Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan
Toshihiro Hasegawa A J , Hidemitsu Sakai A , Takeshi Tokida A , Hirofumi Nakamura B , Chunwu Zhu A H , Yasuhiro Usui A , Mayumi Yoshimoto A , Minehiko Fukuoka A , Hitomi Wakatsuki A , Nobuko Katayanagi A , Toshinori Matsunami C , Yoshihiro Kaneta D , Takashi Sato D , Fumiaki Takakai D , Ryoji Sameshima E I , Masumi Okada F , Tadahiko Mae G and Amane Makino GA National Institute for Agro-Environmental Sciences, Tsukuba, Ibaraki 305-8604, Japan.
B Taiyokeiki Co. Ltd, Kita-ku, Tokyo 114-0032, Japan.
C Akita Prefectural Agricultural Experiment Station, Akita, Akita 010-1231, Japan.
D Akita Prefectural University, Akita, Akita 010-0146, Japan.
E National Agricultural Research Organisation, National Agricultural Research Center for Tohoku Region, Morioka, Iwate 020-0198, Japan.
F Iwate University, Morioka, Iwate 020-8550, Japan.
G Tohoku University, Sendai, Miyagi 981-8555, Japan.
H Present address: Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210 008, PR China.
I Present address: Hokkaido University, Sapporo, Hokkaido 060-8589, Japan.
J Corresponding author. Email: thase@affrc.go.jp
Functional Plant Biology 40(2) 148-159 https://doi.org/10.1071/FP12357
Submitted: 26 November 2012 Accepted: 17 December 2012 Published: 30 January 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
There is some evidence that rice cultivars respond differently to elevated CO2 concentrations ([CO2]), but [CO2] × cultivar interaction has never been tested under open-field conditions across different sites. Here, we report on trials conducted at free-air CO2 enrichment (FACE) facilities at two sites in Japan, Shizukuishi (2007 and 2008) and Tsukuba (2010). The average growing-season air temperature was more than 5°C warmer at Tsukuba than at Shizukuishi. For four cultivars tested at both sites, the [CO2] × cultivar interaction was significant for brown rice yield, but there was no significant interaction with site-year. Higher-yielding cultivars with a large sink size showed a greater [CO2] response. The Tsukuba FACE experiment, which included eight cultivars, revealed a wider range of yield enhancement (3–36%) than the multi-site experiment. All of the tested yield components contributed to this enhancement, but there was a highly significant [CO2] × cultivar interaction for percentage of ripened spikelets. These results suggest that a large sink is a prerequisite for higher productivity under elevated [CO2], but that improving carbon allocation by increasing grain setting may also be a practical way of increasing the yield response to elevated [CO2].
Introduction
The atmospheric CO2 concentration ([CO2]) has risen from 280 to 390 µmol mol−1 over the past 200 years. The rate of increase has accelerated during the last 50 years, increasing from an average of 0.85 µmol mol−1 year−1 in the 1960s to 2.0 µmol mol−1 year−1 in 2001–2010 (http://www.esrl.noaa.gov/gmd/ccgg/trends/, accessed 8 November 2012). The CO2 concentration is projected to rise further for the next 50 years even if various efforts are made to reduce carbon emissions (Fisher et al. 2007). Increases in greenhouse gas concentrations will be the major cause of global changes, which could have large impacts on crop production because of possible increases in abiotic stress.
During the last century, the production of staple food crops has more than tripled, mainly due to an increase in production per unit land area (FAOSTAT, http://faostat.fao.org/, accessed 23 April 2012). The rate of increase was large enough to keep pace with the rapidly growing food demand, which has almost doubled over the past 50 years. The annual rate of yield increase has slowed over the past few decades (Bruinsma 2009). The demand for staple food is projected to increase further, mainly due to the increasing population. The production of major crops will need to increase by 70% by 2050 (Bruinsma 2009) to meet the growing demand for crops. These production increases must be achieved under changing climate conditions, so crop improvement programs must take these changing conditions into account.
Among the various climate changes expected to occur, an increase in [CO2] is one of few that will have positive influences on crop production by promoting photosynthetic rates and possibly reducing crop water use. The mechanisms and magnitudes of elevated [CO2] effects on crop productivity have been studied for decades using various research facilities, including growth chambers, open-top chambers and free-air CO2 enrichment (FACE) facilities. The yield enhancements caused by elevated [CO2] have frequently been reviewed and more recent articles have presented meta-analyses. These reviews indicate that major C3 cereals show a similar yield response to elevated [CO2], i.e. 10–20% greater yield under 550–660 µmol mol−1 CO2 than under 380–400 µmol mol−1 CO2 (Kimball et al. 2002; Long et al. 2004). Identifying and developing cultivars that respond well to elevated [CO2] can be an important option for adaptation to climate change (Tausz et al. 2011; Ziska et al. 2012) and may lead to higher resource use efficiency (Drake et al. 1997).
Rice (Oryza sativa L.) is unequivocally the most important food crop, feeding about a half of the world’s population (Maclean et al. 2002). As in other cereals, the rate of yield increase is slowing and even plateauing in various countries (Horie et al. 2005), yet the demand for grain continues to grow. Furthermore, the area available for rice planting is expected to decrease, so the rate of yield increase must exceed that of the demand increase (Bruinsma 2009). Rice productivity must therefore be increased by all available means. Improvement of the response to elevated [CO2] has the potential to increase yields but has not been given enough attention until recently (Ziska et al. 2012).
The response of rice to elevated [CO2] is similar to that of the other major C3 cereals (see Ainsworth 2008 for a recent review). Some studies have examined the response of different rice cultivars; for example, glasshouse or environmentally controlled chamber studies have shown a range of yield responses to elevated [CO2] (Ziska 1996; Baker 2004). In a field study, (Moya et al. 1998) conducted an open-top chamber experiment using three indica cultivars that showed a range of CO2 responses. Field trials using the FACE facility in Jiangsu Province, China, showed a large yield response to elevated [CO2] in two indica hybrid rice cultivars (Liu et al. 2008; Yang et al. 2009). A FACE experiment at Shizukuishi, in northern Japan, demonstrated a significant [CO2] × cultivar interaction among four japonica cultivars adapted to cool climates (Shimono et al. 2009). These results suggest that responses to higher [CO2] can be genetically improved, but the mechanisms underlying the [CO2] × cultivar interaction are still not fully understood and might differ from one study to another (Ziska et al. 2012). Understanding the traits that can confer better adaptability to elevated [CO2] is crucial for genetic improvement of rice productivity under future climate conditions (Tausz et al. 2011). It is also important to test whether these adaptability traits function under different environmental conditions. The results of field experiments are often field-specific, but the issues are global. Thus, results obtained in one environment must be confirmed by multi-environment experiments, but FACE technology is available at only a limited number of research institutions. Because of these challenges, the genotype × CO2 interaction has not been previously tested at different sites using the same set of cultivars in open-field trials. Unfortunately, no more than four genotypes have been tested under field conditions in any one experiment, and the diversity of genotypes tested is relatively narrow.
