Properties of the halophyte microbiome and their implications for plant salt tolerance
Silke Ruppel A B , Philipp Franken A and Katja Witzel AA Leibniz-Institute of Vegetable- and Ornamental Crops Grossbeeren/Erfurt e.V., Theodor-Echtermeyer-Weg 1, 14979 Grossbeeren, Germany.
B Corresponding author. Email: ruppel@igzev.de
This paper originates from a presentation at the COST WG2 Meeting ‘Putting halophytes to work – genetics, biochemistry and physiology’ Hannover, Germany, 28–31 August 2012.
Functional Plant Biology 40(9) 940-951 https://doi.org/10.1071/FP12355
Submitted: 23 November 2012 Accepted: 6 March 2013 Published: 9 April 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Saline habitats cover a wide area of our planet and halophytes (plants growing naturally in saline soils) are increasingly used for human benefits. Beside their genetic and physiological adaptations to salt, complex ecological processes affect the salinity tolerance of halophytes. Hence, prokaryotes and fungi inhabiting roots and leaves can contribute significantly to plant performance. Members of the two prokaryotic domains Bacteria and Archaea, as well as of the fungal kingdom are known to be able to adapt to a range of changes in external osmolarity. Shifts in the microbial community composition with increasing soil salinity have been suggested and research in functional interactions between plants and micro-organisms contributing to salt stress tolerance is gaining interest. Among others, microbial biosynthesis of polymers, exopolysaccharides, phytohormones and phytohormones-degrading enzymes could be involved.
Additional keywords: Archaea, Bacteria, fungi, microbial community, microbial–plant interaction, PGPB, salt stress.
Introduction
Saline habitats cover a wide area of our planet and halophytes, which are often able to grow in areas with salt concentration higher than ~400 mM (Flowers 2004; English and Colmer 2011), are increasingly used for human benefits. Salinity tolerance of these plants is genetically and physiologically very complex. It is based on genes whose effects are to limit the rate of salt uptake from the soil and the transport of salt through the plant, that adjust the ionic and osmotic balance of cells in roots and shoots, and that regulate leaf development and the onset of senescence (Munns 2005). Most studies on halophytes have concentrated solely on physiological and genetic regulation of salinity resistance. However, plant tolerance is also connected with complex ecological processes within their rhizosphere and phyllosphere. Thus, micro-organisms inhabiting roots and leaves of halophytes may contribute significantly to their well-being and salinity tolerance. Since microbial–plant interactions in saline habitats are sparsely reported, this review focuses on the contribution of micro-organisms to the plant salinity adaptation process. First, we give an overview of how micro-organisms adapt to high surrounding salinity since these mechanisms enable microbes to establish in the same habitats as halophytic plants. Next, we present a summary of studies aimed at characterising the halophyte microbiome. We highlight the modes of interaction between the micro-organisms and the plant’s rhizosphere and phyllosphere and conclude with a discussion on how these findings can be translated into agriculture.
Microbial salt tolerance and their adaptation mechanisms
Micro-organisms including fungi and prokaryotes, members of the two domains Bacteria and Archaea, are able to adapt to a range of changes in external osmolarity. Until recently it was assumed that under extreme salt concentrations at or near NaCl saturation, Archaea of the family Halobacteriaceae were the only active aerobic heterotrophs (Oren 2002). It is now suggested that bacteria also contribute to the aerobic heterotrophic prokaryotic community at the highest salt concentrations. Salt-tolerant bacteria and cyanobacteria have been isolated from a wide range of biotopes at all latitudes. A salt-tolerant bacterium, Staphylococcus xylosus, was isolated from a plant pickled in ~7.2% salt (Abou-Elela et al. 2010). Salt-tolerant bacterial strains isolated from an extreme alkali-saline soil in north-east China belong to the genera Bacillus, Nesterenkonia, Zhihengliuella, Halomonas, Stenotrophomonas, Alkalimonas and Litoribacter (Shi et al. 2012) and isolates from the desert of north-western China were identified as Mesorhizobium alhagi (Zhou et al. 2012). A new genus and species, Salinibacter ruber, an extremely halophilic bacterium, has been recently described and isolated from solar saltern crystalliser ponds in Alicante (Anton et al. 2002). Even a yeast-like fungus Hortaea werneckii is highly halophilic and survives in nearly salt-saturated solutions (Gunde-Cimerman et al. 2000).
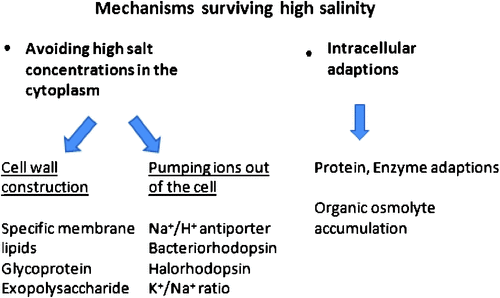
The mechanisms that allow micro-organisms to grow and survive in saline habitats are mostly similar among different taxa. The main strategies include avoiding high salt concentrations via specific membrane or cell wall constructions, pumping ions out of the cell by ‘salting out’ processes or adjusting their intracellular environment by accumulating non-toxic organic osmolytes and the adaptation of proteins and enzymes to high concentrations of solute ions (Fig. 1).
|
Cell wall constructions
The first strategy in surviving high salinity is to avoid high salt concentrations in the cytoplasm and to prevent water loss and plasmolysis through a specific cell wall construction and composition, as known for Archaea and for Cyanobacteria. The cytoplasmic membrane of a halophilic Archaea contains unique ether lipids that cannot easily be degraded, are temperature- and mechanically resistant and highly salt tolerant. Thermophilic and extreme acidophilic Archaea possess membrane-spanning tetraether lipids that form a rigid monolayer membrane that is nearly impermeable to ions and protons. These properties make the archaeal lipid membranes more suitable for life and survival in extreme environments than the ester-type bilayer lipids of Bacteria or Eukaryota (van de Vossenberg et al. 1998). The non-coccoid representatives of the Halobacteriales possess a cell wall of an S-layer, whose main constituent is a high molecular weight glycoprotein regularly arranged on a two dimensional lattice, with 4- or 6-fold symmetry. Glycoprotein makes up to 40–50% of the wall protein (Oren 2006) and requires high NaCl concentrations for stability. Similar to most other proteins of halophilic Archaea, the wall protein denatures when suspended in distilled water.
Cyanobacteria are surrounded by two membrane systems. The cell wall is enclosed by an outer membrane that surrounds the periplasmic space, whereas the cytoplasmic membrane surrounds the cytoplasm. Inside the cytoplasm, cyanobacteria contain a third membrane system, thylakoid membranes, which originate from the cytoplasmic membrane and contain the photosynthetic complex. The respiratory electron transport chain is also mostly situated at the thylakoid membranes (Hagemann 2011). In contrast with the heterotrophic prokaryotes, the mainly photoautotrophic cyanobacteria provide the enhanced energy demand for the extrusion of sodium by an increased photosynthetic activity (Joset et al. 1996). Halococcus species possess a thick sulfated heteropolysaccharide cell wall that does not require high salt concentrations to maintain its rigidity (Steber and Schleifer 1975). The coccoid Natronococcus occultus also has a thick cell wall that retains its shape in the absence of salt, but its structure differs greatly from that of the cell wall polymer of Halococcus, consisting of repeating units of a poly(L-glutamine) glycoconjugate (Niemetz et al. 1997). Several rhizobacterial species excrete massive amounts of exopolysacchrides which help to mitigate salinity stress by unknown processes (Upadhyay et al. 2011). The importance of extracellular polysaccharides (capsular and released polysaccharides) in reducing salt stress was demonstrated by Yoshimura et al. (2012) in the cyanobacterium Nostoc sp., where the composition ratio of sugars in the extracellular polysaccharide hardly changed under NaCl stress in comparison to normal culture conditions.
Fungal cell walls can become melanised and this has been commonly observed under abiotic stress, when it has been suggested to reduce the loss of compatible solutes (Plemenitaś et al. 2008). Comparing different fungi revealed the composition of cell membranes as another adaptation to hypersalinity. In contrast to halosensitive Saccharomyces cerevisiae and some halotolerant filamentous fungi, Hortaea werneckii is able to maintain its sterol-to-phospholipid ratio at a constant level (Turk et al. 2004). This is probably due to the expression of particular fatty acid-modifying enzymes upon salt stress (Gostinčar et al. 2009).
Pumping ions out of the cell
Several bioenergetics processes and ion pumps are involved in the regulation of intracellular ionic concentrations and osmotic adjustment. A proton electrochemical gradient is the driving force for the extrusion of Na+ from the cell, keeping intracellular Na+ concentrations relatively low, using Na+/H+ antiporter (Oren 2006). Membranes from halophilic Archaea possess a very high activity of an electrogenic Na+/H+ antiporter. A key role of Na+ exclusion by different types of Na+/H+ antiporters were also described for the cyanobacterium Synechococcus sp. PCC7942 (Waditee et al. 2002), the cyanobacterium Aphanothec halophytica (Wutipraditkul et al. 2005) and the bacterium Alkalimonas amylolytica (Zhong et al. 2012). In A. amylolytica the Na+/H+ antiporter encoding gene AaNhaD was able to increase salt tolerance in transgenic tobacco BY-2 cells indicating that AaNhaD even in plant cells functions as a pH-dependent tonoplast Na+/H+ antiporter, thus, presenting a new avenue for the genetic improvement of salinity/alkalinity tolerance (Zhong et al. 2012).
