Plant cell walls: the skeleton of the plant world
Monika S. Doblin A , Filomena Pettolino A and Antony Bacic A B CA Plant Cell Biology Research Centre, School of Botany, University of Melbourne, Vic. 3010, Australia.
B Australian Centre for Plant Functional Genomics, School of Botany, University of Melbourne, Vic. 3010, Australia.
C Corresponding author. Email: abacic@unimelb.edu.au
Functional Plant Biology 37(5) 357-381 https://doi.org/10.1071/FP09279
Submitted: 16 November 2009 Accepted: 2 February 2010 Published: 30 April 2010
Abstract
Plants are our major source of renewable biomass. Since cell walls represent some 50% of this biomass, they are major targets for biotechnology. Major drivers are their potential as a renewable source of energy as transport fuels (biofuels), functional foods to improve human health and as a source of raw materials to generate building blocks for industrial processes (biobased industries). To achieve sustainable development, we must optimise plant production and utilisation and this will require a complete understanding of wall structure and function at the molecular/biochemical level. This overview summarises the current state of knowledge in relation to the synthesis and assembly of the wall polysaccharides (i.e. the genes and gene families encoding the polysaccharide synthases and glycosyltransferases (GlyTs)), the predominant macromolecular components. We also touch on an exciting emerging role of the cell wall–plasma membrane–cytoskeleton continuum as a signal perception and transduction pathway allowing plant growth regulation in response to endogenous and exogenous cues.
Additional keywords: cell wall–plasma membrane–cytoskeleton continuum, glycosyltransferase, polysaccharide structure and biosynthesis, synthase.
Plant cell walls
A distinguishing feature of plant cells is the presence of a polysaccharide-rich wall. The wall encloses each cell while at the same time allowing the transfer of solutes and signalling molecules between cells via specific structures such as plasmodesmata (symplastic transport), or pores within the gel-like matrix of the wall itself (apoplastic movement). Similar to the skeleton of animals, the plant wall is a key determinant of overall plant form, growth and development. In contrast to having a specialised skeletal system as occurs in animals, the shape and strength of plants rely on the properties of the wall. The wall also plays a significant role in plant defence and responses to environmental stresses. Plant walls are important not simply because they are integral to plant growth and development but also because they determine the quality of plant-based products. The texture, nutritional and processing properties of plant-based foods for human and animal consumption is heavily influenced by the characteristics of the wall. Fibre for textiles, pulp and paper manufacture, and timber products and increasingly for fuel and composite manufacture is largely composed of walls, or derived from them. Due to their relevance to these industries, the chemical structures of the constituent polymers (polysaccharides, (glyco)proteins, polyphenolics) and the biochemical processes involved in the synthesis, maturation and turnover of the wall have been the subjects of research for many years. This research is now intensifying with the prospect of using plant ligno-cellulosic biomass as a viable and sustainable alternative to fossil fuels in the production of transportation fuels. Advances are likely to occur through molecular biological and functional genomics approaches, including public availability of genome sequences for various species including Arabidopsis, Populus, Vitus (grapevine), Oryza (rice), Brachypodium and Sorghum, coupled with extensive, associated genomics resources. To date, a combination of these and traditional biochemical technologies has facilitated the identification of genes and enzymes involved in wall biosynthesis and is likely to continue to generate progress in the years to come. This review will cover current work that is being undertaken to elucidate the genes and gene families encoding polysaccharide synthases and glycosyltransferases (designated GlyTs) required for the synthesis of wall polysaccharides. The processes involved in polysaccharide modification by non-glycosyl constituents (e.g. methylation, acetylation and phenolic acids) and wall modification by wall-residing proteins and enzymes, although immensely important, are outside the scope of this review. We will also briefly overview an exciting emerging role of the wall as an external sensor of the environment and the proposed mechanisms by which the external signals are sensed/perceived and then transduced across the plasma membrane (PM) to the cytoskeleton/cytoplasm where signalling pathways are activated. Many important aspects of wall biology, including their dynamic re-modelling during growth and development as well as in response to abiotic and biotic stresses are omitted but are critical to understanding the roles of walls in these processes. We recommend some excellent reviews covering these topics (Baskin 2005; Cosgrove 2005; Obroucheva 2008; Szymanski and Cosgrove 2009).
Cell wall structure
Primary plant walls are composed predominantly of a complex array of polysaccharides (~90%) and some protein (~10%). In all cell-types, rigid cellulose microfibrils are embedded in a gel-like matrix of non-cellulosic polysaccharides and pectins. These polysaccharides are intimately associated with one another, both non-covalently and covalently, and often with proteins and lignins. Walls are complex, diverse and dynamic, changing throughout the processes of cell division, growth and differentiation. The types of polysaccharides present in the wall vary depending on the plant species, cell type and location, developmental stage and history of responses to biotic and abiotic stresses (Carpita et al. 2001b; Trethewey and Harris 2002). Growing cells are surrounded by thin (100 nm or less), highly hydrated (~60% of wet weight) primary walls that are sufficiently flexible to yield to the hydrostatic forces exerted by the protoplast that drives growth. Primary walls of dicots, gymnosperms and non-commelinoid monocots (commonly refered to as Type I walls) consist of a cellulosic network embedded in a matrix of complex polysaccharides, of which xyloglucans (XyGs) and pectic polysaccharides are most abundant (Fig. 1a; Fincher and Stone 1986; Bacic et al. 1988; Carpita and Gibeaut 1993; McCann and Roberts 1994). Primary walls of the Poales and related commelinoid monocots (commonly refered to as Type II walls) are organised in essentially the same way except that glucuronoarabinoxylans (GAXs) and (1,3;1,4)-β-d-glucans predominate in the matrix phase of these species, and levels of pectic polysaccharides and XyGs are relatively low (Bacic et al. 1988; Carpita and Gibeaut 1993; McCann and Roberts 1994; Smith and Harris 1999). The concept of Type I/II walls has been extremely useful to guide wall researchers. However, it is now becoming somewhat redundant as surveys across the plant kingdom, particularly driven by the availability of high-throughput antibody profiling techniques of wall polymers (Moller et al. 2007), suggests a continuum of wall structures rather than clearly delineated wall types (Harris et al. 1997; Harris 2005).

|
Cells that have ceased enlarging and are required to withstand large compressive forces mature by depositing secondary walls (up to several microns thick) that increase the strength of the cell and reduce flexibility (Fig. 1b). During secondary wall development cellulose, and matrix phase polysaccharides with a lower degree of backbone substitution, such as heteroxylans and heteromannans, are deposited in a highly ordered pattern. Together with the deposition of lignin this results in a dehydration of the wall compartment, which becomes increasingly hydrophobic in nature. In some cell types, lignin is also deposited throughout the wall during secondary development. Hydrophobic lignins overlie and encrust the cellulose microfibrils and matrix phase polysaccharides, and can also be covalently complexed with wall polysaccharides. To date much effort has centred on manipulating the composition and levels of lignins. This work is extensive and important to understanding wall structure and biosynthesis (Boerjan et al. 2003; Boudet et al. 2003; Vanholme et al. 2008), but lignin biology will not be addressed here.
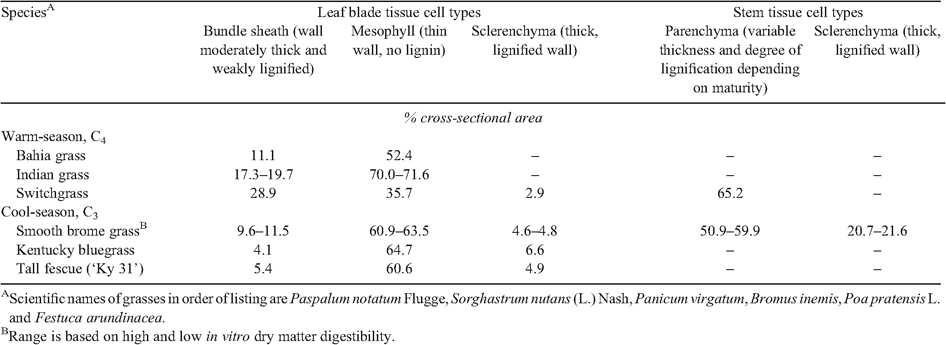
The lignin–polysaccharide associations (together with the dehydration process described above) are factors contributing to the ‘recalcitrance’ of lignified walls to enzymatic digestion and must be broken as a preliminary to the enzymatic digestion, or ‘deconstruction’ phase in ‘cellulosic’ bioethanol production and in ruminant digestion. The more thoroughly this can be achieved, the higher the yields of fermentable sugars. Given the surge in interest in plant biomass for bioethanol production it is important to recognise that the degree of ‘recalcitrance’ is not only influenced by the chemistry of the cell walls but also by the anatomatical structures of the cells and tissues that are the primary feedstock. Although these factors are only now being considered for biofuels research, the exhaustive research findings from fibre digestion and utilisation of forage crops (particularly grasses and legumes) by ruminant animals provides some useful guidance. A summary of the chemical composition of the walls of the plant groups that are the major sources of feedstocks is in Table 1 and summarised in an excellent review by Harris and Stone (2008). The extent and type of lignification is a major factor affecting degradability and, in general, non-lignified walls are easily degraded whereas lignified walls are much less degradable (Table 2; Buxton and Redfearn 1997; Wilson and Hatfield 1997). Other physical and chemical structures, such as the presence of waxes/cuticles and a high proportion of cells with a high surface area : volume ratio (e.g. thick-walled parenchyma and sclerenchyma) all contribute to a lower degradability (Table 2). Plant anatomy at the cellular level also affects degradability and different cell types and their proportions influences outcomes (e.g. Chesson 1993; Buxton and Redfearn 1997; Wilson and Hatfield 1997). Pretreatment of feedstocks that cause either physical disruption of the tissue/cells (e.g. high pressure/temperature), chemical modification (e.g. alkali causes both non-covalent (swelling and dissociation of polysaccharide chains) and covalent (ester saponification) modification) are essential in mitigating the influence of these anatomical and chemical characteristics of different feedstocks on enzymic saccharification.

|
|
The basic structures of plant wall polysaccharides are depicted schematically in Fig. 2. Cellulose microfibrils are composed of bundles of ~36 linear (1,4)-β-glucan chains (Fig. 2a) held tightly together by hydrogen bonding (Somerville 2006). The width, degree of polymerisation and crystallinity of the cellulose microfibrils are highly variable and depend on the source and age of the tissue (Bacic et al. 1988). The molecular backbone of XyGs (Fig. 2b) is similar in structure to cellulose, but the xylosyl (Xylp) side chains and their extensions prevent hydrogen bonding along the length of the substituted chain yielding a more flexible and soluble macromolecule. The side chains of XyGs are quite diverse and vary in frequency and structure depending on the tissue and species (Fry et al. 1993; Peña et al. 2008). The (1,3;1,4)-β-glucans (Fig. 2c) are also similar in structure to cellulose, except that single (1,3)-β-d-Glcp residues are located between blocks of multiple (1,4)-β-d-Glcp residues, introducing ‘kinks’ into the polysaccharide chain. Adjacent (1,4)-β-d-oligoglucosyl residues (cellodextrin units) usually consist of cellotriosyl and cellotetraosyl units (~90% of the molecule) arranged at random with longer cellodextrin units (DP 5–20) comprising the remainder of the polysaccharide (Fincher 2009a).