In an attempt to overcome some of the shortcomings in the previous CO2 studies in rice, we established a new FACE site in Tsukuba, Ibaraki, Japan, ~430 km south of the Shizukuishi FACE site, which was the first rice FACE site (established in 1998). The annual mean temperature at the Tsukuba FACE site is more than 4°C warmer than at the Shizukuishi site (Table 1), so FACE experiments using the same set of cultivars at both sites provide a unique opportunity to test whether the CO2 responses of the cultivars are similar over widely different environments. In addition, the choice of cultivars that can be grown at Shizukuishi is limited to short-duration cultivars, because long-duration ones fail to mature in the cool climate there. In contrast, at Tsukuba, more diverse genotypes can be tested. To enable more cultivars to be tested for CO2 response at Tsukuba, we made the area for CO2 treatment twice as large as at Shizukuishi (Nakamura et al. 2012).
|
Growth and yield enhancement can be influenced by environmental factors other than [CO2], among which the most important is temperature. Long (1991) showed theoretically that stimulation of photosynthesis by elevated [CO2] can be greater under warmer conditions. In contrast, some chamber experiments showed that high temperature reduced yield enhancement by elevated [CO2], possibly owing to increased spikelet sterility (Baker et al. 1992; Kim et al. 1996; Matsui et al. 1997). An open-field trial showed that yield enhancement by elevated [CO2] was less in a cool year in a cool climate (Shimono et al. 2008). These results suggest that the effect of [CO2] on grain yield can be influenced by temperature, but that the magnitudes and even directions of the changes can vary. Nevertheless, the yield gain caused by elevated [CO2] is principally due to enhanced photosynthesis. To better utilise additional atmospheric carbon, a large sink capacity is a likely prerequisite for higher yield regardless of other environmental conditions, but this assumption has not been tested under open-field conditions. Therefore, in this study we conducted FACE experiments at two sites using the same set of four cultivars, including some with large sink size, to examine the interaction among CO2, sites, and cultivars. Four additional cultivars were included at Tsukuba to explore possible sources of productivity improvement under elevated [CO2]
Materials and methods
Study sites
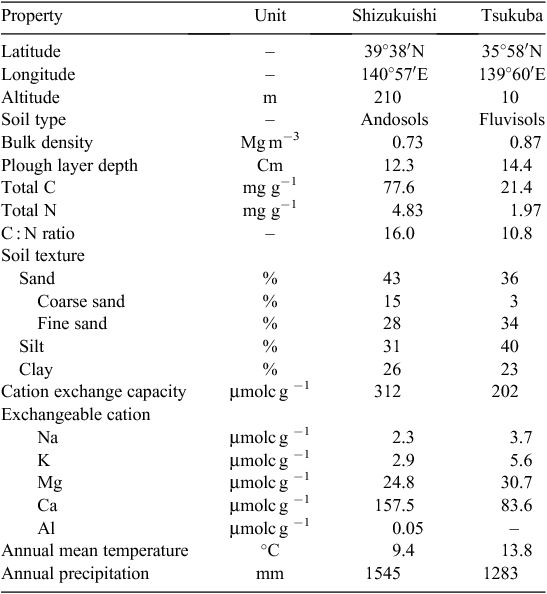
We conducted the FACE experiments at the Shizukuishi FACE site in 2007 and 2008 and at the Tsukuba FACE site in 2010. The Shizukuishi FACE site is located in Shizukuishi Town, Iwate Prefecture (39°38′N, 140°57′E, 210 m above sea level), in a humid continental climate zone with an average temperature of 9.4°C and precipitation of 1545 mm. The soil is an Andosol, which is typical of volcanic areas (see Table 1 for soil properties). Additional site descriptions are given by Kim et al. (2003). The Tsukuba FACE site was established in farmers’ rice fields in Tsukubamirai City, Ibaraki Prefecture, Japan (35°58′N, 139°60′E, 10 m above sea level). The climate is humid subtropical with an average temperature of 13.8°C and annual precipitation of 1280 mm. The soil is a Fluvisol, which is typical of alluvial areas.
Weather conditions
At Shizukuishi, the 2007 growing season was slightly warmer and wetter than the 2008 season, but overall, both seasons were near normal for that region. In contrast, in 2010, the Tsukuba site experienced the hottest summer ever recorded for that region (Table 2). The mean air temperature averaged over the growing season was 25.1°C, ~2°C higher than in an average year. The 2010 season at Tsukuba was also characterised by high solar radiation and limited rainfall (22% higher and 56% less than in a normal year respectively). The unusually hot, dry weather at Tsukuba in 2010 resulted in a larger-than-expected difference in the weather conditions between the two sites.
CO2 treatments
The method for controlling [CO2] in open fields was essentially the same at the two sites. Full descriptions are presented by Okada et al. (2001) for Shizukuishi and by Nakamura et al. (2012) for Tsukuba. Briefly, the elevated [CO2] treatments were imposed on octagonal plots (‘FACE rings’ hereafter) in the fields. Pure CO2 was supplied from polyethylene tubing (Kiriko type-R, MKV Dream Co., Ltd, Tokyo, Japan) installed horizontally on the edges of the FACE rings at ~30 cm above the rice canopy. The emission tubes had small holes of 0.3–0.5 mm in diameter at 40-mm intervals on the lower half of each tube, from which CO2 was released into the air. We monitored [CO2] at the centre of each FACE ring and at ambient control positions. A proportional-integral-derivative algorithm regulated the amounts and locations of CO2 emission, which depended on the wind speed and direction: CO2 was released from the windward sides of the FACE ring. The target [CO2] was 200 μmol mol−1 above the ambient [CO2], and CO2 was supplied during daylight hours (from the sunrise to sunset). The major difference between the two sites was the size of the treatment area: at Tsukuba, each FACE ring was 240 m2 in area and 17 m in inside diameter, whereas at Shizukuishi, it was 120 m2 and 12 m in diameter. Nevertheless, [CO2] control at the Tsukuba site was comparable or even better than at the Shizukuishi site (Nakamura et al. 2012). The season-long daytime average [CO2] was 570 (2007), 576 (2008) and 584 (2010) µmol mol−1 in the FACE plots and 379 (2007), 376 (2008) and 386 (210) µmol mol−1 in the ambient plots. The target achievement ratio, defined as the fraction of time that the 1-min average [CO2] deviated by <10 or <20% from the target [CO2] (TAR10 and TAR20), averaged 0.68 (2007) and 0.67 (2008) for TAR10 and 0.91 (2007) and 0.90 (2008) for TAR20 at Shizukuishi (Tokida et al. 2010). At Tsukuba, TAR10 was 0.74 and TAR20 was 0.91 (Nakamura et al. 2012).
At Shizukuishi, six rectangular fields measuring 100 × 30 m were used for the experiments. These six fields were grouped into three blocks with similar agronomic histories and characteristics (n = 3). In each block, one field was assigned to the FACE ring and the other to the ambient control. The FACE and ambient plots were at least 90 m apart. At Tsukuba, we selected four rectangular fields for their uniformity in growth and yield (n = 4). The longer side of each field measured 100 m and the shorter side ranged from 30 to 70 m. In each field, we established a pair of FACE and ambient control plots with their centres 75 m apart to minimise contamination of the ambient control by CO2 applied to the FACE plots.
