marginally higher in control plants (Fig. 4).
Stem WSCs
Significant genotype and treatment effects (P < 0.001) were observed for WSC content averaged over all time points in all stem segments. However, the G × T effect was significant only for the peduncle (P = 0.007, 0.300 and 0.179 for the peduncle, penultimate and lower internodes, respectively).
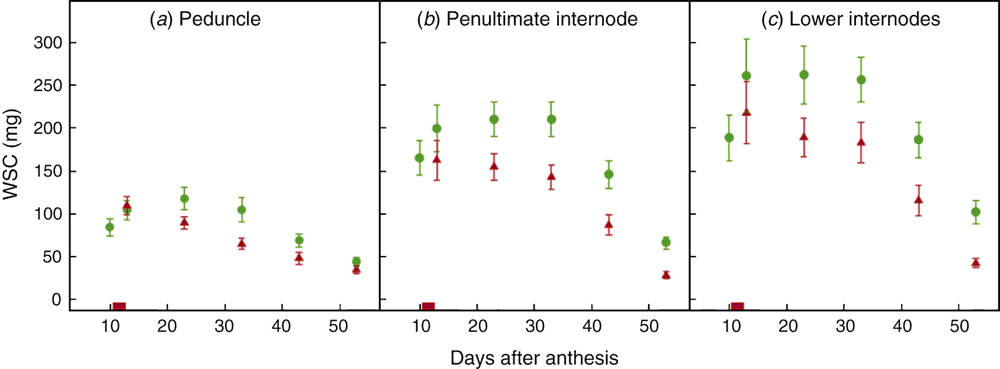
The time course for total WSC content in the various stem segments, averaged over all genotypes, is illustrated in Fig. 5. In control plants, WSC content increased in all stem segments at the time of the heat treatment and peaked ~10–17 days after the heat treatment. Heat treatment decreased WSC content relative to the control, indicating heat-triggered mobilisation of these reserves, a reduction in WSC deposition in the stems or both. In the penultimate and lower internodes, major heat-associated reductions in WSC content occurred during the heat treatment; in the peduncle, reductions mainly occurred after the heat treatment, on average (Fig. 5).
For all stem segments, WSCmax showed a significant genotype effect (P < 0.001) but no significant treatment effect (P > 0.05). For WSCmin, a significant genotype effect was seen in all stem segments (P < 0.001) and a treatment effect was significant in the penultimate and lower internodes (P < 0.001) but not in the peduncle (P > 0.05). WSCmin showed no significant G × T effect in any stem segment (P > 0.05). Mobilised WSC and WSC mobilisation efficiency, which are parameters based on differences between WSCmax and WSCmin, showed no significant treatment effects, perhaps due to matched reductions in WSCmax and WSCmin (Fig. S5).
In line with their relative masses, the peduncle, penultimate internode and lower internodes accounted for different proportions of the total stem WSC pool (18%, 36% and 46%, respectively, based on peak height in the control plants). Reductions in WSC content due to heat (as a proportion of the WSC content in the control) were similar across the three stem segment types (39%, 32% and 29%, respectively, based on maximum differences in WSC contents between control and heat-treated plants).
Stem WSC content data for the individual genotypes and stem segments are presented in Fig. S6 (time course) and Fig. 6 (averaged over all times). Reductions in WSC content due to the heat treatment (based on averages over all time points and the sum of all stem segments) was greatest in Frame, followed by Lyallpur-73 and Reeves.
In cases where WSC content was most dynamic or heat responsive (or both), changes in WSC content (Fig. S6) showed a resemblance to those of the corresponding stem segment DW (Fig. S7). This supports the notion that the facile stem DW measurement has some value as a surrogate for the direct measurement of WSC content.
Associations between heat responses of traits
Table S3 summarises the results of pairwise tests for correlations between the heat responses of traits obtained in Experiment 2, across the nine genotypes.
As was already suggested in the aforementioned descriptions of traits in the genotypes classified as tolerant and intolerant, the heat responses of final SGW were correlated positively with those of GFD and the related trait time to inflection point, as well as the responses of chlorophyll content averaged over all time points (TotChlav, averaged chla and averaged chlb) (r = 0.87–0.95; P < 0.01–0.001). This was consistent with a link between stay-green and maintenance of grain weight under heat stress, via a stabilisation of GFD.