A crucial function of ion transporters is to maintain and retain favourable cytosolic K+/Na+ ratios in the face of low K+/Na+ ratios in the environment. K+ is an important monovalent cation inside the cell, where it is not only crucial for salt or turgor acclimation but is also involved in membrane energetics and the regulation of pH, enzyme activities and gene expression (Hagemann 2011). K+ and Na+ show similar physiological structures, as the smaller ion Na+ together with its rather large hydration shell mimics the size of K+. Therefore, uptake systems for K+ have difficulties discriminating between these ions, and high Na+ concentrations in the solution may result in K+ deficiency (Hagemann 2011). However, the much higher concentration of intracellular K+ than extracellular K+ points to an active uptake of this important cation from the solution. Possible regulatory systems are reviewed by Hagemann (2011). Several of the Dead Sea organisms possess unusual properties. A Halobacterium sp, has extremely high intercellular K+ concentration (up to 4.8 M) and extraordinary specificity for K+ over Na+. They adapt to the environment by adjusting their internal inorganic ionic strength, instead if ionic composition, to that of the medium (Nissenbaum 1975).
In contrast with the monovalent cations K+ and Na+, much less is known about the transport of Cl–. Similar to Na+, Cl– enters cyanobacterial cells in almost equimolar amounts after sudden salt shocks of ≥300 mM NaCl. Its export, however, is slower than that of Na+ (Reed et al. 1985). In E. coli, the majority of Cl– transport is done by H+/Cl– exchange transporter and not via channels (Accardi and Miller 2004).
As described for the prokaryotic organisms, fungi use two general strategies to deal with high salt concentrations in the environment. Exclusion of ions have been postulated for fungi, if they are exposed to high salt concentrations and this strategy seems to be more effective for the halophilic H. werneckii compared with the only halotolerant Aureobasidium pullulans (Kogej et al. 2005). Corresponding proteins or genes have not been yet identified, but in Saccharomyces cerevisiae gene duplication was detected mostly for genes encoding stress-responsive and transport proteins (Kondrashov et al. 2002).
Intracellular adaptations
The presence of high concentrations of solute ions is generally devastating to proteins and other macromolecules, causing aggregation to structural collapse. This is due to the enhancement of hydrophobic interactions, interference with essential electrostatic interactions within or between macromolecules because of charge shielding, and reducing the availability of free water below that required to sustain essential biological processes (Oren 2006). Therefore, the organisms activate various processes: (i) uptake and endogenous biosynthesis of compatible solutes, the nature and amount of which are strain- and salt concentration-dependent; (ii) increased energetic capacity; and (iii) protein and enzyme adaptations. Compatible solutes are low-molecular mass organic compounds, which usually do not have net charge and can be accumulated in high (molar) amounts without negatively interfering with cellular metabolism. The action of compatible solutes is currently best explained by the water exclusion hypothesis (Hagemann 2011). Freshwater cyanobacterial strains with low halotolerance accumulate sucrose or trehalose as their major compatible solutes. Moderately halotolerant (marine) strains are characterised by glycosylglycerol (GG) as their main compatible solute and sometimes glycosylglycerate (GGA) as a secondary compatible solute, whereas halophilic strains that are able to grow in saturated salt concentrations usually synthesise glycine betaine (GB) or glutamate betaine (Hagemann 2011). Only the mostly photoautotrophic cyanobacteria are described as improving their salt tolerance by an increased photosynthetic activity, which serves as energy source to meet the enhanced energy demand for the extrusion of Na+ (Joset et al. 1996).
Halophilic organisms have to maintain their protein structure and enzymatic activity at high salt concentrations. When comparing amino acid composition of proteins of the Halobacteriaceae to proteins from non-halophilic micro-organisms, they contain: (i) a large excess of the acidic amino acids glutamate and aspartate, (ii) a low content of the basic amino acids lysine and arginine, and (iii) a low content of hydrophobic amino acid residues, which is often offset by an increased content of the borderline hydrophobic amino acids serine and threonine (Lanyi 1974). A good reason for increasing the content of acidic amino acids may be the fact that glutamate has the greatest water binding ability of any amino acid residue. This may have important implications when considering the need of any functional protein to maintain a proper hydration shell (Oren 2006). Recently, Bardavid and Oren (2012) performed a comparative analysis of the genome sequences of anaerobic halophilic fermentative bacteria belonging to the order Halanaerobiales, the alkaliphilic Halanaerobium hydrogeniformans, and the thermophilic Halothermothrix orenii to assess the amino acid composition of their proteins. Earlier studies demonstrated that members of the Halanaerobiales accumulate KCl rather than organic compatible solutes for osmotic balance and, therefore, the presence of a dominantly acidic proteome was predicted. Past reports indeed showed a large excess of acidic over basic amino acids in whole-cell hydrolysates of selected members of the order. However, the genomic analysis rarely showed unusually high contents of acidic amino acids or low contents of basic amino acids. The apparent excess of acidic amino acids in these anaerobic halophiles reported earlier is due to the high content in their proteins of glutamine and asparagine, which yield glutamate and aspartate upon acid hydrolysis. It is thus suggested that the proteins of the Halanaerobiales, which are active in the presence of high intracellular KCl concentrations, do not possess the typical acidic signature of the ‘halophilic’ proteins of the Archaea of the order Halobacteriales or of the extremely halophilic bacterium.
Recent analyses of salt-induced changes in proteome maps revealed a more complex insight into the osmolyte accumulation processes of micro-organisms. For example, Halomonas sp. AAD12 showed significant variations in the expression of proteins involved in osmoregulation, stress response, energy generation and transport under salt stress (Ceylan et al. 2012).
Ceylan and colleagues (2012) measured an increase in proline and hydroxyectoine but a decrease in ectoine accumulation at elevated salinity. Fungi also accumulate compatible solutes for dealing with internal high salt concentrations. This has been analysed in the salt sensitive yeast S. cerevisiae and in some halotolerant filamentous fungi; it was shown that mostly glycerol is accumulated upon increasing salt concentrations (Blomberg and Adler 1992). Glycerol is also the compatible solute found in highest concentrations in the halophilic black yeast H. werneckii (Kogej et al. 2007). Other modifications upon exposure to high salt concentrations concern the microsomal HMG-CoA reductase (Vaupotic and Plemenitas 2007) and the glycerol-3-phosphate dehydrogenase (Lenassi et al. 2011). Non-targeted approaches have been also applied for elucidating mechanisms underlying the response of fungi to hypersalinity (e.g. Li et al. 2003). By using subtractive hybridisation, numerous H. werneckii genes that respond to moderately and extremely high salt concentrations were identified (Vaupotic and Plemenitas 2007). Among those, 13 did not show any similarity to sequences in databases suggesting very specific adaptations of the black yeast to hypersalinity. Further work could aim to discover the functions of the corresponding proteins. The mechanisms of how high salinity is sensed by fungal organisms has been investigated mainly in S. cerevisiae. The pathway involved is called ‘high osmolarity glycerol’ (HOG) and involves a well-studied mitogen-activated protein kinase cascade (Parmar et al. 2011). Such a sensing system has been also identified in H. werneckii (Plemenitaś et al. 2008). However, exactly how this contributes to the tolerance to extremely high salt concentrations is unclear.
Interaction of micro-organisms with host plants
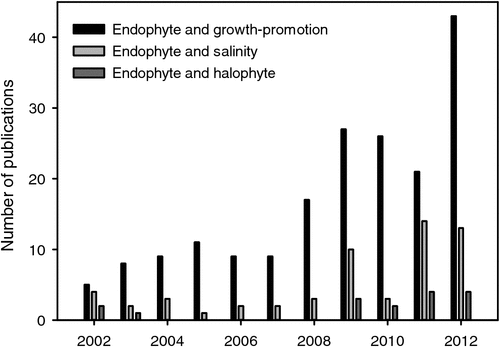
The microbiome of glycophytes under normal growth conditions or under salinity stress has become a research focus over the last 10 years (Rosenblueth and Martinez-Romero 2006). However, the prokaryotic community composition of halophytes has only rarely been investigated and the phyllosphere even more sparsely than the rhizosphere (Fig. 2). Therefore, here we can only refer to a limited number of investigations that tried to classify (taxonomically) and count microbial species and numbers (Table 1). The halophyte Halocnemum strobilaceum, naturally inhabiting hypersaline coastal areas of the Arabian Gulf, harbour up to 8.1 × 104 g–1 and 3 × 102 g–1 extremely halophilic oil-utilising micro-organisms in the rhizosphere and phyllosphere respectively (Al-Mailem et al. 2010). Frequent genera in the rhizosphere were affiliated to the Archaea Halobacterium sp. and Halococcus sp., the firmicute Brevibacillus borstenlensis, and the proteobacteria Pseudoalteromonas ruthenica and Halomonas sinaensis. The phyllospheric microflora consisted of the dimorphic yeast Candida utilis and the two proteobacteria Ochrobactrum sp. and Desulfovibrio sp. All the strains, except C. utilis, which could not tolerate salinities >2 M NaCl, grew also in media with salinities ranging between 1 and 4 M NaCl, with optimum growth between 1 and 2 M NaCl (Al-Mailem et al. 2010). That means bacteria inhabiting that halophytic plant rhizosphere and phyllosphere seem to be adapted to high salinities.
|

|
Similar salt-related adaptations were assumed in the rhizobium population of Acacia spp. (Thrall et al. 2009). Studies of Acacia spp. in Australia and Algeria, where the shrubby legumes dominate many ecosystems where dryland salinity is a major issue, suggested a high phylogenetic bacterial diversity (Thrall et al. 2009; Boukhatem et al. 2012). Based on 16S rRNA gene sequence comparisons, 48 isolates ranked into 10 phylogenetic groups representing five bacterial genera, namely, Ensifer, Mesorhizobium, Bradyrhizobium and Ochrobactrum (Boukhatem et al. 2012). The genetic identification of novel species suggested that the diversity of rhizobia associated with Australian Accacia spp. is significantly greater than previously expected and documented a community differentiation in relation to salt stress (Thrall et al. 2009). Rueda-Puente et al. (2010) detected, for the first time, Rhizobium spp. and Bacillus spp. species in the rhizosphere of the halophyte Salicornia bigelovii (Chenopodiaceae; now in the Amaranthaceae). Recently, the roots and rhizosphere of halophytes have been found to be inhabited by salt-tolerant bacteria. Haloferula luteola sp. nov., a gram-negative, non-spore-forming, endophytic (living within plant tissues) bacterium (strain YC6886t) was isolated from the root of the halophyte Rosa rugosa, which inhabits coastal areas of Namhae Island of the southern coast of Korea and grows optimally with 300–500 mM NaCl (Bibi et al. 2011). Another salt-tolerant (growth up to 680 mM NaCl) bacterial strain, Brachybacterium saurashtrense sp. nov., was isolated from the roots of Salicornia brachiata, an extreme halophyte (Gontia et al. 2011).