|
Heteroxylans (Fig. 2d) are composed of a backbone of (1,4)-β-linked Xylp of which ~10% are substituted at C(O)3 and C(O)2 with α-l-arabinofuranosyl (Araf) residues, α-d-glucuronic acid (GlcpA; and 4-O-methyl GlcpA) or a combination of both to give arabinoxylans (AXs), (methyl)glucuronoxylan (GXs) and glucuronarabinoxylans (GAXs), respectively. GXs are the most abundant non-cellulosic polysaccharides in dicot secondary walls whereas GAXs are minor components of the secondary walls of soft woods. In comparison, AXs are the predominant xylans in grass cell walls, with GAXs being less abundant (York and O’Neill 2008). The backbone Xylp residues can also be acetylated at the C(O)3 and C(O)2 positions or sometimes both (York and O’Neill 2008). The Ara sidechains of the grass wall heteroxylans can be esterified with ferulic and/or p-coumaric acid, enabling crosslinking between polysaccharide molecules and between polysaccharides and lignin (Iiyama et al. 1993). GXs from birch, spruce and Arabidopsis, but not grasses, have been shown to contain a distinct glycosyl sequence 4)-β-d-Xylp-(1,4)-β-d-Xylp-(1,3)-α-l-Rhap-(1,2)-α-d-GalpA-(1,4)-d-Xylp at the reducing end (Peña et al. 2007; York and O’Neill 2008). Heteroxylans comprise approximately one-third of the biomass of wood and as the current microorganisms (yeast) are unable to ferment pentoses into alcohol, their conversion to a fermentable sugar or the discovery of microorganisms that can efficiently utilise heteroxylans as a carbon source is now a high priority to make ligno-cellulosics an effective and sustainable form of alternate transport fuels (Somerville 2006).
The heteromannans (Fig. 2e) consist of the (1,4)-β-mannopyranosyl (Manp)-containing family of wall polysaccharides including mannans, galactomannans, glucomannans and galactoglucomannans. Galactomannans are polymers consisting of a backbone of (1,4)-β-Manp that can be substituted at C(O)6 with β-d-Galp. In glucomannans, the (1,4)-β-Manp backbone is interrupted by (1,4)-β-Glcp residues at irregular intervals. Glucomannans can be acetylated at C(O)2 or C(O)3 of the Manp residues and also substituted with α-d-Galp attached through (1,6)-linkages (galactoglucomannan) (Matheson 1990).
Pectins comprise some of the most complex families of polysaccharides and include the homogalacturonans (Fig. 2f) (HG; homopolymers of (1,4)-α-GalpA); xylogalacturonan (XG; HG substituted at C(O)3 with one or two (1,4)-β-d-Xylp residues); rhamnogalacturonan I (Fig. 2g) (RGI; backbone of [-α-d-GalA-(1,2)-α-l-Rha-(1–4)-]n with Rha residues substituted at C(O)2 or C(O)4 with linear or branched polymers of (1,4)-β-d-galactan, (1,5)-α-arabinan, type I arabino-4-galactans, type II (arabino-3,6-galactans or smaller oligosaccharides containing α-l-Araf, β-d-Galp, or α-l-Fucp); and rhamnogalacturonan II (Fig. 2h) (RGII; short HG backbone substituted with complex side chains containing combinations of Rhap, GalpA, GlcpA, Api, Fucp, Galp, MeXylp, Kdo, Dha and Ace) (Bacic 2006; Mohnen 2008). Structural complexity is increased by the addition of acetyl and methyl groups to GalpA, Fucp, Xylp and Ace residues, which impact on the physical characteristics and molecular interactions within the wall and in industrial applications. Chemical evidence suggests that these pectic polysaccharides are covalently linked to one another, and to other wall polysaccharides (Mohnen 2008) although the physical arrangement of these different molecules within the wall is unknown.
Wall (glyco)proteins, which comprise less than 10% of the dry weight of the primary wall, are critical to the structural and functional elements of both the wall and the PM–cytoskeleton continuum (see below). In addition to serving a structural role, wall proteins contribute to wall assembly and remodelling during growth and development, and in stress responses. Such proteins include enzymes such as hydrolases, proteases, glycosidases, peroxidases, esterases, as well as expansins, wall-associated kinases (WAKs), hydroxyproline-rich glycoproteins (HRGPs) that include extensins, arabinogalactan-proteins (AGPs) and proline-rich proteins (PRPs) and the glycine-rich proteins (GRPs). Bioinformatic analysis of genomic sequences has revealed that most of these wall proteins are encoded by multigene families. A detailed discussion of the wall proteins is beyond the scope of this overview and readers are referred to several recent comprehensive reviews (Rose 2003).
Since the AGPs are implicated in sensing at the cell surface a brief description of their structure is provided. AGPs are highly glycosylated proteoglycans, usually containing 1–10% protein (Johnson et al. 2003). AGP protein backbones are typically rich in Hyp/Pro, Ala, Ser and Thr. The Hyp residues are usually substituted by type II arabino-3,6-galactans (AGs; 5–25 kDa), although short arabinosides are found (Nothnagel 1997). Most AGPs are anchored, sometimes transiently, to the plasma membrane by glycosylphosphatidylinositol (GPI) anchors (Youl et al. 1998). AGPs are secreted from plant cells in which they are either anchored to the plasma membrane or are soluble in the extracellular matrix or cell wall. In Arabidopsis thaliana (L.), at least 47 genes are predicted to encode AGP protein backbones, and most of these are predicted to be GPI anchored (Johnson et al. 2003). These have been divided into several subclasses on the basis of their biased amino acid composition and their modular structure, and include 13 classical AGPs, 12 AG-peptides (mature protein backbones of 10–17 amino acids), three basic AGPs that include a short Lys-rich region and 21 FLAs (fasciclin-like AGPs) that have, in addition to predicted AGP-like glycosylated regions, putative cell adhesion domains known as fasciclin domains (Johnson et al. 2003; Schultz et al. 2004). The recent identification of a salt overly sensitive5 (sos5) mutant with a mis-sense mutation in the fasciclin domain of AtFLA4 (Shi et al. 2003) indicates that these domains are important for FLA function.
Biosynthesis of wall polysaccharides
Polysaccharide synthesis can be broken down into four distinct stages: (1) production of activated nucleotide-sugar donors, (2) initiation of polymer synthesis, (3) polymer elongation, and (4) termination of synthesis (Delmer and Stone 1988). Both the initiation and termination of synthesis are poorly understood whereas there has been significant progress on the production of nucleotide sugar donors and the polymer elongation processes. The key enzymes in wall biogenesis are the polysaccharide synthases and glycosyltransferases (GlyTs) that catalyse formation of the bonds between adjacent monosaccharides from activated nucleotide-sugar donors and are the focus of this review. The specificity of these enzymes determines the sequence of sugar residues within a polysaccharide, and the branching pattern. Figure 3 shows where different polysaccharides are synthesised in an idealised plant cell.

|
The polysaccharides, cellulose and the (1,3)-β-d-glucan callose, are synthesised by polysaccharide synthases directly at the PM (Kudlicka and Brown 1997; Delmer 1999). The rosette, the enzyme complex responsible for cellulose synthesis, has a diameter of ~25 nm and comprises six identical, spherical subunits that aggregate together in a hexagonal arrangement, forming a central pore through which the glucan chains are extruded (Fig. 3). Rosettes have been visualised in transit from the Golgi apparatus (GA) to their destination in the PM using freeze fracture, transmission electron microscopy, and more recently immunofluorescence techniques (Haigler and Brown 1986; Gardiner et al. 2003; Somerville 2006; Wightman et al. 2009). In contrast, all other non-cellulosic and pectic polysaccharides are synthesised within the endoplasmic reticulum (ER) and GA (Fincher and Stone 1981; Gibeaut and Carpita 1994) using both polysaccharide synthases and GlyTs. The assembled polysaccharides are then transported to the cell surface via Golgi-derived secretory vesicles and incorporated into the wall. Recent progress in understanding the assembly of non-cellulosic polysaccharides suggests that this process may occur in both the Golgi compartment and within the apoplast (see below).
Polysaccharide synthases/glycosyltransferases
Sequence similarity, the presence of certain motifs, hydrophobic cluster analysis (HCA) and catalytic specificity, have been used to categorise polysaccharide synthases and GlyTs into more than 90 families within the overall ‘glycosyltransferase’ (GT) class of carbohydrate modifying enzymes collated in the Carbohydrate Active enZymes (CAZy) database (Cantarel et al. 2009). Whilst the human genome estimated to contain ~200 GT genes (Scheible and Pauly 2004), in Arabidopsis and rice (Oryza sativa L.), currently 455 and 565 genes encode CAZy-classified GTs, respectively (July 22, 2009). The sequences of both species are grouped into the same 41 families. This large number of GTs is thought to contribute to the structural and tissue-specific complexity of plant polysaccharides and glycoconjugates (Coutinho et al. 2003). The function/s and biochemical activities of most of the Arabidopsis and other plant GTs have not yet been characterised.
GTs are broadly divided into two enzyme classes, the type III polysaccharide synthases and the type II GlyTs. Type III polysaccharide synthases are integral membrane proteins with multiple predicted trans-membrane domains (TMDs) that catalyse the transfer of glycosyl residues from a sugar nucleotide donor to construct the main polymeric backbone of wall polysaccharides in a processive manner. These enzymes are involved in the biosynthesis of homopolysaccharides such as cellulose, callose, (1,3;1,4)-β-d-glucan and the backbones of most of the other matrix phase heteropolysaccharides, including XyGs and heteromannans, but seemingly excluding the pectins and heteroxylans.
The multigene families of the cellulose synthase (CesA) and the nine cellulose synthase-like (Csl) groups (CslA-H and J) (Fig. 4), and the glucan synthase-like (Gsl) genes (Brownfield et al. 2009) encode members of the type III polysaccharide synthases. The CesA and the related Csl enzymes are members of Family GT2 (Table 3) and encode large, integral membrane proteins, (~500–1200 amino acid residues) that contain between three to six predicted TMDs towards the COOH terminus and one to two towards the NH2 terminus. They share a common D, D, D, QxxRW motif found in all processive GT2 family members, including the chitin and hyaluronan synthases, that is believed to be involved in substrate binding and catalytic activity of the enzyme (Richmond and Somerville 2000). The Gsl proteins belonging to Family GT48 (Table 3) are involved in callose biosynthesis, have 13–19 predicted TMDs and molecular masses of greater than 200 kDa, but lack the D, D, D, QxxRW motif (Brownfield et al. 2009).

