Rhizosphere bacteria containing 1-aminocyclopropane-1- carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation
Qiyuan Wang A , Ian C. Dodd B , Andrey A. Belimov C and Fan Jiang A DA Beijing Key Laboratory of Gene Resource and Molecular Development, College of Life Sciences, Beijing Normal University, Beijing, 100 875, China.
B The Lancaster Environment Centre, Lancaster University, Lancaster LA1 4YQ, UK.
C All-Russia Research Institute for Agricultural Microbiology, Podbelskogo Sh. 3, Pushkin-8, 196 608, Saint Petersburg, Russian Federation.
D Corresponding author. Email: jiangfan@bnu.edu.cn
Functional Plant Biology 43(2) 161-172 https://doi.org/10.1071/FP15200
Submitted: 17 July 2015 Accepted: 5 November 2015 Published: 6 January 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Although plant salt tolerance has been improved by soil inoculation with rhizobacteria containing the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase (which metabolises ACC, the immediate precursor of the phytohormone ethylene), it is not always clear whether ion homeostasis and plant water relations are affected. When pea (Pisum sativum L. cv. Alderman) was grown with 70 and 130 mM NaCl, the ACC-deaminase containing rhizobacterium Variovorax paradoxus 5C-2 increased total biomass by 25 and 54% respectively. Nutrient flow modelling showed that V. paradoxus 5C-2 increased K uptake and root to shoot K flow, but decreased Na flow and increased Na deposition in roots. Thus, shoot K+ : Na+ ratio increased following V. paradoxus 5C-2 inoculation. At 70 and 130 mM NaCl, rhizobacterial inoculation decreased stomatal resistance by 14 and 31% and decreased xylem balancing pressure by 7 and 21% respectively. Furthermore, rhizobacterial inoculation improved photosynthetic efficiency (Fv/Fm) by 12 and 19% and increased maximal electron transport rate (ETR) by 18 and 22% at 70 and 130 mM NaCl respectively. Thus V. paradoxus 5C-2 mitigates salt stress by improving water relations, ion homeostasis and photosynthesis of pea plants, and may provide an economic means of promoting growth of plants exposed to salt stress.
Additional keywords: ion homeostasis, maximal electron transport rate, nutrient flow modelling, photosynthetic efficiency, water relations.
Introduction
Salinity is a major factor responsible for reducing plant growth and productivity, causing the abandonment of land. The Food and Agriculture Organisation (FAO) reported that more than 800 million ha of land throughout the world were affected by salinity (FAO 2005). Most crops tolerate salinity to a threshold level, but exceeding this level decreases crop productivity due to osmotic and ion-specific effects.
For most glycophytes, salt stress often leads to an increased stomatal resistance, a significant inhibition of photosynthesis and specific ion (Na+) toxicity (Rajasekaran et al. 1998; Munns and Tester 2008; Pandolfi et al. 2012). Salt stress increases external osmotic pressure (Munns 2002), which decreases leaf water potential and turgor, and ultimately causes stomatal closure (Munns and Tester 2008). Na+ in the transpiration stream is transported to the leaves where excessive Na+ accumulates (Munns 2002). Excessive Na+ concentrations in plants result in K+ deficiency and interrupt multiple physiological processes mediated by K+, including protein synthesis, stomatal movement and photosynthesis (Adams and Shin 2014). Excessive accumulation of Na+ and diminished K+ status are the major characteristics of plants under salt stress (Maathuis and Amtmann 1999; Chen et al. 2007).
Unfortunately, transgenic approaches and molecular breeding programs for improving crop tolerance to salt stress have generally not brought promising results in farmers’ fields (Wang et al. 2003; Bhatnagar-Mathur et al. 2008; James et al. 2008) with some notable exceptions (Munns et al. 2006). Therefore, alternative approaches such as the use of soil micro-organisms such as mycorrhizal fungi and plant growth-promoting rhizobacteria (PGPR) may be exploited (Dodd and Pérez-Alfocea 2012). The use of PGPR containing 1-aminocyclopropane-1-carboxylate (ACC) deaminase is one of the most promising approaches to promote growth of plants exposed to salt stress (Mayak et al. 2004; Glick 2014), by diminishing stress-induced ethylene production (Ali et al. 2014). These bacteria hydrolyse ethylene precursor ACC into ammonia and α-ketobutyrate (Glick et al. 1997). Since ethylene inhibits plant growth via multiple mechanisms (Pierik et al. 2006), lowering the ethylene levels can increase growth of plants exposed to environmental stress (Mayak et al. 2004; Barnawal et al. 2012; Ali et al. 2014). However, there are alternative non-hormonal mechanisms by which ACC deaminase containing rhizobacteria can alleviate salt stress and increase growth of their host plants (Mayak et al. 2004; Ali et al. 2014).
Maintaining tissue water status is a key strategy to reduce the harmful impacts of salt stress on plant growth. Some ACC deaminase containing PGPR can increase osmolyte (e.g. proline) accumulation (Bharti et al. 2013) and water uptake (Belimov et al. 2009) under optimal conditions, which may enhance tolerance to salt stress. However, there are few measurements of plant water potential of salinised plants that have been inoculated with ACC deaminase containing PGPR (Mayak et al. 2004; Nadeem et al. 2007, 2010). Although these bacteria can increase stomatal conductance (Jiang et al. 2012) and maintain higher photosynthetic ability (Fv/Fm ratio) of plants grown under optimal conditions (Gamalero et al. 2008), their impacts on stomatal and non-stomatal limitation of photosynthesis of salinised plants are not clear. These rhizobacteria can also maintain ion homeostasis by improving plant N, P and K uptake (Jiang et al. 2012; Safronova et al. 2012) or enhancing the K+ : Na+ ratio (Nadeem et al. 2009; Chang et al. 2014). However, previous measurements of the ionic status of salinised plants inoculated with ACC-deaminase containing PGPR have simply measured foliar Na+ and K+ concentrations (Nadeem et al. 2007, 2010), without considering elemental flows within the entire plant. When there is excessive Na+ in the rhizosphere, the partitioning, cycling and recycling of K+ and Na+ between shoot and root are altered (Wolf et al. 1990). By using the nutrient modelling method, this communication between root and shoot can be studied.
