Evans Review No. 2: The hot and the cold: unravelling the variable response of plant respiration to temperature
Owen K. Atkin A , Dan Bruhn B , Vaughan M. Hurry C and Mark G. Tjoelker DA Department of Biology (Area 2), The University of York, PO Box 373, York YO10 5YW, UK. Corresponding author. Email: OKA1@york.ac.uk
B Cooperative Research Centre for Green House Accounting, Ecosystem Dynamics Group, Research School of Biological Sciences, Australian National University, Canberra, ACT 0200, Australia.
C Umeå Plant Science Centre, Department of Plant Physiology, Umeå University, 901 87 Umeå, Sweden.
D Department of Forest Science, Texas A & M University, 2135 TAMU, College Station, TX 77843-2135, USA.
This paper is part of The Evans Review series, named for Dr Lloyd Evans. The series contains reviews that are critical, state-of-the-art evaluations that aim to advance our understanding, rather than being exhaustive compilations of information, and are written by invitation.
Functional Plant Biology 32(2) 87-105 https://doi.org/10.1071/FP03176
Submitted: 30 September 2003 Accepted: 14 December 2004 Published: 24 February 2005
Abstract
When predicting the effects of climate change, global carbon circulation models that include a positive feedback effect of climate warming on the carbon cycle often assume that (1) plant respiration increases exponentially with temperature (with a constant Q10) and (2) that there is no acclimation of respiration to long-term changes in temperature. In this review, we show that these two assumptions are incorrect. While Q10 does not respond systematically to elevated atmospheric CO2 concentrations, other factors such as temperature, light, and water availability all have the potential to influence the temperature sensitivity of respiratory CO2 efflux. Roots and leaves can also differ in their Q10 values, as can upper and lower canopy leaves. The consequences of such variable Q10 values need to be fully explored in carbon modelling. Here, we consider the extent of variability in the degree of thermal acclimation of respiration, and discuss in detail the biochemical mechanisms underpinning this variability; the response of respiration to long-term changes in temperature is highly dependent on the effect of temperature on plant development, and on interactive effects of temperature and other abiotic factors (e.g. irradiance, drought and nutrient availability). Rather than acclimating to the daily mean temperature, recent studies suggest that other components of the daily temperature regime can be important (e.g. daily minimum and / or night temperature). In some cases, acclimation may simply reflect a passive response to changes in respiratory substrate availability, whereas in others acclimation may be critical in helping plants grow and survive at contrasting temperatures. We also consider the impact of acclimation on the balance between respiration and photosynthesis; although environmental factors such as water availability can alter the balance between these two processes, the available data suggests that temperature-mediated differences in dark leaf respiration are closely linked to concomitant differences in leaf photosynthesis. We conclude by highlighting the need for a greater process-based understanding of thermal acclimation of respiration if we are to successfully predict future ecosystem CO2 fluxes and potential feedbacks on atmospheric CO2 concentrations.
Keywords: carbon fluxes, climate change, respiration, temperature.
Introduction
Mitochondrial respiration plays a pivotal role in determining the growth and survival of plants, and has a profound impact on net ecosystem CO2 exchange and the concentration of CO2 in the atmosphere (Gifford 2003). Much of the energy and carbon skeletons necessary for biosynthesis and cellular maintenance are produced by plant respiration. Under some conditions (e.g. excess irradiance), respiration (R) may also help minimise the formation of potentially damaging reactive oxygen species (ROS) through oxidation of excess cellular redox equivalents (Saradadevi and Raghavendra 1992; Shyam et al. 1993; Purvis and Shewfelt 1993; Hurry et al. 1995; Maxwell et al. 1999). R is also crucial for (1) the production of ascorbate (vitamin C; Millar et al. 2003), a necessary component of the protective xanthophyll and glutathinone cycles, (2) the maintenance of photosynthetic activity, largely because of the energy demands of sucrose synthesis (Krömer 1995), and (3) regulating pathogen defence processes (Noctor et al. 2004). R also plays an important role in determining the carbon budget of individual plants and the concentration of CO2 in the atmosphere. Between 30 and 80% of the CO2 taken up by photosynthesis (P) each day is subsequently respired (Poorter et al. 1990; Atkin et al. 1996; Tjoelker et al. 1999a; Amthor 2000; Loveys et al. 2002). Thus, plant R contributes up to 65% of the total CO2 released into the atmosphere at the ecosystem level; with the remaining CO2 being derived from heterotrophic soil R (Xu et al. 2001; Reichstein et al. 2002). Globally, plant R releases approximately 60 Gt of carbon into the atmosphere each year. Although largely balanced by carbon uptake through photosynthesis, annual respiratory carbon flux to the atmosphere is approximately ten times the CO2 released by fossil fuel burning (Raich and Schlesinger 1992; Amthor 1997; Field 2001). However, the extent of respiratory CO2 release may change in the future in response to global climate change. Coupled climate and carbon cycle models need to incorporate variability in rates of plant R if they are to accurately predict future atmospheric CO2 concentrations.
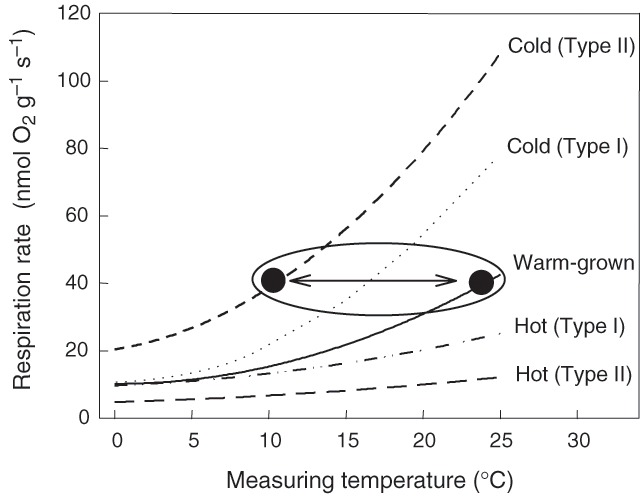
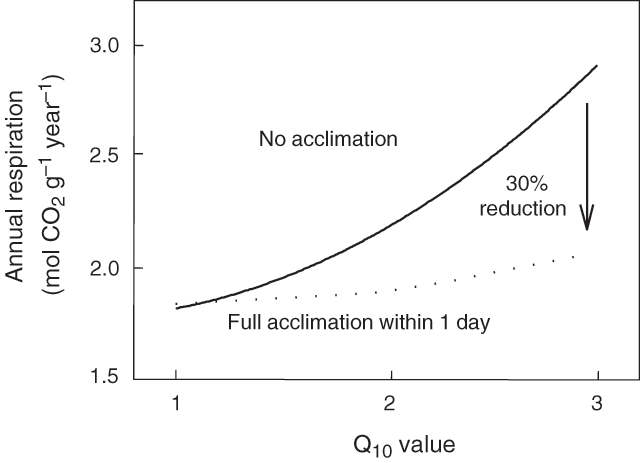
One of the most important environmental parameters affecting rates of plant R is temperature (James 1953; Forward 1960; Berry and Raison 1981). It is often assumed that the relationship between plant R and temperature is exponential with a constant Q10 (i.e. proportional change in R with a 10°C increase in temperature, typically around 2) (e.g. Atkin and Day 1990; Ryan 1991; Raich and Schlesinger 1992). As a result, most simulation models such as Biome-BGC, Century (Schimel et al. 1997), PnET (Aber and Federer 1992) and several dynamic vegetation models (White et al. 2000; Cramer et al. 2001) assume that R responds to short- and long-term changes in temperature in a fixed, exponential manner (Q10 = 2.0). Based on such assumptions, coupled global circulation models (GCMs) predict that global warming will result in increased rates of respiratory CO2 efflux into the atmosphere, which in turn will compound the greenhouse effect. For example, mean annual land surface air temperatures are predicted to be 2.5°C higher by the year 2100 in positive feedback models that incorporate the effects of increased respiratory CO2 efflux than in models that do not include a feedback component (Cox et al. 2000). In the Cox et al. (2000) coupled model, it is also assumed that R will not acclimate to long-term changes in temperature (i.e. rates of R at a given measurement temperature remain constant over time). However, there is growing evidence that the response of R to temperature is dynamic, with plant R often acclimating to long-term changes in temperature (Fig. 1). Moreover, neither Q10 values nor degrees of acclimation are constant; rather, both vary in response to the surrounding environment and / or the metabolic status of the plant. Consequently, GCMs that fail to take into account such variability in Q10 and degrees of temperature acclimation of R are likely to result in large over-estimates of annual respiratory CO2 release into the atmosphere (e.g. Fig. 2; Atkin et al. 2000a; Wythers et al. 2005) and consequently over-estimate the extent to which atmospheric CO2 concentrations will rise over long periods (Luo et al. 2001). The importance of understanding variability in the Q10 is highlighted by predicted changes in the amplitude of diurnal temperatures experienced by plants (e.g. nights are increasing to a greater extent than day time temperatures; Easterling et al. 1997).
|
|
Although recent reviews have considered aspects of the temperature response of plant R, focussing on roots in Atkin et al. (2000a) and the mechanisms responsible for variability in Q10 values in Atkin and Tjoelker (2003), no review has yet provided a detailed account of the extent of variability in Q10 values in both leaves and roots under various environmental conditions, or focussed on biochemical mechanisms underpinning variability in the degree of temperature acclimation. The current review discusses these issues in detail, starting with variability in the short- and long-term temperature response of leaf and root respiration. It then addresses the question of whether thermal acclimation represents a passive response to changes in respiratory substrate supply and / or an active process to provide the energy necessary for growth and maintenance processes following large changes in growth temperature and / or protect plants against oxidative damage. Finally, the impact of acclimation on the balance between R and P is considered. Although our review does not consider the direct and indirect effects of atmospheric CO2 concentration on plant R per se [see Gonzàlez-Meler et al. (2004) for thorough review of this topic], we do consider the effects of growth CO2 concentration on the Q10 of leaf R. For a recent review of how plant respiration is represented in terrestrial carbon models see Gifford (2003).
Variation in the Q10 of respiration