Heat responses of final SGW and GFD (but not grain filling rate) also showed (weaker) positive correlations with the response of WSC (and stem DW) in the peduncle, averaged over all time points (average WSC content and DWav; r = 0.71–0.77; P < 0.05; Table S3). Accordingly, WSC responses in the peduncle were also positively correlated with chlorophyll responses in the flag leaves (for average WSC content and DWav; r = 0.73–0.83; P < 0.01–0.05). These correlations between the responses of WSCs and final SGW were in the opposite direction to what was expected if heat-induced mobilisation of WSC in the peduncle contributed to maintenance of final SGW under heat.
The penultimate internode and lower internodes behaved more similarly to one another than to the peduncle with regard to WSC heat responses, as indicated by the stronger and more frequent (positive) significant correlations between the former two stem segments (Table S3).
In the lower stem internodes, responses of grain growth rate (MGR and SGR) to heat showed (weak) positive correlations with WSC responses (average WSC content, WSCmax, mobilised WSC and MSCME; r = 0.67–0.76; P < 0.05; Table S3). Responses of these WSC traits in the lower internode did not correlate significantly (positively or negatively) with the final SGW response, indicating that the effects were not large enough to influence variation in grain size response among the genotypes. The directions of the correlations were also the opposite to what was expected if mobilisation of WSCs in the lower internodes contributed to the stability of grain filling rate (and final SGW) under heat.
Separate from the analysis represented in Table S3, the maximum Fv/Fm response during treatment in Experiment 1 was found to be well correlated (r = 0.91; P < 0.01) with the chlorophyll content response based on the measurements taken directly after the treatment in Experiment 2.
Relationships between trait potentials and heat responses of traits
Correlations between the potential values of the traits under control conditions in Experiment 2 and the heat responses of the traits were also examined (Table S4). The genotypes that tended to have a high flag leaf chlorophyll content under control conditions also tended to lose a smaller proportion of their chlorophyll, final SGW, GFD and peduncle DW due to heat (as indicated by significant positive correlations between the chlorophyll potentials and these responses). Genotypes with higher stem WSC content and greater WSC mobilisation under control conditions also tended to lose more WSC, chlorophyll and grain weight under heat (as indicated by the significant negative correlations between the WSC content and mobilisation potentials, and these responses). Genotypes with higher grain filling rates and grain size in control plants also tended to lose more chlorophyll and stem WSC under heat (Table S4).
Discussion
In this study, leaf chlorophyll content and functionality, WSC content and mobilisation, together with components of grain filling dynamics, were tested concurrently for their responses to a brief heat stress applied at early grain filling in a panel of nine contrasting wheat genotypes. The heat stability of chlorophyll content and PSII functionality were the variables most highly correlated with grain weight stability (and the ability to maintain GFD), identifying these characteristics as being potentially linked with grain weight stability in the heat-tolerant genotypes.
Chlorophyll content and functionality
In relation to grain size and chlorophyll responses to the heat stress treatment, the nine genotypes fell into two groups: Gladius, Millewa, Sunco, Waagan and Young (tolerant) v. Drysdale, Frame, Lyallpur-73 and Reeves (intolerant), contrasted for losses of final grain mass due to heat (≤8% v. ≥21%) and loss of flag leaf chlorophyll content (≤19% v. ≥26%, averaged over all time points). Furthermore (except for Frame, for which no Fv/Fm data were obtained), they contrasted for losses in PSII functionality during the heat treatment (approximately twofold higher Fv/Fm responses in the intolerant group) and for recovery of PSII functionality by 2 days after the heat treatment (complete v. partial recovery of Fv/Fm). The associations between grain weight and chlorophyll stability were further supported by the correlation analysis (r = 0.94, P < 0.001 for final SGW v. TotChlav).
A decline in Fv/Fm under heat signifies reductions in the maximum photochemical efficiency of the PSII complex within the chloroplasts and hence a reduction in photosynthetic capacity. The reasons why PSII should be particularly prone to heat damage probably relate to the heat lability of the thylakoid membranes on which PSII resides (see Georgieva 1999 for a review). Haque et al. (2014) also reported that heat-triggered reductions in Fv/Fm in bread wheat (for treatments below 45°C) were reversible within several days of relief from heat stress but with the most severely affected genotypes being slower to recover.