Variovorax paradoxus 5C-2 was described as a root-associated bacterium containing ACC deaminase and promoting plant growth in the presence of toxic cadmium concentrations (Belimov et al. 2005). Previous work has shown that soil inoculation with V. paradoxus 5C-2 benefited plant growth, especially when plants were exposed to drying soil, by influencing multiple physiological processes including decreasing ACC concentrations in the rhizosphere of potato (Belimov et al. 2015) and xylem sap of pea (Belimov et al. 2009), and enhancing the nitrogen fixing symbiosis between pea and rhizobia (Belimov et al. 2009). Enhanced nutrient uptake by pea (Jiang et al. 2012) and decreased concentrations of abscisic acid in tomato seedlings (Belimov et al. 2014) were also observed in inoculated plants. However, whether V. paradoxus 5C-2 can protect plants from the effects of salt stress has not been tested.
To provide new insights into how ACCd-containing PGPR affect growth of salinised pea plants, we used a range of physiological techniques that hitherto have not been used to study plant–PGPR interactions under salt stress. Nutrient flow modelling (Wolf et al. 1990; Pate et al. 1979) was used to determine nutrient deposition, transport and flows in plants (rather than simple instantaneous measurements of nutrient content), while Mini-Pam was used to measure photosynthetic electron transport rates in vivo (Hoshida et al. 2000) and a whole-plant pressure chamber was used to determine xylem balancing pressure (Termaat et al. 1985). We hypothesised that decreased shoot Na+ accumulation (due to increased Na+ deposition in roots and decreased xylem Na+ export from roots) benefited photosynthesis, thereby mitigating salt stress in pea plants.
Materials and methods
Bacterial culture and salt tolerance
The PGPR strain Variovorax paradoxus 5C-2 containing ACC deaminase (Belimov et al. 2005) was obtained from the Russian Collection of Agricultural Microorganisms (St Petersburg, Russian Federation) and maintained on Bacto-Pseudomonas F (BPF) medium as previously described (Belimov et al. 2005). Briefly, bacteria were incubated on agar BPF medium for 72 h at 28°C, cells were scraped from the agar surface (to minimise transfer of nutrient-rich agar to the pots) and suspended to a final concentration of 108 cells ml–1 in a nutrient solution (μM): KNO3, 2800; Ca(NO3)2•4H2O, 1600; MgSO4•7H2O, 1000; NH4NO3, 2000; NaH2PO4, 600; and microelements NaFeEDTA, 40; H3BO3, 10; ZnSO4, 2; MnSO4•4H2O, 2; CuSO4•5H2O, 0.5; Co(NO3)2•6H2O, 0.2; H2MoO4, 0.08.
To determine salt tolerance of V. paradoxus 5C-2, bacteria were incubated for 5 days at 28°C on agar BPF medium supplemented with increasing NaCl concentrations in steps of 20 mM. Threshold growth-inhibitory and threshold lethal concentrations of NaCl were estimated visually.
ACC deaminase activity of V. paradoxus 5C-2 in the presence of toxic NaCl concentrations was determined by monitoring the amount of α-ketobytyrate (αKB) generated enzymatically via hydrolysis of ACC (Saleh and Glick 2001) as previously described (Safronova et al. 2012). The protein concentration of disrupted cell suspensions was determined by the method of Bradford (1976) using the Bio-Rad protein reagent (Bio-Rad Laboratory, Hercules, CA, USA).
Plant culture and growth conditions
Pea (Pisum sativum L. cv. Alderman) seeds (Moles Seeds, Colchester, UK) were selected for homogeneity of seed weight, then surface-sterilised with 10% H2O2 for 10 min, rinsed carefully with sterile water, and germinated in well washed quartz sand and irrigated daily with distilled water. Seven-day-old seedlings were washed with sterile water to remove quartz sand from the roots. Afterwards, plants of similar size and developmental stage were transplanted individually (one plant per pot) into 4 L plastic pots (19 cm diameter, 14 cm height) containing carefully washed quartz sand. Each group was watered daily with half-strength nutrient solution for 5 days, then full-strength nutrient solution supplied. The experiment was carried out in a naturally lit glasshouse. The temperature was 12°C (night) and 27°C (day) and photosynthetically active radiation (at noon) was 1300 μmol m–2 s–1. Ten days after transplanting, plants were divided into six groups. In groups 1–2 seedlings were irrigated daily with full-strength nutrient solution without NaCl. Seedlings in groups 3–4 and 5–6 were irrigated daily with full-strength nutrient solution containing 70 mM and 130 mM NaCl, respectively. Meanwhile, plants in groups 2, 4 and 6 were additionally supplied with 4 mL of a suspension of V. paradoxus 5C-2 (108 cells mL–1) every 4 days, and irrigated on intervening days with nutrient solutions at the required salt concentrations. In comparison, field experiments with this organism have applied single doses of 1010 cells mL–1 at transplanting (Teijeiro et al. 2011).
Leaf physiology
At the end of the study (30 days after transplanting, 20 days after inoculation of V. paradoxus 5C-2 and salt treatments), leaf stomatal resistance of fully expanded leaves was measured with a transient-time porometer (Model AP4, Delta-T Devices, Cambridge, UK) between 0900 and 1100 hours.
The same leaves were used to quantify components of chlorophyll fluorescence in situ with a portable modulated fluorometer (Mini-Pam Photosynthesis Yield Analyser; Walz, Effeltrich, Germany) (Bilger et al. 1995). Maximal fluorescence, Fm, was measured after a 0.8 s saturating white light pulse (2318 μmol m–2 s–1) and the minimal fluorescence, F0, was measured after 30 min dark-adaptation. Maximal variable fluorescence was calculated as Fv = Fm – F0. The PSII photochemical efficiency was calculated as Fv/Fm. The efficiency of electron transport rate (ETR) was also calculated as previously described (Genty et al. 1989). Rapid light curves (RLCs) were measured by a software controlled protocol with 10s illumination times and intensities increasing in eight steps (Bilger et al. 1995).