To what extent does the Q10 of leaf and root R vary? Here, we show that although atmospheric CO2 concentration influences the Q10 of R in some studies, a survey of literature indicates that growth CO2 concentration does not, on average, increase Q10 values. The Q10 of leaf R is often, but not always, reduced in the light compared with the Q10 of leaf R in the dark. Q10 values are often lower in water-stressed plants than in their fully-watered counterparts. Roots and leaves can also differ in their Q10 values, as can upper and lower canopy leaves. Q10 of both root and leaf R dark generally decrease with increasing measurement temperature. The assumption that Q10 values are constant and equal to 2.0 is not, therefore, supported by the literature. As such, the consequences of variable Q10 values (v. using a fixed Q10 of 2.0) need to be fully explored in carbon modelling.
Atmospheric CO2 concentration
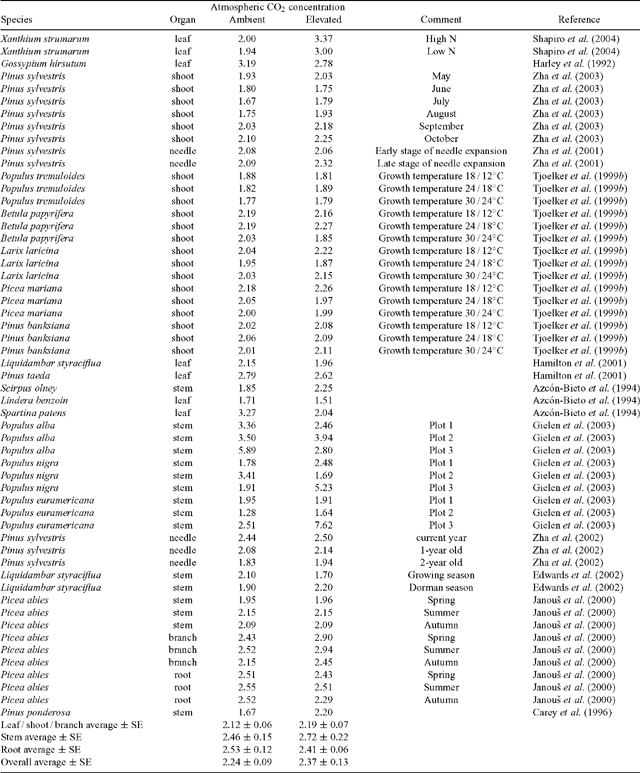
Growth CO2 concentration does not appear to have a predictable, systematic effect on the Q10 of dark respiration of leaves, stems, or roots (Table 1). The effect of atmospheric CO2 concentration during growth on Q10 values of R measured in darkness for above and below ground organs differs among studies, increasing by more than 10% in 14 of the cases shown in Table 1, decreasing by more than 10% in seven cases, or remaining unchanged (i.e. change less than 10%) in 34 cases; overall, elevated CO2 had little impact on the average Q10 values shown in Table 1. Nevertheless, there are cases where the Q10 of dark respiration is greater in plants grown under elevated atmospheric CO2 concentration. For example, Shapiro et al. (2004) found that growth under elevated CO2 increased both Q10 / dark (Table 1) and Q10 / light values of leaf respiration in Xanthium strumarium. Moreover, Q10 values also increased by elevated CO2 in late stage of needle expansion in Pinus sylvestris (Zha et al. 2001) [but see Zha et al. (2005) for contrasting results]. Overall, however, the literature results suggest that growth CO2 concentration does not on average alter the temperature sensitivity of dark respiration in roots, leaves, or shoots, among the studies shown in Table 1.
|
Water and nutrient availability
In the short term, water stress results in a reduction in leaf and root R. Root R declines during drought (Bryla et al. 1997, 2001; Burton et al. 1998). Under field conditions the relationship between soil drying and root R is often further complicated with occurrence of increased soil temperatures during drought. However, in a greenhouse study in which roots of citrus trees were maintained at constant temperatures, root R declined with decreasing soil water content over a 10-d drying period (Bryla et al. 2001). In addition, drought-induced reductions in root R were greater in warmer soils (25 and 35°C) than in a cooler soil (15°C) as soil water contents fell below 6%. Comparing the proportional differences in R at the three soil temperatures suggests that Q10 declined concurrently with soil drying. Moreover, this study demonstrated that root R acclimated to both soil moisture and soil temperatures (>23°C).
Although leaf R declines in response to short-term water deficits, less is known concerning the effects of long-term water deficits on respiratory function and Q10. In a study of three deciduous tree species growing in two sites of contrasting water availability, Q10 differed amongst species (1.5–2.1) and was lower at the drier than the wetter site (Turnbull et al. 2001). In addition, both area- and mass-based leaf R were higher and light-saturated rates of P lower at the drier than at the wetter site, suggesting that leaf-level net carbon gain was reduced at the dry site. These findings suggest that longer-term adjustments in leaf structure under low soil water availability may result in increased R but a lower temperature-sensitivity (Q10) of dark R.
Irradiance
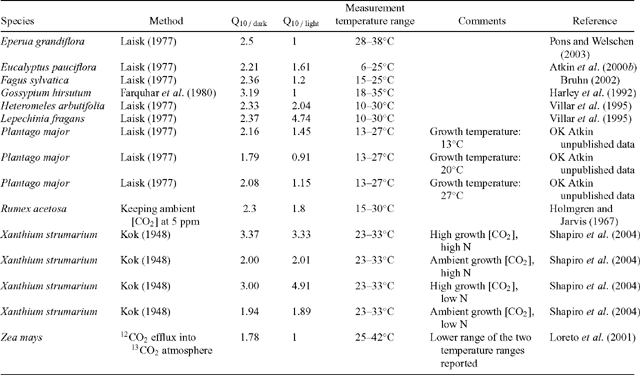
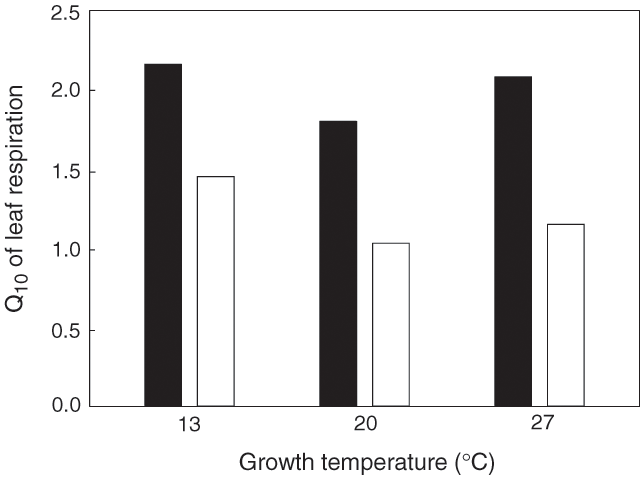
There is growing evidence that the Q10 of leaf respiratory CO2 release is often lower in light than in darkness, regardless of growth temperature (Table 2; Fig. 3). Although Q10 values in the light and dark are highly variable, a majority of studies have found that Q10 values in the light (Q10 / light) are lower than those in darkness (Q10 / dark) (e.g. 10 of the 15 comparisons shown in Table 2). In some cases, Q10 / light is close to 1 [i.e. temperature insensitive; e.g. Gossypium hirsutum, (Harley et al. 1992); Zea mays, (Loreto et al. 2001); Fagus sylvatica, (Bruhn 2002); Eperua grandiflora, (Pons and Welschen 2003)], while no differences in Q10 / light and Q10 / dark values were found in three species [Spinacia oleracea, (Brooks and Farquhar 1985), Heteromeles arbutifolia and Lepechinia fragans; (Villar et al. 1995)]. Shapiro et al. (2004) also reported that Q10 / light and Q10 / dark values were similar in Xanthium strumarium experiencing several growth treatments (with the exception of plants grown under elevated CO2 and low N, where Q10 / light was greater than Q10 / dark). Thus, while light often reduces the Q10 of R, not all species exhibit lower Q10 values in the light.
|
|
Underpinning the effect of irradiance on the Q10 (where it occurs) is the inhibitory effect of light on respiratory CO2 release per se, particularly at high measuring temperatures. All of the above studies have applied the Kok (1948) and Laisk (1977) methods, except from Loreto et al. (1999, 2001) who made use of a new technique based on the insensitivity to 13C by an infrared gas analyser. This inhibitory effect by light has been confirmed by other techniques as well, including a 14C-labelling technique that takes into account refixation of respiratory CO2 by Rubisco (Pärnik and Keerberg 1995; McCashin et al. 1988). In contrast, using the 14C method, Hurry et al. (1996) found that light stimulated R in winter rye by 31%, showing that light does not necessarily inhibit R in all species.
Leaf position within a canopy
Recent studies have shown that there is considerable within-canopy variability in the Q10 of leaf R in some trees (Bolstad et al. 1999; Griffin et al. 2002; Turnbull et al. 2003). In some studies, leaves in the lower part of the canopy exhibit higher Q10 values than leaves in the upper canopy (Griffin et al. 2002; Turnbull et al. 2003). As a result, scaling leaf respiratory CO2 loss to the whole canopy level tends to underestimate CO2 loss if the assumptions are based on the Q10 values of lower canopy leaves alone (Turnbull et al. 2003). In contrast, in a survey of 18 broad-leaved forest tree species in the southern Appalachians of North America, Bolstad et al. (1999) found no consistent trend of Q10 values with respect to canopy position. Further work is needed to assess the extent to which upper and lower canopy leaves differ systematically in their Q10 values and how it may be linked to photosynthesis.
Leaves v. roots
A survey of the literature shows that Q10 values (typically within the 10–30°C measurement temperature range) range from 1.4 to 4.2 for leaf R (Azcón-Bieto 1992; Larigauderie and Körner 1995; Tjoelker et al. 2001) and 1.1 to 4.6 for root R (Higgins and Spomer 1976; Boone et al. 1998; Tjoelker et al. 1999b). This suggests that there is little difference in overall range of Q10 values exhibited by leaves and roots. However, when Loveys et al. (2003) compared 16 species under the same growth conditions (hydroponics in growth cabinets) they found that the mean Q10 values exhibited by mature leaves (2.03–2.39) were generally higher than those of whole root systems or root segments (1.58–1.61) when determined over the 15–25°C measurement temperature range. This general response may suggest that the ability of leaves to more readily access recently fixed carbon increases the temperature response of leaf R compared with that of root R.
One factor that needs to be considered when comparing published leaf and root Q10 values is the nature of the tissue used in different studies. In most studies, rates of leaf R are measured using fully expanded individual leaves. In contrast, measurements of R in roots are made using whole root systems (e.g. Smakman and Hofstra 1982; Bouma et al. 1997; Covey-Crump et al. 2002; Loveys et al. 2003) or root segments of differing age or function (e.g. Higgins and Spomer 1976; Crawford and Palin 1981; Sowell and Spomer 1986; Weger and Guy 1991; Zogg et al. 1996; Pregitzer et al. 1997, 1998; Burton et al. 2002; Comas and Eissenstat 2004). Recent studies with leaves have shown that the Q10 of mature fully expanded leaves is higher than that of immature leaves (A Armstrong, OK Atkin unpublished data). In contrast, Zha et al. (2001) reported that maintenance respiration of fully expanded Scots pine needles was more sensitive to increases in growth temperature than growth respiration in expanding needles. Q10 values of 1.5 for coarse woody roots and 2.0 for fine roots (<2 mm diameter) were reported in a Pinus radiata stand (Ryan et al. 1996). Thus, although there is variability in the temperature response of young v. old tissues, estimates of Q10 in whole root or shoot systems can clearly depend on the proportion of the root or shoot system represented by immature and mature roots or leaves and the Q10 of each developmental stage.
Seasonal variation