Cultural practices and growth conditions
At Shizukuishi, we tested four rice (Oryza sativa L.) cultivars in 2007 and 2008: Akitakomachi, Akita 63, Koshihikari, and Takanari. Akitakomachi is a standard japonica cultivar bred in Akita Prefecture (adjacent to the prefecture of the test site). Akita 63 is a large-grain high-yielding japonica cultivar with a large sink capacity also bred in Akita (Mae et al. 2006). Koshihikari is another standard japonica cultivar and the most widely planted cultivar in Japan, but it matures later and is adapted to warmer regions than is Akitakomachi. Takanari is a high-yielding indica cultivar with a large number of grains that performs well under a range of environments, including northern Japan (Takai et al. 2006; Taylaran et al. 2009; Fukushima and Shiratsuchi 2011). Full descriptions of plant management are given by Tokida et al. (2010). Seedlings were raised in two different chambers, under ambient [CO2] and elevated [CO2] (ambient + 200 μmol mol−1) respectively. Seedlings were transplanted by hand on 23 May 2007 and 22 May 2008 at a spacing of 17.5 × 30 cm (19.1 hills m−2), following the farmers’ practices in the region. Note that, in transplanted rice, two or more seedlings are transplanted to one spot, and a group of these seedlings is called a ‘hill’. In this study, we planted three seedlings per hill. All fertilisers were applied as basal dressing. Nitrogen was supplied at 9 g m−2 (3 g m−2 of N as ammonium sulfate and 6 g m−2 as coated urea; LP-70, JCAM Agri. Co. Ltd, Tokyo, Japan), potassium at 12.5 g m−2 of K (7.5 g as KCl and 5.0 g as potassium silicate), and phosphorous at 13.1 g m−2 of P as fused magnesium phosphate.
At Tsukuba, we tested eight cultivars in 2010. These included the four cultivars used at Shizukuishi and an additional four cultivars – Akihikari, Aikoku, Norin 8, and Akidawara – which differ in maturity and yield potential. We sowed pre-germinated seeds in seedling trays on 26 April; each tray had 448 circular cells (16 mm in diameter and 25 mm in depth, Minoru Pot 448, Minoru Industrial Co. Ltd, Okayama, Japan) filled with sterilised soil amended with fertiliser (0.4 g of N, 0.35 g of P, and 0.5 g of K per kg of soil). Three pre-germinated seeds were planted in each cell. After emergence, we raised seedlings in the puddled open field, with a tunnel cloche or floating mulch for the first 2 weeks. We manually transplanted these seedlings on 26 May at a spacing of 30 × 15 cm (22.2 hills m−2). The area planted to each cultivar differed but was not smaller than 3 m2.
All the plots at Tsukuba received equal amounts of fertiliser: we applied PK compound fertiliser at 4.36 g m−2 of P and 8.30 g m−2 of K on 9 April, before ploughing. Fields were kept submerged after 25 April. We applied a total of 8 g m−2 of N fertilisers: 2 g m−2 as urea, 4 g m−2 as one controlled-release fertiliser (type LP100, JCAM Agri. Co. Ltd), and 2 g m−2 as another controlled-release fertiliser (type LP140, JCAM Agri. Co. Ltd). Right after N application, we puddled (tilled) the field for uniformity on 19 May.
Just before transplanting, seedlings were treated with a combined pesticide containing 1.5% clothianidin (insecticide) and 7.0% orysastrobin (fungicide) at 25 g per seedling tray. On 15 June, we applied granular herbicide containing 1.5% dymron, 0.17% bensulfuron methyl, and 3.5% mefenacet at 640 mg m−2. On 23 June, we applied a granular insecticide containing 5.0% cycloprothrin at a rate of 480 mg m−2. A combined fungicide and insecticide containing 4.0% cartap hydrochloride, 4.0% BPMC (2-s-butylphenyl N-methylcarbamate) and 8.0% Propenazol at 3 g m−2 on 3 July. At the stem elongation phase, we installed plant support netting made of polyethylene, with an aperture size of 30 × 30 cm, horizontally at ~70 cm above the soil surface to prevent plants from lodging. The field was kept flooded until ~20 August, when the surface water was drained for harvesting. We applied flush irrigations on several occasions to keep the soil moist.
Measurements
The methods for yield determination were essentially the same at each site. At physiological maturity, we sampled aboveground biomass from an area of at least 0.945 m2 avoiding edge rows for each of the four cultivars tested at both sites; the sampled areas correspond to 18 hills at Shizukuishi and 21 hills at Tsukuba. For the other four cultivars tested at Tsukuba, we sampled eight hills per cultivar to measure grain yield components. After drying the material under a rain shelter, we first measured total aboveground plant weight and panicle number and then the total weight of the spikelets after threshing. The spikelet sample was then split into three subsamples. A half of the spikelets were dehulled to determine the brown rice weight. A quarter of the spikelets were used to determine the proportion of ripened spikelets by sorting in an ammonium sulfate solution of specific gravity (SG) = 1.06. At Tsukuba, we further sorted the unfilled (floating) spikelets into three classes by using water (SG = 1) and an 80% ethanol solution with an SG of 0.86 to determine the level of grain filling (Kobata et al. 2010); we counted those that floated on the ethanol solution (SG ≤ 0.86), those that floated in the water but sank in the ethanol solution (0.86 ≤ SG < 1.0) and those that floated in the ammonium sulfate but sank in water (1.0 ≤ SG < 1.06). The other quarter of the spikelets was stored for future chemical analyses. We measured the moisture content of the grains with a grain moisture tester (Riceter f, Kett Electric Laboratory, Tokyo, Japan) and that of the rice straw by the gravimetric method after oven-drying at 80°C. Brown rice yield and single-grain mass were expressed on a 15% moisture content basis. We also calculated the sink capacity defined as the product of total number of spikelets and single-grain mass (Akita 1989).
At Tsukuba, we monitored panicle emergence of 6–8 hills in each plot every other day and considered the heading date to be the date when 50% of the productive tillers reached panicle-tip emergence.
Statistical analyses
We first conducted an analysis of variance (ANOVA) for the four cultivars tested at both sites, applying a split-split-plot design, where site-year was treated as the main factor, [CO2] as the split factor, and cultivar as the split-split factor, using the general linear model procedure of SPSS 14.0 for Windows (SPSS Japan Inc.; now IBM, Tokyo, Japan). We treated site and year as fixed effects because the site selection was not random. We also examined the relative importance of yield components for the multi-site data by multiple regression analysis based on the response ratio (FACE : ambient). We used the natural logarithm transformation because the relationships between yield and its components were multiplicative.
We then performed an ANOVA for the main factors of [CO2] and cultivars on the yield traits measured at Tsukuba in 2010. We also attempted to determine the relative importance of yield components in the yield response to elevated [CO2] by multiple regression based on the log-transformed response ratio (FACE : ambient).