Measurement of transpiration and xylem balancing pressure
Whole-plant transpiration was measured gravimetrically daily (before and after the daily addition of nutrient solution and drainage) by weighing pots of each treatment during the last six days of the study period (from 25 days after transplanting to 30 days after transplanting). Whole plant transpiration was summed during the last 6 days of the experiment, with corrections applied for the water loss from pots without plants (Jiang et al. 2001).
Xylem balancing pressure of 2–3 pea plants was measured (once per plant) each day during the last 10 days of the study period (from 21 to 30 days after transplanting). The entire pot (including the plant) was placed in the pressure chamber (25 cm in diameter and 16 cm in length) and external pressure applied until the appearance of the first drop of xylem sap on the cut surface of the youngest fully expanded leaf, which was recorded as the xylem balancing pressure (Liang et al. 1996). Since preliminary experiments had established that additional overpressure did not yield sufficient xylem sap for ionic analysis, the entire shoot was removed at the stem base to facilitate xylem sap collection from the roots. All xylem sap samples were immediately frozen in liquid nitrogen after collection and stored at −20°C.
Plant harvests and ion analysis
At the beginning and the end of the study (20 and 30 days after transplanting, 10 and 20 days after inoculation of V. paradoxus 5C-2 and salt treatments), FW was determined, then all samples were frozen in liquid nitrogen. Dry tissues were weighed after being lyophilised, then ground into powder and kept for further analyses. Ionic composition of different tissues (and xylem sap) were analysed using an ICP-OES (inductively coupled plasma-optical emission spectrometer - JY Plus, Division d’ Instruments SA, Longjumeau, France).
Modelling plant internal flows
Based on the assumption that calcium is transported in the xylem only and mass flow occurs in the xylem, net xylem potassium flow and sodium flow (μmol plant–1) from root to shoot (JK,X) and (JNa,X) were calculated from the ratio of potassium to calcium (K : Ca)X and (Na : Ca)X in xylem sap and the increment of calcium in the shoot, ΔCa (Armstrong and Kirkby 1979):
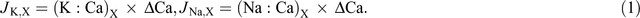
Net potassium flow and sodium flow in the phloem (JK,P) and (JNa,P) were calculated from the difference between the potassium increment ΔK and sodium increment ΔNa in each organ and the net xylem import to the organ, Jk,X and JNa,X;

The content of each element in the organs in μmol plant–1 and increments in moles per plant over the study period were then calculated from the element concentrations and the DW (Jiang et al. 2012).
Bacterial root colonisation
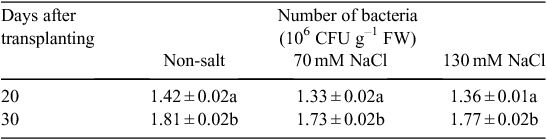
To determine the persistence of V. paradoxus 5C-2 on the root surface, bacterial colonisation was assayed at the beginning and the end of the study (20 and 30 days after transplanting, corresponding to 10 and 20 days after inoculation of V. paradoxus 5C-2 and salt treatments). Roots were removed from the pots in each treatment group and shaken gently to remove adhering sand particles. Main roots and lateral roots were homogenised in sterile tap water with a sterile mortar and pestle, the homogenates serially diluted in 10-fold steps, and 50 μL aliquots plated in duplicate on BPF agar supplemented with rifampicin 20 mg L–1, kanamycin 30 mg L–1 (to which V. paradoxus 5C-2 shows resistance) and nystatin 40 mg L–1 (to prevent the growth of fungi). Then the numbers of colony forming units (CFU) were counted after incubation for 4 days at 28°C.
Statistics
Two-way analysis of variance (ANOVA) was performed to determine effects of salt, inoculation and their interactions, using SPSS version 19 (SPSS, Chicago, IL, USA). One-way ANOVA with Tukey’s test (P < 0.05) was used to discriminate means. Analysis of correlations was made using the Microsoft Excel statistical software (Microsoft Corporation, Seattle, WA, USA).
Results
Incubating V. paradoxus 5C-2 on NaCl-supplemented agar showed that this strain possessed threshold growth-inhibitory and threshold lethal concentrations of 140 and 280 mM NaCl respectively. This is consistent with the salt treatments (70 and 130 mM NaCl) having no significant effects on root colonisation of V. paradoxus 5C-2 (Table 1). Moreover, these salt concentrations had no inhibitory effect on ACC deaminase activity of V. paradoxus 5C-2 in vitro, but a higher salt concentration (260 mM) significantly decreased ACCd activity (Fig. 1). A residual amount of αKB was detected in the presence of 520 mM NaCl, probably due to the activity of a trace amount of ACC deaminase in the bacterial inoculum.
|

|
Salt stress significantly (P < 0.05) decreased whole plant biomass, by 34% and 60% at 70 and 130 mM NaCl respectively (Fig. 2). V. paradoxus 5C-2 improved plant growth significantly independently of salt treatment (no significant salt × inoculation interaction), with inoculation increasing whole plant biomass by 36% at 70 mM NaCl and by 58% at 130 mM NaCl (Fig. 2). Salinity stress inhibited shoot growth more strongly than root growth. Shoot growth of uninoculated plants was reduced by 36 and 62% when treated with 70 mM and 130 mM NaCl, respectively, but by only 30 and 48% in inoculated plants, respectively. Although 70 mM NaCl had no significant impact (P < 0.05) on root biomass, 130 mM NaCl decreased root biomass of non-inoculated plants by 29%. In contrast, root biomass of inoculated plants grown at 130 mM NaCl was not statistically different to uninoculated plants grown in the absence of salt.