Under field conditions, the Q10 of leaf R is higher in winter and autumn than in summer in evergreen species [Chamaecyparis obtuse (Paembonan et al. 1991); Picea abies (Stockfors and Linder 1998); Eucalyptus pauciflora (Atkin et al. 2000b); Pinus sylvestris (Zha et al. 2003), 2005 and Pinus banksiana (MG Tjoelker, J Oleksyn, PB Reich unpublished data)], even when compared at the same measurement temperatures. However, Eucalyptus pauciflora showed little seasonal variation in Q10 over much of the year (Atkin et al. 2000b). Q10 values were only greater on days when daily average and minimum air temperatures were below 6°C and –1°C, respectively. Also, Q10 of branch R in a Fagus sylvatica stand was found to be relatively constant (around 1.7) throughout the course of the year (Damesin et al. 2002). Zha et al. (2004) found that Q10 values of stem respiration were highest in the growing season.
Growth temperature
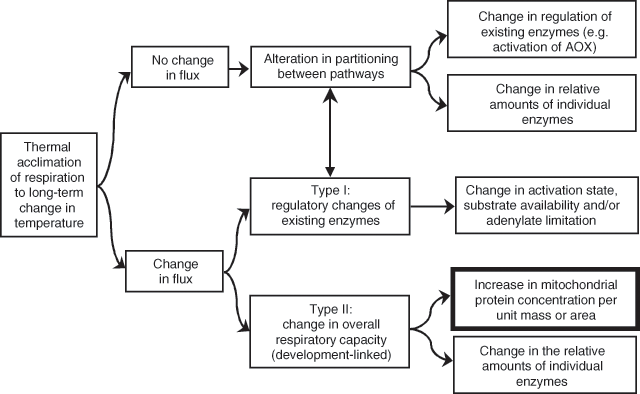
Several studies have shown that changing growth temperature also affects the Q10 of leaf and root R (e.g. schematically shown in Fig. 1; Wager 1941; Atkin et al. 2000b; Covey-Crump et al. 2002; Zha et al. 2002; Loveys et al. 2003). Differences in Q10 (for a common measurement temperature interval) between plants grown at contrasting growth temperatures ultimately arises from changes in the shape of the short-term temperature-response function. In some cases, a growth temperature-dependent change in the Q10 reflects changes in the availability of respiratory substrate and / or degree of adenylate restriction of R (Fig. 4; Atkin and Tjoelker 2003). Support for this suggestion comes from the fact that exposure to elevated atmospheric CO2 (which increases photosynthesis and steady-state carbohydrate concentrations) also increases the Q10 of leaf or needle R (Zha et al. 2002; Shapiro et al. 2004).
|
Growth-temperature effects on Q10 also appear to differ between plants exposed to a new thermal regime for several days and leaves and roots that develop under contrasting temperatures. Covey-Crump et al. (2002) found that the Q10 (between 15–23°C) of Plantago lanceolata root R was greater at low measurement temperatures in plants exposed to 15°C for 7 d than in plants kept at 23°C. Similarly, Rook (1969) found that the Q10 (between 15–30°C) of leaf R in Pinus radiata seedlings grown at 33 / 28°C increased following a 2-d exposure to 15 / 10°C (rates of R measured at 15–30°C increased significantly, whereas there was no change in R measured at 8°C). Conversely, shifting of 15 / 10°C grown plants to 33 / 28°C resulted in the Q10 decreasing within 2 d (again, no change in R at 8°C was observed). However, in another study shifting from a growth temperature of 25 / 20°C to 15 / 10°C for 7 d had no effect on the Q10 of root R of several species (calculated using rates of R measured at 15 and 25°C; Loveys et al. 2003). Moreover, no growth-temperature-dependent differences in Q10 values were apparent in plant leaves or roots that had developed at different temperatures (i.e. were not shifted; Tjoelker et al. 1999b; Loveys et al. 2003). Thus, the effect of growth temperature on the Q10 of R (and, thus, the degree of acclimation) is variable, being dependent on species and on the range of growth temperatures being compared.
Inter-biome variation
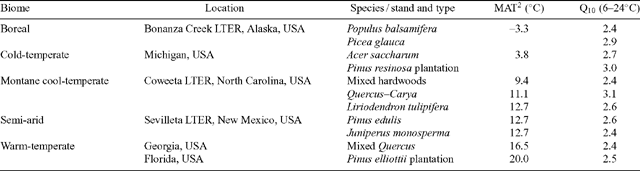
To successfully predict annual CO2 release in contrasting biomes, the extent to which Q10 values differ between biomes may be an important issue in modelling. When Q10 values are determined at measurement temperatures experienced by plants in the field, plants growing in cold climates exhibit higher Q10 values than their warm-climate counterparts [e.g. Tjoelker et al. (2001) found that the mean Q10 values of arctic and tropical plants were 2.56 and 2.14, respectively]. Because of this, short-term changes in temperature can have a greater effect on respiratory flux in plants growing in the arctic than in plants grown in tropical regions.
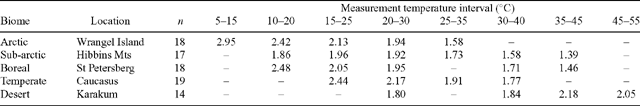
To what extent are the inter-biome differences in Q10 a reflection of inherent differences between plant species characteristic of the contrasting biomes? To assess this question, comparisons of Q10 values need to be made over a common measurement temperature range (owing to temperature dependence of the Q10; see Tjoelker et al. 2001 and Atkin and Tjoelker 2003). Criddle et al. (1994) found that temperature-corrected Q10 values varied with climate of origin amongst woody species, but not amongst annuals or herbaceous perennials. However, in a controlled-environment experiment, Larigauderie and Körner (1995) found no evidence that temperature-corrected leaf Q10 values depend on altitude of plant origin. There was no systematic variation in leaf or root Q10 values amongst species characteristic of alpine, temperate and arid environments in the study by Loveys et al. (2003). When Tjoelker et al. (2001) compared Q10 values of plants in arctic, boreal, and temperate biomes over a common temperature range, they found that the mean Q10 values were relatively similar (2.42 for arctic, 2.22 for boreal, an 2.31 for temperate biomes), compared with when Q10 values were measured over the temperatures experienced by plants in the field. Moreover, Burton et al. (2002) found that the Q10 of fine root respiration of forest trees was similar in North American biomes when compared at a common temperature (Table 3). Thus, even though some differences in Q10 occur amongst biomes when compared at low and high measurement temperatures (Tjoelker et al. 2001; Table 4), overall the large inter-biome differences in Q10 are likely the result of differences in measuring temperature in the thermally contrasting biomes. Thus, differences in Q10 values amongst biomes are unlikely to reflect inherent differences in the temperature sensitivity of R for species characteristic of each biome (i.e. there has been no adaptive change in the Q10 of R).
|
|
Further support for the suggestion that Q10 values do not differ systematically among contrasting plant species comes from the fact that most species exhibit Q10 values that fall within a narrow range when values are compared at a common measurement temperature interval. For example, Ivanova et al. (1989) found that the mean Q10 for leaf R in 34 temperate and Arctic plant species was 2.45 (between 10 and 20°C measurement temperature range) with the upper and lower 95% confidence intervals being 2.62 and 2.27, respectively. Similarly, the mean leaf Q10 of 59 species (15°C midpoint) reported by Tjoelker et al. (2001) was 2.50, with the upper and lower 95% confidence intervals of 2.62 and 2.39, respectively. In a review of published values of 125 species, Larigauderie and Körner (1995) found that the majority of species exhibited leaf Q10 values between 2.0 and 2.5, with the overall mean being 2.3. Thus, despite large differences between the highest and lowest Q10, contrasting species often exhibit relatively similar Q10 values over a given measurement temperature range.
Acclimation: characteristics, variability, mechanisms and consequences for R / P
As stated earlier, most GCMs assume that the changes in the long-term growth temperature will not alter rates of R at a common measurement temperature (i.e. R does not acclimate). Rather, most simulation models assume that R responds to short- and long-term changes in temperature in a fixed, exponential manner (Q10 = 2.0). This might not be a problem if plant R did not acclimate to long-term changes in temperature. However, plant R often does acclimate. Here, we outline the characteristics of acclimation and the extent to which acclimation varies among and within plant species.
Overview
An example of thermal acclimation is shown in Fig. 1, where exposure of a warm-grown plant to the cold for several days results in an increase in the rate of R at a common measurement temperature (Rook 1969; Chabot and Billings 1972; Pisek et al. 1973; Larigauderie and Körner 1995; Körner 1999; Atkin et al. 2000b; Covey-Crump et al. 2002; Zha et al. 2002, 2005; Bolstad et al. 2003). Conversely, exposure to high temperatures results in a decrease in the rate of R at a common temperature (Fig. 1). Differences in the rate of R at standard measurement temperatures are also commonly exhibited by plants that grew and developed under contrasting temperature regimes (either in the laboratory or in the field) (e.g. Figs 1, 3; Billings and Mooney 1968; Chabot and Billings 1972; Körner and Larcher 1988; Collier and Cummins 1990; Semikhatova et al. 1992; Collier 1996; Goldstein et al. 1996; Arnone and Körner 1997; Zha et al. 2002). In some cases, acclimation is associated with a change in the rate of R primarily at moderate to high measuring temperatures, with little or no change in R at low measuring temperatures (i.e. the short-term Q10 value changes) (Fig. 1; see below). Atkin and Tjoelker (2003) defined this as ‘Type I acclimation’; it appears to reflect a change in the availability of respiratory substrate and / or degree of adenylate restriction of R (Fig. 4). Type I acclimation R can occur within a 1–2-d period following a change in ambient temperature (Rook 1969; Billings et al. 1971; Chabot and Billings 1972; Atkin et al. 2000b; Covey-Crump et al. 2002; Bolstad et al. 2003), raising the possibility that plant R may dynamically acclimate to changes in thermal environment with an onset of the acclimation processes within perhaps hours. Changes in gene expression may also occur, but are not essential for the overall change in respiratory flux (Fig. 4). In other cases, acclimation is associated with an increase in the rate of R over a wide range of measurement temperatures (‘Type II acclimation’; Fig. 1). Type II acclimation is likely associated with temperature-mediated changes in respiratory capacity that can be maximally realised through growth of new tissues with altered morphology and biochemistry (Fig. 4; Atkin and Tjoelker 2003). Total respiratory capacity might be altered as a result of changes in the density of mitochondria (Miroslavov and Kravkina 1991) and / or amount of total protein invested in the respiratory chain (Klikoff 1966, 1968). Type II acclimation could be associated with changes in the relative amounts of particular enzymes (e.g. AOX v. complex IV; Ribas-Carbó et al. 2000). Intermediate cases of acclimation (i.e. between Types I and II) are likely, particularly in individual plants that experience long-term changes in temperature depending on the extent to which respiratory capacity is altered in pre-existing and newly formed leaves and roots. Another characteristic of acclimation (particularly Type II acclimation) is that it can result in respiratory homeostasis [i.e. identical rates of R in plants grown and measured in contrasting temperatures (Körner and Larcher 1988; Semikhatova et al. 1992; Goldstein et al. 1996; Arnone and Körner 1997; Körner 1999; Atkin et al. 2000b)] (Fig. 1). A good example comes from the work of Xiong et al. (2000), who showed that two Antarctic species, Colobanthus quitensis and Deschampsia antarctica, are capable of maintaining constant rates of R (measured at their respective daytime growth temperature) when grown at three different temperatures. Such changes can result in annual respiratory CO2 release being substantially reduced in leaves and roots that exhibit a high degree of thermal acclimation of R compared with that of tissues that do not acclimate (Fig. 2; Atkin et al. 2000a).