Results
Four-cultivar trial at the Shizukuishi and Tsukuba FACE sites
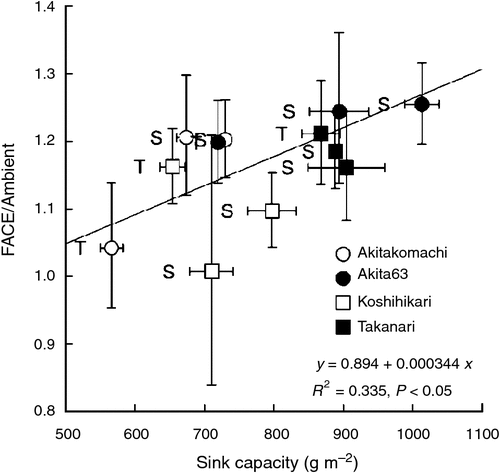
The response of brown rice yield (BRY) of the four cultivars planted at both sites to elevated [CO2] averaged 17% (P < 0.001) across the three environments (2 years at Shizukuishi and 1 year at Tsukuba; Table 3). Brown rice yield differed significantly among the cultivars (P < 0.001), but there was a strong site-year (SY) × cultivar interaction (P < 0.001); for example, Akitakomachi performed better than Koshihikari at Shizukuishi but their ranks were reversed at Tsukuba. The effect of elevated [CO2] on BRY was also different among cultivars (CO2 × cultivar interaction, P < 0.05). Akita 63 and Takanari showed greater enhancement due to elevated [CO2] in all three environments (average BRY enhancement of 23 and 19% respectively) than Akitakomachi (15%) and Koshihikari (9%). The BRY enhancement of the four cultivars was positively correlated with the sink capacity (Fig. 1, P < 0.05), supporting the importance of sink size in the response to elevated [CO2]. It is worth noting that both Akita 63 and Takanari are in general higher yielding than the other two cultivars, so the net increases in BRY due to the FACE treatment were substantially higher for Akita 63 and Takanari (averaging 151 and 112 g m−2 respectively) than for Akitakomachi (87 g m−2) and Koshihikari (51 g m−2). This ranking was generally consistent over the three environments, resulting in a non-significant CO2 × SY interaction.
|
Among the yield components examined, the effects of elevated [CO2] were most apparent and positive for spikelet density (Table 3). Panicle density (PD) generally accounted for more of the increase in spikelet density than did spikelets per panicle (SPP). There was a significant interaction among CO2, SY and cultivar (P < 0.05) for PD: the response of Koshihikari was consistent across the three environments, whereas that of Akitakomachi depended highly on the environment, suggesting that complicated mechanisms are involved in the enhancement of PD due to elevated [CO2] for different cultivars under different environments. As shown in Fig. 1, however, enhancement of PD had a significantly negative correlation with enhancement of SPP (P < 0.05), suggesting that a large increase in PD is offset by a limited response in SPP.
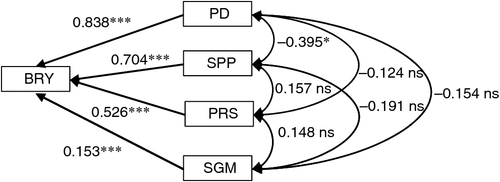
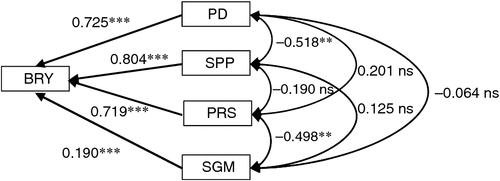
The relative contribution to the enhancement of BRY due to elevated [CO2] was largest for PD followed by SPP (Fig. 2). Two components related to grain filling – percentage ripened spikelets (PRS) and single-grain mass (SGM) – also had significant positive effects on BRY, but the effects were less than those of the components related to sink size (i.e. PD and SPP). Correlations among yield components were not significant except for a significant negative correlation between PD and SPP.
|
Eight-cultivar trial at the Tsukuba FACE site in 2010
The eight cultivars tested at Tsukuba in 2010 differed significantly in the number of days from transplanting to heading (DTH), with a range from earliest to latest of about three weeks (P < 0.001, Table 4). The effect of the FACE treatment was significant (P < 0.01), shortening DTH by 1 day on average, without a significant CO2 × cultivar interaction. Brown rice yield (BRY) increased significantly due to the FACE treatment (19% on average, P < 0.01), but the enhancement of yield differed significantly among the cultivars, ranging from 3 to 36% (P < 0.05). Among the yield components, the main effect of the FACE treatment was significantly positive for spikelet density and PRS (P < 0.05) but marginally negative for SGM (P = 0.068). All the yield components showed a highly significant main effect of cultivar (P < 0.001).
The main effect of elevated [CO2] on PD was not significant, but the effect on PD differed among cultivars, as evidenced by a significant CO2 × cultivar interaction (P < 0.01, Table 4). The cultivars with a limited enhancement of PD, however, showed a large response of SPP; in fact, the correlation between relative enhancements of PD and SPP due to elevated [CO2] was significant and negative (P < 0.01, Fig. 2). As a result, the main effect of elevated [CO2] on spikelet density (the product of PD and SPP) was significant, but there was no significant CO2 × cultivar interaction.
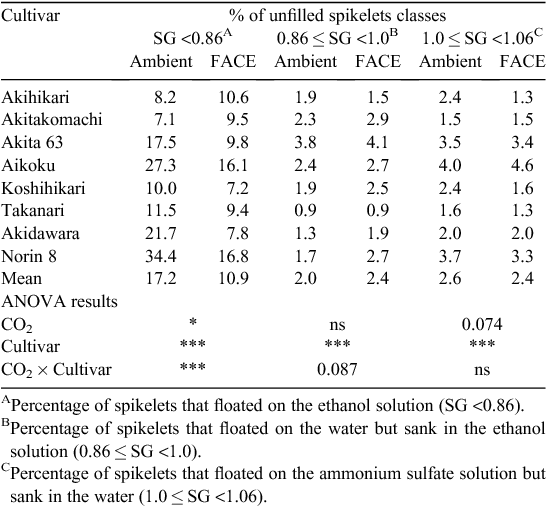
The effect of elevated [CO2] on PRS was generally positive but differed substantially among cultivars, with a highly significant CO2 × cultivar interaction (P < 0.001, Table 4): the difference in PRS between FACE and ambient [CO2] ranged from −3% to +17%. Further sorting of unfilled spikelets showed that the percentage of spikelets in the lightest category accounted for most of the variation in the percentage of unfilled spikelets (Table 5), with a modestly significant CO2 main effect (P < 0.05) and a highly significant CO2 × cultivar interaction (P < 0.001). The level of statistical significance for the interaction became weaker as the specific gravity of the sorting medium increased (P = 0.087 for 0.86 < SG <1.0 and not significant for 1.0 < SG <1.06). The main effect of elevated [CO2] on the percentage of floating spikelets in the 1.0 < SG <1.06 category was close to being significant (P = 0.074), but there was no significant CO2 × cultivar interaction for this trait.