|

|
The type II class of GlyTs, transfer single glycosyl residues from the donor to a polysaccharide backbone, although the recently identified (1,4)-α-d-galacturonosyltransferases (GAUTs) that participate in HG synthesis and the putative CAZy GT43 xylan synthases, IRX9 and IRX14, are exceptions (Table 3). They are anchored to the membrane via a single TMD that spans the membrane, have a short cytosolic NH2 terminus, an extended hydrophilic stem region and a globular catalytic domain towards the COOH terminus of the protein that resides in the Golgi lumen (Keegstra and Raikhel 2001). Examples include the α-d-GalTs that add single α-d-Galp substituents to the (1,4)-β-d-mannan backbone of galactomannans (Edwards et al. 1999, 2004) and the α-d-XylTs that add single α-d-Xylp residues to the (1,4)-β-d-glucan backbone of XyGs (Faik et al. 2002; Cavalier and Keegstra 2006; Cavalier et al. 2008).
Recent developments in understanding wall biosynthesis
Increasingly, genetic manipulation and conventional breeding techniques are being applied in attempts to modify the quality and quantity of individual cell wall components for broader commercial applications. The best candidates for this manipulation are the genes, enzymes and biochemical pathways involved in wall polysaccharide biosynthesis. It has been estimated that well over 2000 different gene products are involved in making and maintaining the wall (Carpita et al. 2001a). Until recently, little was known about the polysaccharide synthases and GlyTs involved in wall biosynthesis, mostly due to the difficulty in obtaining active enzymes, particularly the polysaccharide synthases, by traditional biochemical methods. There has now been significant progress in describing some of the genes that are involved in the biosynthesis of cellulose and matrix phase polysaccharides and these genes, and the evidence for their functionality, are summarised in Table 3 and discussed below. A cautionary note, however, is that plants demonstrate incredible plasticity and hence disruption of genes in one pathway results in pleiotrophic effects making interpretation of some gene disruption experiments difficult.
Cellulose
Before 1996, attempts to isolate and purify the cellulose synthase enzyme complex (CSC) were largely unsuccessful as the activity of the enzyme is rapidly lost with cell rupture and detergent solubilisation and the predominant product synthesised from UDP-Glc is callose; this considerably hampered progress towards identifying the catalytic protein(s). A highly significant breakthrough occurred through the use of a bioinformatic approach when Delmer and colleagues made a cDNA library of cotton fibre transcripts at a time of maximal cellulose synthesis and randomly sequenced a small subset of the resulting clones (Pear et al. 1996). Two genes, later renamed GhCesA1 and 2, were identified that shared low but significant sequence similarity with the catalytic subunits of the bacterial cellulose synthases. Unequivocal proof of their involvement in cellulose synthesis came with the identification and characterisation of cellulose-deficient Arabidopsis mutants, most notably the root swelling (rsw) (Baskin et al. 1992; Arioli et al. 1998) and irregular xylem (irx) (Turner and Somerville 1997; Taylor et al. 1999) mutants, with lesions in CesA genes (Desprez et al. 2007).
It is now clear that there are multiple CesA genes in the genome of each plant species and that there is functional specialisation (and in some cases partial redundancy) between isoforms. Mutant analyses in Arabidopsis (but since verified in other species) have shown that a functional CSC has at least three different, non-redundant CesA proteins: CesAs 1, 3 and 6 or 6-like (CesAs 2, 5 and 9 are partially redundant to 6) and CesAs 4, 7 and 8 are required for primary and secondary wall cellulose synthesis, respectively (Taylor et al. 1999, 2000, 2003; Gardiner et al. 2003; Desprez et al. 2007; Persson et al. 2007b). The exact biochemical activity of each CesA isoform, although speculated upon (Read and Bacic 2002), has not yet been shown experimentally apart from one instance in which yeast-expressed GhCesA1 was able to convert sitosterol-β-glucoside to sitosterol cellodextrins, thus confirming their ability to synthesise (1,4)-β-glucan linkages (Peng et al. 2002). The CesA isoforms interact directly and when all three are present, they form higher order oligomeric structures involving homodimers (Kurek et al. 2002; Gardiner et al. 2003; Atanassov et al. 2009). Through the use of tagged CesA proteins, it may now be possible to purify CSCs from detergent-soluble extracts and establish whether any other proteins are permanent components of the complex. CSCs are also being isolated in lipid rafts with a view to identifying their components and understanding their functions of the complex partners (V. Bulone, pers. comm.). While the in vitro cellulose synthase assay conditions have been significantly improved (Lai-Kee-Him et al. 2002; Pelosi et al. 2003; Colombani et al. 2004), synthesis rates are still quite low compared with those observed in vivo (~10%) and assay conditions need to be empirically defined and optimised for each plant system.
Several studies have implicated other proteins in the CSC. These include the membrane-bound KORRIGAN-type cellulases, as well as KOBITO (KOB/ELD1/ABI8), a protein of unknown function, the glycosylphosphatidyl inositol (GPI)-anchored protein COBRA and the chitinase-like CTL1/POM1/ELP1/HOT2 (Robert et al. 2004; Somerville 2006), and enzymes involved in N-linked glycan maturation, for example knopf, and rsw3, glycosidase I and II mutants, respectively. It is not always clear how or why these mutations impact on cellulose assembly. More recently, correlation analyses of microarray data have been used to search for other genes that may be involved in cellulose synthesis, regulation and/or deposition (Brown et al. 2005; Persson et al. 2005). Associations between the CSCs and the cytoskeleton are also critical to the orientation of wall cellulose and are further discussed in the cell wall–PM–cytoskeleton continuum section.
The cellulose synthase-like D (CslD) family has also been linked with cellulose biosynthesis, although the evidence for their involvement is currently inconclusive. The CslD genes are the most similar in sequence to the CesAs in the regions surrounding the D, D, D, QxxRW motif and the cysteine-rich domain near the NH2 terminus shown to be important for CesA isoform interaction (Kurek et al. 2002; Atanassov et al. 2009). In Arabidopsis, Nicotiana spp. and rice, CslD expression is associated with tip-growing cells (root hairs, pollen tubes, xylem fibres) and in moss, protonema cells (Doblin et al. 2001; Favery et al. 2001; Wang et al. 2001; Becker et al. 2003; Honys and Twell 2003; Bernal et al. 2007, 2008; Kim et al. 2007; Roberts and Bushoven 2007), providing correlative evidence for this proposal. CslD proteins have been located in the ER (Favery et al. 2001; Bernal et al. 2007), GA (Dunkley et al. 2006; Bernal et al. 2008; Zeng and Keegstra 2008) and PM (Natera et al. 2008), making deductions of function from these data impossible. Analysis of mutants has not allowed the function of CslDs to be elucidated unambiguously, although it has confirmed that these genes play an important role in tip-growing cells. Apart from the proposed function in cellulose microfibril synthesis, the CslDs have also been suggested to be involved in both the synthesis of non-crystalline cellulose (Manfield et al. 2004) and, surprisingly, in xylan synthesis (Bernal et al. 2007). Clearly, additional avenues to link the CslDs to a function need to be explored to clarify the exact role of this gene family.
In a recent study of the CslC gene family in barley (Hordeum vulgare L.), indirect evidence that also included immunocytochemical location (PM) was provided that is consistent with some family members (HvCslC2) being involved in the synthesis of cellulose (Dwivany et al. 2009). Unlike HvCslC3 and HvCslC1, the expression profiles of HvCslC2 and HvCslC4 were not found to be highly correlated with those of putative XylTs or XET/XTHs, enzymes involved in XyG assembly or re-modelling, suggesting that these genes are not involved in XyG synthesis (Dwivany et al. 2009) (see ‘XyG’ section). Corroborative evidence of these divergent functions is that the HvCslC2 sequence has a PM location and, is in a clade separate to AtCslC4 (Dwivany et al. 2009), the CslC protein with (1,4)-β-glucan activity and a Golgi location (Cocuron et al. 2007). From a biochemical perspective, the proposed functional diversification within the CslC family is far from implausible as XyG backbone synthases and cellulose synthases both make a (1,4)-β-glucan chain.
Heteromannan
Two different types of enzymes involved in the biosynthesis of mannan polysaccharides have been identified to date: a (1,4)-β-(gluco)mannan synthase (GlcManS) that polymerises the polysaccharide backbone, and a (1,6)-α-galactomannan galactosyltransferase (GMGT) that adds Gal residues to the backbone. The fenugreek (Trigonella foenum-graecum L.) seed endosperm GMGT is one of only three type II GTs that have been biochemically purified (Edwards et al. 1999) enabling the gene (TfGalT) to be cloned from NH2 terminal peptide sequence (Table 3). TfGalT is a member of Family GT34 containing both galactomannan (1,6)-α-GalTs and XyG (1,6)-α-XylTs. Bioinformatic analyses have shown that Arabidopsis may contain two putative galactomannan (1,6)-α-GalTs (Faik et al. 2002) and at least one in barley (Doblin et al. 2009). However, these genes and their encoded enzymes have not yet been functionally characterised, either in vitro or in vivo.
The first gene isolated that encoded a mannan synthase was CtManS, a CslA from guar (Cyamopsis tetragonoloba (L.)) endosperm (Dhugga et al. 2004). Several other CslA genes have since been tested for mannan (and other) synthase activities. In Drosophila Schneider 2 (S2) cells, recombinant AtCslA9, the Arabidopsis CslA with highest sequence similarity to the guar enzyme, and AtCslA2 both displayed ManS and glucomannan synthase (GlcManS) activities in vitro when assayed with GDP-Man, or both GDP-Man and GDP-Glc, respectively (Liepman et al. 2005). These observations indicate that multiple members of the Arabidopsis CslA gene family encode β-(gluco)mannan synthases and that a single CslA gene is capable of all three ManS, GlcS, and GlcManS activities (Liepman et al. 2005). Heterologous expression of CslA family members from diverse plant species has yielded proteins with ManS and/or GlcManS activity, at least in vitro (Suzuki et al. 2006; Liepman et al. 2007). As yet, none of the rice, maize and barley CslA genes in the cereal-specific clade of the CslA family have been shown experimentally to encode ManS (van Erp and Walton 2009).
Xyloglucan (XyG)
Genes encoding the (1,4)-β-glucan synthases involved in synthesising the XyG backbone as well as the (1,6)-α-Xylp-, (1,2)-β-Galp- and (1,2)-α-Fucp transferases that participate in XyG sidechain addition have been identified. These enzymes have been classified into GT families 2, 34, 47 and 37, respectively (Table 3). There are multiple members within each family with only some biochemically characterised with respect to substrate specificity (Fig. 5).