Variation in the degree of acclimation
Inter-specific variation
There is growing evidence that the degree of respiratory acclimation in leaves and roots varies substantially, both within and amongst individual species (Larigauderie and Körner 1995; Tjoelker et al. 1999a; Loveys et al. 2003). For example, Frantz et al. (2004) reported no acclimation of whole-plant respiration in young, rapidly growing plant communities experiencing contrasting night temperatures for a 20-d period. Moreover, Larigauderie and Körner (1995) found that growth at low temperatures resulted in little or no acclimation of leaf R in several alpine (Poa alpina, Leucanthemopsis alpina, Luzula alpino-pilosa, Carex foetida, Cirsium alpinum and Saxifraga biflora) and lowland (Luzula campestris, Carex caryophyllea and Cirsium acaule) species. In contrast, acclimation of leaf R occurred in Ranunculus acris, Anthoxanthum odoratum, Leucanthemum alpinum, Poa pratensis, Taraxacum alpinum, T. officinale (Larigauderie and Körner 1995) and Ranunculus glacialis (Arnone and Körner 1997).
Are there systematic differences amongst plant taxa in the degree to which leaf R acclimates? Tjoelker et al. (1999a) found that broad-leaved tree species exhibited a lower degree of acclimation of leaf R than selected conifer species, suggesting that acclimation might be predicted using structural and / or functional traits. Differences in ability to acclimate were also observed amongst six of the eight genera used by Larigauderie and Körner (1995). For example, the two Taraxacum species exhibited a greater degree of acclimation than did the two Cirsium species. However, Larigauderie and Körner (1995) found no evidence that within a given genus, alpine and lowland plant species differ in their extent of leaf R acclimation to contrasting growth temperatures. In contrast, Loveys et al. (2003) showed that slow-growing species exhibited a higher degree of leaf R acclimation than their fast-growing counterparts in four of six genera. The degree of acclimation was not, however, related to inherent differences in whole plant maximum RGR when all 16 species were considered (Loveys et al. 2003). Thus, while there is some evidence that the degree of acclimation differs systematically amongst taxa in some studies, there are also many results that contradict this.
The degree of thermal acclimation of root R is also highly variable. Acclimation of root respiration occurs in Plantago lanceolata (Smakman and Hofstra 1982; Loveys et al. 2002, 2003), Zostera marina (Zimmerman et al. 1989), Citrus volkameriana (Bryla et al. 1997, 2001), Festuca ovina, Juncus squarrosus, Nardus stricta (Fitter et al. 1998), Bellis perennis, Poa annua (Gunn and Farrar 1999) and Holcus lanatus (Edwards et al. 2004). In contrast, there is little or no acclimation of root R in field-grown Acer saccharum and Pinus resinosa to seasonal changes in temperature (Burton and Pregitzer 2003). Moreover, there was no obvious acclimation in roots of two Picea species (Sowell and Spomer 1986; Weger and Guy 1991) and Abies lasiocarpa (Sowell and Spomer 1986). Similarly, while acclimation to changes in growth temperature results in near-perfect homeostasis of R in Citrus volkameriana in wet soils, no acclimation occurs in roots of the same species growing in dry soils (Bryla et al. 1997). Even in species where root R does acclimate, the degree of acclimation is variable. For example, in a comparison of root R of five cold-grown (18 / 12°C) and warm-grown (30 / 24°C) boreal tree species at a set measuring temperature (18°C), warm-grown plants exhibited root R rates that were 50–74% of that exhibited by the cold-grown plants (Tjoelker et al. 1999a). Similarly, the degree of acclimation of root R was highly variable amongst 16 species in the study by Loveys et al. (2003).
Nitrogen-dependent variation
Low N availability does not appear to influence the degree of respiratory temperature acclimation exhibited by plants transferred from one growth temperature to another for several days. Atkinson and Atkin (LJ Atkinson, OK Atkin unpublished data) grew several herbaceous plant species at 25 / 20°C, at both high and low N availability (2000 and 25 μm, respectively); these plants were then shifted to 15 / 10°C for 7 d, and the degree of acclimation of root R was determined using methods described by Loveys et al. (2003). Although homeostasis was not observed in any of the species, plants grown at high and low N availability exhibited significant and similar degrees of temperature acclimation of root R. Low N supply did, however, result in a slower specific rate of R in all species; consequently, the absolute change in R following extended exposure to low temperature was lower in the low-N plants.
Variations that are development-dependent
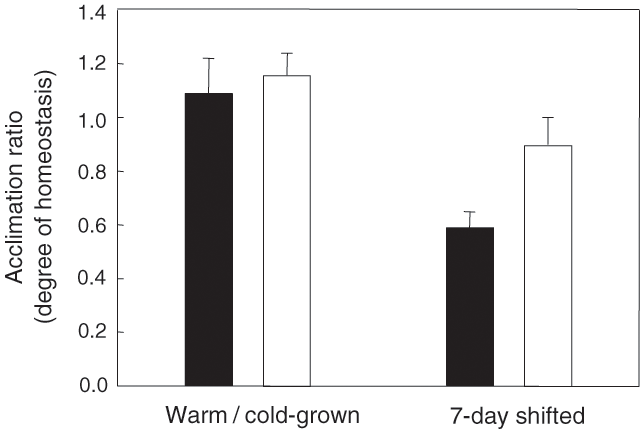
There is evidence that the degree of temperature acclimation of R is lower in pre-existing plant tissues shifted from one temperature to another (an example of ‘Type I’ acclimation; Fig. 1) than in leaves and roots that develop at the growth temperature (‘Type II’ acclimation) (Atkin and Tjoelker 2003). In a comparison of nine species, Loveys et al. (2003) found that the degree of respiratory acclimation was greater in leaves and roots that had developed under contrasting temperatures (18, 23 and 28°C) than in 25°C-grown plants shifted to 15°C for 7 d (Fig. 5). Moreover, acclimation of R to 5°C was substantially greater in Arabidopsis thaliana leaves that developed at 5°C than that of warm-grown leaves shifted to 5°C for several days (Talts et al. 2004). A similar requirement for the development of new tissues has been reported for thermal acclimation of P; studies with winter rye and Arabidopsis have shown that for full acclimation of P to a low growth temperature, new leaves need to be formed in the cold (Hurry et al. 1995; Strand et al. 1997, 2003). Cold-developed leaves are thicker, more dense, exhibit higher nitrogen concentrations and have higher transcript and activity levels of photosynthetic and sucrose synthesis enzymes than their warm-grown counterparts (Stitt and Hurry 2002).
|
There are two other areas where stage of development needs to be considered when dealing with acclimation of plant R to temperature. First, the effect of growth temperature on rates of R at a set temperature may depend on the age of plant leaves or roots. Recently, Armstrong and Atkin (A Armstrong, OK Atkin unpublished data) found that immature leaves of A. thaliana exhibited near-identical rates of R at any given temperature (no acclimation), regardless of whether the tissue developed under 25 or 5°C. In contrast in mature leaves, rates of leaf R at any given temperature were faster in cold-acclimated than warm-acclimated leaves (see above). These findings suggest that although thermal environment during development likely leads to long-term effects on R response to temperature (i.e. acclimation), the full extent of the acclimation response is only evident in fully developed leaves or roots. Second, the question of whether roots and leaves of the same plant differ in their ability to acclimate R to contrasting temperatures also depends on development. For example, Loveys et al. (2003) found no evidence that leaves and roots differ in their magnitude of acclimation when both tissues develop at the prevailing growth temperature (Fig. 5). Similarly, Tjoelker et al. (1999a) also reported no systematic difference in the degree of acclimation of roots and leaves in tree seedlings that develop under contrasting temperatures. However, Loveys et al. (2003) found that roots exhibited a higher degree of acclimation than leaves when plants were shifted from 25 to 15°C for 7 d. As Loveys et al. (2003) measured whole root systems (i.e. young and old roots), development of new roots at the new growth temperature could account for a gradual acclimation of the whole root system.
Component of the daily temperature regime to which R acclimates