|
Multiple regression of enhancement of BRY due to elevated [CO2] on that of four yield components (PD, SPP, PRS and SGM) showed that all four components had significant positive effects on BRY enhancement (Fig. 3). The relative contribution to BRY enhancement was largest for SPP, followed by PD and PRS. However, if we use spikelet density as an explanatory variable in place of SPP and PD, PRS showed the largest standardised partial regression coefficient (0.744, P < 0.001), followed by spikelet density (0.706, P < 0.001) (data not shown in Fig. 3). The contribution of SGM was also significantly positive but low.
|
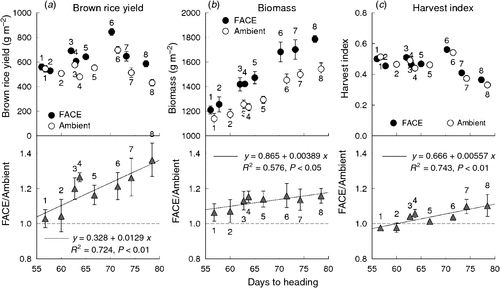
The BRY enhancement due to elevated [CO2] increased with DTH of the cultivars (Fig. 4a, P < 0.01). Aboveground biomass at harvest was larger in the late-maturing cultivars than in the early-maturing ones, and its enhancement by elevated [CO2] increased linearly with DTH (Fig. 4b, P < 0.05). The effect of FACE treatment on harvest index (HI) was also pronounced in the late-maturing cultivars, and revealed an even stronger dependence on DTH than did biomass (Fig. 4c, P < 0.01).
|
Discussion
CO2 × cultivar interaction across the two FACE sites: importance of sink capacity
The two rice FACE sites established in Japan differed significantly in climatic conditions, particularly temperature: growing-season mean air temperatures differed by more than 5°C. The FACE experimental setups were essentially the same, except that the treatment area at Tsukuba was twice the size of that at Shizukuishi. We have confirmed that [CO2] control at Tsukuba was even slightly better than that at Shizukuishi, even though the ring at Tsukuba was larger (Nakamura et al. 2012). This allowed us to test for the first time the effects of FACE treatment on the same cultivars at different sites, and the results could be highly relevant to the combined effects of global warming and rising [CO2] on different cultivars.
We confirmed a significant positive effect of elevated [CO2] on grain yield, which averaged 17% over the four common cultivars and was similar to yield increases of japonica cultivars typically observed in previous rice experiments (Kim et al. 2003; Yang et al. 2006). Panicle density made the largest contribution to enhancement of the yield of the four common cultivars at the two sites, followed by SPP (Fig. 1), which together produced a larger spikelet density. This result was also consistent with previous rice FACE studies (Kim et al. 2003; Yang et al. 2006; Shimono et al. 2009), confirming the importance of sink formation for yield responsiveness to elevated [CO2].
We also observed a significant CO2 × cultivar interaction: Takanari and Akita 63, which are high-yielding cultivars but not planted widely due to their poor eating quality, showed greater yield enhancement under elevated [CO2] than did Akitakomachi and Koshihikari, which are major cultivars planted widely across different regions of Japan. The net increase due to elevated [CO2] differed substantially among the cultivars, ranging from 51 to 151 g m−2 when averaged over the three environments. This suggests the potential for increasing both yield capacity and response to elevated [CO2]. Takanari and Akita 63 are well known for their high yield capacity (Takai et al. 2006; Taylaran et al. 2009; Fukushima and Shiratsuchi 2011), with a large sink capacity.
Importantly, we did not detect SY × CO2 × cultivar interactions for spikelet density and BRY. This suggests that the advantages of large spikelet density and BRY under elevated [CO2] are consistent over different environments and that they could be valuable materials for the improvement of both yield and yield responses to higher [CO2] over widely different conditions. Takanari and Akita 63 have a larger sink capacity than Akitakomachi and Koshihikari, which had a positive influence on the yield gain due to elevated [CO2] (Fig. 1, P < 0.05), but this capacity is achieved through different pathways. Takanari had an average SPP more than 90% greater than those of the other cultivars, whereas Akita 63 had an average SGM 40% greater than those of the other cultivars. These results suggest that both pathways are capable of providing the capacity for rice yield enhancement.
It is also worth noting that Takanari and Akita 63 are well known not just for sink capacity, but also for higher N use efficiency (Mae et al. 2006; Taylaran et al. 2009). It is still not clear how resource use efficiency influences the sink capacity of the cultivars, but there is ample evidence that N nutrition has a key role in determining rice responsiveness to elevated [CO2] (Stitt and Krapp 1999; Kim et al. 2003; Sakai et al. 2006).
Year-to-year variation in the yield responses to elevated [CO2] for cultivar ‘Akitakomachi’
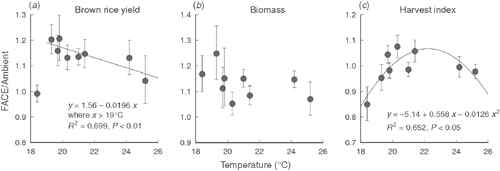
We did not observe a significant CO2 × SY interaction in the three years of this study, but a previous study showed that the yield enhancement due to elevated [CO2] was significantly lower in a year with a cool summer than in a typical year (Shimono et al. 2008). We further analysed the effect of interannual variation in yield response to elevated [CO2] by analysing nine years of FACE data obtained for Akitakomachi, which was planted in all of the cropping seasons tested (Fig. 5). Yield enhancement due to elevated [CO2] in the nine cropping seasons averaged 13%, but the hottest and coolest seasons recorded smaller yield responses to elevated [CO2] than did those in the medium temperature range (Fig. 5a). Moreover, with the exception of the coolest season (2003), there was a negative correlation between BRY and growing-season temperature. No significant response of biomass to elevated [CO2] was observed over the nine seasons tested (Fig. 5b). In contrast, harvest index showed a quadratic relationship with growing-season temperature (Fig. 5c, P < 0.05), suggesting that biomass allocation to the grain accounted for the variation in BRY enhancement. We previously showed that yield enhancement was limited under cool conditions, but this analysis showed that yield enhancement might also be reduced under hot conditions.
|
In earlier studies using chambers, Kim et al. (1996) and Matsui et al. (1997) showed that spikelet sterility increased with high temperatures around flowering time, and that elevated [CO2] often exacerbated this heat-induced sterility. Yoshimoto et al. (2005b) showed that heat stress can be exacerbated under elevated [CO2] because of the warmer canopy and panicle temperatures caused by reduced stomatal conductance and transpirational cooling in the FACE canopy, suggesting that these factors could reduce the harvest index. In fact, percent of unfilled spikelets of the lightest category (SG <0.86) increased by 2.4 percentage points (Table 5), which partially accounted for the limited response of harvest index and grain yield to elevated [CO2] in the hot summer of 2010, when the maximum temperature remained between 33 and 35°C for almost a month, covering the flowering-period window of all the cultivars tested.