|
The GT Family 2 gene AtCslC4 is proposed to encode a XyG glucan backbone synthase. (Cocuron et al. 2007) used transcription profiling of developing nasturtium (Tropaeolum majus (L.)) seeds in which XyG is the primary seed storage polysaccharide to identify TmCslC, a gene with highest sequence similarity to Arabidopsis AtCslC4. When either TmCslC or AtCslC4 was expressed in Pichia cells, short chains of (1,4)-β-glucan accumulated and, upon co-expression of AtCslC4 with AtXXT1 (a XylT), a longer (1,4)-β-glucan was synthesised (Cocuron et al. 2007). Strong correlative evidence of AtXXT1 involvement in XyG biosynthesis, as well as the fact that AtCSCL4 and AtXXT1 are coordinately expressed and co-locate to the GA (Dunkley et al. 2006; Cocuron et al. 2007; Zabotina et al. 2008), provide support that both AtCslC4 (and TmCslC) are involved in XyG backbone synthesis and appear to interact either directly or indirectly with their respective XylTs. Whether all members of the CslC clade in dicots/gymnosperms participate in XyG synthesis needs to be confirmed, particularly in light of the recent study by Dwivany et al. (2009) proposing a role for HvCslC2 in cellulose synthesis (see ‘Cellulose’ section).
The addition of the side chain residues (monosaccharides and oligosaccharides) to the (1,4)-β-d-glucan backbone to form XyG by the GlyTs (XylTs/GalTs/FucTs) has been intensively studied using a combination of heterologous expression of the proteins and biochemical characterisation of their substrate specificity together with mutant analysis. A detailed description is beyond the scope of this review but the salient features and relevant references are summarised in Table 3 and Fig. 5. These elegant studies demonstrate the exquisite substrate specificity of GTs as well as their plasticity under abnormal conditions and reinforce the need to perform biochemical studies on each of the members of multigene families.
(1,3;1,4)-β-d-glucan
Like the cellulose synthases, attempts to isolate the components of the (1,3;1,4)-β-d-glucan synthase by biochemical means have been ineffective. More recently, a comparative genomics approach has, however, been successfully applied to uncover putative polysaccharide synthase genes. The identification of a quantitative trait locus contributing to (1,3;1,4)-β-d-glucan content in ungerminated barley grain (Han et al. 1995) allowed the syntenic region in the rice genome to be located, leading to the discovery of a cluster of six rice OsCslF genes (Burton et al. 2006). Given the earlier finding that the CslF family is one of three cereal-specific Csl families (the others are the CslHs and CslJs; Hazen et al. 2002; Fincher 2009b) and the fact that (1,3;1,4)-β-d-glucan is a polysaccharide unique to the walls of cereals, the CslF genes became prime candidates for encoding (1,3;1,4)-β-d-glucan synthases (Burton et al. 2006). This possibility was tested by ‘gain-of-function’ experiments in transgenic Arabidopsis plants, a species that contains no (1,3;1,4)-β-d-glucan in its walls nor has any CslF genes. Heterologous expression of rice OsCslF2, 4 or 9 in Arabidopsis resulted in the appearance of immunologically detectable levels of (1,3;1,4)-β-d-glucan in leaf epidermal walls, and therefore the CslF family probably encodes (1,3;1,4)-β-d-glucan synthases (Burton et al. 2006). This conclusion is consistent with the temporal and spatial expression patterns of the seven CslF genes in barley (Burton et al. 2008).
Similar experiments in which barley CslH1 was expressed in transgenic Arabidopsis plants and the plants analysed immuncytochemically and biochemically to confirm polysaccharide structures have shown that the CslH proteins are also capable of generating (1,3;1,4)-β-d-glucan in leaf walls (Doblin et al. 2009), suggesting that there are two types of (1,3;1,4)-β-d-glucan synthases in cereals. The transcript level of the single barley CslH gene is very low and is restricted to cells that have either mature primary walls (e.g. starchy endosperm) or secondary wall thickenings (e.g. leaf interfascicular sclerenchymal fibre and xylem cells) and hence, it has been suggested that its main role is in (1,3;1,4)-β-d-glucan synthesis during secondary wall synthesis (Doblin et al. 2009). That two Csl families appear to be involved in (1,3;1,4)-β-d-glucan synthesis is curious. Why have two similar protein families, not just one, evolved to provide a (1,3;1,4)-β-d-glucan synthesis function within the restricted Poalean plant lineage? The answer may lie in the different functional roles of (1,3;1,4)-β-d-glucan in primary v. secondary walls, as a mobilisable, storage or structural polysaccharide, respectively (Burton and Fincher 2009). This hypothesis and others remain to be tested.
The CslF and CslH gain-of-function experiments in Arabidopsis suggest that both genes encode synthases with the same biochemical activity and produce a type of (1,3;1,4)-β-d-glucan that is similar to barley (Doblin et al. 2009). Whilst the simplest explanation is that both CslF and CslH proteins synthesise both the (1,3)-β- and (1,4)-β-linkages, the cumulative evidence is most consistent with the interpretation that both types of Csl protein produce only the (1,4)-β-linkage. First, all other Csl proteins characterised to date make only (1,4)-β-linkages (see previous sections). Second, the levels of (1,3;1,4)-β-d-glucan deposited in Arabidopsis leaf walls were very low (<0.02% w/w) in comparison to barley (0.4% w/w). The restricted pattern of deposition in only certain leaf cell walls despite transgene expression being driven by the constitutive CaMV35S promoter implies that additional protein/s (or other factors) are likely to be involved in (1,3;1,4)-β-d-glucan synthesis (Burton et al. 2006; Doblin et al. 2009). A second GlyT to make the (1,3)-β-linkage would explain some of the idiosyncrasies of (1,3;1,4)-β-d-glucan structure such as the random arrangement of the cellotriosyl and cellotetraosyl units within (1,3;1,4)-β-d-glucan chains (Staudte et al. 1983) and the observation that in cells actively making (1,3;1,4)-β-d-glucan, this polysaccharide, unlike AX and heteromannans, is never observed by immunoEM within the GA (Wilson et al. 2006), the proposed location of (1,3;1,4)-β-d-glucan synthesis (Henry et al. 1983; Gibeaut and Carpita 1993). Given the Golgi location of the HvCslH1 protein, a novel mechanism has been proposed for (1,3;1,4)-β-d-glucan assembly in which cellodextrins are synthesised by CslF or CslH proteins in the Golgi and joined together by (1,3)-β-linkages by another GlcT at the PM (Burton and Fincher 2009; Doblin et al. 2009; Fincher 2009a, 2009b). The Gsl proteins, the XyG endotransglycosylases (XETs) or another as yet unidentified GlcT might be able to catalyse such a reaction (Doblin et al. 2009; Fincher 2009b). Co-expression analyses have begun to be used to identify other genes that might be involved in the (1,3;1,4)-β-d-glucan synthesis and assembly process (Schreiber et al. 2008; Burton and Fincher 2009). The next step will be to test the function of these candidate genes through various means, including heterologous expression in combination with the CslF and/or H proteins.
Pectin
Homogalacturonan (HG)
Based on structure, a total of ~67 GlyTs, methyltransferases and acetyltransferases are predicted to be required for pectin synthesis (Ridley et al. 2001). Assays for some of these activities have been described (Ridley et al. 2001; Mohnen 2008) but to date, there is conclusive experimental evidence supporting a role in pectin biosynthesis for only a few genes (Scheller et al. 2007; Mohnen 2008). Only one pectin biosynthetic enzyme has been biochemically isolated. The type II HG (1,4)-α-GalpAT, termed GAUT1, has been partially purified from solubilised membrane extracts of Arabidopsis suspension-cultured cells (Sterling et al. 2006). GAUT1 antibodies immuno-adsorbed HG (1,4)-α-GalpAT activity from these preparations and when expressed in mammalian HEK293 cells, GAUT1 transfers GalA from UDP-α-d-GalpA onto the non-reducing end of HG polysaccharide and oligosaccharide acceptors with a preference for DP > 9 (Sterling et al. 2006). GAUT1 was one of two genes, together with GAUT7, identified by peptide sequencing of the partially purified HG (1,4)-α-GalpAT. Both genes belong to a family of 25 genes in Arabidopsis that consist of 15 GAUT and 10 GAUT1-like (GATL) genes clustered in CAZy Family GT8 (Lao et al. 2003; Sterling et al. 2006). To date, mutants in three GAUT1-related family genes have been studied and the observed phenotypes suggest that the encoded proteins, in addition to GAUT1, are involved in the synthesis of wall polysaccharides and may have GalAT activity. The Arabidopsis QUASIMODO1 (QUA1), IRX8 and PARVUS/GLZ1 proteins are encoded by the GAUT8, GAUT12 and GATL1 genes, respectively. Interestingly, the mutant phenotypes of all three genes include effects on both HG and xylan content and/or synthase activity, thus preventing their definitive functional identification (Shao et al. 2004; Orfila et al. 2005; Brown et al. 2007; Lee et al. 2007b; Peña et al. 2007; Persson et al. 2007a). While pectins and heteroxylans are generally thought of as separate polymers, structural analyses have suggested that they may be covalently linked (Nakamura et al. 2002). Hence, it has been proposed that a lack of an enzymatic activity may affect both polymers through the absence of a specific linkage (Persson et al. 2007a; Mohnen 2008). Other explanations that have been proposed to account for the dual polysaccharide effects in the mutants are that HG and xylan synthesis are associated through either a common biosynthetic complex/es or regulatory mechanism, altered polysaccharide deposition or impaired scaffolding between the xylan polymer and specific pectic polymers in the secondary wall (Persson et al. 2007a; Mohnen 2008).
A XylT that adds Xylp residues onto the HGA backbone has recently been identified by mutant analysis of the Arabidopsis CAZy GT47 members (Jensen et al. 2008). In the xgd1–1 mutant, less terminal Xylp residues were found in the pectic fraction compared with wild type, indicating a reduction in xylose-substituted HG (XGA). Transformation of the xgd1–1 mutant with the wild-type gene complemented the XGA-deficient phenotype and when XGD1 was transiently expressed via Agrobacterium in leaves of Nicotiana benthamiana (Domin.), the protein catalysed the transfer of Xylp from UDP- Xylp onto oligogalacturonides and endogenous acceptors (Jensen et al. 2008). The reaction products were hydrolysable with an XGA-specific hydrolase, confirming that XGD1 is a XGA XylT. Additional biochemical analysis of the XGD1 protein is, however, necessary to determine the exact linkage formed by the enzyme (Jensen et al. 2008).
Rhamnogalacturonan I (RG I)
The ARAD1 protein, also a CAZy Family GT47 member (Fig. 6), appears to be involved in the biosynthesis of arabinan side-chains of RG I. Walls of ARAD1 mutants contain significant decreases in leaf and stem Ara (75% and 46%, respectively, of wild-type levels) (Harholt et al. 2006). Immunohistochemical and linkage analysis indicated a specific decrease in the level but not structure of arabinan side chains of RGI with no change in other pectic domains or wall glycoproteins, indicating that it is likely that the decreased arabinan content is a direct result of the mutation. Transformation of mutant plants with ARAD1 driven by the 35S promoter led to full restoration of the Ara-deficient phenotype, suggesting that ARAD1 is an arabinan (1,5)-α-arabinosyltransferase. A demonstration of this activity by the ARAD1 protein is required as proof of its function, however.

|
Rhamnogalacturonan (RG II)
Two homologous plant-specific Arabidopsis genes, RGXT1 and RGXT2, encode novel type II, Golgi-localised XylTs and are likely to participate in the formation of the A chain in RG II (Egelund et al. 2006). These sequences, originally identified in a bioinformatic search aimed at discovering unclassified cell wall GlyTs, seeded the new CAZy GT family 77, of which there are 18 and 15 members in Arabidopsis and rice, respectively. Truncated, soluble forms of the corresponding proteins expressed in insect cells displayed XylT activity, transferring d-Xyl from UDP-α-d-Xyl to l-Fuc in a (1,3)-α-linkage, demonstrating the proteins to be (1,3)-α-d-XylTs. As this particular linkage is only known to exist in RG II, it was surmised that RGXT1 and RGXT2 transfer Xylp to the Fucp residue in side chain A of RG II (Egelund et al. 2006). In phylogenetic analyses, two other sequences form a cluster with RGXT1 and 2, sharing 68–75% identity with these proteins, hence, they too may be Fuc-specific XylTs. However, the function of the remaining Arabidopsis sequences cannot be inferred with any accuracy as they only share low (<19%) identity with the RGXTs.
Another gene that most likely participates in the synthesis of RG II is GUT1 from Nicotiana plumbaginifolia (Viv.), yet another member of CAZy Family GT47 (Fig. 6). A T-DNA insertion mutant of NpGUT1, nolac-H18 (non-organogenic callus with loosely attached cells), has only 14% GlcpA content of normal callus cell walls (Iwai et al. 2002). The presence of undetectable levels of GlcpA and a 50% decrease in Gal in RG II isolated from the mutant together with an ~82% reduction in RG II dimer formation in vitro suggest that the NpGUT1 protein is an RG II (1,4)-β-GlcAT that transfers GlcpA onto the l-Fucp in RG-II side chain A (Iwai et al. 2002), the same Fuc acceptor substrate for RGXT1 and 2. This proposed function for NpGUT1 has not yet been substantiated by an in vitro enzyme assay. This is particularly important in this case, as the two Arabidopsis proteins with highest sequence similarity to NpGUT1, IRX10 and IRX-LIKE with 86% and 92% amino acid identity, respectively, have recently been implicated in xylan chain elongation (Brown et al. 2009; Wu et al. 2009). If the biochemical activities corroborate these conclusions, then a close sequence comparison of these proteins may yield information regarding what sequence motifs direct donor and/or acceptor specificity and potentially the control of single v. multiple sugar additions.
Heteroxylan
GX biosynthesis is likely to involve multiple GTs responsible for the formation of the backbone, the reducing-end sequence (see ‘Cell wall structure’ section), at least for dicots and gymnosperms, as well as enzymes that add side chains and modify them. No single gene has been unambiguously described as encoding a xylan synthase, although there is mounting evidence implicating several GlyTs in having such a function, as well as others that appear to be involved in the synthesis of the reducing-end primer sequence. To date in Arabidopsis, four genes (IRX9 and IRX14, IRX10 and IRX10-like) and three genes (IRX7/FRA8, IRX8/GAUT12, PARVUS/GLZ1/GATL1) have been hypothesised to be involved in xylan backbone synthesis and synthesis of the reducing-end sequence, respectively (Table 3).
The IRX and PARVUS genes and their homologues in poplar and rice were all initially identified by correlative expression analyses using EST or microarray data aimed at identifying genes that function in secondary wall polysaccharide synthesis, including xylan (Aspeborg et al. 2005; Brown et al. 2005; Persson et al. 2005; Geisler-Lee et al. 2006; Mitchell et al. 2007). Biochemical analysis of the corresponding mutants has shown that all have a secondary wall phenotype and exhibit a deficiency in xylan, with two classes of enzymes involved in xylan biosynthesis being identified. NMR and MALDI–TOF MS analysis of xylan extracted from irx7/fra8, irx8 and parvus has shown an absence of the reducing-end oligosaccharide sequence, yet xylan synthase activity in these mutants is similar to wild-type (Brown et al. 2007; Lee et al. 2007a, 2007b; Peña et al. 2007). This suggests an important role for the reducing-end sequence in xylan biosynthesis, and has led to the prediction that IRX7/FRA8, IRX8 and PARVUS catalyse the linkages found within this reducing-end oligosaccharide (Brown et al. 2007; Lee et al. 2007b; Peña et al. 2007). In contrast, the reducing-end sequence is present in irx9, irx14 and the irx10 irx10-like double mutant (a mild or no phenotype is observed in the single mutants, respectively, suggesting considerable functional redundancy between these genes) but xylan synthase activity (measured by the ability to transfer [14C]Xyl from UDP-[14C]Xyl onto (1,4)-β-xylooligosaccharides) is greatly reduced (Brown et al. 2007; Lee et al. 2007b; Peña et al. 2007). A decreased xylan chain length has also been observed in irx9 (Peña et al. 2007) and the irx10 irx10-like double mutant (Brown et al. 2009). These data imply that IRX9, IRX14, IRX10 and IRX10-LIKE are required for xylan chain elongation.
IRX9 and IRX14 belong to CAZy Family GT43 and IRX10 and IRX10-LIKE are yet further members of GT47 (Fig. 6). The findings that expression of IRX10 and to a lesser extent IRX10-LIKE is predominantly in cells undergoing secondary wall thickening, that there is no loss of cell adhesion and the majority of RG II is dimerised in the irx10 irx10-like double mutant and there appears to be no difference compared with wild-type in either the A or B sidechain of RG II argues against these genes having a function similar to NpGUT1 (Brown et al. 2009; Wu et al. 2009). However, the recent complementation of the irx10 irx10-like mutant with a chimeric IRX10-NpGUT1 protein in which the NH2-terminal 71 amino acids of IRX10 were fused to the NH2-terminus of NpGUT1 (Wu et al. 2009) calls into question the presumed role of NpGUT1 in RG II biosynthesis. Further work is required to resolve this discrepancy. Whether the individual IRX9/14/10/10-LIKE proteins have the same mechanism of action and whether they are associated in a biosynthetic complex in a similar way to the CesA proteins is unclear (Peña et al. 2007; Brown et al. 2009). IRX7/FRA8 is also member of the GT47 family (Fig. 6); hence has been proposed to catalyse either the formation of one of several β-linkages of Xylp, for example, Xylp to Rha using UDP-α-Xylp, or the addition of α-linked Rhap to GalpA using UDP-β-Rhap (Peña et al. 2007).
Since IRX8 and PARVUS are both members of the GAUT1-related family they have both been hypothesised to catalyse the addition of the α-d-GalA residue to the O-4 of the reducing Xyl residue of the reducing end oligosaccharide, possibly acting redundantly (Peña et al. 2007). However, YFP-tagged PARVUS protein is found to predominantly localise to the ER (Lee et al. 2007b), a subcellular location distinct from FRA8, IRX8 and IRX9, which have been shown to be Golgi-located (Zhong et al. 2005; Peña et al. 2007). This difference is consistent with trans-membrane predictions indicating that whereas IRX7/FRA8, IRX8 and IRX9 are type II membrane proteins, PARVUS contains a hydrophobic signal peptide sequence but no trans-membrane helices (Lee et al. 2007b). The ER localisation of PARVUS suggests that it catalyses an earlier step that is different from IRX8 in GX biosynthesis (Lee et al. 2007b). It has been hypothesised that the reducing-end sequence might function as either a primer or as a chain terminator for GX biosynthesis (Peña et al. 2007; York and O’Neill 2008). The finding that PARVUS is located in the ER and is required for the synthesis of the oligosaccharide sequence at the reducing end of GX favours the reducing end sequence having a primer function (Lee et al. 2007b).
Despite the plausible roles for IRX7/8/9/10/10-LIKE/14 and PARVUS in GAX biosynthesis, experimental proof of their precise enzymatic function is lacking. Attempts to demonstrate the function(s) of FRA8 and IRX9 by heterologous expression in Pichia cells have been unsuccessful as no activity was detected (Zhong et al. 2005; Peña et al. 2007). Hence, further work is required to demonstrate their biochemical activity.
Nucleotide sugar interconversion
Apart from cellulose and callose, the synthesis of the non-cellulosic polysaccharides and pectins occurs within the Golgi apparatus. The active site of the participating polysaccharide synthases (Golgi lumen or cytosol) and GlyTs (Golgi lumen only) may face either the cytosol or the Golgi lumen depending on their membrane topology (Doblin et al. 2003). In the latter case, the enzymes are separated from their substrates and cofactors in the cytosol and hence their availability to the synthesis machinery relies on transporters resident in Golgi cisternal membranes as well as the appropriate nucleotide sugar interconversion enzymes (NSIEs). Substantial progress has been made in identifying and characterising the genes and enzymes utilised in this process. These advancements have recently been reviewed (Seifert 2004; Reiter 2008; Reyes and Orellana 2008) and will not be discussed in detail here. Analyses of various mutants with defects in NSIEs have confirmed the essential role for these proteins in wall biosynthesis. It has been hypothesised that transcriptional and post-translational control of NSIEs regulates wall biosynthesis in response to developmental, metabolic and other environmental stimuli (Seifert 2004). Apart from characterising additional NSIEs and transporters, future work will focus upon elucidating the role of NSIEs in regulating wall synthesis, addressing issues such as feedback inhibition and redox sensing, the significance of differing kinetic properties and subcellular location between isoforms and their ability to form multimeric complexes (Seifert 2004; Reiter 2008). Another area of research will be to further understand the role of NSIEs in growth, development and defence responses. Mutants of Arabidopsis UXE1/MUR4/HSR8, for example, have reduced L-Ara levels due to impaired enzymatic function but also display altered sugar response phenotypes affecting cell division and expansion (Burget and Reiter 1999; Burget et al. 2003; Li et al. 2007). Such studies will aid in determining the signalling mechanisms linking wall changes to cellular responses (see the wall–PM–cytoskeleton continuum section below).
The cell wall–plasma membrane–cytoskeleton continuum
The cell wall–PM-cytoskeleton continuum is vital for the perception of signals from the external environment and for coordinated cell growth and expansion during plant development. To date, several (glyco)proteins that have been implicated in regulating/sensing the external environment of the cell (Humphrey et al. 2007). These include the AGPs (Du et al. 1996; Nothnagel 1997; Schultz et al. 1998; Majewska-Sawka and Nothnagel 2000; Seifert and Roberts 2007), cellulose synthases (Somerville 2006; Hématy et al. 2007; Hématy and Höfte 2008) and more recently PM receptor kinases including the WAKs (Kohorn 2000; Riese et al. 2003), the Ser/Thr CrRLK1 L (Catharanthus roseus receptor-like-kinase-1-like) subfamily (Hématy et al. 2007) and a clade of the leucine-rich repeat (LRR) RLK family (Xu et al. 2008). These are summarised schematically in Fig. 7 and briefly discussed below.