Salt significantly decreased whole plant transpiration by 44 and 65% at 70 and 130 mM NaCl respectively (Table 2). Inoculation with V. paradoxus 5C-2 significantly increased transpiration by 31, 42 and 85% at 0, 70 and 130 mM NaCl respectively (Table 2).
The effect of salt on stomatal resistance was moderated by V. paradoxus 5C-2 inoculation, as indicated by a significant salt × inoculation interaction (Fig. 3a). Inoculation of V. paradoxus 5C-2 decreased stomatal resistance by 19, 14 and 31% when 0, 70 and 130 mM NaCl was applied respectively. In contrast, only salinity had a significant (P < 0.05) effect on xylem balancing pressure (Fig. 3b). Nevertheless, V. paradoxus 5C-2 inoculation decreased the xylem balancing pressure by 9, 7 and 21% when 0, 70 and 130 mM NaCl was applied respectively.
In all non-inoculated plants, salt stress significantly (P < 0.05) increased shoot and root Na+ concentrations at both the beginning and the end of the study period (Fig. 4a–d). Over the same interval, V. paradoxus 5C-2 inoculation significantly decreased shoot Na+ concentrations at both NaCl levels. Importantly, rhizobacterial effects on limiting shoot Na+ concentration were greater with increasing salt concentration (as indicated by a significant salt × inoculation interaction).
Although salt stress significantly decreased both root and shoot K+ concentrations (P < 0.05) when measured at the beginning and end of the study period, rhizobacterial inoculation had no significant effect. Averaged across both measurement occasions, 70 and 130 mM NaCl decreased shoot K+ concentrations by 7 and 10%, and root K+ concentrations by 30 and 45% respectively (Fig. 4e–h).
In both salt treatments, the K+ : Na+ ratio was decreased in both shoots and roots (P < 0.05) (Fig. 4i–l). In comparison, rhizobacterial inoculation significantly increased the K+ : Na+ ratios in shoot tissues (Fig. 4i, j) whereas no significant differences were observed in root tissues. In shoot tissues of salinised plants, the inoculated plants grown at 70 mM NaCl had the highest K+ : Na+ ratio and non-inoculated plants grown at 130 mM NaCl had the lowest.
The effects of salinity and the inoculation of V. paradoxus 5C-2 on K, Na, Ca, Mg and P accumulation are shown in Table 2. Two-way ANOVA showed that both salt and rhizobacterial inoculation had a significant (P < 0.05) effect on accumulation of total K, Na, Ca, Mg and P in whole plant (Table 2). Inoculation with V. paradoxus 5C-2 increased the accumulation of Ca, Mg and P in 70 mM NaCl-stressed plants by 52, 167 and 23% respectively (Table 2).
Rhizobacterial effects on nutrient budgets were investigated by constructing flow models of K and Na (Fig. 5). Salt stress decreased K flows from root to shoot in xylem by 5 and 92% in 70 and 130 mM NaCl, respectively (Fig. 5a, c, e). Rhizobacterial inoculation increased total K uptake by 26 and 28% at 0 and 70 mM NaCl (Fig. 5b, d). Rhizobacterial inoculation increased xylem K flows by 14, 42 and 220% and increased phloem K flows by 45, 59 and 19% at 0, 70 and 130 mM NaCl treatment respectively (Fig. 5a–f). Nevertheless, phloem export exceeded root K+ deposition in the roots, indicating K recycling back to the shoot. Under salt stress, K+ uptake and K+ retrieved from roots were allocated to shoot. Interestingly, at 130 mM NaCl treatment, 20 μmol K was effluxed from the root of non-inoculated plants (Fig. 5e), which may be due to cell membrane damage caused by high salt stress (Cuin and Shabala 2005; Shabala and Cuin 2008).
In non-inoculated plants, salt clearly increased total Na uptake by 15- and 19-fold at 70 and 130 mM NaCl respectively (Fig. 5g, i, k). Even greater changes were detected in xylem sap Na+ concentration, which increased from 0.35 to 6 to 197 mM at 0, 70 and 130 mM external NaCl respectively (Table 3). We noted that rhizobacterial inoculation increased root Na deposition and decreased Na flow from root to shoot by 17 and 9%. Moreover, such inoculation decreased shoot Na+ deposition by 13 and 6% under 70 and 130 mM NaCl respectively (Fig. 5i, j), which may be related to enhanced performance in the presence of salt. Rhizobacterial inoculation had no statistically significant effect on xylem sap Na+ concentration at 0 and 70 mM NaCl, but at 130 mM NaCl decreased it by 65% (Table 3).

|
All measured chlorophyll fluorescence variables were significantly, independently affected by both salt stress and rhizobacterial inoculation. Salt treatments increased F0 accompanied by a decrease in Fm; however, inoculated plants had higher Fm with a lower F0 (Fig. 6a, b). In uninoculated plants, Fv/Fm ratio significantly decreased (P < 0.05) by 12 and 24% when 70 and 130 mM NaCl was applied, whereas inoculation significantly increased the Fv/Fm ratio by 12 and 19%, respectively (Fig. 6c), at these salt concentrations.
Salt treatments decreased PSII ETR, especially at 130 mM NaCl. The maximal PSII ETR decreased by 16 and 64% when 70 and 130 mM NaCl was applied respectively. However, rhizobacterial inoculation increased the maximal PSII ETR by 18 and 22% of at 70 and 130 mM NaCl respectively (Fig. 7a–c). Even in non-stressed plants, rhizobacterial inoculation increased ETR.
Significant negative correlations (P < 0.01) were found between chlorophyll fluorescence parameters (Fv/Fm, maximal ETR) and stomatal resistance (r = –0.8, –0.94), shoot Na+ concentration (r = –0.81, –0.92) and xylem balancing pressure (r = –0.85, –0.76) (Table 4). Stomatal resistance was significantly (P < 0.01) positively correlated with both shoot Na+ concentration (r = 0.93) and xylem balancing pressure (r = 0.75). Xylem balancing pressure was also significantly positively correlated (P < 0.01) with shoot Na+ concentration (r = 0.8) (Table 4).