Factors associated with the large difference in the response to elevated [CO2] among the eight cultivars at the Tsukuba FACE site
The variation in the response of grain yield to elevated [CO2] was much larger in the eight-variety comparison at Tsukuba in 2010 than in the cross-site comparison involving only four cultivars: the yield enhancement among the eight cultivars at Tsukuba ranged from 3 to 36%. The range was smaller than those observed in enclosure studies (Ziska 1996; Baker 2004), but larger than we previously observed (Shimono et al. 2009). Liu et al. (2008) and Yang et al. (2009) showed a large yield response of Chinese indica hybrid cultivars to [CO2] in their FACE studies. Our results here demonstrate the potential for inbred japonica cultivars to show large yield responses to elevated [CO2].
The BRY enhancement due to elevated [CO2] increased with days to heading (DTH) of the cultivars (Fig. 4a). Both biomass (Fig. 4b) and HI (Fig. 4c) accounted for this enhancement, the latter showing a higher correlation with DTH. Multiple regression analysis of yield enhancement against yield components of the eight cultivars tested showed a highly significant contribution by both SPP and PD (Fig. 3); this was similar to that observed in the cross-site analysis (Fig. 2). The contribution of PRS to yield enhancement at Tsukuba in 2010 was comparable to those of sink capacity (SPP and PD), whereas in the cross-site analysis, PRS made less of a contribution overall. At Tsukuba in 2010, PRS showed a highly significant CO2 × cultivar interaction, and the difference between the CO2 treatments (FACE – ambient) ranged from −3 to +17% (Table 4). This was reflected in the varietal difference in the response of HI to [CO2] (Fig. 4c).
One possible explanation for the different responses to PRS and HI among cultivars is that different cultivars would be exposed to different temperature conditions depending on their heading dates. The weather in the summer of 2010, however, was exceptionally and consistently hot over a full month. This resulted in cultivars with different heading dates experiencing very similar temperature conditions. In fact, the average air temperature for the 20-day period from the heading stage, encompassing the critical period for grain growth and quality determination, ranged only from 27.4 to 27.8°C; this result suggesting that all eight cultivars were exposed to similar temperature conditions during grain filling.
Reduction in PRS can arise from any or all of a failure of grain set, early cessation of grain development, and insufficient starch accumulation. Sorting of spikelets in solutions of different specific gravities suggested that the effect of elevated [CO2] occurred early in grain development, possibly at the stage of fertilisation or grain setting, because variation in the percentage of very light spikelets accounted for most of the variation in PRS (Table 5). Short-term temperature records showed that the maximum average air temperature in the 5 days after heading exceeded 33°C for the two early cultivars (Akihikari and Akitakomachi), but was between 30 and 32°C for the rest of the cultivars. The slightly negative responses of HI and PRS to elevated [CO2] in the two early cultivars could be related to the higher maximum temperature at the critical time for grain setting. As discussed previously, the canopy tends to be warmer under FACE than under ambient [CO2] because of reduced stomatal conductance and transpiration. At the Shizukuishi site in 1999, FACE increased canopy temperature by 0.2–1°C (Yoshimoto et al. 2005a). In the present study, air temperature at panicle height in the Koshihikari canopy was ~0.44°C higher in the FACE treatment than in the ambient air (M. Yoshimoto, M. Fukuoka and T. Hasegawa, unpubl. data). We did not measure the canopy temperatures of the two early cultivars, but a previous study showed that panicle transpiration conductance of Akitakomachi was similar to that of Koshihikari (Fukuoka et al. 2012) and that a reduction in stomatal conductance due to elevated [CO2] is common across many crop species (Ainsworth and Rogers 2007). For these reasons, we expect that the magnitude of the difference in canopy temperature of the early cultivars was not much different from that measured in the Koshihikari canopy. It is well known that grain setting is very sensitive to temperatures above a threshold of around 34–35°C (Satake and Yoshida 1978; Kim et al. 1996), so a slight increase in canopy and panicle temperatures caused by elevated [CO2] could explain the limited responses of the two early cultivars, as was suggested in chamber studies (Kim et al. 1996; Matsui et al. 1997).
The positive effect of elevated [CO2] on HI and PRS for the later-maturing cultivars was somewhat unexpected, but Ziska (1996) and Baker (2004) previously showed in chamber studies that some cultivars show a positive effect of elevated [CO2] on HI. Yang et al. (2009) showed in a FACE study in China that PRS of a high-yielding indica hybrid increased under FACE and they suggested that a better carbon supply could be the reason; this could be one plausible explanation for why some cultivars showed increased PRS in the present study. However, the effect of elevated [CO2] occurred early in grain development, possibly by preventing failure of fertilisation or grain setting. During this phase, the absolute amount of carbon demand for these processes is rather small, so the increased quantity of carbon under elevated [CO2] may not be the sole reason for the increased PRS.
It is worth noting that the increase in PRS due to elevated [CO2] occurred in cultivars that are high in sink capacity but low in PRS under ambient conditions. The elevated [CO2] condition could increase the grain-setting rate at high temperatures for some cultivars but not for others, such as Akitakomachi, which showed a non-significant but negative response of PRS to elevated [CO2]. Also, no significant positive effect on PRS was measured in any of the four cultivars tested at Shizukuishi. For cultivars that showed a large positive response of PRS and therefore BRY, there might be mechanisms that ameliorate the reduction in spikelet fertilisation and grain setting at high temperatures. Further studies to improve our understanding of reproductive physiology under high temperature and [CO2] are essential to enhance yield potential and to reduce the negative effects of high temperature in the future.
Biomass enhancement by elevated [CO2] was also correlated with DTH (P < 0.05, Fig. 4), but this modestly significant correlation was attributable mainly to the low level of enhancement of the two early cultivars. The reasons for the limited biomass response in the two early cultivars are not clear. Previously, in a comparison of four cultivars at Shizukuishi, we did not find a relationship between biomass and maturity group; in that study, DTH ranged from 65 to 92 (Shimono et al. 2009). In the present study, DTH of the two early cultivars were 56 and 58 in the FACE plot, much shorter than for any cultivars in the previous study. The shorter DTH meant that the time for vegetative growth was much more limited than in the previous study. Indeed, the response of PD of the two early cultivars was also smaller than that of the later cultivars, resulting in a significant CO2 × cultivar interaction (Table 4). This might have also limited the enhancement of leaf area, which could be a reason for the smaller biomass response of these cultivars. Although we need to confirm whether the response of early cultivars to elevated CO2 is limited, this finding could raise a concern that future warming, which reduces DTH, might also reduce the potential growth response to elevated [CO2].