Most fungi detected in halophytes belong to the phylum Glomeromycota, which live with the roots of land plants in an obligate biotrophic symbiosis called arbuscular mycorrhiza (Newman and Reddell 1987). Although spore germination, hyphal growth and root colonisation of arbuscular mycorrhizal (AM) fungi is inhibited by high salt concentrations (Juniper and Abbott 1993; Juniper and Abbott 2006), plants in saline habitats can be highly colonised. The roots of, for example, sea aster (Aster tripolium) in marsh lands appeared to be fully colonised by AM fungal structures (Hildebrandt et al. 2001), but also plants from other saline ecosystems such as mangroves, in a river delta or in a desert riparian forest were colonised (Sengupta and Chaudhuri 2002; Wang et al. 2004; Yang et al. 2008). A survey along a salt gradient showed a negative correlation between salt concentrations and mycorrhization of roots of the saltbush Atriplex spp. (Aguilera et al. 1998). However, cluster analysis in a more detailed investigation revealed that not soil salinity and ion concentrations, but soil pH, the percentage of clay and available P correlates with AM fungal spore numbers (Aliasgharzadeh et al. 2001). Another factor that has to be taken into account in such habitats is drought, which can be more important than salt for the abundance of AM fungi in halophytes (Füzy et al. 2008). Attempts to identify the AM fungal species associated with plants in saline habitats revealed that at least in Europe, most isolates belong to the Glomus geosporum/Glomus caledonium cluster (Hildebrandt et al. 2001; Landwehr et al. 2002; Sonjak et al. 2009).
In addition to arbuscular mycorrhiza, some papers report the occurrence of so-called dark septate endophytes (DSEs). These root colonisers can be found in diverse habitats (Jumpponen and Trappe 1998) and have been detected in saline environments (Barrow and Aaltonen 2001; Sonjak et al. 2009). These associations can be obligate: it is, for example, impossible to remove DSEs from the fourwing salt bush even if the plants have been grown from calli (Barrow et al. 2004). Fungi of the order Sebacinales (Basidiomycota) are also present in roots of all ecosystems (Weiß et al. 2011) and further work is needed to screen plants growing in saline habitats for species of this order. One representative is the model endophyte Piriformospora indica, which has been isolated from the Thar desert in India (Verma et al. 1998). Therefore, it might also tolerate higher salt concentrations as it has been shown that it can confer salt tolerance to plants (Waller et al. 2005).
One study conducted in a Mediterranean salt marsh from semiarid south-eastern Spain to determine the influence of eight halophytes (Asteriscus maritimus, Arthrocnenium macrostachyum, Frankenia corymbosa, Halimione portulacoides, Limonium cossonianum, Limonium caesium, Lygeum spartum and Suaeda vera Forsskal) on the soil microbiological and biochemical properties of the rhizosphere (labile C fractions, biomass C, oxidoreductases, hydrolases) and aggregate stabilisation. Results showed that soil microbial activity and microbial-related soil properties, such as aggregate stability, were determined by the species of the halophyte (Caravaca et al. 2005). However, microbial community composition and microbial counts were not recorded. From the halophyte Prosopis strombulifera, grown under extreme salinity, 29 different bacterial strains were detected, which are grouped into seven clusters according to similarity (Sgroy et al. 2009).
From these first microbial investigations of halophytes it seems that, compared with glycophytes, an accumulation of specialised micro-organisms living in the rhizosphere and inside halophytic plants exists. Comprehensive studies and comparison of the halophyte and glycophyte microbiomes are required to evaluate that assumption. Currently the low number of investigations does not allow a general conclusion regarding the microbial impact on the environmental adaptation processes of halophytes. An improved understanding of the microbial community composition in halophilic plants, however, may open new opportunities to broaden plant growth in fragile environments.
Influence of micro-organisms on salinity tolerance of halophytes
The rhizosphere and phyllosphere of plants is colonised by a range of micro-organisms and growing investments in this research aims at unravelling the mechanisms underlying these beneficial or pathogenic interactions. Microbes are attracted to plant roots by a specific blend of root exudates containing sugars, amino acids and organic acids, which are assimilated and metabolised. The composition of actively and passively released primary and secondary metabolites varies with plant species, developmental stage and environmental conditions. It has been demonstrated that the plant influences the soil microbial communities and its colonising microbiota via the exudate composition (Bais et al. 2006; Faure et al. 2009; Doornbos et al. 2012). The beneficial effects provoked by endophytes result from nitrogen fixation, phytohormone production, supply of nutrients and pathogen suppression (Rosenblueth and Martinez-Romero 2006; Hardoim et al. 2008) and those mechanisms also account for the alleviating effects of micro-organisms when host plants face unfavourable environmental conditions. Two recent reviews are devoted to the protective microbial processes conferring abiotic stress tolerance to plants (Dimkpa et al. 2009; Dodd and Perez-Alfocea 2012). Halophytic plants harbour a variety of micro-organisms and in the following we summarise the contribution of micro-organisms to salt tolerance in this specific plant group.
Plant nutritional status
The growth-promoting effect of most micro-organisms emanates from their ability to increase the availability and uptake of nitrogen, carbon, and minerals. Bacteria residing in halophytic plants are known to (i) produce exopolysaccharide and form rhizosheaths, where mucilage binds a layer of sand grains tightly to the root, providing a special habitat for dinitrogen fixing bacteria (Bergmann et al. 2009); (ii) fix atmospheric nitrogen and provide ammonium for plant metabolic processes; and (iii) enrich C and N (Nabeel et al. 2010). Hence, the presence of the dinitrogenase reductase (nif) gene family is frequently used in microbiota screens for identification of beneficial strains (Juraeva et al. 2006). The nifH gene has been found in bacterial isolates from roots of Salicornia brachiata belonging to the genera Brachybacterium, Brevibacterium and Zhihengliuella (Jha et al. 2012).
Other micro-organisms are also referred to as biofertilisers because of their ability to solubilise phosphates and mobilise iron, thereby facilitating plant growth (Vessey 2003). Ca3(PO4)2, AlPO4 and FePO4 solubilising bacteria have been isolated from various halophytic plants, from the rhizosphere of four halophytic weeds grown in the Pakistanian Khewra salt range (Yasmin and Bano 2011), the mangrove Avicennia marina rhizosphere (El-Tarabily and Youssef 2010) and the oilseed halophyte Salicornia bigelovii (Bashan et al. 2000). Among the rarely identified bacterial strains are Arthrobacter, Bacillus (Banerjee et al. 2010), Azospirillum, Vibrio, Bacillus, Phyllobacterium species (Bashan et al. 2000) and Oceanobacillus picturae (El-Tarabily and Youssef 2010). When the oilseed halophyte Salicornia bigelovii was inoculated with eight species of halotolerant bacteria, including Azospirillum, Vibrio, Bacillus and Phyllobacterium, phosphate content in foliage increased compared with non-inoculated plants (Bashan et al. 2000). A screen of the mangrove Avicennia marina rhizosphere identified 129 bacterial strains with the ability to solubilise rock phosphate, with Oceanobacillus picturae being able to mobilise 97% of this mineral (El-Tarabily and Youssef 2010). These activities provide major components in ameliorating the growth-restraining effects of salinity.
The central feature of mycorrhizal symbiosis is the exchange of nutrients between the partners of the symbiosis. This enables plants to receive mineral nutrients even at high salt concentrations where usually the uptake is hampered (for review see Evelin et al. 2009). Mycorrhizal plants harbour higher phosphorus contents and are able to keep a better K+ : Na+ ratio than their non-mycorrhizal counterparts (Giri et al. 2007). For chloride ions, however, differing results are reported. Mycorrhization of roots can reduce their uptake of Cl– (Zuccarini and Okurowska 2008), but enhanced accumulation of Cl– has been also detected (Buwalda et al. 1983). Salt stress impacts on chlorophyll biosynthesis and one reason is the reduced uptake of magnesium. Giri et al. (2003) showed that this can be at least partially overcome in mycorrhizal plants. Mineral nutrition in the interaction between plants and other root-endophytic fungi is a matter of debate and the uptake of mineral nutrients by halophytes has not yet been specifically analysed.
Plant hormone status
The production or metabolism of plant hormones and their precursors is widely documented for plant-associated micro-organisms and it is an astonishing demonstration of the close evolution between plants and micro-organisms. A large body of physiological data related to the modulation of abscisic acid (ABA), auxins, cytokinins, ethylene, gibberellins, jasmonic acid (JA) and salicylic acid (SA) in plants upon infection with bacteria proves its significance to plant performance (Dodd et al. 2010).