|
AGPs are situated at the PM wall interface and have long been thought to play a central role as a mediator of these processes. Mounting evidence indicates that AGPs have a specific function during root formation, the promotion of somatic embryogenesis, pollen tube guidance, alternation between sporophytic and gametophytic transitions in the ovules, resistance to root-dependent transformation by Agrobacterium, hormone signalling and xylem differentiation/vascular function in Zinnia and Arabidopsis (Seifert and Roberts 2007). Physical connections between the wall and the PM were originally inferred from microscopic observations of the presence of AGPs as a fuzzy ‘glycocalyx’ occupying the PM-wall interface (Roberts 1990) and then as discrete connections (Hechtian strands), particularly in plasmolysed cells upon salt stress (Carpita and Gibeaut 1993; Gens et al. 2000; Lamport et al. 2006; Pickard 2008). Since AGPs were know to be highly water soluble protoeglycans it was unclear how they might fulfill this role. The discovery that most AGPs have a GPI membrane anchor provided the evidence for their mechanism of association with the PM (Youl et al. 1998; Oxley and Bacic 1999). Since GPI anchors do not traverse the entire PM, GPI anchored proteins have been implicated in mediating cell–cell interactions in mammalian systems by interacting with other proteins (Du et al. 1996; Plun-Favreau et al. 2001; Johnson et al. 2003). A sub-class of AGPs, the fasciclin-like AGPs (FLAs) are likely candidates as they have, in addition to predicted AGP-like glycosylated regions in their protein backbones, putative cell adhesion domains known as fasciclin domains (Johnson et al. 2003; Schultz et al. 2004). In other eukaryotes (Elkins et al. 1990; Kawamoto et al. 1998) and Volvox (Huber and Sumper 1994), fasciclin domain-containing proteins are involved in cell adhesion. Interestingly, SOS5/FLA4, a GPI-anchored fasciclin-type AGP, has been proposed as a ligand of the LRR-RLK family members FEI1 and FEI2 (Xu et al. 2008). fei1 fei2 double mutants have defects in cell expansion primarily in roots, are cellulose-deficient and hypersensitive to isoxaben in the presence of sucrose or salt (Xu et al. 2008). Genetic analyses indicate that FEI1 and FEI2 act within the same pathway as SOS5/FLA4 but in a pathway independent of COBRA or PROCUSTE1/CESA6 to regulate wall expansion (Fig. 7). Classical AGPs that are GPI-anchored have been implicated in controlling cell shape via a connection, as yet undefined, with the cytoskeleton, due to their involvement in orienting cortical microtubules and influencing F-actin organisation (Andème-Onzighi et al. 2002; Sardar et al. 2006).
Wall associated kinases (WAKs) are a family of transmembrane proteins that, unlike AGPs, possess the necessary structure to provide a physical link between the wall and the PM and to be directly involved in subcellular signalling (Kohorn 2000; Riese et al. 2003; Humphrey et al. 2007). They are transmembrane proteins that have a conserved cytoplasmic Ser/Thr protein kinase domain as well as an extracellular wall domain that differs between family members (Fig. 7). An Arabidopsis GRP, AtGRP-3 is predicted to be a wall protein that interacts with WAKs, specifically binding to WAK1 both in vitro and in vivo (Park et al. 2001). Both AtGRP-3 and WAKs have conserved Cys residues, and it is possible these are responsible for the interaction between AtGRP-3, WAK1 and other proteins (Park et al. 2001). WAKs are also bound to the wall in part by a covalent association with pectin (Anderson et al. 2001). The ability of WAKs to bind both GRPs and pectins in their phosphorylated form is proposed to relate to their role in regulating cell expansion (Fig. 7).
The CrRLK1 L family includes THESEUS1 (THE1), which appears to be involved in sensing the integrity of the wall through the perturbation of the CSC (Hématy et al. 2007; Hématy and Höfte 2008), FERONIA (FER) required for growth cessation of compatible pollen tubes (Escobar-Restrepo et al. 2007) and AmRLK involved in the control of the polar conical outgrowth of epidermal cells in Antirrhinium petals (Hématy and Höfte 2008). The potential mechanism/s by which these novel Ser/Thr receptor kinases are involved in growth regulation was recently summarised by Hématy and Höfte (2008) and will not be discussed further here as no ligands for these receptors are yet identified.
Evidence accumulated over many decades, including the observations of co-alignment of cortical microtubules and cellulose microfibrils and a loss of directed cell expansion and CSC mobility upon microtubule disruption (Gardiner et al. 2003; Somerville et al. 2004; Paredez et al. 2006; DeBolt et al. 2007; Paredez et al. 2008) confirm a link between the cortical cytoskeleton and the wall through the cellulose synthase rosette complexes in the PM and the central role this plays in cell growth and expansion. The link between the cytoskeleton and cellulose synthesis is further demonstrated by the fact that CesA mutants have disrupted cytoskeletal organisation and cytoskeleton mutants having disrupted cellulose deposition (Burk and Ye 2002; Chu et al. 2007; Paredez et al. 2008). However, this relationship is not completely mutually dependent. For example, cellulose microfibril deposition is normal in roots of the temperature-sensitive microtubule organisation1 (mor1–1) mutant that causes cortical microtubule disassembly (Sugimoto et al. 2003; Wasteneys 2004), supporting the notion that cellulose microfibrils can self-align in the absence of normal microtubule organisation. By analogy, it is likely that another polysaccharide synthase also located at the PM, callose synthase, could have a similar role as it assembles callose in a similar directed manner and is rapidly activated upon physical or biotic stresses although a link between the cytoskeleton and the synthase complex is yet to be demonstrated.
Future directions
The focus of this review has been on plant wall biosynthesis and, in particular, the synthesis of wall polysaccharides as well as the emerging realisation of the importance of the apoplast, plasma membrane, cytoskeleton in sensing/perceiving the external environment leading to cytoplasmic signalling cascades that regulate plant growth processes. Polysaccharide synthesis and wall assembly occurs through a complex and intricate series of steps that often begin in an intracellular compartment and end in the wall itself, after the polysaccharides have been deposited. The great structural diversity of wall polysaccharides is due to the large number of possible constituent sugars and non-sugar components, the variety of ways these can be linked together, and the many ways in which linear polymers can be branched or modified. Regulation of these steps is central to cell development, because the polysaccharide composition of the wall changes during cell division, elongation and differentiation. At a larger scale, for the plant to assemble its mature organs and tissues in an orderly manner, the stages of wall synthesis in the range of different cell types must be integrated through time and space and must also respond to internal and external developmental cues; how this coordinate regulation of wall synthesis is achieved is unknown and remains a major future research question in plant biology.
The wall is now recognised to be a metabolically active ‘organelle’ that also plays a critical role in sensing and responding to external signals during growth and development as well as in response to abiotic/biotic stresses. Identifying the (glyco)proteins located at the PM involved in the interaction between the wall, PM and cytoskeleton and understanding their mechanism(s) of signal transduction will be important in devising strategies to optimise growth for plant production.
In the post-genomic era, the greatest challenge is to demonstrate the enzymatic activity/specificity of each GT gene product implicated in wall biosynthesis. GT mutants have proven useful in some instances, providing a phenotype from which a function could be deduced, however, in many cases, an association with a specific biochemical activity has not been forthcoming. This means that additional approaches and tools – bioinformatic, biochemical, genetic, molecular biological, proteomic and others – need to be used in future studies to gather evidence of function. One approach is heterologous expression of GTs within organisms that provide a ‘null’ background and where a robust enzyme assay and unequivocal product characterisation can be provided. In other cases, the road to determining a definitive activity for a GT will be more difficult. The use (and availability) of appropriate acceptors and donors in biochemical reactions to test enzymatic activity will be vital in these instances. Importantly, care must be taken in the extrapolation of data obtained from ‘proof-of-function’ experiments to other GT family members, and in assuming that an enzymatic activity shown in vitro is the same as its function in vivo; for example, the complexity of the GlyTs in CAZy Family GT47 described above. Continued perseverance of elucidating the suite of genes and enzymes that participate in wall polysaccharide and (glyco)protein biosynthesis is critical, given the rich rewards in terms of the benefits to human society in using plant products for food, fibre, feed and fuel.
Acknowledgements
The authors acknowledge the generous support of the Australia Research Council (current grant ARC LP0989478) and Grains Research and Development Corporation over many years and more recently the Commonwealth Scientific and Research Organisation Flagship Collaborative Research Program, provided to the High Fibre Grains Cluster via the Food Futures Flagship, that has enabled us to pursue research, fundamental and strategic, into wall structure and function.
Andème-Onzighi C,
Sivaguru M,
Judy-March J,
Baskin T, Driouich A
(2002) The reb1–1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta 215, 949–958.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Anderson CM,
Wagner TA,
Perret M,
He Z-H,
He D, Kohorn BD
(2001) WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Molecular Biology 47, 197–206.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Arioli T,
Peng L,
Betzner AS,
Burn J, Wittke W ,
et al
.
(1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Aspeborg H,
Schrader J,
Coutinho PM,
Stam M, Kallas Å ,
et al
.
(2005) Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiology 137, 983–997.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Atanassov II,
Pittman JK, Turner SR
(2009) Elucidating the mechanisms of assembly and subunit interaction of the cellulose synthase complex of Arabidopsis secondary cell walls. The Journal of Biological Chemistry 284, 3833–3841.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bacic A
(2006) Breaking an impasse in pectin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 103, 5639–5640.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Baskin TI
(2005) Anisotropic expansion of the plant cell wall. Annual Review of Cell and Developmental Biology 21, 203–222.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Baskin TI,
Betzner AS,
Hoggart R,
Cork A, Williamson RE
(1992) Root morphology mutants in Arabidopsis thaliana. Australian Journal of Plant Physiology 19, 427–437.
| Crossref | GoogleScholarGoogle Scholar |