Inter-annual variation in biomass enhancement of the later cultivars was much less than that in BRY and HI. This rather limited response of biomass enhancement to elevated [CO2] suggests that improving source response to [CO2] may be more difficult than improving sink response. In a glasshouse study comparing 17 cultivars, (Ziska 1996) showed highly significant differences in biomass enhancement in response to [CO2], ranging from 9 to 263% at 29/21°C (day/night temperature) and from −20% to +181% at 37/29°C for widely-spaced potted plants. This large difference might be difficult to repeat under full-canopy conditions, because the major source of biomass enhancement of widely spaced is increased tillering and leaf area that can take full advantage of solar radiation. Nevertheless, as shown in Fig. 4, the increase in biomass caused by the FACE treatment (i.e. FACE − ambient) was apparently larger for the cultivars with large yield enhancement, ensuring larger grain weight. It is also worth noting that the present study indicates that improving rice productivity under higher [CO2] can be achieved by improving grain setting and thereby carbon allocation to the grains, although we need to further explore possibilities for improving relative responses of biomass to elevated [CO2]
Conclusions
Trials of four cultivars conducted at each of two FACE sites showed a significant [CO2] × cultivar interaction in brown rice yield. Higher-yielding cultivars generally showed a larger [CO2] response, and both large panicles and grain size are promising traits for adaptation to higher [CO2] under different climatic conditions. The Tsukuba experiment using eight cultivars revealed an even wider range of yield due to elevated [CO2], which ranged from 3 to 36%. All of the measured yield components contributed to the larger yield enhancement. Panicle density and spikelets per panicle were important in both studies, but only the Tsukuba trial showed a highly significant CO2 × cultivar interaction for percentage of ripened spikelets; in that trial, the effects of elevated [CO2] ranged from −3% to +17%, depending on the cultivar. These results suggest that a large sink is an effective means of obtaining higher productivity under elevated [CO2], but improving carbon allocation by increasing grain setting can also be a practical way of increasing the yield response to elevated [CO2]. Further studies are needed to understand the mechanisms of [CO2] × cultivar interaction with respect to reproductive growth.
Acknowledgements
We thank Dr Rota Wagai of the National Institute for Agro-Environmental Sciences for his help in the soil properties determination at the Tsukuba site. This work was in part supported by the Ministry of Agriculture, Forestry and Fisheries, Japan, through a research project entitled ‘Development of technologies for mitigation and adaptation to climate change in Agriculture, Forestry and Fisheries’ and in part by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 22114515).
References
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology 14, 1642–1650.| Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration.Crossref | GoogleScholarGoogle Scholar |
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270.
| The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtlemu78%3D&md5=e7e789bd6ed2f1abf505f3139bacfcfdCAS |
Akita S (1989) Improving yield potential in tropical rice. In ‘Progress in irrigated rice research’. pp. 41–73. (International Rice Research Institute: Manila, Philippines)
Baker JT (2004) Yield responses of southern US rice cultivars to CO2 and temperature. Agricultural and Forest Meteorology 122, 129–137.
| Yield responses of southern US rice cultivars to CO2 and temperature.Crossref | GoogleScholarGoogle Scholar |
Baker JT, Allen LH, Boote KJ (1992) Temperature effects on rice at elevated CO2 concentration. Journal of Experimental Botany 43, 959–964.
| Temperature effects on rice at elevated CO2 concentration.Crossref | GoogleScholarGoogle Scholar |
Bruinsma J (2009) The resource outlook to 2050. By how much do land, water use and crop yields need to increase by 2050. In ‘Technical papers from the expert meeting on ‘How to feed the world in 2050’’. pp. 1–33. (FAO: Rome)
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48, 609–639.
| More efficient plants: a consequence of rising atmospheric CO2?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1eltbY%3D&md5=050ad0f7b4f2a39c914080ee055d75b9CAS |
Fisher BS, Nakicenovic N, Alfsen K, Corfee Morlot J, de la Chesnaye F, Hourcade J-Ch, Jiang K, Kainuma M, LaRovere E, Matysek A, Rana A, Riahi K, Richels R, Rose S, van Vuuren D, Warren R (2007) Issues related to mitigation in the long term context. In ‘Climate change 2007: mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-Governmental Panel on Climate Change’. (Eds B Metz, OR Davidson, PR Bosch, R Dave and LA Meyer) pp. 169–250. (Cambridge University Press: Cambridge)
Fukuoka M, Yoshimoto M, Hasegawa T (2012) Varietal range in transpiration conductance of flowering rice panicle and its impact on panicle temperature. Plant Production Science 15, 258–264.
| Varietal range in transpiration conductance of flowering rice panicle and its impact on panicle temperature.Crossref | GoogleScholarGoogle Scholar |
Fukushima A, Shiratsuchi H (2011) Varietal differences in morphological traits, dry matter production and yield of high-yielding rice in the Tohoku Region of Japan. Plant Production Science 14, 47–55.
| Varietal differences in morphological traits, dry matter production and yield of high-yielding rice in the Tohoku Region of Japan.Crossref | GoogleScholarGoogle Scholar |
Horie T, Shiraiwa T, Homma K, Katsura K, Maeda S, Yoshida H (2005) Can yields of lowland rice resume the increases that they showed in the 1980s? Plant Production Science 8, 259–274.
| Can yields of lowland rice resume the increases that they showed in the 1980s?Crossref | GoogleScholarGoogle Scholar |
Kim H, Horie T, Nakagawa H, Wada K (1996) concentration and high temperature on growth and yield of rice. 1: The effect on development, dry matter production and some growth characteristics. Nihon Sakumotsu Gakkai Kiji 65, 634–643.
| concentration and high temperature on growth and yield of rice. 1: The effect on development, dry matter production and some growth characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtlGjtw%3D%3D&md5=71a17a7293ef2bba59d701a02177ebadCAS |
Kim H-Y, Lieffering M, Kobayashi K, Okada M, Mitchell MW, Gumpertz M (2003) Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Research 83, 261–270.
| Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops.Crossref | GoogleScholarGoogle Scholar |
Kimball B, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Advances in Agronomy 77, 293–368.
| Responses of agricultural crops to free-air CO2 enrichment.Crossref | GoogleScholarGoogle Scholar |
Kobata T, Akiyama Y, Kawaoka T (2010) Convenient estimation of unfertilized grains in rice. Plant Production Science 13, 289–296.
| Convenient estimation of unfertilized grains in rice.Crossref | GoogleScholarGoogle Scholar |
Liu H, Yang L, Wang Y, Huang J, Zhu J, Yunxia W, Dong G, Liu G (2008) Yield formation of CO2-enriched hybrid rice cultivar Shanyou 63 under fully open-air field conditions. Field Crops Research 108, 93–100.
| Yield formation of CO2-enriched hybrid rice cultivar Shanyou 63 under fully open-air field conditions.Crossref | GoogleScholarGoogle Scholar |
Long SP (1991) Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell & Environment 14, 729–739.
| Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyns7s%3D&md5=aef20ae7edcbb9febebd7cd28e15e186CAS |
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology 55, 591–628.