The presence of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase is frequently described for halotolerant bacteria residing in plants. The enzyme converts the ethylene precursor ACC into ammonia and α-ketobutyrate, thus, lowering the ethylene concentration within plant tissues and its constraining effect on root elongation and general plant growth. A screen of 140 halotolerant bacterial isolates from coastal soil of the South Korea Yellow Sea for plant-growth-promoting effects led to the identification of 25 strains exhibiting different levels of ACC deaminase activity and those belonging to the genera of Arthrobacter, Bacillus, Brevibacterium, Corynebacterium, Exiguobacterium, Halomonas, Micrococcus, Oceanimonas, Planococcus and Zhihengliuella (Siddikee et al. 2010). Recent reports on halotolerant ACC-deaminase-producing bacteria isolated from halophytic plants indicate that this is an essential mechanism for salt stress alleviation. Six out of 29 endophytic strains isolated from the halophyte Prosopis strombulifera tested positive for ACC deaminase activity (Sgroy et al. 2009). Novel diazotrophic halotolerant bacteria from roots of Salicornia brachiata featured ACC deaminase activity and isolates included Brachybacterium saurashtrense, Brevibacterium casei, Cronobacter sakazakii, Haererehalobacter, Halomonas, Mesorhizobium, Pseudomonas, Rhizobium radiobacter, Vibrio and Zhihengliuella (Jha et al. 2012). Additionally, reinoculation of axenically cultured S. brachiata with Brachybacterium saurashtrense and Pseudomonas increased growth parameters significantly under salt stress. The screening of root, stalk and leaf of Suaeda salsa allowed for the identification of four ACC deaminase-containing strains: Pantoea agglomerans, Pseudomonas oryzihabitans, Pseudomonas putida, and Pseudomonas sp. (Teng et al. 2010). Sixty-two different bacterial isolates were extracted from the rhizosphere of the mangrove Avicennia marina. One of those, Pseudomonas maricaloris, showed high levels of ACC deaminase activity, decreased endogenous levels of ACC in seedlings under salinity stress and improved plant performance (El-Tarabily and Youssef 2011). A mutant strain of P. maricaloris without ACC deaminase failed to ameliorate the effects of salinity in mangrove seedlings, demonstrating the impact of modulating the plant’s ethylene status to confer salt tolerance.
Production of auxins, especially indole-3-acetic acid (IAA), is frequently found in growth-promoting endophytes (Witzel et al. 2012). Auxins play a cardinal role in elevation of root growth and development, and act antagonistically to ethylene. Thus, managing auxin production in halophytic plants by endophytic microbes might be an important tool in conferring salt tolerance. IAA production has been found in (i) salt tolerant rhizobacteria (Arthrobacter sp., Bacillus pumilus, Halomonas sp., Nitrinicola lacisaponensis, and Pseudomonas mendocina) isolated from highly saline habitats (Tiwari et al. 2011) and (ii) species of Bacillus, Brevundimonas, Exiguobacterium, Halobacillus, Oceanobacillus, Serratia, Staphylococcus and Vibrio originating from four halotolerant plants from a Chinese coastal sandbank (Bian et al. 2011). Production of IAA, ABA and gibberellins has also been identified in as yet unclassified bacterial strains isolated from the rhizosphere of halophytic weeds from the Pakistani Khewra salt range (Naz et al. 2009) as well as from the halophyte Prosopis strombulifera (Piccoli et al. 2011). Tiwari and colleagues (2011) demonstrated an increase in the fitness of wheat plants grown in salt-affected soil when they were inoculated with salt tolerant IAA producing rhizobacteria.
Mycorrhizal fungi exert impact on nearly all phytohormones and this has been shown for jasmonate and abscisic acid (Hause et al. 2007; Herrera-Medina et al. 2007). The involvement of phytohormones in the interaction between plants and the root endophyte Piriformospora indica is also well investigated (Qiang et al. 2012). The role of phytohormones for increasing salt tolerance has, however, neither been analysed for mycorrhizal not for other root endophytic fungi.
Plant antioxidant status
Plants have evolved a range of tools in order to protect their cells from reactive oxygen species formed on the onset of osmotic and salt stress. Oxidation of membrane lipids, proteins or DNA is prevented by scavenging enzymes, including superoxide dismutase, catalase, and ascorbate peroxidase. Micro-organisms use similar approaches to cope with oxidative stress. A recent review focuses on the mediation of reactive oxygen species in plants by fungal endophytes (Hamilton et al. 2012). It is likely that micro-organisms use the same mechanisms to alleviate salt stress effects in halophytes as in other plants; however, detailed information remains scarce. The halophytic Sesuvium portulacastrum was grown on sterilised and non-sterilised soil during salinity treatment. Plants grown on non-sterilised soil, containing bacteria from the genera Bacillus, Aeromonas, Pseudomonas, Corynebacterium and Escherichia, revealed lower levels of antioxidant enzymes indicating a reduced degree of oxidative stress (Anburaj et al. 2012). Organisms described to alleviate salinity in halophytes are likewise known to colonise glycophytes and often they are described as improving plant growth and plant immunity against pathogens and abiotic stress (Shin et al. 2007; Bibi et al. 2012).
The production of antioxidants and the expression of enzymes involved in scavenging of reactive oxygen species is probably the mechanism most analysed in seeking explanations of how mycorrhizal fungi are able to increase abiotic stress tolerance of plants (Porcel et al. 2012). Mycorrhizal plants contain higher concentrations of ascorbate and glutathione and harbour a greater activity of superoxide dismutase, catalase and ascorbate peroxidase than the corresponding non-mycorrhizal control plants (Wu et al. 2010; Borde et al. 2011; Latef and Chaoxing 2011). Since plants show this response to mycorrhization even before they are treated with high salt concentrations, one can suggest that colonisation of the roots by an AM fungus makes the plants more tolerant to high soil salinity and also to other osmotic stresses. Similar responses have been observed in plants when colonised by the non-mycorrhizal endophyte P. indica where it was proposed as being involved in general abiotic stress tolerance (Baltruschat et al. 2008).
Production of exopolysaccharides
Both plant and the plant colonising bacteria may produce exopolysaccharides as a mechanism to shield the root from excessive salt concentrations. Mangroves and some other halophytic plants produce a mixture of viscous exopolysaccharides, so called mucilage, in xylem vessels of roots and shoots that have high water-binding capacity and may act as water reserves during exposure to salinity. Production of mucilage in the halophyte Kosteletzkya virginica is positively correlated with salt levels and composition of polysaccharides differs between plant organs and treatments (Ghanem et al. 2010). Equally, micro-organisms produce exopolysaccharides, mainly during biofilm formation (Danese et al. 2000), but also during the establishment of symbiotic interactions (Jones et al. 2007). Microbial biofilms are found on roots of every terrestrial plant (Danhorn and Fuqua 2007) and proper adhesion of microbes to roots through exopolysaccharides may also aid shielding the root form excessive salt concentrations. Exopolysaccharide-producing bacterial strains, including Aeromonas hydrophila/caviae and Bacillus sp., were isolated from roots of salt-adapted wheat plants and reinoculation restricted the Na+ uptake by roots (Ashraf et al. 2004). Similarly, exopolysaccharide-producing rhizobacteria isolated from salt-adapted wheat plants reduced the plants Na+ availability and conferred salt tolerance upon inoculation in stress experiments (Upadhyay et al. 2011). Although the application of mucilage-producing bacteria might be a promising tool in alleviating salt stress effects, many of the underlying mechanisms still remain unresolved.
Biocontrol
The induction of plant systemic resistance by growth-promoting micro-organisms through various pathways led to the application of selected strains for diseases control (van Loon et al. 1998). Mechanisms of how microbes reduce plant diseases include production of antibiotics, competition with pathogens for nutrients, and induction of systemic resistance in the host (Lugtenberg and Kamilova 2009). A screen of 17 phyllosphere endophytes isolated from the Karangkadu mangrove ecosystem in India identified eight strains with a broad spectrum of antibacterial activity (Sundaram et al. 2011a, 2011b). Induction of resistance against pathogens has been also described for the interaction of plant roots with mycorrhizal fungi (Jung et al. 2012), with dark septate endophytes (Andrade-Linares et al. 2011) and with the endophyte Piriformospora indica (Waller et al. 2005). Although a large body of data was generated for the trilateral relationship between pathogens, growth-promoting microbes, and glycophytes, respective investigations concerning interactions with halophytes are scarce (Fig. 2). As the use of micro-organisms to counteract pathogen attack is environmentally friendly and locally effective, more research activity in this field is expected within the next years.
Other mechanisms
Another important mechanism of facing osmotic stress is the accumulation of organic solutes and this has been also found in halophytes (Flowers and Colmer 2008); consequently the amino acid proline has been the subject of research into understanding increases in salt tolerance after colonisation of plants with endophytes. Results with AM fungi, however, have been variable and suggest that proline accumulation is, in most cases, not the cause but the effect of salt tolerance (Ruiz-Lozano et al. 2012). Osmoregulation can be also achieved with betaines and with sugars. Both are increased in mycorrhizal plants and were suggested as being involved in salt tolerance (Porcel and Ruiz-Lozano 2004; Manchanda and Garg 2011).
In the case of fungi, the growth of hyphae in the rhizosphere has a particular impact on soil physical properties (Augé et al. 2001). Soil aggregates are more stable if colonised with hyphae, this in turn influences water retention (Rillig et al. 2010) and ultimately has an impact on the tolerance of plants to osmotic stresses (Augé et al. 2007).