Becker JD,
Boavida LC,
Carneiro J,
Haury M, Feijo JA
(2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology 133, 713–725.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bernal AJ,
Jensen JK,
Harholt J,
Sørensen S, Moller I ,
et al
.
(2007) Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. The Plant Journal 52, 791–802.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bernal AJ,
Yoo C-M,
Mutwil M,
Jensen JK,
Hou G,
Blaukopf C,
Sørensen I,
Blancaflor EB,
Scheller HV, Willats WGT
(2008) Functional analysis of the cellulose synthase-like genes CSLD1, CSLD2, and CSLD4 in tip-growing Arabidopsis cells. Plant Physiology 148, 1238–1253.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boerjan W,
Ralph J, Baucher M
(2003) Lignin biosynthesis. Annual Review of Plant Biology 54, 519–546.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Boudet AM,
Kajita S,
Grima-Pettenati J, Goffner D
(2003) Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends in Plant Science 8, 576–581.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bouton S,
Leboeuf E,
Mouille G,
Leydecker M-T,
Talbotec J,
Granier F,
Lahaye M,
Hofte H, Truong H-N
(2002) QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. The Plant Cell 14, 2577–2590.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brown DM,
Zeef LAH,
Ellis J,
Goodacre R, Turner SR
(2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. The Plant Cell 17, 2281–2295.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brown DM,
Goubet F,
Wong VW,
Goodacre R,
Stephens E,
Dupree P, Turner SR
(2007) Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. The Plant Journal 52, 1154–1168.
| Crossref |
PubMed |

Brown DM,
Zhinong Z,
Stephens E,
Dupree P, Turner SR
(2009) Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. The Plant Journal 57, 732–746.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Brownfield L,
Ford K,
Doblin MS,
Newbigin E,
Read S, Bacic A
(2007) Proteomic and biochemical evidence links the callose synthase in Nicotiana alata pollen tubes to the product of the NaGSL1 gene. The Plant Journal 52, 147–156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Burget EG, Reiter W-D
(1999) The mur4 mutant of Arabidopsis is partially defective in the de novo synthesis of uridine diphospho L-arabinose. Plant Physiology 121, 383–390.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Burget EG,
Verma R,
Molhoj M, Reiter W-D
(2003) The biosynthesis of L-arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-d-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. The Plant Cell 15, 523–531.
| Crossref | d-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis.&journal=The Plant Cell&volume=15&pages=523-531&publication_year=2003&author=W%2DD%20Reiter&hl=en&doi=10.1105/tpc.008425" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Burk DH, Ye Z-H
(2002) Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. The Plant Cell 14, 2145–2160.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Burton RA, Fincher GB
(2009) (1,3;1,4)-β-d-glucans in cell walls of the poaceae, lower plants, and fungi: a tale of two linkages. Molecular Plant 2, 873–882.
| Crossref | d-glucans in cell walls of the poaceae, lower plants, and fungi: a tale of two linkages.&journal=Molecular Plant&volume=2&pages=873-882&publication_year=2009&author=GB%20Fincher&hl=en&doi=10.1093/mp/ssp063" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Burton RA,
Wilson SM,
Hrmova M,
Harvey AJ,
Shirley NJ,
Medhurst A,
Stone BA,
Newbigin EJ,
Bacic A, Fincher GB
(2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-d-glucans. Science 311, 1940–1942.
| Crossref | d-glucans.&journal=Science&volume=311&pages=1940-1942&publication_year=2006&author=GB%20Fincher&hl=en&doi=10.1126/science.1122975" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Burton RA,
Jobling SA,
Harvey AJ,
Shirley NJ,
Mather DE,
Bacic A, Fincher GB
(2008) The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiology 146, 1821–1833.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Buxton DR, Redfearn DD
(1997) Plant limitations to fiber digestion and utilization. The Journal of Nutrition 127, 814S–818S.
| PubMed |

Cantarel BL,
Coutinho PM,
Rancurel C,
Bernard T,
Lombard V, Henrissat B
(2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Research 37, D233–D238.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Carpita NC, Gibeaut DM
(1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal 3, 1–30.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Carpita N,
Tierney M, Campbell M
(2001a) Molecular biology of the plant cell wall: searching for the genes that define structure, architecture and dynamics. Plant Molecular Biology 47, 1–5.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Carpita NC,
Defernez M,
Findlay K,
Wells B,
Shoue DA,
Catchpole G,
Wilson RH, McCann MC
(2001b) Cell wall architecture of the elongating maize coleoptile. Plant Physiology 127, 551–565.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cavalier DM, Keegstra K
(2006) Two xyloglucan xylosyltransferases catalyze the addition of multiple xylosyl residues to cellohexaose. The Journal of Biological Chemistry 281, 34197–34207.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cavalier DM,
Lerouxel O,
Neumetzler L,
Yamauchi K, Reinecke A ,
et al
.
(2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell 20, 1519–1537.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chu Z,
Chen H,
Zhang Y,
Zhang Z, Zheng N ,
et al
.
(2007) Knockout of the AtCESA2 gene affects microtubule orientation and causes abnormal cell expansion in Arabidopsis. Plant Physiology 143, 213–224.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cocuron J-C,
Lerouxel O,
Drakakaki G,
Alonso AP,
Liepman AH,
Keegstra K,
Raikhel N, Wilkerson CG
(2007) A gene from the cellulose synthase-like C family encodes a β-1,4 glucan synthase. Proceedings of the National Academy of Sciences of the United States of America 104, 8550–8555.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Colombani A,
Djerbi S,
Bessueille L,
Blomqvist K,
Ohlsson A,
Berglund T,
Teeri TT, Bulone V
(2004)
In vitro synthesis of (1 → 3)-β-d-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen. Cellulose 11, 313–327.
| Crossref | d-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen.&journal=Cellulose&volume=11&pages=313-327&publication_year=2004&author=V%20Bulone&hl=en&doi=10.1023/B:CELL.0000046404.25406.19" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |

Cosgrove DJ
(2005) Growth of the plant cell wall. Nature Reviews. Molecular Cell Biology 6, 850–861.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Coutinho PM,
Starn M,
Blanc E, Henrissat B
(2003) Why are there so many carbohydrate-active enzyme-related genes in plants? Trends in Plant Science 8, 563–565.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cui XJ,
Shin HS,
Song C,
Laosinchai W,
Amano Y, Brown RM
(2001) A putative plant homolog of the yeast beta-1,3-glucan synthase subunit FKS1 from cotton (Gossypium hirsutum L.) fibers. Planta 213, 223–230.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

DeBolt S,
Gutierrez R,
Ehrhardt DW,
Melo CV,
Ross L,
Cutler SR,
Somerville C, Bonetta D
(2007) Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proceedings of the National Academy of Sciences of the United States of America 104, 5854–5859.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Delmer DP
(1999) Cellulose biosynthesis: exciting times for a difficult field of study. Annual Review of Plant Physiology and Plant Molecular Biology 50, 245–276.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Desprez T,
Juraniec M,
Crowell E,
Jouy H,
Pochylova Z,
Parcy F,
Höfte H,
Gonneau M, Vernhettes S
(2007) Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 104, 15572–15577.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dhugga KS,
Barreiro R,
Whitten B,
Stecca K,
Hazebroek J,
Randhawa GS,
Dolan M,
Kinney AJ,
Tomes D,
Nichols S, Anderson P
(2004) Guar seed β-mannan synthase is a member of the cellulose synthase super gene family. Science 303, 363–366.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Doblin MS,
De Melis L,
Newbigin E,
Bacic A, Read SM
(2001) Pollen tubes of Nicotiana alata express two genes from different β-glucan synthase families. Plant Physiology 125, 2040–2052.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Doblin MS,
Pettolino FA,
Wilson SM,
Campbell R,
Burton RA,
Fincher GB,
Newbigin E, Bacic A
(2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-d-glucan synthesis in transgenic Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 106, 5996–6001.
| Crossref | d-glucan synthesis in transgenic Arabidopsis.&journal=Proceedings of the National Academy of Sciences of the United States of America&volume=106&pages=5996-6001&publication_year=2009&author=A%20Bacic&hl=en&doi=10.1073/pnas.0902019106" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Dong X,
Hong Z,
Sivaramakrishnan M,
Mahfouz M, Verma DPS
(2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. The Plant Journal 42, 315–328.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Du H,
Clarke AE, Bacic A
(1996) Arabinogalactan-proteins: a class of extracellular matrix proteoglycans involved in plant growth and development. Trends in Cell Biology 6, 411–414.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dunkley TPJ,
Hester S,
Shadforth IP,
Runions J, Weimar T ,
et al
.
(2006) Mapping the Arabidopsis organelle proteome. Proceedings of the National Academy of Sciences of the United States of America 103, 6518–6523.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dwivany FM,
Yulia D,
Burton RA,
Shirley NJ,
Wilson SM,
Fincher GB,
Bacic A,
Newbigin E, Doblin MS
(2009) The Cellulose-Synthase Like C (CSLC) family of barley includes members that are integral membrane proteins targeted to the plasma membrane. Molecular Plant 2, 1025–1039.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Edwards ME,
Dickson CA,
Chengappa S,
Sidebottom C,
Gidley MJ, Reid JSG
(1999) Molecular characterisation of a membrane-bound galactosyltransferase of plant cell wall matrix polysaccharide biosynthesis. The Plant Journal 19, 691–697.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Edwards ME,
Choo TS,
Dickson CA,
Scott C,
Gidley MJ, Reid JSG
(2004) The seeds of Lotus japonicus lines transformed with sense, antisense, and sense/antisense galactomannan galactosyltransferase constructs have structurally altered galactomannans in their endosperm cell walls. Plant Physiology 134, 1153–1162.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Egelund J,
Petersen BL,
Motawia MS,
Damager I,
Faik A,
Olsend CE,
Ishii T,
Clausen H,
Ulvskov P, Geshi N
(2006)
Arabidopsis thaliana RGXT1 and RGXT2 encode Golgi-localized (1,3)-α-d-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II. The Plant Cell 18, 2593–2607.
| Crossref | d-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II.&journal=The Plant Cell&volume=18&pages=2593-2607&publication_year=2006&author=N%20Geshi&hl=en&doi=10.1105/tpc.105.036566" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Elkins T,
Hortsch M,
Bieber AJ,
Snow PM, Goodman CS
(1990)
Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. The Journal of Cell Biology 110, 1825–1832.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Enns LC,
Kanaoka MM,
Torii KU,
Comai L,
Okada K, Cleland RE
(2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Molecular Biology 58, 333–349.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Escobar-Restrepo JM,
Huck N,
Kessler S,
Gagliardini V,
Gheyselinck J,
Yang WC, Grossniklaus U
(2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317, 656–660.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Faik A,
Bar-Peled M,
DeRocher AE,
Zeng W,
Perrin RM,
Wilkerson C,
Raikhel NV, Keegstra K
(2000) Biochemical characterization and molecular cloning of an α-1,2-fucosyltransferase that catalyzes the last step of cell wall xyloglucan biosynthesis in pea. The Journal of Biological Chemistry 275, 15082–15089.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Faik A,
Price NJ,
Raikhel NV, Keegstra K
(2002) An Arabidopsis gene encoding an α-xylosyltransferase involved in xyloglucan biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 99, 7797–7802.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Favery B,
Ryan E,
Foreman J,
Linstead P,
Boudonck K,
Steer M,
Shaw P, Dolan L
(2001) KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes & Development 15, 79–89.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fincher GB
(2009a) Exploring the evolution of (1,3;1,4)-β-d-glucans in plant cell walls: comparative genomics can help! Current Opinion in Plant Biology 12, 140–147.
| Crossref | d-glucans in plant cell walls: comparative genomics can help!&journal=Current Opinion in Plant Biology&volume=12&pages=140-147&publication_year=2009&author=GB%20Fincher&hl=en&doi=10.1016/j.pbi.2009.01.002" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Fincher GB
(2009b) Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiology 149, 27–37.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fry SC,
York WS,
Albersheim P,
Darvill A, Hayashi T ,
et al
.
(1993) An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiologia Plantarum 89, 1–3.
| Crossref | GoogleScholarGoogle Scholar |