The mechanisms by which heat accelerates chlorophyll loss are not known but could relate to damage to the thylakoid membranes, given that chlorophyll loss and decline in Fv/Fm due to heat were positively correlated. Ristic et al. (2007, 2008), using the chlorophyll fluorescence parameter O/P (F0/Fm, which is numerically related to Fv/Fm; Fv being calculated as Fm – F0), also observed strong positive correlations between losses in PSII functionality and chlorophyll content under heat in both hexaploid and tetraploid wheat. The TotChlav in control plants, which reflected rates of flag leaf senescence under nonstress conditions, was positively correlated with the heat response of TotChlav (r = 0.85; P < 0.01; Table S4), suggesting that the mechanisms of heat-accelerated chlorophyll loss could be related to senescence processes in nonstressed plants. Indeed, Harding et al. (1990), who followed various activities in leaves of heat-stressed and nonstressed wheat plants, found that both showed an initial breakdown of thylakoid membranes. Alternatively, the temperatures in the greenhouse in which the control plants were grown (up to 29.7°C on some days during late grain filling) may have been high enough to induce low-level expression of chlorophyll loss mechanisms that become accentuated under higher temperatures.
Chlorophyll a and b differed from one another in their heat responses, in that the effects of heat on chlb were proportionately greater than those of chla from during the heat treatment up to 20 days after the heat treatment and then became proportionately less from 30 days after the heat treatment (Fig. 4). The greater initial response for chlb could be explained by heat damage to PSII, because PSII is more heat labile than PSI (Georgieva 1999) and most of the chlb is found in PSII. However, there is no obvious explanation for the shift towards greater chla loss at later time points. Al‐Khatib and Paulsen (1984) also reported differences in the dynamics of chla and chlb in heat-treated wheat plants.
It is generally thought that grain filling in wheat is not limited by source (assimilate) supply to the developing grain under mild growing conditions, but assimilate supply can become limiting under conditions such as drought or heat that reduce photosynthetic capacity (Abbad et al. 2004). Under nonstress conditions, flag leaf photosynthesis contributes a significant proportion of the assimilates used in grain filling (e.g. 22%) (Araus et al. 1993). Heat caused flag leaves of the intolerant varieties Lyallpur-73, Frame and Reeves to lose >50% of their chlorophyll by 20 DAA, and effects of Fv/Fm were manifested from 11 DAA (during the heat treatment) and shortly thereafter. The timing of these effects preceded the point when heat-triggered levelling off of grain filling became evident at ~30 DAA (Fig. 1). These associations between flag leaf chlorophyll and grain filling responses to heat would be consistent with the notion that supply became limiting for grain filling in the intolerant varieties due to losses in photosynthetic capacity and, conversely, that chlorophyll stability was a significant determinant of grain weight stability in the tolerant varieties (although see an argument to the contrary in the next section). The ability to limit chlorophyll loss under stress (stay-green) has been previously reported to correlate with the yield and grain weight performance of wheat grown under heat stress conditions in the field (Kumari et al. 2007; Lopes and Reynolds 2012; Talukder et al. 2014) and in a pot experiment similar to ours (Vignjevic et al. 2015).
Grain growth and development
The most prominent effect of heat on grain growth was a premature levelling off of grain filling in the intolerant genotypes, which effectively shortened the GFD by up to 24% (Fig. 1). The heat response of GFD showed a positive correlation with response of final SGW under heat (r = 0.87, P < 0.01; Table S3). By contrast, the maximum grain growth rate after the heat treatment (SGR and MGR) showed smaller responses to heat, which were positive or negative depending on the variety (+18% to –17%); these responses were not significantly correlated with the responses of final SGW (Table S3). A similar impact of heat on grain filling (i.e. GFD was affected with little effect on the grain filling rate) was observed by Stone and Nicolas (1995) after applying a similar heat treatment to ours (40°C : 19°C day : night at 15 DAA for 5 days).