Agronomic and environmental prospects
Comparative population studies indicate that plants in different habitats contain different microbiomes (Berendsen et al. 2012). The identification of halophyte-associated bacteria and fungi, the analysis of their interaction with the host and how this interaction contributes to the survival of both partners will be essential to develop strategies for protecting these plants. Most examples of increased salt tolerance are reported where glycophytes have been inoculated with plant growth promoting bacteria or mycorrhizal fungi (Nabti et al. 2010; del Amor and Cuadra-Crespo 2012; Dodd and Perez-Alfocea 2012). Many experiments have been conducted with standard inocula meaning that the micro-organisms being used were not well adapted to hypersaline conditions. Direct comparisons, however, showed (for example, for mycorrhizal fungi) that an isolate from a saline habitat is more able to improve the tolerance of a plant than a corresponding reference strain (Ruiz-Lozano and Azcon 2000). Therefore, it seems necessary to exploit the potential of halophytes and their inhabitants because they could be a valuable resource for plant production systems where soils or irrigation water contain high salt concentrations. This will be a prerequisite for expanding efficient plant production areas into salt affected soils and to get less dependent on fresh water, which will be increasingly important in future agriculture in many regions of the world.
Acknowledgements
This research was supported by the COST action FA 0901 ‘Putting halophytes to work – from genes to ecosystems’. The authors would like to thank Professor Tim Flowers for his critical reading and very helpful comments.
References
Abou-Elela SI, Kamel MM, Fawzy ME (2010) Biological treatment of saline wastewater using a salt-tolerant microorganism. Desalination 250, 1–5.| Biological treatment of saline wastewater using a salt-tolerant microorganism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVCqtr%2FP&md5=27c11237d681e2e370689a384c30b0b0CAS |
Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl– channels. Nature 427, 803–807.
| Secondary active transport mediated by a prokaryotic homologue of ClC Cl– channels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsFCis7o%3D&md5=f7cffb37a38cac33d92af01ff834e2eaCAS | 14985752PubMed |
Aguilera LE, Gutierrez JR, Moreno RJ (1998) Vesiculo arbuscular mycorrhizae associated with saltbushes Atriplex spp. (Chenopodiaceae) in the Chilean arid zone. Revista Chilena de Historia Natural 71, 291–302.
Al-Mailem D, Sorkhoh N, Marafie M, Al-Awadhi H, Eliyas M, Radwan S (2010) Oil phytoremediation potential of hypersaline coasts of the Arabian Gulf using rhizosphere technology. Bioresource Technology 101, 5786–5792.
| Oil phytoremediation potential of hypersaline coasts of the Arabian Gulf using rhizosphere technology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlt1Sisbg%3D&md5=b2c5bccb62d86bdac6715d31cd8fb09eCAS | 20303746PubMed |
Aliasgharzadeh N, Rastin NS, Towfighi H, Alizadeh A (2001) Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 11, 119–122.
| Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFWls78%3D&md5=9b47394ed83c667093f5ee80f81284efCAS |
Anburaj R, Nabeel MA, Sivakumar T, Kathiresan K (2012) The role of rhizobacteria in salinity effects on biochemical constituents of the halophyte Sesuvium portulacastrum. Russian Journal of Plant Physiology: a Comprehensive Russian Journal on Modern Phytophysiology 59, 115–119.
| The role of rhizobacteria in salinity effects on biochemical constituents of the halophyte Sesuvium portulacastrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1KrtLbO&md5=8d00da5b9314a20beae4bd5c95ac9b65CAS |
Andrade-Linares DR, Grosch R, Restrepo S, Krumbein A, Franken P (2011) Effects of dark septate endophytes on tomato plant performance. Mycorrhiza 21, 413–422.
| Effects of dark septate endophytes on tomato plant performance.Crossref | GoogleScholarGoogle Scholar | 21184117PubMed |
Anton J, Oren A, Benlloch S, Rodriguez-Valera F, Amann R, Rossello-Mora R (2002) Salinibacter ruber gen. nov., sp nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. International Journal of Systematic and Evolutionary Microbiology 52, 485–491.
Ashraf M, Hasnain S, Berge O, Mahmood T (2004) Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biology and Fertility of Soils 40, 157–162.
| Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVChurc%3D&md5=95ed027abe8a0cf5c11cea3b3df6581dCAS |
Augé RM, Stodola AJW, Tims JE, Saxton AM (2001) Moisture retention properties of a mycorrhizal soil. Plant and Soil 230, 87–97.
| Moisture retention properties of a mycorrhizal soil.Crossref | GoogleScholarGoogle Scholar |
Augé RM, Toler HD, Moore JL, Cho K, Saxton AM (2007) Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo. Journal of Plant Physiology 164, 1289–1299.
| Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo.Crossref | GoogleScholarGoogle Scholar | 17189660PubMed |
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms. Annual Review of Plant Biology 57, 233–266.
| The role of root exudates in rhizosphere interations with plants and other organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKhtr8%3D&md5=20e1fca4ab1fb03870d350786ee981dfCAS | 16669762PubMed |
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel K-H, Schäfer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytologist 180, 501–510.
| Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlKru73E&md5=ef12151c25e3cff5aea1ab8fd5a81f52CAS | 18681935PubMed |
Banerjee S, Palit R, Sengupta C, Standing D (2010) Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Australian Journal of Crop Science 4, 378–383.
Bardavid RE, Oren A (2012) The amino acid composition of proteins from anaerobic halophilic bacteria of the order Halanaerobiales. Extremophiles 16, 567–572.
| The amino acid composition of proteins from anaerobic halophilic bacteria of the order Halanaerobiales.Crossref | GoogleScholarGoogle Scholar |
Barrow JR, Aaltonen RE (2001) Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza 11, 199–205.
| Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and the influence of host physiological activity.Crossref | GoogleScholarGoogle Scholar |
Barrow JR, Osuna-Avila P, Reyes-Vera I (2004) Fungal endophytes intrinsically associated with micropropagated plants regenerated from native Bouteloua eriopoda Torr. and Atriplex canescens (Pursh) Nutt. In Vitro Cellular & Developmental Biology. Plant 40, 608–612.
| Fungal endophytes intrinsically associated with micropropagated plants regenerated from native Bouteloua eriopoda Torr. and Atriplex canescens (Pursh) Nutt.Crossref | GoogleScholarGoogle Scholar |
Bashan Y, Moreno M, Troyo E (2000) Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biology and Fertility of Soils 32, 265–272.
| Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotFyqtrc%3D&md5=033c29997ccaaaabec6eb1607e561baeCAS |
Berendsen RL, Pieterse CMJ, Bakker P (2012) The rhizosphere microbiome and plant health. Trends in Plant Science 17, 478–486.
| The rhizosphere microbiome and plant health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xms12rs70%3D&md5=0b509cdc9868e9b5274fd32bef56e6b3CAS | 22564542PubMed |
Bergmann D, Zehfus M, Zierer L, Smith B, Gabel M (2009) Grass rhizosheaths: Associated bacterial communities and potential for nitrogen fixation. Western North American Naturalist 69, 105–114.
| Grass rhizosheaths: Associated bacterial communities and potential for nitrogen fixation.Crossref | GoogleScholarGoogle Scholar |
Bian G, Zhang Y, Qin S, Xing K, Xie H, Jiang J (2011) Isolation and biodiversity of heavy metal tolerant endophytic bacteria from halotolerant plant species located in coastal shoal of Nantong. Acta Microbiologica Sinica 51, 1538–1547.
Bibi F, Chung EJ, Yoon HS, Song GC, Jeon CO, Chung YR (2011) Haloferula luteola sp nov., an endophytic bacterium isolated from the root of a halophyte, Rosa rugosa, and emended description of the genus Haloferula. International Journal of Systematic and Evolutionary Microbiology 61, 1837–1841.
| Haloferula luteola sp nov., an endophytic bacterium isolated from the root of a halophyte, Rosa rugosa, and emended description of the genus Haloferula.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Gmu7vL&md5=122a28729a78b99f5721196ecd508a8eCAS | 20817839PubMed |
Bibi F, Chung EJ, Khan A, Jeon CO, Chung YR (2012) Rhizobium halophytocola sp nov., isolated from the root of a coastal dune plant. International Journal of Systematic and Evolutionary Microbiology 62, 1997–2003.
| Rhizobium halophytocola sp nov., isolated from the root of a coastal dune plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVKrs73I&md5=0cfc4ae8439fe83de117388d46375eb5CAS | 22021574PubMed |
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Advances in Microbial Physiology 33, 145–212.
| Physiology of osmotolerance in fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhs1CktL8%3D&md5=96771dcd7c73d732a09f7af9b1377a28CAS | 1636508PubMed |
Borde M, Dudhane M, Jite P (2011) Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Protection 30, 265–271.
| Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvV2qsL8%3D&md5=01196037b0a9cdd146cfad631d1a7515CAS |
Boukhatem ZF, Domergue O, Bekki A, Merabet C, Sekkour S, Bouazza F, Duponnois R, de Lajudie P, Galiana A (2012) Symbiotic characterization and diversity of rhizobia associated with native and introduced acacias in arid and semi-arid regions in Algeria. FEMS Microbiology Ecology 80, 534–547.
| Symbiotic characterization and diversity of rhizobia associated with native and introduced acacias in arid and semi-arid regions in Algeria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvVKju7g%3D&md5=f4a69c17b65dcbfccdd67c25290c6239CAS | 22283876PubMed |
Buwalda JG, Stribley DP, Tinker PB (1983) Increased uptake of bromide and chloride by plants infected with vesicular-arbuscular mycorrhizas. New Phytologist 93, 217–225.
| Increased uptake of bromide and chloride by plants infected with vesicular-arbuscular mycorrhizas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXhslKnu7g%3D&md5=914e5128f63837e41dccbc957ce08067CAS |
Caravaca F, Aiguacil MM, Torres P, Roldan A (2005) Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 124, 375–382.
| Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVKmtbbM&md5=2464ff67f5f809b27573038a6bc25c06CAS |
Ceylan S, Yilan G, Akbulut BS, Poli A, Kazan D (2012) Interplay of adaptive capabilities of Halomonas sp AAD12 under salt stress. Journal of Bioscience and Bioengineering 114, 45–52.
| Interplay of adaptive capabilities of Halomonas sp AAD12 under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlyltL7J&md5=c842d1bbcfad61b3c8804de5763b047aCAS | 22575437PubMed |
Danese PN, Pratt LA, Kolter R (2000) Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. Journal of Bacteriology 182, 3593–3596.
| Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjvFajsrc%3D&md5=ad29c461c5c478f53f4a13b8c5be50f8CAS | 10852895PubMed |
Danhorn T, Fuqua C (2007) Biofilm formation by plant-associated bacteria. In ‘Annual Review of Microbiology. Vol. 61’. pp. 401–422. (Annual Reviews: Palo Alto, CA)
del Amor FM, Cuadra-Crespo P (2012) Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Functional Plant Biology 39, 82–90.
| Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVWmsw%3D%3D&md5=528332df3e1671c4b49eaaad347eeb12CAS |
Dimkpa C, Weinand T, Asch F (2009) Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell & Environment 32, 1682–1694.
| Plant-rhizobacteria interactions alleviate abiotic stress conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFKrsrvI&md5=05882d36e703a4c6a0253cec0cdc076dCAS |
Dodd IC, Perez-Alfocea F (2012) Microbial amelioration of crop salinity stress. Journal of Experimental Botany 63, 3415–3428.
| Microbial amelioration of crop salinity stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVSitLg%3D&md5=d72f8ecff9957b6234d90c204c0fb5a8CAS | 22403432PubMed |
Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Annals of Applied Biology 157, 361–379.
| Rhizobacterial mediation of plant hormone status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFelsrvL&md5=af2305d7a6690c804fe96f31c36b02d2CAS |
Doornbos RF, van Loon LC, Bakker P (2012) Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agronomy for Sustainable Development 32, 227–243.
| Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review.Crossref | GoogleScholarGoogle Scholar |
El-Tarabily KA, Youssef T (2010) Enhancement of morphological, anatomical and physiological characteristics of seedlings of the mangrove Avicennia marina inoculated with a native phosphate-solubilizing isolate of Oceanobacillus picturae under greenhouse conditions. Plant and Soil 332, 147–162.
| Enhancement of morphological, anatomical and physiological characteristics of seedlings of the mangrove Avicennia marina inoculated with a native phosphate-solubilizing isolate of Oceanobacillus picturae under greenhouse conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntlGgs78%3D&md5=db500bc494a2380ab23a2cd190cd7a75CAS |
El-Tarabily KA, Youssef T (2011) Improved growth performance of the mangrove Avicennia marina seedlings using a 1-aminocyclopropane-1-carboxylic acid deaminase-producing isolate of Pseudoalteromonas maricaloris. Plant Growth Regulation 65, 473–483.
| Improved growth performance of the mangrove Avicennia marina seedlings using a 1-aminocyclopropane-1-carboxylic acid deaminase-producing isolate of Pseudoalteromonas maricaloris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVaqtb%2FO&md5=c12cfcfe0684ba2c27acf82e340edb9aCAS |
English JP, Colmer TD (2011) Salinity and waterlogging tolerances in three stem-succulent halophytes (Tecticornia species) from the margins of ephemeral salt lakes. Plant and Soil 348, 379–396.
| Salinity and waterlogging tolerances in three stem-succulent halophytes (Tecticornia species) from the margins of ephemeral salt lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Gnt7rO&md5=ea9302da98eb9a46cf34793ee92ed653CAS |
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of Botany 104, 1263–1280.
| Arbuscular mycorrhizal fungi in alleviation of salt stress: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVKnsLjK&md5=4e0f51c6efaf79a134e13b5a2667c81aCAS | 19815570PubMed |
Faure D, Vereecke D, Leveau JHJ (2009) Molecular communication in the rhizosphere. Plant and Soil 321, 279–303.
| Molecular communication in the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1enurc%3D&md5=d13ce9aa569de71742977156dafdae99CAS |
Flowers TJ (2004) Improving crop salt tolerance. Journal of Experimental Botany 55, 307–319.
| Improving crop salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXms1egtQ%3D%3D&md5=ba62fd0b22f1d3b5393d8ae4f78ee465CAS | 14718494PubMed |
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqur%2FE&md5=06dcabcbf1411c34a35d7aed78f04afbCAS | 18565144PubMed |
Füzy A, Bíro B, Toth T, Hildebrandt U, Bothe H (2008) Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. Journal of Plant Physiology 165, 1181–1192.
| Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar | 18155803PubMed |
Ghanem ME, Han RM, Classen B, Quetin-Leclerq J, Mahy G, Ruan CJ, Qin P, Perez-Alfocea F, Lutts S (2010) Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress. Journal of Plant Physiology 167, 382–392.
| Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFSks7w%3D&md5=b2b4216a4944678e2b05aa120abcc48fCAS |
Giri B, Kapoor R, Mukerji KG (2003) Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis Biology and Fertility of Soils 38, 170–175.
| Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis Crossref | GoogleScholarGoogle Scholar |
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microbial Ecology 54, 753–760.
| Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1amtLbE&md5=689136374665c3a6ff893a1b3081fb43CAS | 17372663PubMed |
Gontia I, Kavita K, Schmid M, Hartmann A, Jha B (2011) Brachybacterium saurashtrense sp nov., a halotolerant root-associated bacterium with plant growth-promoting potential. International Journal of Systematic and Evolutionary Microbiology 61, 2799–2804.
| Brachybacterium saurashtrense sp nov., a halotolerant root-associated bacterium with plant growth-promoting potential.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslyjsL8%3D&md5=0b744de9d37c58fb333f84b88246ca0cCAS | 21216918PubMed |
Gostinčar C, Turk M, Plemenitaš A, Gunde-Cimerman N (2009) The expressions of Δ9-, Δ12-desaturases and an elongase by the extremely halotolerant black yeast Hortaea werneckii are salt dependent. FEMS Yeast Research 9, 247–256.
| The expressions of Δ9-, Δ12-desaturases and an elongase by the extremely halotolerant black yeast Hortaea werneckii are salt dependent.Crossref | GoogleScholarGoogle Scholar | 19220869PubMed |
Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitas A (2000) Hypersaline waters in salterns – natural ecological niches for halophilic black yeasts. FEMS Microbiology Ecology 32, 235–240.
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiology Reviews 35, 87–123.
| Molecular biology of cyanobacterial salt acclimation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1Snsg%3D%3D&md5=01bafd36763d04613d15922782531905CAS | 20618868PubMed |
Hamilton CE, Gundel PE, Helander M, Saikkonen K (2012) Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Diversity 54, 1–10.
| Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review.Crossref | GoogleScholarGoogle Scholar |
Hardoim PR, van Overbeek LS, van Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends in Microbiology 16, 463–471.
| Properties of bacterial endophytes and their proposed role in plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SmsrzM&md5=5a6d0b540f135ceaf9e60dfad7ca644fCAS | 18789693PubMed |
Hause B, Mrosk C, Isayenkov S, Strack D (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68, 101–110.
| Jasmonates in arbuscular mycorrhizal interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsFChug%3D%3D&md5=daab11b868d3e6e57e4b13c73bf8ce05CAS | 17097695PubMed |
Herrera-Medina MJ, Steinkellner S, Vierheilig H, Bote JAO, Garrido JMG (2007) Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytologist 175, 554–564.
| Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFSqsbk%3D&md5=9ec1a3d5e4f77a665ee1e57fc562718eCAS | 17635230PubMed |
Hildebrandt U, Janetta K, Ouziad F, Renne B, Nawrath K, Bothe H (2001) Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 10, 175–183.
| Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvFyqtg%3D%3D&md5=e82ea09672673de5aff7b2c29d1d2b11CAS |
Jha B, Gontia I, Hartmann A (2012) The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant and Soil 356, 265–277.
| The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVWlu7g%3D&md5=4ea8a012a5fa5bd132a2f2a8d5b8f8bdCAS |
Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC (2007) How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nature Reviews. Microbiology 5, 619–633.
| How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns12htbc%3D&md5=feb84cc8d622cca5d9a2a6f4b76165eaCAS | 17632573PubMed |
Joset F, Jeanjean R, Hagemann M (1996) Dynamics of the response of cyanobacteria to salt stress: deciphering the molecular events. Physiologia Plantarum 96, 738–744.
| Dynamics of the response of cyanobacteria to salt stress: deciphering the molecular events.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xisleqt78%3D&md5=9740371d927c787b68ceee3581f6e42bCAS |
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytologist 140, 295–310.
| Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi.Crossref | GoogleScholarGoogle Scholar |
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. Journal of Chemical Ecology 38, 651–664.
| Mycorrhiza-induced resistance and priming of plant defenses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xptl2lu7g%3D&md5=45a7b61cfddcf924fab9341541d872f2CAS | 22623151PubMed |
Juniper S, Abbott L (1993) Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza 4, 45–57.
| Vesicular-arbuscular mycorrhizas and soil salinity.Crossref | GoogleScholarGoogle Scholar |
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16, 371–379.
| Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28vit1Oltg%3D%3D&md5=906be3c26527b9290f25c6f096fe16d3CAS | 16525784PubMed |
Juraeva D, George E, Davranov K, Ruppel S (2006) Detection and quantification of the nifH gene in shoot and root of cucumber plants. Canadian Journal of Microbiology 52, 731–739.