Gardiner JC,
Taylor NG, Turner SR
(2003) Control of cellulose synthase complex localization in developing xylem. The Plant Cell 15, 1740–1748.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Geisler-Lee J,
Geisler M,
Coutinho PM,
Segerman B, Nishikubo N ,
et al
.
(2006) Poplar carbohydrate-active enzymes. gene identification and expression analyses. Plant Physiology 140, 946–962.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gens JS,
Fujiki M, Pickard BG
(2000) Arabinogalactan protein and wall-associated kinase in a plasmalemmal reticulum with specialized vertices. Protoplasma 212, 115–134.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gibeaut DM, Carpita NC
(1993) Synthesis of (1→3),(1→4)-β-d-glucan in the Golgi apparatus of maize coleoptiles. Proceedings of the National Academy of Sciences of the United States of America 90, 3850–3854.
| Crossref | d-glucan in the Golgi apparatus of maize coleoptiles.&journal=Proceedings of the National Academy of Sciences of the United States of America&volume=90&pages=3850-3854&publication_year=1993&author=NC%20Carpita&hl=en&doi=10.1073/pnas.90.9.3850" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Gibeaut DM, Carpita NC
(1994) Improved recovery of (1→3),(1→4)-β-d-glucan synthase activity from Golgi apparatus of Zea mays (L.) using differential flotation centrifugation. Protoplasma 180, 92–97.
| Crossref | d-glucan synthase activity from Golgi apparatus of Zea mays (L.) using differential flotation centrifugation.&journal=Protoplasma&volume=180&pages=92-97&publication_year=1994&author=NC%20Carpita&hl=en&doi=10.1007/BF01379227" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |

Haigler CH, Brown RM
(1986) Transport of rosettes from the Golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134, 111–120.
| Crossref | GoogleScholarGoogle Scholar |

Han F,
Ullrich SE,
Chirat S,
Menteur S, Jestin L ,
et al
.
(1995) Mapping of β-glucan content and β-glucanase activity loci in barley grain and malt. Theoretical and Applied Genetics 91, 921–927.
| Crossref | GoogleScholarGoogle Scholar |

Harholt J,
Jensen JK,
Sørensen SO,
Orfila C,
Pauly M, Scheller HV
(2006) ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiology 140, 49–58.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Harris PJ,
Kelderman MR,
Kendon MF, McKenzie RJ
(1997) Monosaccharide compositions of unlignified cell walls of monocotyledons in relation to the occurrence of wall-bound ferulic acid. Biochemical Systematics and Ecology 25, 167–179.
| Crossref | GoogleScholarGoogle Scholar |

Hazen SP,
Scott-Craig JS, Walton JD
(2002) Cellulose synthase-like genes of rice. Plant Physiology 128, 336–340.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hématy K, Höfte H
(2008) Novel receptor kinases involved in growth regulation. Current Opinion in Plant Biology 11, 321–328.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hématy K,
Sado P-E,
Van Tuinen A,
Rochange S,
Desnos T,
Balzergue S,
Pelletier S,
Renou J-P, Höfte H
(2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Current Biology 17, 922–931.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Henry RJ,
Schibeci A, Stone BA
(1983) Localization of β-glucan synthases on the membranes of cultured Lolium multiflorum (ryegrass) endosperm cells. The Biochemical Journal 209, 627–633.
| PubMed |

Honys D, Twell D
(2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology 132, 640–652.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huber O, Sumper M
(1994) Algal-CAMs: isoforms of a cell adhesion molecule in embryos of the alga Volvox with homology to Drosophila fasciclin I. The EMBO Journal 13, 4212–4222.
| PubMed |

Humphrey TV,
Bonetta DT, Goring DR
(2007) Sentinels at the wall: cell wall receptors and sensors. New Phytologist 176, 7–21.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Iwai H,
Masaoka N,
Ishii T, Satoh S
(2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proceedings of the National Academy of Sciences of the United States of America 99, 16319–16324.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Iwai H,
Hokura A,
Oishi M,
Chida H,
Ishii T,
Sakai S, Satoh S
(2006) The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proceedings of the National Academy of Sciences of the United States of America 103, 16592–16597.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jacobs AK,
Lipka V,
Burton RA,
Panstruga R,
Strizhov N,
Schulze-Lefert P, Fincher GB
(2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. The Plant Cell 15, 2503–2513.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jensen JK,
Sorensen SO,
Harholt J,
Geshi N, Sakuragi Y ,
et al
.
(2008) Identification of a xylogalacturonan xylosyltransferase involved in pectin biosynthesis in Arabidopsis. The Plant Cell 20, 1289–1302.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kawamoto T,
Noshiro M,
Shen M,
Nakamasu K,
Hashimoto K,
Kawashima-Ohya Y,
Gotoh O, Kato Y
(1998) Structural and phylogenetic analyses of RGD-CAP/βig-h3, a fasciclin-like adhesion protein expressed in chick chondrocytes. Biochimica et Biophysica Acta (BBA) – Gene Structure and Expression 1395, 288–292.
| Crossref |

Keegstra K, Raikhel N
(2001) Plant glycosyltransferases. Current Opinion in Plant Biology 4, 219–224.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kim CM,
Park SH,
Je BI,
Park SH,
Park SJ,
Piao HL,
Eun MY,
Dolan L, Han C-d
(2007)
OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiology 143, 1220–1230.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kohorn BD
(2000) Plasma membrane-cell wall contacts. Plant Physiology 124, 31–38.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kohorn BD,
Kobayashi M,
Johansen S,
Riese J,
Li-Fen H,
Koch K,
Fu S,
Dotson A, Byers N
(2006) An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. The Plant Journal 46, 307–316.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kudlicka K, Brown RM
(1997) Cellulose and callose biosynthesis in higher plants. I. Solubilization and separation of (1→3)- and (1→4)-beta-glucan synthase activities from mung bean. Plant Physiology 115, 643–656.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kurek I,
Kawagoe Y,
Jacob-Wilk D,
Doblin M, Delmer D
(2002) Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proceedings of the National Academy of Sciences of the United States of America 99, 11109–11114.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lai-Kee-Him J,
Chanzy H,
Muller M,
Putaux JL,
Imai T, Bulone V
(2002)
In vitro versus in vivo cellulose microfibrils from plant primary wall synthases: structural differences. The Journal of Biological Chemistry 277, 36931–36939.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lally D,
Ingmire P,
Tong H-Y, He Z-H
(2001) Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. The Plant Cell 13, 1317–1332.
| Crossref |
PubMed |

Lamport DTA,
Kieliszewski MJ, Showalter AM
(2006) Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyse AGP function. New Phytologist 169, 479–492.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lao NT,
Long D,
Kiang S,
Coupland G,
Shoue DA,
Carpita NC, Kavanagh TA
(2003) Mutation of a family 8 glycosyltransferase gene alters cell wall carbohydrate composition and causes a humidity-sensitive semi-sterile dwarf phenotype in Arabidopsis. Plant Molecular Biology 53, 687–701.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Leboeuf E,
Guillon F,
Thoiron S, Lahaye M
(2005) Biochemical and immunohistochemical analysis of pectic polysaccharides in the cell walls of Arabidopsis mutant QUASIMODO 1 suspension-cultured cells: implications for cell adhesion. Journal of Experimental Botany 56, 3171–3182.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lee C,
O’Neill MA,
Tsumuraya Y,
Darvill AG, Ye Z-H
(2007a) The irregular xylem9 mutant is deficient in xylan xylosyltransferase activity. Plant & Cell Physiology 48, 1624–1634.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lee C,
Zhong R,
Richardson EA,
Himmelsbach DS,
McPhail BT, Ye Z-H
(2007b) The PARVUS gene is expressed in cells undergoing secondary wall thickening and is essential for glucuronoxylan biosynthesis. Plant & Cell Physiology 48, 1659–1672.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Li J,
Burton RA,
Harvey AJ,
Hrmova M,
Wardak AZ,
Stone BA, Fincher GB
(2003) Biochemical evidence linking a putative callose synthase gene with (1→3)-beta-d-glucan biosynthesis in barley. Plant Molecular Biology 53, 213–225.
| Crossref | d-glucan biosynthesis in barley.&journal=Plant Molecular Biology&volume=53&pages=213-225&publication_year=2003&author=GB%20Fincher&hl=en&doi=10.1023/B:PLAN.0000009289.50285.52" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Li Y,
Smith C,
Corke F,
Zheng L,
Merali Z,
Ryden P,
Derbyshire P,
Waldron K, Bevan MW
(2007) Signaling from an altered cell wall to the nucleus mediates sugar-responsive growth and development in Arabidopsis thaliana. The Plant Cell 19, 2500–2515.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Liepman AH,
Wilkerson CG, Keegstra K
(2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proceedings of the National Academy of Sciences of the United States of America 102, 2221–2226.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Liepman AH,
Nairn CJ,
Willats WGT,
Sorensen I,
Roberts AW, Keegstra K
(2007) Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggests diverse roles of mannans in plants. Plant Physiology 143, 1881–1893.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Madson M,
Dunand C,
Li X,
Verma R,
Vanzin GF,
Caplan J,
Shoue DA,
Carpita NC, Reiter W-D
(2003) The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. The Plant Cell 15, 1662–1670.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Majewska-Sawka A, Nothnagel EA
(2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiology 122, 3–10.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Manfield IW,
Orfila C,
McCartney L,
Harholt J,
Bernal AJ,
Scheller HV,
Gilmartin PM,
Mikkelsen JD,
Knox JP, Willats WGT
(2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. The Plant Journal 40, 260–275.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

McCann MC, Roberts K
(1994) Changes in cell wall architecture during cell elongation. Journal of Experimental Botany 45, 1683–1691.

Mitchell RAC,
Dupree P, Shewry PR
(2007) A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiology 144, 43–53.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mohnen D
(2008) Pectin structure and biosynthesis. Current Opinion in Plant Biology 11, 266–277.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Moller I,
Sørensen I,
Bernal AJ,
Blaukopf C, Lee K ,
et al
.
(2007) High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. The Plant Journal 50, 1118–1128.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mouille G,
Ralet M-C,
Cavelier C,
Eland C, Effroy D ,
et al
.
(2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. The Plant Journal 50, 605–614.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nakamura A,
Furata H,
Maeda H,
Takao T, Nagamatsu Y
(2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Bioscience, Biotechnology, and Biochemistry 66, 1301–1313.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Natera SHA,
Ford KL,
Cassin AM,
Patterson JH,
Newbigin EJ, Bacic A
(2008) Analysis of the Oryza sativa plasma membrane proteome using combined protein and peptide fractionation approaches in conjunction with mass spectrometry. Journal of Proteome Research 7, 1159–1187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nishikawa S,
Zinkl GM,
Swanson RJ,
Maruyama D, Preuss D
(2005) Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biology 5, 22.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nishimura MT,
Stein M,
Hou B-H,
Vogel JP,
Edwards H, Somerville SC
(2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301, 969–972.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nothnagel EA
(1997) Proteoglycans and related components in plant cells. International Review of Cytology 174, 195–291.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Obroucheva N
(2008) Cell elongation as an inseparable component of growth in terrestrial plants. Russian Journal of Developmental Biology 39, 13–24.