| Rising atmospheric carbon dioxide: plants FACE the future.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvFeisb8%3D&md5=90372ae3a3ad173142e8eb82fdb1595aCAS |
Maclean JL, Dawe D, Hardy B, Hettel GP (2002) ‘Rice almanac.’ (CABI Publishing: Wallingford UK)
Mae T, Inaba A, Kaneta Y, Masaki S, Sasaki M, Aizawa M, Okawa S, Hasegawa S, Makino A (2006) A large-grain rice cultivar, Akita 63, exhibits high yields with high physiological N-use efficiency. Field Crops Research 97, 227–237.
| A large-grain rice cultivar, Akita 63, exhibits high yields with high physiological N-use efficiency.Crossref | GoogleScholarGoogle Scholar |
Matsui T, Namuco OS, Ziska LH, Horie T (1997) Effects of high temperature and CO2 concentration on spikelet sterility in indica rice. Field Crops Research 51, 213–219.
| Effects of high temperature and CO2 concentration on spikelet sterility in indica rice.Crossref | GoogleScholarGoogle Scholar |
Moya TB, Ziska LH, Namuco OS, Olszyk D (1998) Growth dynamics and genotypic variation in tropical, field-grown paddy rice (Oryza sativa L.) in response to increasing carbon dioxide and temperature. Global Change Biology 4, 645–656.
| Growth dynamics and genotypic variation in tropical, field-grown paddy rice (Oryza sativa L.) in response to increasing carbon dioxide and temperature.Crossref | GoogleScholarGoogle Scholar |
Nakamura H, Tokida T, Yoshimoto M, Sakai H, Fukuoka M, Hasegawa T (2012) Performance of the enlarged rice-FACE system using pure CO2 installed in Tsukuba, Japan. Journal of Agricultural Meteorology 68, 15–23.
| Performance of the enlarged rice-FACE system using pure CO2 installed in Tsukuba, Japan.Crossref | GoogleScholarGoogle Scholar |
Okada M, Lieffering M, Nakamura H, Yoshimoto M, Kim HY, Kobayashi K (2001) Free-air CO2 enrichment (FACE) using pure CO2 injection: system description. New Phytologist 150, 251–260.
| Free-air CO2 enrichment (FACE) using pure CO2 injection: system description.Crossref | GoogleScholarGoogle Scholar |
Sakai H, Hasegawa T, Kobayashi K (2006) Enhancement of rice canopy carbon gain by elevated CO2 is sensitive to growth stage and leaf nitrogen concentration. New Phytologist 170, 321–332.
| Enhancement of rice canopy carbon gain by elevated CO2 is sensitive to growth stage and leaf nitrogen concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltFamtLg%3D&md5=75c0146099cf8cd3ede9a9a46914320dCAS |
Satake T, Yoshida S (1978) High temperature-induced sterility in indica rices at flowering. Proceedings of the Crop Science Society of Japan 47, 6–17.
| High temperature-induced sterility in indica rices at flowering.Crossref | GoogleScholarGoogle Scholar |
Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2008) Rice yield enhancement by elevated CO2 is reduced in cool weather. Global Change Biology 14, 276–284.
| Rice yield enhancement by elevated CO2 is reduced in cool weather.Crossref | GoogleScholarGoogle Scholar |
Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2009) Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group. Journal of Experimental Botany 60, 523–532.
| Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivFSmtrY%3D&md5=b6eb0221da34e60397e1b3ace56c5515CAS |
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell & Environment 22, 583–621.
| The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksVartLo%3D&md5=d8439abfb5b5d5f341f971f4cf2da7dcCAS |
Takai T, Matsuura S, Nishio T, Ohsumi A, Shiraiwa T, Horie T (2006) Rice yield potential is closely related to crop growth rate during late reproductive period. Field Crops Research 96, 328–335.
| Rice yield potential is closely related to crop growth rate during late reproductive period.Crossref | GoogleScholarGoogle Scholar |
Tausz M, Tausz-Posch S, Norton RM, Fitzgerald GJ, Nicolas ME, Seneweera S (2011) Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations. Environmental and Experimental Botany
| Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations.Crossref | GoogleScholarGoogle Scholar |
Taylaran R, Ozawa S, Miyamoto N (2009) Performance of a high-yielding modern rice cultivar Takanari and several old and new cultivars grown with and without chemical fertilizer in a submerged paddy field. Plant Production Science 12, 365–380.
| Performance of a high-yielding modern rice cultivar Takanari and several old and new cultivars grown with and without chemical fertilizer in a submerged paddy field.Crossref | GoogleScholarGoogle Scholar |
Tokida T, Fumoto T, Cheng W, Matsunami T, Adachi M, Katayanagi N, Matsushima M, Okawara Y, Nakamura H, Okada M, Sameshima R, Hasegawa T (2010) Effects of free-air CO2 enrichment (FACE) and soil warming on CH4 emission from a rice paddy field: impact assessment and stoichiometric evaluation. Biogeosciences 7, 2639–2653.
| Effects of free-air CO2 enrichment (FACE) and soil warming on CH4 emission from a rice paddy field: impact assessment and stoichiometric evaluation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFCns7vN&md5=27da0544c6154a0f0e8de93051b15ddfCAS |
Yang L, Huang J, Yang H, Zhu J, Liu H, Dong G, Liu G, Han Y, Wang Y (2006) The impact of free-air CO2 enrichment (FACE) and N supply on yield formation of rice crops with large panicle. Field Crops Research 98, 141–150.
| The impact of free-air CO2 enrichment (FACE) and N supply on yield formation of rice crops with large panicle.Crossref | GoogleScholarGoogle Scholar |
Yang L, Liu H, Wang YY, Zhu J, Huang J, Liu G, Dong G (2009) Yield formation of CO2-enriched inter-subspecific hybrid rice cultivar Liangyoupeijiu under fully open-air field condition in a warm sub-tropical climate. Agriculture, Ecosystems & Environment 129, 193–200.
| Yield formation of CO2-enriched inter-subspecific hybrid rice cultivar Liangyoupeijiu under fully open-air field condition in a warm sub-tropical climate.Crossref | GoogleScholarGoogle Scholar |
Yoshimoto M, Oue H, Kobayashi K (2005a) Energy balance and water use efficiency of rice canopies under free-air CO2 enrichment. Agricultural and Forest Meteorology 133, 226–246.
| Energy balance and water use efficiency of rice canopies under free-air CO2 enrichment.Crossref | GoogleScholarGoogle Scholar |
Yoshimoto M, Oue H, Takahashi N (2005b) The effects of FACE (free-air CO2 enrichment) on temperatures and transpiration of rice panicles at flowering stage. Journal of Agricultural Meteorology 60, 597–600.
Ziska L (1996) Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: growth and yield response of 17 cultivars. Journal of Experimental Botany 47, 1353–1359.
| Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: growth and yield response of 17 cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmslWlsr8%3D&md5=52e619a6aa8174fc1d475a99e89c1615CAS |
Ziska LH, Bunce JA, Shimono H, Gealy DR, Baker JT, Newton PCD, Reynolds MP, Jagadish KSV, Zhu C, Howden M, Wilson LT (2012) Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proceedings of the Royal Society Biological Sciences 279, 4097–4105.
| Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide.Crossref | GoogleScholarGoogle Scholar |