Thermal acclimation of R has to be taken into account when modelling responses of R to a warmer climate. The most straightforward approach may be to assume that R acclimates to the daily mean temperature, and then use forecasted daily mean temperatures to model, e.g. an annual release of CO2 for a specific type of species or vegetation. So far, only a few studies have addressed the question of which component of the daily temperature regime R acclimates to. For the sake of simplicity, the diurnal fluctuations in temperature can be divided into a daily minimum, mean, and maximum temperature. The general conclusion is that R does not acclimate to the daily mean temperature in all species and / or tissues (Fitter et al. 1998; Atkin et al. 2000b). Will (2000) and Covey-Crump et al. (2002) examined the response of R in Pinus taeda leaves and Plantago lanceolata roots, respectively. Niether found that respiration acclimates to the daily average temperature. Covey-Crump et al. (2002) concluded that root respiration acclimates to the night-time minimum temperature, a conclusion that is supported in part by work on field grown Eucalyptus pauciflora leaves in south eastern Australia (Atkin et al. 2000b). However, night temperature was not the dominant factor in the study by Will (2000). Moreover, in a study by Bruhn (2002) assessing whether generalisations could be made among and / or within 10 contrasting genotypes, the temperature regime that R acclimated to was highly variable after transfer to different thermal environments. Thus, while there is some evidence that nighttime temperatures may be of particular importance, the results of Will (2000) and Bruhn (2002) suggest that generalisations are not yet possible.
Mechanisms responsible for variability in acclimation
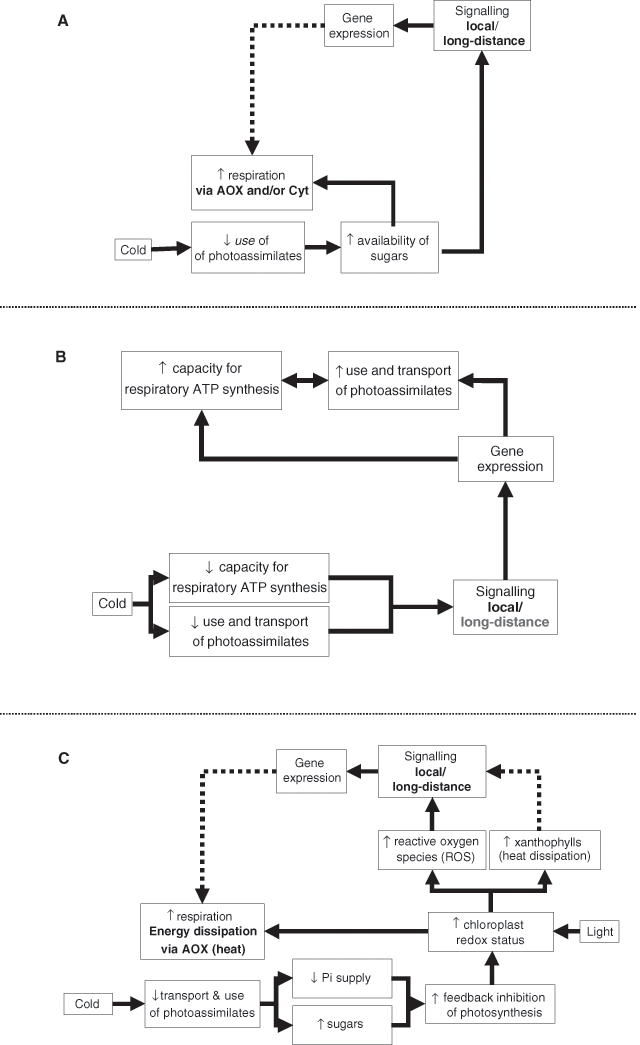
What factors are responsible for the variability in degrees of acclimation? Given the link between developmental plasticity and cold acclimation and respiration (see previous section), it seems likely that interspecific differences in Type II acclimation may reflect differences in the plasticity of contrasting species when challenged with a new growth temperature. This leads to the prediction that plant species that produce long-lived leaves and roots and that are relatively slow at generating new tissues may exhibit a relatively limited ability to acclimate at the whole-plant level to long-term changes in temperature (Atkin and Tjoelker 2003). In species that are highly plastic, Type II acclimation is likely associated with changes in glycolytic and / or mitochondrial proteins when new leaves develop following a change in growth temperature. What is less obvious, however, are the molecular and biochemical events that lead to the developmentally linked changes in R. Some factors that may contribute are the extent to which acclimation is (1) a response to temperature-dependent changes in substrate availability (Fig. 6A), (2) a result of the maintenance of homeostatic levels of ATP synthesis across a range of contrasting growth temperatures (to support growth, maintenance and / or ion exchange processes) (Fig. 6B) and / or (3) a reduction in the production of reactive oxygen species (ROS; through avoiding accumulation of excess redox equivalents) (Fig. 6C). Reactive oxygen species are produced by aerobic metabolism in chloroplasts and mitochondria and can damage proteins, lipids and DNA (Møller 2001). Here, we deal with each of these possibilities.
|
(1) Responding to substrate availability: Changes in growth temperature often result in a change in the concentration of soluble sugars and thus availability of substrates to the respiratory system (Mooney and Billings 1965; Warren Wilson 1966; Farrar and Williams 1991; Hurry et al. 1994; Atkin et al. 2000b; Oleksyn et al. 2000; Covey Crump et al. 2002). Why is this? Although changes in growth temperature can affect the rate of CO2 fixation and resultant sucrose synthesis, growth temperature often has a greater relative affect on the rate of substrate use (by growth and maintenance processes, as well as respiration per se) and translocation (Körner 1999). As a result, the balance between substrate production and supply is altered, which in turn results in a change in the steady-state concentration of respiratory substrate (e.g. Atkin et al. 2000b). In tissues where R is substrate limited, such changes in substrate availability may alter rates of R (e.g. cold acclimation often results in an increase in leaf and root soluble sugar concentrations and concomitant increases in R; Tjoelker et al. 1999b; Atkin et al. 2000b; Covey Crump et al. 2002). Changes in soluble sugar concentrations may also affect gene expression (Sheen 1994; Koch 1996), either locally or in remote tissues as a result of systemic signalling, resulting in enhanced mitochondrial transcript and protein levels (Fig. 6A). This may explain, in part, why respiratory capacity appears to be greater in cold acclimated tissues than their warm-grown counterparts. Whether substrate-dependent changes in respiratory flux are coupled to the synthesis of ATP depends, however, on the relative partitioning of electrons between the alternative oxidase (AOX) and cytochrome pathway, as well as the extent to which protons diffuse through the inner mitochondrial membrane through plant uncoupling mitochondrial proteins (PUMP). As PUMP protein (Nantes et al. 1999) and AOX protein / engagement (Vanlerberghe and McIntosh 1992; Gonzàlez-Meler et al. 1999) increase in cold-acclimated tissues, it seems likely that a portion of the increased capacity is not coupled to the production of ATP.
(2) Responding to the demand for ATP Thermal acclimation may also be linked to ATP demand and synthesis (Fig. 6B; Kurimoto et al. 2004a, b). Upon initial exposure to a new growth temperature, demand for ATP will be altered as a result of temperature-dependent changes in the rate of growth, maintenance processes and / or ion uptake. Consequently, temperature-mediated changes in R might result from concomitant changes in adenylate restriction of glycolysis and / or mitochondrial electron transport (Atkin et al. 2000a, d; Atkin and Tjoelker 2003). Moreover, at very low temperatures, limitations in the Vmax of respiratory enzymes can limit potential rates of ATP synthesis (Atkin and Tjoelker 2003). However, both the demand for ATP and ability to synthesise ATP (through increases in cytochrome pathway proteins) could recover following long-term exposure to a new growth temperature (Kurimoto et al. 2004b). For example, rates of ion uptake and growth often recover (either partially or fully) following a change in growth temperature (e.g. Bigot and Boucaud 1996; Clarkson et al. 1988; Ziska and Bunce 1998). If so, this will re-establish a demand for ATP similar to the demand before the change in growth temperature. Changes in the demand for ATP by processes associated with photosynthetic thermal acclimation (e.g. sucrose synthesis) and general maintenance processes could also contribute (Hoefnagel et al. 1998; Atkin et al. 2000d). To meet an increased demand for ATP, respiratory flux can be increased through removal of adenylate restriction of glycolysis and the mitochondrial electron transport chain (at complexes I, III and IV), particularly in tissues where flux is not limited by respiratory capacity. However, whenever the Vmax of respiratory enzymes limits ATP synthesis (e.g. in the cold; Atkin and Tjoelker 2003), increases in respiratory capacity are necessary; the greater respiratory capacity exhibited by cold-acclimated plants may, therefore, partly reflect changes in gene expression triggered by the increased demand for ATP following acclimation of growth, maintenance and / or ion uptake to the cold (Fig. 6B). Variations in the degree of acclimation might, therefore, reflect inter and intra-specific differences in the extent to which growth and maintenance processes maintain a homeostatic demand for ATP (Fig. 6B); support for this hypothesis comes from recent work by Kurimoto et al. (2004a, b) which shows that plants capable of maintaining growth at similar rates across a wide range of temperatures also exhibit higher degrees of acclimation than their less flexible counterparts.
(3) Reducing the production of reactive oxygen species imbalances in cellular redox potential (and thus levels of ROS) are another factor that may contribute to variations in the degree of acclimation. ROS is known to be an important signalling agent, both at the site of ROS production (Wagner 1995; Foyer et al. 1997) and in remote parts of the plant (Karpinski et al. 1999). Wagner (1995) proposed that any constraint on the respiratory electron transport chain [e.g. low temperature inhibition of the cytochrome pathway by cold (Prasad et al. 1994)] would lead to increased ROS production. ROS could signal for increased synthesis of enzymes that lower ROS production [e.g. AOX and PUMP (Møller 2001)]. If correct, this suggests that variations in the degree of acclimation might reflect variations in ROS production, which in turn may reflect differences in the environmental cues that lead to ROS production (e.g. irradiance / temperature combinations; Fig. 6C).