Orfila C,
Sørensen SO,
Harholt J,
Geshi N,
Crombie H,
Truong H-N,
Reid JSG,
Knox JP, Scheller HV
(2005)
QUASIMODO1 is expressed in vascular tissue of Arabidopsis thaliana inflorescence stems, and affects homogalacturonan and xylan biosynthesis. Planta 222, 613–622.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oxley D, Bacic A
(1999) Structure of the glycosylphosphatidylinositol anchor of an arabinogalactan protein from Pyrus communis suspension-cultured cells. Proceedings of the National Academy of Sciences of the United States of America 96, 14246–14251.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Paredez AR,
Somerville CR, Ehrhardt DW
(2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Paredez AR,
Persson S,
Ehrhardt DW, Somerville CR
(2008) Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiology 147, 1723–1734.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Park AR,
Cho SK,
Yun UJ,
Jin MY,
Lee SH,
Sachetto-Martins G, Park OK
(2001) Interaction of the Arabidopsis receptor protein kinase wak1 with a glycine-rich protein, AtGRP-3. The Journal of Biological Chemistry 276, 26 688–26 693.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pear JR,
Kawagoe Y,
Schreckengost WE,
Delmer DP, Stalker DM
(1996) Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proceedings of the National Academy of Sciences of the United States of America 93, 12 637–12 642.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pelosi L,
Imai T,
Chanzy H,
Heux L,
Buhler E, Bulone V
(2003) Structural and morphological diversity of (1→3)-β-d-glucans synthesized in vitro by enzymes from Saprolegnia monoica. Comparison with a corresponding in vitro product from blackberry (Rubus fruticosus). Biochemistry 42, 6264–6274.
| Crossref | d-glucans synthesized in vitro by enzymes from Saprolegnia monoica. Comparison with a corresponding in vitro product from blackberry (Rubus fruticosus).&journal=Biochemistry&volume=42&pages=6264-6274&publication_year=2003&author=V%20Bulone&hl=en&doi=10.1021/bi0340550" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | PubMed |

Peña MJ,
Zhong R,
Zhou G-K,
Richardson EA,
O’Neill MA,
Darvill AG,
York WS, Ye Z-H
(2007)
Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. The Plant Cell 19, 549–563.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peña MJ,
Darvill AG,
Eberhard S,
York WS, O’Neill MA
(2008) Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology 18, 891–904.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Peng LC,
Kawagoe Y,
Hogan P, Delmer D
(2002) Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 295, 147–150.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Perrin RM,
DeRocher AE,
Bar-Peled M,
Zeng W,
Norambuena L,
Orellana A,
Raikhel NV, Keegstra K
(1999) Xyloglucan fucosyltransferase, an enzyme involved in plant cell wall biosynthesis. Science 284, 1976–1979.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Persson S,
Wei H,
Milne J,
Page G, Somerville C
(2005) Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proceedings of the National Academy of Sciences of the United States of America 102, 8633–8638.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Persson S,
Caffall KH,
Freshour G,
Hilley MT,
Bauer S,
Poindexter P,
Hahn MG,
Mohnen D, Somerville C
(2007a) The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. The Plant Cell 19, 237–255.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Persson S,
Paredez A,
Carroll A,
Palsdottir H,
Doblin M,
Poindexter P,
Khitrov N,
Auer M, Somerville C
(2007b) Genetic evidence for three unique components in primary cell wall cellulose synthase complexes in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 104, 15566–15571.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pickard BG
(2008) ‘Second extrinsic organizational mechanism’ for orienting cellulose: modeling a role for the plasmalemmal reticulum. Protoplasma 233, 7–29.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Plun-Favreau H,
Elson G,
Chabbert M,
Froger J, deLapeyrière O ,
et al
.
(2001) The ciliary neurotrophic factor receptor α component induces the secretion of and is required for functional responses to cardiotrophin-like cytokine. The EMBO Journal 20, 1692–1703.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Read SM, Bacic A
(2002) Prime time for cellulose. Science 295, 59–60.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Reiter W-D
(2008) Biochemical genetics of nucleotide sugar interconversion reactions. Current Opinion in Plant Biology 11, 236–243.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Reyes F, Orellana A
(2008) Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Current Opinion in Plant Biology 11, 244–251.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Richmond TA, Somerville CR
(2000) The cellulose synthase superfamily. Plant Physiology 124, 495–498.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ridley BL,
O’Neill MA, Mohnen D
(2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Robert S,
Mouille G, Höfte H
(2004) The mechanism and regulation of cellulose synthesis in primary walls: lessons from cellulose-deficient Arabidopsis mutants. Cellulose 11, 351–364.
| Crossref | GoogleScholarGoogle Scholar |

Roberts K
(1990) Structures at the plant cell surface. Current Opinion in Cell Biology 2, 920–928.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Roberts AW, Bushoven JT
(2007) The cellulose synthase (CESA) gene superfamily of the moss Physcomitrella patens. Plant Molecular Biology 63, 207–219.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sardar HS,
Yang J, Showalter AM
(2006) Molecular interactions of arabinogalactan proteins with cortical microtubules and F-Actin in bright yellow-2 tobacco cultured cells. Plant Physiology 142, 1469–1479.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarria R,
Wagner TA,
O’Neill MA,
Faik A,
Wilkerson CG,
Keegstra K, Raikhel NV
(2001) Characterization of a family of Arabidopsis genes related to xyloglucan fucosyltransferase1. Plant Physiology 127, 1595–1606.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Scheible WR, Pauly M
(2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Current Opinion in Plant Biology 7, 285–295.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Scheller HV,
Jensen JK,
Sørensen SO,
Harholt J, Geshi N
(2007) Biosynthesis of pectin. Physiologia Plantarum 129, 283–295.
| Crossref | GoogleScholarGoogle Scholar |

Schreiber A,
Shirley N,
Burton R, Fincher G
(2008) Combining transcriptional datasets using the generalized singular value decomposition. BMC Bioinformatics 9, 335.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schultz CJ,
Gilson P,
Oxley D,
Youl JJ, Bacic A
(1998) GPI-anchors on arabinogalactan-proteins: implications for signalling in plants. Trends in Plant Science 3, 426–431.
| Crossref | GoogleScholarGoogle Scholar |

Schultz CJ,
Ferguson KL,
Lahnstein J, Bacic A
(2004) Post-translational modifications of arabinogalactan-peptides of Arabidopsis thaliana: endoplasmic reticulum and glycosylphosphatidylinositol-anchor signal cleavage sites and hydroxlation of proline. The Journal of Biological Chemistry 279, 45503–45511.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Seifert GJ
(2004) Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Current Opinion in Plant Biology 7, 277–284.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Seifert GJ, Roberts K
(2007) The biology of arabinogalactan proteins. Annual Review of Plant Biology 58, 137–161.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shao M,
Zheng H,
Hu Y,
Liu D,
Jang J-C,
Ma H, Huang H
(2004) The GAOLAOZHUANGREN1 gene encodes a putative glycosyltransferase that is critical for normal development and carbohydrate metabolism. Plant & Cell Physiology 45, 1453–1460.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shi H,
Kim Y,
Guo Y,
Stevenson B, Zhu J-K
(2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. The Plant Cell 15, 19–32.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Smith BG, Harris PJ
(1999) The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochemical Systematics and Ecology 27, 33–53.
| Crossref | GoogleScholarGoogle Scholar |

Somerville C
(2006) Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology 22, 53–78.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Somerville C,
Bauer S,
Brininstool G,
Facette M, Hamann T ,
et al
.
(2004) Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Staudte RG,
Woodward JR,
Fincher GB, Stone BA
(1983) Water-soluble (1→3),(1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. III. Distribution of cellotriosyl and cellotetraosyl residues. Carbohydrate Polymers 3, 299–312.
| Crossref | d-glucans from barley (Hordeum vulgare) endosperm. III. Distribution of cellotriosyl and cellotetraosyl residues.&journal=Carbohydrate Polymers&volume=3&pages=299-312&publication_year=1983&author=BA%20Stone&hl=en&doi=10.1016/0144-8617(83)90027-9" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |

Sterling JD,
Atmodjo MA,
Inwood SE,
Kolli VSK,
Quigley HF,
Hahn MG, Mohnen D
(2006) Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proceedings of the National Academy of Sciences of the United States of America 103, 5236–5241.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sugimoto K,
Himmelspach R,
Williamson RE, Wasteneys GO
(2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. The Plant Cell 15, 1414–1429.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Suzuki S,
Li L,
Sun Y-H, Chiang VL
(2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiology 142, 1233–1245.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Szymanski DB, Cosgrove DJ
(2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Current Biology 19, R800–R811.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tamura K,
Shimada T,
Kondo M,
Nishimura M, Hara-Nishimura I
(2005) KATAMARI1/MURUS3 is a novel Golgi membrane protein that is required for endomembrane organization in Arabidopsis. The Plant Cell 17, 1764–1776.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Taylor NG,
Scheible WR,
Cutler S,
Somerville CR, Turner SR
(1999) The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. The Plant Cell 11, 769–779.
| Crossref |
PubMed |

Taylor NG,
Laurie S, Turner SR
(2000) Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. The Plant Cell 12, 2529–2539.
| Crossref |
PubMed |

Taylor NG,
Howells RM,
Huttly AK,
Vickers K, Turner SR
(2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proceedings of the National Academy of Sciences of the United States of America 100, 1450–1455.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Töller A,
Brownfield L,
Neu C,
Twell D, Schulze-Lefert P
(2008) Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. The Plant Journal 54, 911–923.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trethewey JAK, Harris PJ
(2002) Location of (1→3),(1→4)-β-d-glucans in vegetative cell walls of barley (Hordeum vulgare) using immunogold labelling. New Phytologist 154, 347–358.
| Crossref | d-glucans in vegetative cell walls of barley (Hordeum vulgare) using immunogold labelling.&journal=New Phytologist&volume=154&pages=347-358&publication_year=2002&author=PJ%20Harris&hl=en&doi=10.1046/j.1469-8137.2002.00383.x" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |

Turner SR, Somerville CR
(1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. The Plant Cell 9, 689–701.
| Crossref |
PubMed |

van Erp H, Walton J
(2009) Regulation of the cellulose synthase-like gene family by light in the maize mesocotyl. Planta 229, 885–897.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vanholme R,
Morreel K,
Ralph J, Boerjan W
(2008) Lignin engineering. Current Opinion in Plant Biology 11, 278–285.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vanzin GF,
Madson M,
Carpita NC,
Raikhel NV,
Keegstra K, Reiter W-D
(2002) The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proceedings of the National Academy of Sciences of the United States of America 99, 3340–3345.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vogel J, Somerville S
(2000) Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proceedings of the National Academy of Sciences of the United States of America 97, 1897–1902.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wagner TA, Kohorn BD
(2001) Wall-associated kinases are expressed throughout plant development and are required for cell expansion. The Plant Cell 13, 303–318.
| Crossref |
PubMed |

Wang X,
Cnops G,
Vanderhaeghen R,
De Block S,
Van Montagu M, Van Lijsebettens M
(2001)
AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiology 126, 575–586.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wasteneys GO
(2004) Progress in understanding the role of microtubules in plant cells. Current Opinion in Plant Biology 7, 651–660.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wightman R,
Marshall R, Turner SR
(2009) A cellulose synthase-containing compartment moves rapidly beneath sites of secondary wall synthesis. Plant & Cell Physiology 50, 584–594.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wilson JR, Hatfield RD
(1997) Structural and chemical changes of cell wall types during stem development: consequences for fibre degradation by rumen microflora. Australian Journal of Agricultural Research 48, 165–180.
| Crossref | GoogleScholarGoogle Scholar |

Wilson S,
Burton R,
Doblin M,
Stone B,
Newbigin E,
Fincher G, Bacic A
(2006) Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224, 655–667.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wu A-M,
Rihouey C,
Seveno M,
Hörnblad E,
Singh SK,
Matsunaga T,
Ishii T,
Lerouge P, Marchant A
(2009) The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. The Plant Journal 57, 718–731.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Xu S-L,
Rahman A,
Baskin TI, Kieber JJ
(2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. The Plant Cell 20, 3065–3079.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

York WS, O’Neill MA
(2008) Biochemical control of xylan biosynthesis – which end is up? Current Opinion in Plant Biology 11, 258–265.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Youl JJ,
Bacic A, Oxley D
(1998) Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphaticylinositol membrane anchors. Proceedings of the National Academy of Sciences of the United States of America 95, 7921–7926.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zabotina O,
Malm E,
Drakakaki G,
Bulone V, Raikhel N
(2008) Identification and preliminary characterization of a new chemical affecting glucosyltransferase activities involved in plant cell wall biosynthesis. Molecular Plant 1, 977–989.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zeng W, Keegstra K
(2008) AtCSLD2 is an integral Golgi membrane protein with its N-terminus facing the cytosol. Planta 228, 823–838.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhong R, Ye Z-H
(2003) Unraveling the functions of glycosyltransferase family 47 in plants. Trends in Plant Science 8, 565–568.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhong R,
Peña MJ,
Zhou G-K,
Nairn CJ,
Wood-Jones A,
Richardson EA,
Morrison WH,
Darvill AG,
York WS, Ye Z-H
(2005)
Arabidopsis Fragile Fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. The Plant Cell 17, 3390–3408.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