Sofield et al. (1977) tested the effect of source supply on grain filling dynamics by subjecting wheat to three different light intensities during grain filling from 4 DAA onwards. An effect on grain filling rate was observed within a few days, with the lowest and highest light intensities giving the lowest and highest grain filling rates, respectively, whereas there were minimal effects on GFD. These findings challenge the notion that the rapid reduction in photosynthetic capacity during the heat treatment in the susceptible genotypes (Drysdale, Frame, Lyallpur-73 and Reeves) directly reduced their grain weights (by limiting source supply), as the responses of their developing grains to heat were characterised by a reduction in GFD rather than a reduction in grain filling rate. Similarly, the consistent trend for heat to increase grain filling rate during the treatment, and the absence of a significant reduction in MGR following heat treatment (except in Waagan) argues against damage to the grain soluble starch synthase as being very influential under these conditions, despite other evidence that this enzyme is heat-sensitive (Jenner 1994). An alternative explanation for why grain filling was cut short in these varieties is that heat accelerated senescence in the grain, curtailing the development of the grains and their ability to convert the delivered sugars into starch. Thus the susceptible genotypes may have been prone to heat-induced acceleration of senescence in both the leaves and grain, due to coordination by a common signal at the top of the plant (e.g. related to the plant senescence hormone ethylene). Hays et al. (2007) found heat-triggered ethylene production in grains and leaves to be 7- to 12-fold higher in a heat-sensitive wheat variety than in a tolerant variety, and observed that application of the ethylene receptor inhibitor 1-methylcyclopropane abrogated the heat-triggered truncation of grain filling otherwise seen in the intolerant variety after exposure to a 2-day treatment of 38°C at 10 DAA.
The four intolerant varieties were also distinguished by having larger grains than the five tolerant varieties under control conditions (with the exception of Gladius and Reeves, which had a similar grain size in the controls in Experiment 1; Fig. S2 and Fig. 2), which seemed to be achieved more by having a long GFD in Drysdale, and a high grain filling rate in Frame, Lyallpur-73 and Reeves. However, we have identified quantitative trait loci in wheat that control the SGW response to heat without affecting grain weight under control conditions (Shirdelmoghanloo et al. 2016), indicating that a connection between grain weight potential and the heat stability of grain size is not a necessary feature of (all) heat tolerance loci. This is an important point, as the usefulness of a heat tolerance gene in breeding would be seriously limited if it limited yield in the absence of heat stress.
All of the varieties tended to show a reversible acceleration of grain filling during the heat treatment (Fig. 1) but because this was confined to a short period, it had little opportunity to affect final SGW. In other studies where (milder) heat treatments were applied throughout grain filling, the degree to which grain filling was accelerated by heat showed genetic variation that manifested in differential responses of final SGW (e.g. Sofield et al. 1977; Wardlaw and Moncur 1995; Zahedi and Jenner 2003). In our study, there were a few cases where significant growth rate effects manifested after the heat treatment, namely decreases for MGR and SGR in Waagan, and increases for SGR in Millewa and Gladius (Fig. 2). Among the five tolerant varieties, GFD was reduced by heat the least in Waagan and the most in Millewa and Gladius, which would have partially offset the effects of heat on grain filling rate in these varieties. There were no obvious patterns of chlorophyll, WSC or grain weight potential among these varieties (differences in Waagan v. Millewa and Gladius) that might have explained these grain filling rate responses.
Water soluble carbohydrate
Stem reserves (WSCs) also contribute to grain filling in wheat (Blum et al. 1994), and their relative contribution to final grain mass can increase under stress conditions such as drought that reduce photosynthetic capacity (e.g. rising from 13% under irrigation to 27% under drought) (Bidinger et al. 1977). However, the correlations of final SGW responses to WSC traits were in opposite directions to those expected if WSCs contributed to grain weight maintenance under heat: there was a negative correlation between final SGW response and control values for WSC content and mobilisation parameters for all stem segments (Table S4). In the peduncle, there was a positive (weak, P < 0.05) correlation between the final SGW response and the heat responses of WSC content averaged over all time points (average WSC content) and the related parameter DWav (Table S3). Similarly, in the lower internodes, the heat responses of several WSC content or mobilisation parameters were positively correlated with the heat responses of grain growth rate (MGR and SGR; P < 0.05).