Discussion
A recurrent question with the use of PGPR to enhance plant growth is whether their effects are consistent in a range of environments (Dodd and Ruiz-Lozano 2012), or are magnified under specific stresses. Although V. paradoxus 5C-2 enhanced plant growth similarly under all salt treatments (Fig. 2 - as indicated by a non-significant inoculation × salt interaction), it seems that the relative importance of some physiological mechanisms differed according to the salinity level. Notably, shoot Na+ concentration was alleviated by V. paradoxus 5C-2 at high salinity (Fig. 4a, b – as indicated by a significant inoculation × salt interaction), as was the salinity-induced increase of stomatal resistance (Fig. 3a). Although the highly significant correlation between shoot Na+ concentration and stomatal resistance (Table 4) suggests a potential regulatory mechanism whereby apoplastic Na+ concentration causes stomatal closure (Perera et al. 1995), other factors may also regulate stomatal responses. Although stomatal closure acts to limit transpiration and maintain leaf water status, xylem balancing pressure (Fig. 3b) increased in response to salinity as reported previously (Termaat et al. 1985). Inoculation with V. paradoxus 5C-2 decreased xylem balancing pressure (Fig. 3b), which may result from increased root hydraulic conductance (L), since many rhizobacteria can increase L under drought and salinity (Groppa et al. 2012). This is especially likely in ACCd-containing rhizobacteria such as V. paradoxus 5C-2 that decrease root ethylene synthesis, since increased ethylene synthesis may limit hydraulic conductance by inhibiting aquaporin activity (Li et al. 2009).
Although the correlation of xylem balancing pressure with stomatal resistance (Table 4) may be causative, maintaining salinised plants at full turgor (xylem balancing pressure was set to ensure sap was on the verge of bleeding from an incision in the leaf) via root pressurisation did not alleviate salt-induced restriction of transpiration (Termaat et al. 1985), suggesting non-hydraulic regulation of stomatal responses. Indeed, well fertilised pea plants inoculated with V. paradoxus 5C-2 had significantly lower root ABA concentrations and enhanced ABA degradation in lower leaves, which likely explained the lower stomatal resistance of inoculated plants (Jiang et al. 2012). V. paradoxus 5C-2 also decreased shoot ABA concentrations of tomato seedlings grown in vitro (Belimov et al. 2014). Attenuation of liming-induced stomatal closure in the ABA-deficient wilty pea mutant (Rothwell et al. 2015) suggests an important role for ABA in mediating stomatal responses of pea to changes in rhizosphere elemental status, requiring further studies in salinised plants. Nevertheless, distinguishing the relative importance of phytohormonal and nutritional effects on stomatal closure can be challenging (Rothwell et al. 2015).
Although increased shoot K+ : Na+ ratio in response to ACC-deaminase containing PGPR has been demonstrated previously (Nadeem et al. 2007; Nadeem et al. 2010), our study showed that it was at least partially due to increased deposition of Na+ in roots (Fig. 5). In contrast, although root K+ uptake increased significantly (Table 2), inoculation did not greatly improve K+ concentrations in plant tissues, likely due to the diluting effect of the increased plant biomass. Nutrient modelling (Fig. 5) has allowed the evaluation of dynamic flows and partitioning of K+ and Na+ via phloem and xylem during the study period. Rhizobacterial inoculation increased K+ uptake by roots and xylem flows of K+ from root to shoot, which provided a sufficient supply of potassium via xylem (Fig. 5a–f) to sustain K+ deposition in the shoot and a higher growth rate. When plants were salt stressed, a negative K+ accumulation in roots indicated that K was remobilised from roots into xylem and transported into shoots.
Salt stress increased Na+ uptake by 15- and 19-fold at 70 and 130 mM NaCl treatments respectively. Although inoculation also increased Na+ uptake slightly, the xylem stream became depleted in Na+ due to increased Na+ deposition in roots (Fig. 5g–l). The increased Na+ deposition in roots of inoculated plants may be due to changes in gene expression (such as the sodium transporters HKT1 and SOS1) (Shi et al. 2002; Zhang et al. 2008). Under NaCl stress, inoculation decreased Na+ deposition in shoot tissue, which is simply due to the decreased xylem Na+ flow from root to shoot, despite lower stomatal resistance. Future studies need to consider both the molecular regulation of Na+ uptake and xylem loading of Na+ in inoculated pea roots.
Besides the positive effects of V. paradoxus 5C-2 on the K and Na balance, the bacterium also enhanced the uptake of Ca, Mg and P (Table 2). Similarly, Lotus edulis plants inoculated with V. paradoxus 5C-2 and grown in heavy metal contaminated soil had increased Ca, Mg and P content and increased shoot accumulation of several nutrient elements including K (Safronova et al. 2012), which may be simply due to improved root growth. Improved uptake of K and other nutrients by the plants inoculated with V. paradoxus 5C-2 may enhance photosynthesis.
As is well known, salinity induced stomatal closure by an osmotic stress and displaced essential cations from the endo-membrane structure and degraded thylakoid membrane proteins (Flowers and Yeo 1981), thereby causing photoinhibition. In our study, salt stress decreased photosynthetic efficiency (Fv/Fm) and ETR of pea plants while inoculation with V. paradoxus 5C-2 mitigated these declines (Figs 6, 7). Limitation of Na+ transport into, and accumulation in, the shoot (Fig. 5) may also contribute to the enhanced photosynthesis by alleviating the damage of photosynthetic apparatus caused by excessive Na+ (Seemann and Critchley 1985). Although chlorophyll fluorescence is correlated with both shoot Na+ concentration and stomatal resistance, resolving the contributions of both stomatal and non-stomatal limitations to photosynthesis requires further work (Table 4).