| Detection and quantification of the nifH gene in shoot and root of cucumber plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVKnsb3N&md5=7af55870a607d98a218185fd393beeafCAS | 16917531PubMed |
Kogej T, Ramos J, Plemenitas A, Gunde-Cimerman N (2005) Halophilic fungus Hortaea werneckii and the halotolerant fungus Aureobasidium pullulans maintain low intracellular cation concentrations in hypersaline environments. Applied and Environmental Microbiology 71, 6600–6605.
| Halophilic fungus Hortaea werneckii and the halotolerant fungus Aureobasidium pullulans maintain low intracellular cation concentrations in hypersaline environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1ektbbI&md5=cf77c70e748966e34a95f737b0e43bcfCAS | 16269687PubMed |
Kogej T, Stein M, Volkmann M, Gorbushina AA, Galinski EA, Gunde-Cimerman N (2007) Osmotic adaptation of the halophilic fungus Hortaea werneckii: role of osmolytes and melanization. Microbiology 153, 4261–4273.
| Osmotic adaptation of the halophilic fungus Hortaea werneckii: role of osmolytes and melanization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmt1Q%3D&md5=5f3c220d29201e13979857d6f9c08ee0CAS | 18048939PubMed |
Kondrashov FA, Rogozin IB, Wolf YI, Koonin EV (2002) Selection in the evolution of gene duplications. Genome Biology 3, research0008–research0008.9.
| Selection in the evolution of gene duplications.Crossref | GoogleScholarGoogle Scholar | 11864370PubMed |
Landwehr M, Hildebrandt U, Wilde P, Nawrath K, Toth T, Biro B, Bothe H (2002) The arbuscular mycorrhizal fungus Glomus geosporum in European saline, sodic and gypsum soils. Mycorrhiza 12, 199–211.
| The arbuscular mycorrhizal fungus Glomus geosporum in European saline, sodic and gypsum soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xmt1WisL0%3D&md5=afa544c3c20b835517c664d3b8c8ad8cCAS | 12189475PubMed |
Lanyi JK (1974) Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriological Reviews 38, 272–290.
Latef AAHA, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Scientia Horticulturae 127, 228–233.
| Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress.Crossref | GoogleScholarGoogle Scholar |
Lenassi M, Zajc J, Gostincar C, Gorjan A, Gunde-Cimerman N, Plemenitas A (2011) Adaptation of the glycerol-3-phosphate dehydrogenase GPD1 to high salinities in the extremely halotolerant Hortaea werneckii and halophilic Wallemia ichthyophaga. Fungal Biology 115, 959–970.
| Adaptation of the glycerol-3-phosphate dehydrogenase GPD1 to high salinities in the extremely halotolerant Hortaea werneckii and halophilic Wallemia ichthyophaga.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ajtLrP&md5=16e1d9b825778727432edd5aee4a0761CAS | 21944208PubMed |
Li JX, Steen H, Gygi SP (2003) Protein profiling with cleavable isotope-coded affinity tag (cICAT) reagents – the yeast salinity stress response. Molecular & Cellular Proteomics 2, 1198–1204.
| Protein profiling with cleavable isotope-coded affinity tag (cICAT) reagents – the yeast salinity stress response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsFGgu7o%3D&md5=f6f8718112067b47286636a0c0f3b34eCAS |
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting Rhizobacteria. In ‘Annual Review of Microbiology. Vol. 63.’ pp. 541–556. (Annual Reviews: Palo Alto, CA)
Manchanda G, Garg N (2011) Alleviation of salt-induced ionic, osmotic and oxidative stresses in Cajanus cajan nodules by AM inoculation. Plant Biosystems 145, 88–97.
| Alleviation of salt-induced ionic, osmotic and oxidative stresses in Cajanus cajan nodules by AM inoculation.Crossref | GoogleScholarGoogle Scholar |
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytologist 167, 645–663.
| Genes and salt tolerance: bringing them together.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVGisbfP&md5=a2d23c6d5faa06931cb8a05ae3e289bcCAS | 16101905PubMed |
Nabeel MA, Kathiresan K, Rajendran N, Ohnishi H, Hamaoka H, Omori K (2010) Contribution by microbes to the foodweb of a mangrove biotope: the approach of carbon and nitrogen stable isotopes. African Journal of Marine Science 32, 65–70.
| Contribution by microbes to the foodweb of a mangrove biotope: the approach of carbon and nitrogen stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Nabti E, Sahnoune M, Ghoul M, Fischer D, Hofmann A, Rothballer M, Schmid M, Hartmann A (2010) Restoration of growth of durum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca. Journal of Plant Growth Regulation 29, 6–22.
| Restoration of growth of durum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFWltLo%3D&md5=dfbbd709e0b980b2501abb0aa9aa6d2cCAS |
Naz I, Bano A, Tamoor Ul H (2009) Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. African Journal of Biotechnology 8, 5762–5768.
Newman EI, Reddell P (1987) The distribution of mycorrhizas among families of vascular plants. New Phytologist 106, 745–751.
| The distribution of mycorrhizas among families of vascular plants.Crossref | GoogleScholarGoogle Scholar |
Niemetz R, Karcher U, Kandler O, Tindall BJ, Konig H (1997) The cell wall polymer of the extremely halophilic archaeon Natronococcus occultus. European Journal of Biochemistry 249, 905–911.
| The cell wall polymer of the extremely halophilic archaeon Natronococcus occultus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntl2hsbY%3D&md5=d9d12bc33055564414ea82e147b70c94CAS | 9395342PubMed |
Nissenbaum A (1975) The microbiology and biogeochemistry of the Dead Sea. Microbial Ecology 2, 139–161.
| The microbiology and biogeochemistry of the Dead Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28Xht1KmsLc%3D&md5=e6076b1fba065ee29432f7c63861ed8cCAS |
Oren A (2002) Molecular ecology of extremely halophilic Archaea and Bacteria. FEMS Microbiology Ecology 39, 1–7.
| Molecular ecology of extremely halophilic Archaea and Bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhslWitr0%3D&md5=37642b9f5e2fec9c5f881e2b556509adCAS | 19709178PubMed |
Oren A (2006) The order Halobacteriales. In ‘The prokaryotes. Vol. 3.’ (3rd edn) (Eds M Dworkin, S Falkow, E Rosenberg, K-H Schleifer, E Stackebrandt) pp. 113–164. (Springer: Singapore)
Parmar JH, Bhartiya S, Venkatesh KV (2011) Characterization of the adaptive response and growth upon hyperosmotic shock in Saccharomyces cerevisiae. Molecular BioSystems 7, 1138–1148.
| Characterization of the adaptive response and growth upon hyperosmotic shock in Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFGhtrc%3D&md5=1b009fbee34f0e472761c0efa7297fccCAS | 21234493PubMed |
Piccoli P, Travaglia C, Cohen A, Sosa L, Cornejo P, Masuelli R, Bottini R (2011) An endophytic bacterium isolated from roots of the halophyte Prosopis strombulifera produces ABA, IAA, gibberellins A(1) and A(3) and jasmonic acid in chemically-defined culture medium. Plant Growth Regulation 64, 207–210.
| An endophytic bacterium isolated from roots of the halophyte Prosopis strombulifera produces ABA, IAA, gibberellins A(1) and A(3) and jasmonic acid in chemically-defined culture medium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlvVSktL8%3D&md5=08699017d6b3fdde24c8dce5f91dfb62CAS |
Plemenitaś A, Vaupotić T, Lenassi M, Kogej T, Gunde-Cimerman N (2008) Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: a molecular perspective at a glance. Studies in Mycology 61, 67–75.
| Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: a molecular perspective at a glance.Crossref | GoogleScholarGoogle Scholar | 19287528PubMed |
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany 55, 1743–1750.
| Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntValt7s%3D&md5=35d293f469e0c354d54be0d021b72666CAS | 15208335PubMed |
Porcel R, Aroca R, Ruiz-Lozano JM (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agronomy for Sustainable Development 32, 181–200.
| Salinity stress alleviation using arbuscular mycorrhizal fungi. A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFSitLk%3D&md5=bc20cf28a96c86f52c9f21aa8e126535CAS |
Qiang X, Weiss M, Kogel KH, Schafer P (2012) Piriformospora indica a mutualistic basidiomycete with an exceptionally large plant host range. Molecular Plant Pathology 13, 508–518.
| Piriformospora indica a mutualistic basidiomycete with an exceptionally large plant host range.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVant7nP&md5=80d00e29e5568373e197fd087fef704fCAS | 22111580PubMed |
Reed RH, Warr SRC, Richardson DL, Moore DJ, Stewart WDP (1985) Multiphasic osmotic adjustment in a euryhaline cyanobacterium. FEMS Microbiology Letters 28, 225–229.
| Multiphasic osmotic adjustment in a euryhaline cyanobacterium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXltF2rsbY%3D&md5=a60e738f16f9d0a6ffda844c8d19d00fCAS |
Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010) Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biology & Biochemistry 42, 1189–1191.
| Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVeiu7k%3D&md5=1e1b7254bd7f8346f4bfa1ec45f947f8CAS |
Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Molecular Plant-Microbe Interactions 19, 827–837.
| Bacterial endophytes and their interactions with hosts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnslOqsr0%3D&md5=1bcd3fb6ff644731e9c0eef7ee88ef95CAS | 16903349PubMed |
Rueda-Puente E, Castellanos-Cervantes T, Diaz de Leon-Alvarez J, Preciado-Rangel P, Almaguer-Vargas G (2010) Bacterial community of rhizosphere associated to the annual halophyte Salicornia bigelovii (Torr.). Terra Latinoamericana 28, 345–353.