The genotypes that showed the greatest responses of WSCs to heat treatment (Frame, Lyallpur-73 and Reeves, based on averages over all time points and stem segments; Fig. 6) also showed the most drastic losses in flag leaf chlorophyll (Figs S3 and S4). Hence, the responses of stem WSC content observed in these varieties may have been driven mostly by reduced deposition of new WSC in the stem during this period (due to reduced photosynthesis), rather than a tendency for these varieties to remobilise more stem WSCs to support grain filling under heat. Such an effect may have masked correlative evidence that stem WSC remobilisation contributed to SGW stability under heat. This hypothesis could be tested by using 14CO2 labelling and other detailed physiological measurements on these genotypes to more accurately apportion changes in net stem WSC content to deposition v. loss, and losses to mobilisation to the grains v. losses to other processes such as respiration.
In contrast to our work, Talukder et al. (2013, 2014) found a positive correlation between WSCs mobilised from the peduncle and the ability to maintain grain weight after exposure to a single day of heat stress applied at flowering or 7–10 DAA. None of the six genotypes used in that study (including the tolerant variety Gladius) showed a noticeable decline in flag leaf chlorophyll during the heat treatment, applied using drop-on chambers in the field – a factor that may have allowed the detection of the positive WSC effect. The lack of a large initial chlorophyll response may be attributable to the milder heat stress treatment applied (1 day with a maximum of 35°C v. 3 days with a maximum of 37°C in our study), different growing conditions or the absence of very susceptible genotypes.
Conclusions
Rapid losses in chlorophyll content and functionality in flag leaves associated with a 3-day heat treatment at early grain filling were found to be strongly correlated with losses in final SGW. A parallel quantitative trait locus mapping study in wheat (Shirdelmoghanloo et al. 2016) confirmed that this association can derive from control of these two processes by the same locus. This raises the possibility of using nondestructive measurement of chlorophyll fluorescence or content changes in field plots over the duration of a brief heat wave (e.g. using instrumentation described by Deery et al. (2014) to assist in selecting heat-tolerant varieties.
An acceleration of grain growth observed after the heat treatment in the varieties Gladius and Millewa deserves further investigation as a potential tolerance mechanism for achieving stable grain weight under heat. However, the truncating effects of heat on GFD emerged as the main determinant of grain weight responses among the nine wheat genotypes, pointing to senescence in the grain more than source limitation as the underlying driver of grain weight losses. Blocking heat-triggered premature senescence in grains could therefore be considered as an appropriate focus in efforts to improve the heat stability of grain size in wheat.
Losses in stem WSC and grain weight under heat were positively correlated, perhaps because they were both promoted by strong senescence responses in the intolerant genotypes. Hence, avoidance of genotypes or conditions that lead to strong senescence responses would be recommended in studies that aim to quantify positive contributions of stem WSC to grain weight stability under heat.
Acknowledgements
This project was funded by the Grains Research and Development Corporation (GRDC; project UA00123), with additional support from the Australian Centre for Plant Functional Genomics (ACPFG). ACPFG is funded mainly by the GRDC, the Australian Research Council, the Government of South Australia and the University of Adelaide. We thank staff of The Plant Accelerator, Australian Plant Phenomics Facility, for plant care and growth facilities. The Plant Accelerator is supported by the Australian Government under the National Collaborative Research Infrastructure Strategy and the University of Adelaide.
References
Abbad H, El Jaafari S, Bort Pie J, Araus Ortega JL (2004) Comparison of flag leaf and ear photosynthesis with biomass and grain yield of durum wheat under various water conditions and genotypes. Agronomie 24, 19–28.| Comparison of flag leaf and ear photosynthesis with biomass and grain yield of durum wheat under various water conditions and genotypes.Crossref | GoogleScholarGoogle Scholar |
Al‐Khatib K, Paulsen GM (1984) Mode of high temperature injury to wheat during grain development. Physiologia Plantarum 61, 363–368.
| Mode of high temperature injury to wheat during grain development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlt1OnsLo%3D&md5=3dc1498f21f29ec53f59dfd9150628eaCAS |
Araus J, Brown H, Febrero A, Bort J, Serret M (1993) Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant, Cell & Environment 16, 383–392.
| Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlslOlt7k%3D&md5=b895be9ae35ecd054ade6ffb50ca97bcCAS |
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology 24, 1–15.
| Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaH1MXhtFaqtg%3D%3D&md5=f047f798fba7c2e7b043de4bb1179c39CAS | 16654194PubMed |
Asseng S, Foster IAN, Turner NC (2011) The impact of temperature variability on wheat yields. Global Change Biology 17, 997–1012.