The potential for ROS production by chloroplasts and mitochondria increases at low temperatures due to over-reduction of the photosynthetic and respiratory electron transport chains (Purvis and Shewfelt 1993; Purvis 1997; Foyer and Noctor 2000; Møller 2001). In illuminated chloroplasts, cold decreases the turnover of NADPH with the result that the photosynthetic electron transport chain becomes highly reduced and ROS production increases (Fig. 6C; Fryer et al. 1998) . Similarly, low temperature inhibition of electron transport through the respiratory electron transport chain may increase the potential for mitochondrial ROS production (Purvis and Shewfelt 1993; Møller 2001). Regardless of the site of ROS production, low temperature-mediated increases in ROS production could be alleviated, in part, by increases in respiratory capacity, particularly through increases in flux through non-phosphorylating pathways such as the AOX (Purvis and Shewfelt 1993). Dutilleul et al. (2003) reported that there is effective crosstalk between mitochondria and other organelles to maintain homeostasis of cellular redox potential. Maxwell et al. (1999) showed that overexpression of the AOX decreased the ROS by half, whereas antisensing the AOX increased the production of ROS 5-fold. Similarly, inhibition of the AOX increased ROS production in isolated mitochondria (Popov et al. 1997). Increases in AOX thus help avoid over-reduction of the respiratory electron transport chain and the production of mitochondrial derived ROS. However, increases in AOX also appear to reduce the redox potential of the chloroplast. For example, several studies have shown that mitochondria can oxidise excess photosynthetic redox equivalents (Saradadevi and Raghavendra 1992; Shyam et al. 1993; Raghavendra et al. 1994; Hurry et al. 1995); this is achieved through the chloroplast to mitochondrion malate / oxaloacetate (OAA) shuttle mechanism (see Hoefnagel et al. 1998). However, for this system to operate successfully in the cold, respiratory flux needs to increase in response to the increased demand for oxidation of redox equivalents. In particular, AOX levels must not be limiting (this may explain why AOX protein levels increase in cold-acclimated leaves; e.g. Vanlerberghe and McIntosh 1992). Thus, Type II acclimation in cold-grown plants may, in part, represent a mechanism to increase the capacity for oxidation of redox equivalents and in turn decrease the potential for ROS generation (Fig. 6C).
Impact of acclimation on the balance between respiration and photosynthesis
What impact does thermal acclimation have on the balance between R and photosynthesis? In individual leaves, the short-term temperature sensitivity of light-saturated P (Psat) typically differs from that of leaf R (in darkness). For example, a decline in temperature from 25°C to 15°C reduces leaf R and Psat by 55% and 21%, respectively, in Eucalyptus pauciflora (Atkin et al. 2000c). As a result, the balance between dark leaf R and Psat varies with short-term changes in temperature. However, prolonged exposure to a new growth temperature can result in photosynthetic and respiratory acclimation, with the result that the balance between leaf R and Psat is re-established (Dewar et al. 1999; Gifford 1995, 2003; Loveys et al. 2003). The maintenance of a balance probably reflects the fact that dark leaf R and Psat are interdependent, with R in darkness relying on photosynthesis for substrate, whereas photosynthesis depends on R in darkness and in the light for a range of compounds, such as carbon skeletons for protein synthesis and ATP for sucrose synthesis and repair of photosynthetic proteins; (Krömer 1995, Hoefnagel et al. 1998, Atkin et al. 2000c, Padmasree et al. 2002). What is surprising, however, is the extent to which contrasting species often exhibit similar leaf R to Psat ratios, at least when grown and measured at moderate-high temperatures (e.g. 18, 23 and 28°C; Loveys et al. 2003). Leaves of plant species from contrasting habitats differ substantially in chemical composition, metabolic fluxes and physical structures; such differences might result in differences between species and growth temperature in the amount of leaf R needed to support photosynthesis and vice versa. Thus, even though the relationship between leaf R and Psat is affected by environmental factors such as water availability (Turnbull et al. 2001), the available data suggests that temperature-mediated differences in dark leaf R are closely linked to concomitant differences in leaf P. Further research such as that by Zha et al. (2004) is needed to test this idea in natural variable conditions in the field, where photosynthesis under ambient irradiance instead of Psat is measured.
Does acclimation result in the proportion of daily fixed carbon released by respiratory activity in whole plants being constant across a range of growth temperatures (when measured at the growth temperature)? Few studies have actually measured in situ rates of whole plant R and P. Gifford (1995) found that R / P was constant for wheat (Triticum aestivum) grown at constant temperatures ranging from 15 to 30°C (when measured at the respective growth temperatures). Likewise, soybean (Glycine max) grown at a range of growth temperatures between 20 and 35°C showed no differences in R / P ratios, owing to acclimation of R to temperature (Ziska and Bunce 1998). However, in that study, growth under an elevated concentration of CO2 (700 µl l–1) did result in reduced R / P, compared with growth at ambient CO2 (350 µl l–1). A study of seedlings of five boreal tree species (Tjoelker et al. 1999a) showed small increases in the proportion of daily fixed carbon used in R in plants grown in warmer compared with colder growth environments. For example, total R ranged from 24 to 55% of total daily CO2 uptake amongst species grown at 18 / 12°C, and increased to 38–74% in plants grown at 30 / 24°C. However, the increases were less than would have occurred without acclimation of both respiration and photosynthesis to growth temperature. Moreover, in that study species differed in overall R losses as a proportion of daily net CO2 uptake. Compared with the faster-growing broad-leaved species Populus tremuloides and Betula papyrifera, slower-growing conifers Larix laricina, Pinus banksiana, and Picea mariana used a larger proportion of net daily CO2 uptake in R, especially in roots.
Although R / P in whole plants is often homeostatic at moderate growth temperatures, changes can occur when plants are grown at unfavourably high temperatures. For example, although Loveys et al. (2002) found no difference in balance between daily whole plant R and P amongst Silene uniflora grown at 18, 23 and 28°C, four other species did exhibit higher daily whole plant R / P values at 28°C than at 18 and 23°C. R / P is also unlikely to remain homeostatic when plants are grown at very low temperatures. Why? First, R and P do not exhibit identical temperature responses when initially exposed to low temperatures (e.g. Atkin et al. 2000c) and second, R and P may differ in their ability to acclimate to low temperatures. For example, P exhibits greater acclimation to the cold than does leaf R in Plantago major (OK Atkin, I Scheurwater, TL Pons unpublished data).
Concluding statements
Our review has highlighted the evidence, including long-standing evidence, that challenges widely held assumptions about the temperature sensitivity of plant R, namely that the Q10 is around 2.0, that the Q10 remains constant, and that R response through time in contrasting thermal environments can be described by a simple exponential model. We have shown that the sensitivity of plant R to short- and long-term changes in temperature is highly variable and that to successfully model future rates of R at a range of scales from individual leaves to global carbon cycle models, factors such as the temperature dependence of the Q10 and degree and speed of acclimation will likely need to be taken into account. An example of how this can be done is provided in Wythers et al. (2005), where the ecosystem model PnET was run with a temperature driven algorithm that accounts for thermal acclimation and a temperature-dependent Q10 algorithm; incorporation of these algorithms resulted in large decreases in predicted annual foliar respiration and increases in predicted net primary productivity, especially in the context of both respiratory and photosynthetic acclimation to temperature and resulting R / P ratios.
Much is now known about the extent to which Q10 values vary and the underlying mechanisms responsible for that variability. For example, whereas Q10 does not appear to respond to elevated atmospheric CO2 concentrations, temperature, light, and water availability each appear to influence the temperature sensitivity of respiratory CO2 efflux. Moreover, there is growing evidence that the response of R to long-term changes in temperature is highly dependent on the effect of temperature on plant development. It seems likely that variations in the degree of acclimation will also reflect interactive effects of temperature and other abiotic factors (e.g. irradiance, drought and nutrient availability). In some cases acclimation may simply reflect a passive response to changes in respiratory substrate availability whereas in others acclimation may be critical in helping plants grow/survive at contrasting temperatures. Much work is needed, however, before we have a full understanding of the factors that determine the extent of thermal acclimation in plants. Establishing what determines the degree of acclimation is critical if we are to successfully predict future rates of R, ecosystem CO2 fluxes and potential feedbacks on atmospheric CO2 concentrations.
Acknowledgments
This work was supported by grants from NERC in the UK (GR3/11898, NERC/A/S/2001/01186, NERC/B/S/2001/00875; OKA), the National Science Foundation (USA, IBN-9630241; MGT) the Swedish Council for Forestry and Agricultural Research (VH), The Cooperative Research Centre for Greenhouse Accounting, Australia (DB), and the Nordic Academy of Advanced Studies (NorFA) Temperature Stress Network.
Aber JD, Federer CA
(1992) A generalized, lumped-parameter model of photosynthesis, evapotranspiration and net primary production in temperate and boreal forest ecosystems. Oecologia 92, 463–474.
| Crossref | GoogleScholarGoogle Scholar |