Ruiz-Lozano JM, Azcon R (2000) Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 10, 137–143.
| Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXovVOnsbg%3D&md5=8141da4190e9da1672284538600a703aCAS |
Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany 63, 4033–4044.
| Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSlsLnM&md5=ea27eb4288e2704c0e11f64210f30efaCAS | 22553287PubMed |
Sengupta A, Chaudhuri S (2002) Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 12, 169–174.
| Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India.Crossref | GoogleScholarGoogle Scholar | 12189470PubMed |
Sgroy V, Cassan F, Masciarelli O, Del Papa MF, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Applied Microbiology and Biotechnology 85, 371–381.
| Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlKhtrnP&md5=8114ee99e1024d755df29071bc0c40acCAS | 19655138PubMed |
Shi W, Takano T, Liu S (2012) Isolation and characterization of novel bacterial taxa from extreme alkali-saline soil. World Journal of Microbiology & Biotechnology 28, 2147–2157.
| Isolation and characterization of novel bacterial taxa from extreme alkali-saline soil.Crossref | GoogleScholarGoogle Scholar |
Shin D-S, Park MS, Jung S, Lee MS, Lev KH, Bae KS, Kim SB (2007) Plant growth-promoting potential of endophytic bacteria isolated from roots of coastal sand dune plants. Journal of Microbiology and Biotechnology 17, 1361–1368.
Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. Journal of Microbiology and Biotechnology 20, 1577–1584.
| Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVChsb0%3D&md5=69327d698f279ab8de5701c086d6476fCAS | 21124065PubMed |
Sonjak S, Udovic M, Wraber T, Likar M, Regvar M (2009) Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Secovlje salterns. Soil Biology & Biochemistry 41, 1847–1856.
| Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Secovlje salterns.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVenu7%2FP&md5=85d7d32ceb80db80b05d85cf5769682eCAS |
Steber J, Schleifer KH (1975) Halococcus morrhuae sulfated heteropolysaccharide as structural component of bacterial cell wall Archives of Microbiology 105, 173–177.
| Halococcus morrhuae sulfated heteropolysaccharide as structural component of bacterial cell wallCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XjvF2msw%3D%3D&md5=9c2cfbb4bb4618432219aa8d05ff88a1CAS | 1200739PubMed |
Sundaram R, Inbaneson SJ, Muthu U, Priya SR, Andy R, Banerjee MB (2011a) Diversity of endophytic actinomycetes from Karangkadu mangrove ecosystem and its antibacterial potential against bacterial pathogens. Journal of Pharmacy Research 4, 294–296.
Sundaram R, Inbaneson SJ, Muthu U, Ramakrishnan K, Andy R, Banerjee MB, Jayaprakasham R (2011b) Antibacterial activity of heterotrophic endophytes from Karangkadu mangrove ecosystem, India. Journal of Pharmacy Research 4, 195–198.
Teng S, Liu Y, Zhao L (2010) Isolation, identification and characterization of ACC deaminase-containing endophytic bacteria from halophyte Suaeda salsa. Weishengwu Xuebao 50, 1503–1509.
Thrall PH, Broadhurst LM, Hoque MS, Bagnall DJ (2009) Diversity and salt tolerance of native Acacia rhizobia isolated from saline and non-saline soils. Austral Ecology 34, 950–963.
| Diversity and salt tolerance of native Acacia rhizobia isolated from saline and non-saline soils.Crossref | GoogleScholarGoogle Scholar |
Tiwari S, Singh P, Tiwari R, Meena KK, Yandigeri M, Singh DP, Arora DK (2011) Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biology and Fertility of Soils 47, 907–916.
| Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlCmtbbJ&md5=e616f427d85b666e4497b565b2f33d10CAS |
Turk M, Mejanelle L, Sentjurc M, Grimalt JO, Gunde-Cimerman N, Plemenitas A (2004) Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles 8, 53–61.
| Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXos1ertA%3D%3D&md5=f041e2c0a08738369312453839773a62CAS | 15064990PubMed |
Upadhyay S, Singh J, Singh D (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21, 214–222.
| Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVyrsL0%3D&md5=9cfb1c5b5ec06005d4bb7ed0118f1cc1CAS |
van de Vossenberg JLCM, Driessen AJM, Konings WN (1998) The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles 2, 163–170.
| The essence of being extremophilic: the role of the unique archaeal membrane lipids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtVOqtrc%3D&md5=944a4d5b99ccfef80e21e2a97250d1e5CAS |
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology 36, 453–483.
| Systemic resistance induced by rhizosphere bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtlaltLo%3D&md5=4bbed2849d26d40d3388148e203845a5CAS | 15012509PubMed |
Vaupotic T, Plemenitas A (2007) Differential gene expression and HogI interaction with osmoresponsive genes in the extremely halotolerant black yeast Hortaea werneckii. BMC Genomics 8, 280
| Differential gene expression and HogI interaction with osmoresponsive genes in the extremely halotolerant black yeast Hortaea werneckii.Crossref | GoogleScholarGoogle Scholar | 17705830PubMed |
Verma S, Varma A, Rexer KH, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütehorn B, Franken P (1998) Piriformospora indica, gen. nov. sp. nov., a new root-colonizing fungus. Mycologia 90, 896–903.
| Piriformospora indica, gen. nov. sp. nov., a new root-colonizing fungus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmvFGntLk%3D&md5=ba844a645995ee61b3d01ae4e64ab8f1CAS |
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255, 571–586.
| Plant growth promoting rhizobacteria as biofertilizers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXns1Ojsr0%3D&md5=b8236efc70c9661453e1e21da8436942CAS |
Waditee R, Hibino T, Nakamura T, Incharoensakdi A, Takabe T (2002) Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proceedings of the National Academy of Sciences of the United States of America 99, 4109–4114.
| Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xis1Kks78%3D&md5=87864016ce575865a65540edae3ea32aCAS | 11891307PubMed |
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, Franken P, Kogel K-H (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proceedings of the National Academy of Sciences of the United States of America 102, 13 386–13 391.
| The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVygsbnJ&md5=ca8ebe026aacab08dbf42f8d90875ad2CAS |
Wang FY, Liu RJ, Lin XG, Zhou JM (2004) Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 14, 133–137.
| Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta.Crossref | GoogleScholarGoogle Scholar | 12827474PubMed |
Weiß M, Sykorova Z, Garnica S, Riess K, Martos F, Krause C, Oberwinkler F, Bauer R, Redecker D (2011) Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PLoS ONE 6, e16 793
| Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes.Crossref | GoogleScholarGoogle Scholar |
Witzel K, Gwinn-Giglio M, Nadendla S, Shefchek K, Ruppel S (2012) Genome sequence of Enterobacter radicincitans DSM16656T, a plant growth-promoting endophyte. Journal of Bacteriology 194, 5469
| Genome sequence of Enterobacter radicincitans DSM16656T, a plant growth-promoting endophyte.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWlu7%2FP&md5=8c42fe381c0e8e6ac733919db8ab8723CAS | 22965092PubMed |
Wu QS, Zou YN, Liu W, Ye XF, Zai HF, Zhao LJ (2010) Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: changes in leaf antioxidant defense systems. Plant, Soil and Environment 56, 470–475.
Wutipraditkul N, Waditee R, Incharoensakdi A, Hibino T, Tanaka Y, Nakamura T, Shikata M, Takabe T (2005) Halotolerant cyanobacterium Aphanothece halophytica contains NapA-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH. Applied and Environmental Microbiology 71, 4176–4184.
| Halotolerant cyanobacterium Aphanothece halophytica contains NapA-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXoslGitr4%3D&md5=6b4bcd101b5b79f556f05df8ba4e08d3CAS | 16085800PubMed |
Yang YH, Chen YN, Li WH (2008) Arbuscular mycorrhizal fungi infection in desert riparian forest and its environmental implications: a case study in the lower reach of Tarim River. Progress in Natural Science 18, 983–991.
| Arbuscular mycorrhizal fungi infection in desert riparian forest and its environmental implications: a case study in the lower reach of Tarim River.Crossref | GoogleScholarGoogle Scholar |
Yasmin H, Bano A (2011) Isolation and characterisation of phosphate solubilizing bacteria from rhizosphere soil of weeds of Khewra salt range and attock. Pakistan Journal of Botany 43, 1663–1668.
Yoshimura H, Kotake T, Aohara T, Tsumuraya Y, Ikeuchi M, Ohmori M (2012) The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance. Journal of Applied Phycology 24, 237–243.
| The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjt1Wms78%3D&md5=d7de11b333aa9d650b69db92071aed25CAS |
Zhong NQ, Han LB, Wu XM, Wang LL, Wang F, Ma YH, Xia GX (2012) Ectopic expression of a bacterium NhaD-type Na+/H+ antiporter leads to increased tolerance to combined salt/alkali stresses. Journal of Integrative Plant Biology 54, 412–421.
| Ectopic expression of a bacterium NhaD-type Na+/H+ antiporter leads to increased tolerance to combined salt/alkali stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Wksb7O&md5=cda376b9e42e5fb0250dede889d048deCAS | 22583823PubMed |
Zhou M, Chen W, Chen H, Wei G (2012) Draft genome sequence of Mesorhizobium alhagi CCNWXJ12–2(Tau), a novel salt-resistant species isolated from the desert of north-western China. Journal of Bacteriology 194, 1261–1262.
| Draft genome sequence of Mesorhizobium alhagi CCNWXJ12–2(Tau), a novel salt-resistant species isolated from the desert of north-western China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtV2it7o%3D&md5=9b533996c9fa7b3d54c2536da06e4468CAS | 22328758PubMed |
Zuccarini P, Okurowska P (2008) Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress. Journal of Plant Nutrition 31, 497–513.
| Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXislWju7g%3D&md5=5355f3fdb8c14d42cc9718b796f94f32CAS |