| The impact of temperature variability on wheat yields.Crossref | GoogleScholarGoogle Scholar |
Bidinger F, Musgrave R, Fischer R (1977) Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 270, 431–433.
| Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Blum A, Sinmena B, Mayer J, Golan G, Shpiler L (1994) Stem reserve mobilisation supports wheat-grain filling under heat stress. Australian Journal of Plant Physiology 21, 771–781.
| Stem reserve mobilisation supports wheat-grain filling under heat stress.Crossref | GoogleScholarGoogle Scholar |
Deery D, Jimenez-Berni J, Jones H, Sirault X, Furbank R (2014) Proximal remote sensing buggies and potential applications for field-based phenotyping. Agronomy 4, 349–379.
| Proximal remote sensing buggies and potential applications for field-based phenotyping.Crossref | GoogleScholarGoogle Scholar |
Dreccer MF, Barnes LR, Meder R (2014) Quantitative dynamics of stem water soluble carbohydrates in wheat can be monitored in the field using hyperspectral reflectance. Field Crops Research 159, 70–80.
| Quantitative dynamics of stem water soluble carbohydrates in wheat can be monitored in the field using hyperspectral reflectance.Crossref | GoogleScholarGoogle Scholar |
Georgieva K (1999) Some mechanisms of damage and acclimation of the photosynthetic apparatus due to high temperature. Bulgarian Journal of Plant Physiology 25, 89–99.
Haque MS, Kjaer KH, Rosenqvist E, Sharma DK, Ottosen CO (2014) Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environmental and Experimental Botany 99, 1–8.
| Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXislKqu74%3D&md5=88ee56cf46e9fb95eb93ce09d2e24797CAS |
Harding SA, Guikema JA, Paulsen GM (1990) Photosynthetic decline from high temperature stress during maturation of wheat I. Interaction with senescence processes. Plant Physiology 92, 648–653.
| Photosynthetic decline from high temperature stress during maturation of wheat I. Interaction with senescence processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhslGrtro%3D&md5=22d9d9dca4d7725d6f8a3209163117e6CAS | 16667329PubMed |
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Science 172, 1113–1123.
| Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFGitLw%3D&md5=163e058a5db7764a4552ca2d4e5ba41dCAS |
Hiscox JT, Israelstam G (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany 57, 1332–1334.
| A method for the extraction of chlorophyll from leaf tissue without maceration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXltlKlsbg%3D&md5=e3be76c8c48580543dfbbbb4e51c106eCAS |
Hurkman WJ, McCue KF, Altenbach SB, Korn A, Tanaka CK, Kothari KM, Johnson EL, Bechtel DB, Wilson JD, Anderson OD (2003) Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Science 164, 873–881.
| Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXivFeqsbc%3D&md5=51b3a26328831ae9ab382b85ac184de5CAS |
Jenner C (1994) Starch synthesis in the kernel of wheat under high temperature conditions. Functional Plant Biology 21, 791–806.
Kumari M, Singh V, Tripathi R, Joshi A (2007) Variation for staygreen trait and its association with canopy temperature depression and yield traits under terminal heat stress in wheat. In ‘Wheat production in stressed environments.’ (Eds HT Buck, JE Nisi, N Salomón) pp. 357–363. (Springer: Dordrecht.)
Lopes MS, Reynolds MP (2012) Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. Journal of Experimental Botany 63, 3789–3798.
| Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVSht7jI&md5=ea34738739d3c02b42ce214fcd56cc43CAS | 22412185PubMed |
Loss S, Kirby E, Siddique K, Perry M (1989) Grain growth and development of old and modern Australian wheats. Field Crops Research 21, 131–146.
| Grain growth and development of old and modern Australian wheats.Crossref | GoogleScholarGoogle Scholar |
Maphosa L, Collins N, Taylor J, Mather D (2014) Post-anthesis heat and a Gpc-B1 introgression have similar but non-additive effects in bread wheat. Functional Plant Biology 41, 1002–1008.
| Post-anthesis heat and a Gpc-B1 introgression have similar but non-additive effects in bread wheat.Crossref | GoogleScholarGoogle Scholar |
McMaster GS (1997) Phenology, development, and growth of wheat (Triticum aestivum L.) shoot apex: a review. Advances in Agronomy 59, 63–118.