Amthor, JS (1997). Plant respiratory response to elevated CO2 partial pressure. In ‘Advances in carbon dioxide effects research’. pp. 35–77. (American Society of Agronomy: Wisconsin)
Amthor JS
(2000) The McCree-de Wit–Penning de Vries–Thornley respiration paradigms, 30 years later. Annals of Botany 86, 1–20.
| Crossref | GoogleScholarGoogle Scholar |

Arnone JA, Körner C
(1997) Temperature adaptation and acclimation potential of leaf dark respiration in two species of Ranunculus from warm and cold habitats. Arctic and Alpine Research 29, 122–125.

Atkin OK, Day DA
(1990) A comparison of the respiratory processes and growth rates of selected Australian alpine and related lowland plant species. Australian Journal of Plant Physiology 17, 517–526.

Atkin OK, Tjoelker MG
(2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8, 343–351.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Atkin OK,
Botman B, Lambers H
(1996) The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Functional Ecology 10, 698–707.

Atkin OK,
Edwards EJ, Loveys BR
(2000a) Response of root respiration to changes in temperature and its relevance to global warming. New Phytologist 147, 141–154.
| Crossref | GoogleScholarGoogle Scholar |

Atkin OK,
Holly C, Ball MC
(2000b) Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature, the importance of changes in the capacity and temperature sensitivity of respiration. Plant, Cell and Environment 23, 15–26.
| Crossref | GoogleScholarGoogle Scholar |

Atkin OK,
Evans JR,
Ball MC,
Pons TL, Lambers H
(2000c) Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiology 122, 915–923.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Atkin, OK ,
Millar, AH ,
Gardeström, P ,
and
Day, DA (2000d). Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In ‘Photosynthesis, physiology and metabolism’. d. pp. 153–175. (Kluwer Academic Publishers: Dordrecht)
Atkin OK,
Zhang QS, Wiskich JT
(2002) Effect of temperature on rates of alternative and cytochrome pathway respiration and their relationship with the redox poise of the quinone pool. Plant Physiology 128, 212–222.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Azcón-Bieto, J (1992). Relationships between photosynthesis and respiration in the dark in plants. In ‘Trends in photosynthesis research’. pp. 241–253. (Intercept Ltd: Andover)
Azcón-Bieto J,
Gonzalelz-Meler MA,
Doherty W, Drake B
(1994) Acclimation of respiratory O2 uptake in green tissues of field-grown native species after long-term exposure to elevated atmospheric CO2. Plant Physiology 106, 1163–1168.
| PubMed |

Berry, JA ,
and
Raison, JK (1981). Responses of macrophytes to temperature. In ‘Physiological plant ecology I. Responses to the physical environment’. pp. 277–338. (Springer-Verlag: Berlin)
Bigot J, Boucaud J
(1996) Short-term responses of Brassica rapa plants to low root temperature, effects on nitrate uptake and its translocation to the shoot. Physiologia Plantarum 96, 646–654.
| Crossref | GoogleScholarGoogle Scholar |

Billings WD, Mooney HA
(1968) The ecology of arctic and alpine plants. Biological Reviews 43, 481–529.

Billings WD,
Godfrey PJ,
Chabot BF, Bourque DP
(1971) Metabolic acclimation to temperature in Arctic and alpine ecotypes of Oxyria digyna. Arctic and Alpine Research 3, 277–289.

Bolstad PV,
Mitchell K, Vose JM
(1999) Foliar temperature-respiration response functions for broad-leaved tree species in the southern Appalachians. Tree Physiology 19, 871–878.
| PubMed |

Bolstad PV,
Reich P, Lee T
(2003) Rapid temperature acclimation of leaf respiration rates in Quercus alba and Quercus rubra. Tree Physiology 23, 969–976.
| PubMed |

Boone RD,
Nadelhoffer KJ,
Canary JD, Kaye JP
(1998) Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 396, 570–572.
| Crossref | GoogleScholarGoogle Scholar |

Bouma TJ,
Nielsen KL,
Eissenstat DM, Lynch JP
(1997) Estimating respiration of roots in soil, interactions with soil CO2, soil temperature and soil water content. Plant and Soil 195, 221–232.
| Crossref | GoogleScholarGoogle Scholar |

Brooks A, Farquhar GD
(1985) Effect of temperature on the CO2–O2 specificity of ribulose-1,5-biphosphate carboxylase / oxygenase and the rate of respiration in the light. Estimates from gas exchange measurements on spinach. Planta 165, 397–406.
| Crossref | GoogleScholarGoogle Scholar |

Bruhn D
(2002) Plant pespiration and climate change effects. PhD thesis.
(Risø National Laboratory, Roskilde:
Denmark)
Bruhn D,
Mikkelsen TN, Atkin OK
(2002) Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiologia Plantarum 114, 57–64.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bryla DR,
Bouma TJ, Eissenstat DM
(1997) Root respiration in citrus acclimates to temperature and slows during drought. Plant, Cell and Environment 20, 1411–1420.
| Crossref | GoogleScholarGoogle Scholar |

Bryla DR,
Bouma TJ,
Hartmond U, Eissenstat DM
(2001) Influence of temperature and soil drying on respiration of individual roots in citrus, integrating greenhouse observations into a predictive model for the field. Plant, Cell and Environment 24, 781–790.
| Crossref | GoogleScholarGoogle Scholar |

Burton AJ, Pregitzer KS
(2003) Field measurements of root respiration indicate little to no seasonal temperature acclimation for sugar maple and red pine. Tree Physiology 23, 273–280.
| PubMed |

Burton AJ,
Pregitzer KS,
Zogg GP, Zak DR
(1998) Drought reduces root respiration in sugar maple forests. Ecological Applications 8, 771–778.

Burton AJ,
Pregitzer KS,
Ruess RW,
Hendrik RL, Allen MF
(2002) Root respiration in North American forests, effects of nitrogen concentration and temperature across biomes. Oecologia 131, 559–568.
| Crossref | GoogleScholarGoogle Scholar |

Carey EV,
DeLucia EH, Ball JT
(1996) Stem maintenance and construction respiration in Pinus ponderosa grown in different concentrations of atmospheric CO2. Tree Physiology 16, 125–130.
| PubMed |

Chabot BF, Billings WD
(1972) Origins and ecology of the Sierran alpine flora and vegetation. Ecological Monographs 42, 163–199.

Clarkson, DT ,
Earnshaw, MJ ,
White, PJ ,
and
Cooper, HD (1988). Temperature dependent factors influencing nutrient uptake, an analysis of responses at different levels of organization. In ‘Plants and temperature’. pp. 281–309. (Company of Biologists: Cambridge)
Collier DE
(1996) No difference in leaf respiration rates among temperate, subarctic, and arctic species grown under controlled conditions. Canadian Journal of Botany 74, 317–320.

Collier DE, Cummins WR
(1990) The effects of low growth and measurement temperature on the respiratory properties of five temperate species. Annals of Botany 65, 533–538.

Comas LH, Eissenstat DM
(2004) Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18, 388–397.
| Crossref | GoogleScholarGoogle Scholar |

Covey-Crump EM,
Attwood RG, Atkin OK
(2002) Regulation of root respiration in two species of Plantago that differ in relative growth rate the effect of short- and long-term changes in temperature. Plant, Cell and Environment 25, 1501–1513.
| Crossref | GoogleScholarGoogle Scholar |

Cox PM,
Betts RA,
Jones CD,
Spall SA, Totterdell IJ
(2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cramer W,
Bondeau A,
Woodward FI,
Prentice IC, Betts RA , et al.
(2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change, results from six dynamic global vegetation models. Global Change Biology 7, 357–373.
| Crossref | GoogleScholarGoogle Scholar |

Crawford RMM, Palin MA
(1981) Root respiration and temperature limits to the north-south distribution of four perennial maritime plants. Flora 171, 338–354.

Criddle RS,
Hopkin MS,
McArthur ED, Hansen LD
(1994) Plant distribution and the temperature coefficient of metabolism. Plant, Cell and Environment 17, 233–243.

Damesin C,
Ceschina E,
Le Goff N,
Ottorini J, Dufrêne E
(2002) Stem and branch respiration of beech, from tree measurements to estimations at the stand level. New Phytologist 153, 159–172.
| Crossref | GoogleScholarGoogle Scholar |

Dewar RC,
Medlyn BE, McMurtrie RE
(1999) Acclimation of the respiration / photosynthesis ratio to temperature, insights from a model. Global Change Biology 5, 615–622.
| Crossref | GoogleScholarGoogle Scholar |

Dutilleul C,
Garmier M,
Noctor G,
Mathieu C,
Chetrit P,
Foyer CH, de Paepe R
(2003) Leaf mitochondrial module whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signalling and diurnal regulation. The Plant Cell 15, 1212–1226.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Easterling DR,
Horton B,
Jones PD,
Peterson TC, Karl TR , et al.
(1997) Maximum and minimum temperature trends for the globe. Science 277, 364–366.
| Crossref | GoogleScholarGoogle Scholar |

Edwards EJ,
Benham DG,
Marland LA, Fitter AH
(2004) Root production is determined by radiation flux in a temperate grassland community. Global Change Biology 10, 209–227.
| Crossref |

Edwards NT,
Tschaplinski TJ, Norby RJ
(2002) Stem respiration increases in CO2-enriched sweetgum trees. New Phytologist 155, 239–248.
| Crossref | GoogleScholarGoogle Scholar |

Farquhar GD,
von Caemmerer S, Berry JA
(1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
| Crossref | GoogleScholarGoogle Scholar |

Farrar JF, Williams ML
(1991) The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source-sink relations and respiration. Plant, Cell and Environment 4, 819–830.