Decreased photosynthesis in response to salt stress might be expected to decrease carbohydrate flow to the root system and root exudation, thereby limiting the establishment of symbiotic plant/microbe interactions in the rhizosphere. Although it is well known that high salinity can decrease nodulation of legumes by rhizobia (Steinborn and Roughley 1974; Steil et al. 2003; Barea et al. 2005), effects of salinity on associative PGPR inhabiting the rhizosphere may be variable. Although high salinity increased root colonisation of lettuce with PGPR Pseudomonas mendocina (Steil et al. 2003), pea root colonisation of V. paradoxus 5C-2 was independent of salinity level (Table 1), similar to experiments where soil drying had no effect on, or even increased, colonisation of V. paradoxus 5C-2 (Belimov et al. 2009). Salinity-induced increases of root ACC concentration (Albacete et al. 2008) may enhance root ACC efflux thereby increasing substrate availability for ACC-deaminase containing PGPR. Indeed, several such rhizobacteria were recently shown to decrease rhizosphere ACC concentration (Belimov et al. 2015) but separating the contributions of root ACC efflux from bacterial ACC utilisation remains challenging.
Conclusions
Although ACC-deaminase containing rhizobacteria have previously been shown to improve plant growth under salt stress (Mayak et al. 2004; Bal et al. 2013; Qin et al. 2014), this study is the first to investigate ion homeostasis using nutrient flow modelling, and to demonstrate both stomatal and non-stomatal (Fv/Fm and ETR) effects of ACC-deaminase containing rhizobacteria on photosynthesis. Although the relative importance of these different mechanisms remains to be established, V. paradoxus 5C-2 may be an important and economic means of decreasing the deleterious effects of saline soils on plant growth.
Acknowledgements
We are very grateful to Professor Pu Mou for the valuable advice on the statistics analysis. Financial support was provided to FJ by the Fundamental Research Funds for the Central Universities (2015KJJCB22), the National Natural Science Foundation of China (31421063) and the 111 Project (B13008). This work on bacterial tolerance to NaCl and ACC deaminase activity was supported by the Russian Science Foundation (14-16-00137).
References
Adams E, Shin R (2014) Transport, signaling, and homeostasis of potassium and sodium in plants. Journal of Integrative Plant Biology 56, 231–249.| Transport, signaling, and homeostasis of potassium and sodium in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktlOitL4%3D&md5=60850a7172cd96050ec991b3e5c5e613CAS | 24393374PubMed |
Albacete A, Ghanem ME, Matínez-Andújar C, Acosta M, Sánchez-Bravo J, Matínez V, Lutts S, Dodd IC, Pérez-Alfocea F (2008) Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. Journal of Experimental Botany 59, 4119–4131.
| Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCgt73M&md5=fd6ca33f07a542919fb7a39d053c9994CAS | 19036841PubMed |
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiology and Biochemistry 80, 160–167.
| Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpsl2mt7w%3D&md5=23da92f9b9975229b48558d3eb8674aeCAS | 24769617PubMed |
Armstrong MJ, Kirkby EA (1979) Estimation of potassium recirculation in tomato plants by comparison of the rates of potassium and calcium accumulation in the tops with their fluxes in the xylem stream. Plant Physiology 63, 1143–1148.
| Estimation of potassium recirculation in tomato plants by comparison of the rates of potassium and calcium accumulation in the tops with their fluxes in the xylem stream.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXksFarsbc%3D&md5=5ca3ee78ad96739d56cf839e48c71bc9CAS | 16660872PubMed |
Bal HB, Nayak L, Das S, Adhya TK (2013) Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant and Soil 366, 93–105.
| Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmtlCqt7g%3D&md5=209677ac3d66671c0fed88436fc05d69CAS |
Barea JM, Pozo MJ, Azcon R, Azcon-Aguilar C (2005) Microbial cooperation in the rhizosphere. Journal of Experimental Botany 56, 1761–1778.
| Microbial cooperation in the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpvFKnt7g%3D&md5=919f79743f5980b61fc554dc55963310CAS | 15911555PubMed |
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiology and Biochemistry 58, 227–235.
| 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1emsrjN&md5=fdc7b8e2a4fe423cc5b944df4607dfbaCAS | 22846334PubMed |
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biology & Biochemistry 37, 241–250.
| Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVahu77L&md5=fb29a8c8732ac66fc3203eaab8681ac4CAS |
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronovn VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytologist 181, 413–423.
| Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvV2nsLY%3D&md5=2702ea5cfeade6f0729d37b8f4bd3d10CAS | 19121036PubMed |
Belimov AA, Dodd IC, Safronova VI, Dumova VA, Shaposhnikov AI, Ladatko AG, Davies WJ (2014) Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiology and Biochemistry 74, 84–91.
| Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtF2jsr4%3D&md5=b47c059924e33132bc464a970e04fc3cCAS | 24270514PubMed |
Belimov AA, Dodd IC, Safronova VI, Shaposhnikov AI, Azarova TS, Makarova NM, Davies WJ, Tikhonovich IA (2015) Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Annals of Applied Biology 167, 11–25.
| Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXps12gu7o%3D&md5=ee23034feca265381c3193350df4b8a3CAS |
Bharti N, Deepti Y, Deepti B, Deepamala M, Alok K (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World Journal of Microbiology & Biotechnology 29, 379–387.
| Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptlSqtw%3D%3D&md5=d1f2cc5dd923643ed3062188b43bd268CAS |
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Reports 27, 411–424.
| Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvFygt7k%3D&md5=405f112ae90ea1ae3fd1156830d6cf31CAS | 18026957PubMed |
Bilger W, Schreiber U, Bock M (1995) Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 102, 425–432.
| Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field.Crossref | GoogleScholarGoogle Scholar |
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.
| A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksVehtrY%3D&md5=436242ae9b449e7335b086f00b7e6e5fCAS | 942051PubMed |
Chang P, Gerhardt KE, Huang XD, Yu XM, Glick BR, Gerwing PD, Greenberg BM (2014) Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils. International Journal of Phytoremediation 16, 1133–1147.
| Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1SjtbfF&md5=01ab4a58dcee803c996861ae7d45818fCAS | 24933907PubMed |
Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang G, Shabala S (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Functional Plant Biology 34, 150–162.
| Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhs1yqsbk%3D&md5=4677086b37e605b7d7c1f0739fcc8ef9CAS |
Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant & Cell Physiology 46, 1924–1933.
| Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsVOiug%3D%3D&md5=6216d91ba6cd0c059d20f170efe4accdCAS |
Dodd IC, Pérez-Alfocea F (2012) Microbial amelioration of crop salinity stress. Journal of Experimental Botany 63, 3415–3428.
| Microbial amelioration of crop salinity stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVSitLg%3D&md5=e7ec0fe065826f289e02d2e556aa8e55CAS | 22403432PubMed |
Dodd IC, Ruiz-Lozano JM (2012) Microbial enhancement of crop resource use efficiency. Current Opinion in Biotechnology 23, 236–242.
| Microbial enhancement of crop resource use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltVKnt7s%3D&md5=e56c68dbd047578b90b86ccb132b7703CAS | 21982722PubMed |
FAO (2005) ‘Global network on integrated soil management for sustainable use of salt-affected soils.’ (FAO Land and Plant Nutrition Management Service: Rome, Italy) Available at: http://www.fao.org/ag/agl/agll/spush [Verified 30 November 2015].
Flowers T, Yeo A (1981) Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytologist 88, 363–373.
| Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXlt12ksL8%3D&md5=07f6f660ac61f4f12e7ba55373004434CAS |
Gamalero E, Berta G, Massa N, Glick BR, Lingua G (2008) Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiology Ecology 64, 459–467.
| Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFajtbo%3D&md5=ca16b47ac4f7ef0270805c0c3d8eb0beCAS | 18400004PubMed |
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA) – General Subjects 990, 87–92.
| The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhsFWntL4%3D&md5=b425fb11aa4db57339c98862210509dfCAS |
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiological Research 169, 30–39.
| Bacteria with ACC deaminase can promote plant growth and help to feed the world.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFOnsr3K&md5=727e08d4caae0a437ab31d3b7c1dbe5bCAS | 24095256PubMed |
Glick BR, Liu C, Ghosh S, Dumbroff EB (1997) Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12–2. Soil Biology & Biochemistry 29, 1233–1239.
| Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12–2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksFCnsrY%3D&md5=aa5eef24522177f4b530ce4bd72480a3CAS |
Groppa MD, Benavides MP, Zawoznik MS (2012) Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: a revision. Applied Soil Ecology 61, 247–254.
| Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: a revision.Crossref | GoogleScholarGoogle Scholar |
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tananka A, Takabe T, Takabe T (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Molecular Biology 43, 103–111.
| Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlvVCjsL0%3D&md5=69ca51dfe853747d5b3b8b2d9ec71836CAS | 10949377PubMed |
James RA, von Caemmerer S, Condon AT, Zwart AB, Munns R (2008) Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat. Functional Plant Biology 35, 111–123.
| Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVKktbY%3D&md5=59992d44908cac0c061aadb434fac6e3CAS |
Jiang F, Li C, Jeschke WD, Zhang F (2001) Effect of top excision and replacement by 1‐naphthylacetic acid on partition and flow of potassium in tobacco plants. Journal of Experimental Botany 52, 2143–2150.
Jiang F, Chen L, Belimov AA, Shaposhnikov AI, Gong F, Meng X, Hartung W, Jeschke DW, Davies WJ, Dodd IC (2012) Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C–2 on nutrient and ABA relations of Pisum sativum. Journal of Experimental Botany 63, 6421–6430.
| Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C–2 on nutrient and ABA relations of Pisum sativum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslaktrbE&md5=16785c379b667d58b67eb89acbac48e4CAS | 23136167PubMed |
Li YS, Mao XT, Tian QY, Li LH, Zhang WH (2009) Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environmental and Experimental Botany 67, 172–177.
| Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2qs7rF&md5=e0ae77637f34be654da03ba48bc70668CAS |
Liang J, Zhang J, Wong MH (1996) Stomatal conductance in relation to xylem sap abscisic acid concentrations in two tropical trees, Acacia confusa and Litsea glutinosa. Plant, Cell & Environment 19, 93–100.
| Stomatal conductance in relation to xylem sap abscisic acid concentrations in two tropical trees, Acacia confusa and Litsea glutinosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhs1ejtbk%3D&md5=afa64f3efff21c4c4c4a70a8224400a6CAS |
Maathuis FJ, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany 84, 123–133.
| K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVCgtL4%3D&md5=7b0272dc18f4dea0e544b93e8adad5f5CAS |
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiology and Biochemistry 42, 565–572.
| Plant growth-promoting bacteria confer resistance in tomato plants to salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFyrsrw%3D&md5=27862aedca1fc85f0d43cb90e3211542CAS | 15246071PubMed |
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell & Environment 25, 239–250.
| Comparative physiology of salt and water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhslakurw%3D&md5=ec575b5a94258a1c6a8d564f45b9464eCAS |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=dd68a82a6d2c6414e1266213f98edac3CAS | 18444910PubMed |
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany 57, 1025–1043.
| Approaches to increasing the salt tolerance of wheat and other cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xis1GlsrY%3D&md5=1d6a1d640c63bd0d75ff2231876f67ddCAS | 16510517PubMed |
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2007) Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Canadian Journal of Microbiology 53, 1141–1149.
| Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWnurjL&md5=9838e6457e9602c2c6f54982e27c0b2eCAS | 18026206PubMed |
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2009) Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Canadian Journal of Microbiology 55, 1302–1309.
| Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVOhurvN&md5=4eba33e79b58ffd488015b394824c58eCAS | 19940939PubMed |
Nadeem SM, Zahir ZA, Muhammad N, Asghar HN, Muhammad A (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Science Society of America Journal 74, 533–542.
| Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat.Crossref | GoogleScholarGoogle Scholar |
Pandolfi C, Mancuso S, Shabala S (2012) Physiology of acclimation to salinity stress in pea pisum sativum. Environmental and Experimental Botany 84, 44–51.
| Physiology of acclimation to salinity stress in pea pisum sativum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtV2itrnN&md5=0e9d98439d49e949e669e7d5948245c1CAS |
Pate JS, Atkins CA, Hamel K, McNeil DL, Layzell DB (1979) Transport of organic solutes in phloem and xylem of a nodulated legume. Plant Physiology 63, 1082–1088.
| Transport of organic solutes in phloem and xylem of a nodulated legume.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXkslWrurk%3D&md5=a326942d9c1ac0268e5cf9d94456de2dCAS | 16660861PubMed |
Perera NH, Hartmann E, Holaday AS (1995) Regulation of cotton photosynthesis during moderate chilling. Plant Science 111, 133–143.
| Regulation of cotton photosynthesis during moderate chilling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXptVemtbk%3D&md5=0d969a148868decd3327ab6ee62c2d97CAS |
Pierik R, Tholen D, Poorter H, Visser EJ, Voesenek LA (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends in Plant Science 11, 176–183.
| The Janus face of ethylene: growth inhibition and stimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvVGnsLo%3D&md5=f2fb876cffaa5f6454eed42d48091d89CAS | 16531097PubMed |
Qin S, Zhang YJ, Yuan B, Xu PY, Xing K, Wang J, Jiang JH (2014) Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant and Soil 374, 753–766.
| Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFKjtrzP&md5=5549db542737c670d88b5bd30960d31dCAS |
Rajasekaran LR, Kriedemann PE, Aspinall D, Paleg LG (1998) Physiological significance of proline and glycinebetaine: maintaining photosynthesis during NaCl stress in wheat. Photosynthetica 34, 357–366.
| Physiological significance of proline and glycinebetaine: maintaining photosynthesis during NaCl stress in wheat.Crossref | GoogleScholarGoogle Scholar |
Rothwell SA, Elphinstone ED, Dodd IC (2015) Liming can decrease legume crop yield and leaf gas exchange by enhancing root to shoot ABA signalling. Journal of Experimental Botany 66, 2335–2345.
| Liming can decrease legume crop yield and leaf gas exchange by enhancing root to shoot ABA signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitVOitb7K&md5=a5abebd20da6c778183932d704c962f2CAS | 25740925PubMed |
Safronova VI, Piluzza G, Zinovkina NY, Kimeklis AK, Belimov AA, Bullitta S (2012) Relationships between pasture legumes, rhizobacteria and nodule bacteria in heavy metal polluted mine waste of SW Sardinia. Symbiosis 58, 149–159.
| Relationships between pasture legumes, rhizobacteria and nodule bacteria in heavy metal polluted mine waste of SW Sardinia.Crossref | GoogleScholarGoogle Scholar |
Saleh SS, Glick BR (2001) Involvement of gasS and pros in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and UW4. Canadian Journal of Microbiology 47, 698–705.
| Involvement of gasS and pros in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and UW4.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnt12gur0%3D&md5=0299788aa732be9d34912bfd29e3798fCAS | 11575495PubMed |
Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164, 151–162.
| Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXktlGjs7o%3D&md5=4c98fcd9b6f7873e75fcde5629cb6da9CAS | 24249556PubMed |
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669.
| Potassium transport and plant salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit70%3D&md5=e3b7086a1c0639a11d71b2068d781bd9CAS | 18724408PubMed |
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. The Plant Cell 14, 465–477.
| The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVKgur0%3D&md5=4148f05c0b4a9c726f08d266eaae764eCAS | 11884687PubMed |
Steil L, Hoffmann T, Budde I, Völker U, Bremer E (2003) Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. Journal of Bacteriology 185, 6358–6370.
| Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXosV2ktr0%3D&md5=1cb46510fe86073d4ea097aad4afca8aCAS | 14563871PubMed |
Steinborn J, Roughley RJ (1974) Sodium chloride as a cause of low numbers of rhizobium in legume inoculants. Journal of Applied Bacteriology 37, 93–99.
| Sodium chloride as a cause of low numbers of rhizobium in legume inoculants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXkslCrs74%3D&md5=b59b89fbbee006278f62df2f5ad5b9fbCAS | 4846742PubMed |
Teijeiro RG, Dodd IC, Elphinstone ED, Safronova VI, Belimov AA (2011) From seed to salad: impacts of ACC deaminase-containing plant growth promoting rhizobacteria on lettuce growth and development. Acta Horticulturae 898, 245–252.
| From seed to salad: impacts of ACC deaminase-containing plant growth promoting rhizobacteria on lettuce growth and development.Crossref | GoogleScholarGoogle Scholar |
Termaat A, Passioura JB, Munns R (1985) Shoot turgor does not limit shoot growth of NaCl-affected wheat and barley. Plant Physiology 77, 869–872.
| Shoot turgor does not limit shoot growth of NaCl-affected wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXitFymsrs%3D&md5=8db6b000c8e902a7d59877bd879a8a96CAS | 16664152PubMed |
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14.
| Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovV2ltbo%3D&md5=672213265881cc71567e5478f7f0c669CAS | 14513379PubMed |
Wolf O, Munns R, Tonnet ML, Jeschke WD (1990) Concentrations and transport of solutes in xylem and phloem along the leaf axis of NaCl-treated Hordeum vulgare. Journal of Experimental Botany 41, 1133–1141.
| Concentrations and transport of solutes in xylem and phloem along the leaf axis of NaCl-treated Hordeum vulgare.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtVymt7o%3D&md5=84156a95998e6670438518dc12a9300aCAS |
Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Molecular Plant-Microbe Interactions 21, 737–744.
| Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1.Crossref | GoogleScholarGoogle Scholar | 18624638PubMed |