| Phenology, development, and growth of wheat (Triticum aestivum L.) shoot apex: a review.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2012) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at: http://www.R-project.org [Verified 16 May 2016]
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytologist 153, 185–194.
| An evaluation of noninvasive methods to estimate foliar chlorophyll content.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xns1yrsQ%3D%3D&md5=f9b5e6e4527cf84e0719337ede18613cCAS |
Ristic Z, Bukovnik U, Prasad PV (2007) Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Science 47, 2067–2073.
| Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlSltbbN&md5=05c8e36d2104c8eaa3e381935f7b7a85CAS |
Ristic Z, Bukovnik U, Prasad P, West M (2008) A model for prediction of heat stability of photosynthetic membranes. Crop Science 48, 1513–1522.
| A model for prediction of heat stability of photosynthetic membranes.Crossref | GoogleScholarGoogle Scholar |
Saini H, Aspinall D (1982) Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Annals of Botany 49, 835–846.
Shirdelmoghanloo H, Taylor JD, Lohraseb I, Rabie H, Brien C, Timmins A, Martin P, Mather DE, Emebiri L, Collins NC (2016) A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biology 16, 100
| A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling.Crossref | GoogleScholarGoogle Scholar | 27101979PubMed |
Sofield I, Evans L, Cook M, Wardlaw IF (1977) Factors influencing the rate and duration of grain filling in wheat. Functional Plant Biology 4, 785–797.
Stone P, Nicolas M (1995) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Functional Plant Biology 22, 927–934.
Talukder A, McDonald GK, Gill GS (2013) Effect of short-term heat stress prior to flowering and at early grain set on the utilization of water-soluble carbohydrate by wheat genotypes. Field Crops Research 147, 1–11.
| Effect of short-term heat stress prior to flowering and at early grain set on the utilization of water-soluble carbohydrate by wheat genotypes.Crossref | GoogleScholarGoogle Scholar |
Talukder A, McDonald GK, Gill GS (2014) Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crops Research 160, 54–63.
| Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Tashiro T, Wardlaw I (1990) The response to high temperature shock and humidity changes prior to and during the early stages of grain development in wheat. Functional Plant Biology 17, 551–561.
Telfer P, Edwards J, Kuchel H, Reinheimer J, Bennett D (2013) ‘Heat stress tolerance of wheat.’ (Grains Research and Development Corporation: Barton, ACT) Available at: http://www.grdc.com.au/Research-and-Development/GRDC-Update-Papers/2013/02/Heat-stress-tolerance-of-wheat [Verified 16 May 2016]
van Herwaarden A, Farquhar G, Angus J, Richards R, Howe G (1998) ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser: I. Biomass, grain yield, and water use. Australian Journal of Agricultural Research 49, 1067–1081.
| ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser: I. Biomass, grain yield, and water use.Crossref | GoogleScholarGoogle Scholar |
Vignjevic M, Wang X, Olesen J, Wollenweber B (2015) Traits in spring wheat cultivars associated with yield loss caused by a heat stress episode after anthesis. Journal Agronomy & Crop Science 201, 32–48.
| Traits in spring wheat cultivars associated with yield loss caused by a heat stress episode after anthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXislWmtA%3D%3D&md5=7fd41ae465ccc21cef6c45588a29e510CAS |
Wardlaw I, Moncur L (1995) The response of wheat to high temperature following anthesis. I. The rate and duration of kernel filling. Functional Plant Biology 22, 391–397.
Wardlaw I, Wrigley C (1994) Heat tolerance in temperate cereals: an overview. Functional Plant Biology 21, 695–703.
Wardlaw I, Sofield I, Cartwright P (1980) Factors limiting the rate of dry matter accumulation in the grain of wheat grown at high temperature. Functional Plant Biology 7, 387–400.
Yemm E, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. The Biochemical Journal 57, 508–514.
| The estimation of carbohydrates in plant extracts by anthrone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2cXlvFOmtw%3D%3D&md5=51b32e5bf16c9fb78c2e5384c4ab834dCAS | 13181867PubMed |
Zahedi M, Jenner CF (2003) Analysis of effects in wheat of high temperature on grain filling attributes estimated from mathematical models of grain filling. The Journal of Agricultural Science 141, 203–212.
| Analysis of effects in wheat of high temperature on grain filling attributes estimated from mathematical models of grain filling.Crossref | GoogleScholarGoogle Scholar |