Field CB
(2001) Plant physiology of the ‘missing’ carbon sink. Plant Physiology 125, 25–28.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fitter AH,
Graves JD,
Self GK,
Brown TK,
Bogie DS, Taylor K
(1998) Root production, turnover and respiration under two grassland types along an altitudinal gradient — influence of temperature and solar radiation. Oecologia 114, 20–30.
| Crossref | GoogleScholarGoogle Scholar |

Forward, DF (1960). Effect of temperature on respiration. In ‘Encyclopedia of plant physiology. Vol. 12’. pp. 234–258. (Springer-Verlag: Berlin)
Foyer CH,
Lopez-Delgado H,
Dat JF, Scott IM
(1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum 100, 241–254.
| Crossref | GoogleScholarGoogle Scholar |

Foyer CH, Noctor G
(2000) Tansley Review NO 112. Oxygen processing in photosynthesis, regulation and signalling. New Phytologist 146, 359–388.
| Crossref | GoogleScholarGoogle Scholar |

Frantz JM,
Cometti NN, Bugbee BRUC
(2004) Night temperature has a minimal effect on respiration and growth in rapidly growing plants. Annals of Botany 94, 155–166.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fryer MJ,
Andrews JR,
Oxborough K,
Blowers DA, Baker NR
(1998) Relationship between CO2 assimilation, photosynthetic electron transport, active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology 116, 571–580.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gielen B,
Scarascia-Mugnozza G, Ceulemans R
(2003) Stem respiration of Populus species in the third year of free-air CO2 enrichment. Physiologia Plantarum 117, 500–507.
| Crossref |
PubMed |

Gifford RM
(1995) Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature — long-term versus short-term distinctions for modelling. Global Change Biology 1, 385–396.

Gifford RM
(2003) Plant respiration in productivity models, conceptualisation, representation and issues for global terrestrial carbon-cycle research. Functional Plant Biology 30, 171–186.
| Crossref | GoogleScholarGoogle Scholar |

Goldstein G,
Drake DR,
Melcher P,
Giambelluca TW, Heraux J
(1996) Photosynthetic gas exchange and temperature-induced damage in seedlings of the tropical alpine species Argyroxiphium sandwicense. Oecologia 106, 298–307.
| Crossref | GoogleScholarGoogle Scholar |

Gonzàlez-Meler MA,
Ribas-Carbó M,
Giles L, Siedow JN
(1999) The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiology 120, 765–772.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gonzàlez-Meler MA,
Taneva LINA, Trueman RJ
(2004) Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Annals of Botany 94, 647–656.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Griffin KL,
Turnbull M, Murthy R
(2002) Canopy position affects the temperature response of leaf respiration in Populus deltoides. New Phytologist 154, 609–619.
| Crossref | GoogleScholarGoogle Scholar |

Gunn S, Farrar JF
(1999) Effects of a 4°C increase in temperature on partitioning of leaf area and dry mass, root respiration and carbohydrates. Functional Ecology 13, 12–20.
| Crossref | GoogleScholarGoogle Scholar |

Hamilton JG,
Thomas RB, Delucia EH
(2001) Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant, Cell and Environment 24, 975–982.
| Crossref | GoogleScholarGoogle Scholar |

Harley PC,
Thomas RB, Reynolds J
(1992) Modeling photosynthesis of cotton grown in elevated CO2. Plant, Cell and Environment 15, 271–282.

Higgins PD, Spomer GG
(1976) Soil temperature effects on root respiration and the ecology of alpine and subalpine plants. Botanical Gazette 137, 110–120.
| Crossref | GoogleScholarGoogle Scholar |

Hoefnagel MHN,
Atkin OK, Wiskich JT
(1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochimica et Biophysica Acta 1366, 235–255.

Holmgren P, Jarvis PG
(1967) Carbon dioxide efflux from leaves in light and darkness. Physiologia Plantarum 20, 1045–1051.

Hurry VM,
Malmberg G,
Gardeström P, Öquist G
(1994) Effects of a short-term shift to low temperature and of long-term cold hardening on photosynthesis and ribulose-1,5-bisphosphate carboxylase oxygenase and sucrose phosphate synthase activity in leaves of winter rye (Secale cereale L.). Plant Physiology 106, 983–990.
| PubMed |

Hurry VM,
Tobiæson M,
Krömer S,
Gardeström P, Öquist G
(1995) Mitochondria contribute to increased photosynthetic capacity of leaves of winter rye (Secale cereale L.) following cold-hardening. Plant, Cell and Environment 18, 69–76.

Hurry VM,
Keerberg O,
Pärnik T,
Öquist G, Gardeström P
(1996) Effect of cold hardening on the components of respiratory decarboxylation in the light and in the dark in leaves of winter rye. Plant Physiology 111, 713–719.
| PubMed |

Ivanova, TL ,
Semikhatova, OA ,
Judina, OS ,
and
Leina, GD (1989). The effect of temperature on the respiration of plants from different plant-geographic zones. In ‘Ecophysiological investigations of photosynthesis and respiration in plants’. pp. 140–166. (Nauka Publishing, Nauka: St Petersberg)
James, WO (1953).
Janouš D,
Pokornyý R,
Brossaud J, Marek MV
(2000) Long-term effects of elevated CO2 on woody tissues respiration in Norway spruce studied in open-top chambers. Biologia Plantarum 43, 41–46.
| Crossref | GoogleScholarGoogle Scholar |

Karpinski S,
Reynolds H,
Karpinska B,
Wingsle G,
Creissen G, Mullineaux P
(1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Klikoff LG
(1966) Temperature dependence of the oxidative rates of mitochondria in Danthonia intermedia, Penstemon davidsonii and Sitanion hystrix. Nature 212, 529–530.

Klikoff LG
(1968) Temperature dependence of mitochondrial oxidative rates of several plant species of the Sierra Nevada. Botanical Gazette 129, 227–230.
| Crossref | GoogleScholarGoogle Scholar |

Koch KE
(1996) Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 509–540.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kok B
(1948) A critical consideration of the quantum yield of Chlorella-photosynthesis. Enzymology 13, 1–56.

Kurimoto K,
Day DA,
Lambers H, Noguchi K
(2004a) Effect of respiratory homeostasis on plant growth in cultivars of wheat and rice. Plant, Cell and Environment 27, 853–862.
| Crossref | GoogleScholarGoogle Scholar |

Kurimoto K,
Millar AH,
Lambers H,
Day DA, Noguchi K
(2004b) Maintenance of growth rate at low temperature in rice and wheat cultivars with a high degree of respiratory homeostasis is associated with a high efficiency of respiratory ATP production. Plant and Cell Physiology 45, 1015–1022.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Körner, C (1999).
Körner, C ,
and
Larcher, W (1988). Plant life in cold environments. In ‘Plants and temperature. Symposium of the Society of Experimental Biologists. Vol. 42’. pp. 25–57. (The Company of Biologists Limited: Cambridge)
Krömer S
(1995) Respiration during photosynthesis. Annual Review of Plant Physiology and Molecular Biology 46, 45–70.
| Crossref | GoogleScholarGoogle Scholar |

Laisk, AK (1977).
Larigauderie A, Körner C
(1995) Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Annals of Botany 76, 245–252.
| Crossref | GoogleScholarGoogle Scholar |

Loreto F,
Delfine S, DiMarco G
(1999) Estimation of photorespiratory carbon dioxide recycling during photosynthesis. Australian Journal of Plant Physiology 26, 733–736.

Loreto F,
Velikova V, Di Marco G
(2001) Respiration in the light measured by (CO2)-C12 emission in (CO2)-C13 atmosphere in maize leaves. Australian Journal of Plant Physiology 28, 1103–1108.

Loveys BR,
Scheurwater I,
Pons TL,
Fitter AH, Atkin OK
(2002) Growth temperature influences the underling components of relative growth rate, an investigation using inherently fast- and slow-growing plant species. Plant, Cell and Environment 25, 975–987.
| Crossref | GoogleScholarGoogle Scholar |

Loveys BR,
Atkinson LJ,
Sherlock DJ,
Roberts RL,
Fitter AH, Atkin OK
(2003) Thermal acclimation of leaf and root respiration, an investigation comparing inherently fast- and slow-growing plant species. Global Change Biology 9, 895–910.
| Crossref | GoogleScholarGoogle Scholar |

Luo YQ,
Wan SQ,
Hui DF, Wallace LL
(2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Maxwell DP,
Wang Y, Mcintosh L
(1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences USA 96, 8271–8276.
| Crossref | GoogleScholarGoogle Scholar |

McCashin BG,
Cossins EA, Canvin DT
(1988) Dark respiration during photosynthesis in wheat leaf slices. Plant Physiology 87, 155–161.

Millar AH,
Mittova V,
Kiddle G,
Heazlewood JL,
Bartoli CG,
Theodoulou FL, Foyer CH
(2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiology 133, 443–447.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Miroslavov EA, Kravkina IM
(1991) Comparative analysis of chloroplasts and mitochondria in leaf chlorenchyma from mountain plants grown at different altitudes. Annals of Botany 68, 195–200.

Møller IM
(2001) Plant mitochondria and oxidative stress, electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52, 561–591.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mooney HA, Billings WD
(1965) Effects of altitude on carbohydrate content of mountain plants. Ecology 46, 750–751.

Nantes IL,
Fagian MM,
Catisti R,
Arruda P,
Maia IG, Vercesi AE
(1999) Low temperature and aging-promoted expression of PUMP in potato tuber mitochondria. FEBS Letters 457, 103–106.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Noctor G,
Dutilleul C,
De Paepe R, Foyer CH
(2004) Use of mitochondrial electron transport mutants to evaluate the effects of redox state on photosynthesis, stress tolerance and the integration of carbon / nitrogen metabolism. Journal of Experimental Botany 55, 49–57.
| Crossref |
PubMed |

Oleksyn J,
Zytkowiak R,
Reich PB,
Tjoelker MG, Karolewski P
(2000) Ontogenetic patterns of leaf CO2 exchange, morphology and chemistry in Betula pendula trees. Trees 14, 271–281.
| Crossref | GoogleScholarGoogle Scholar |

Padmasree K,
Padmavathi L, Raghavendra AS
(2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis, optimization of carbon assimilation and protection against photoinhibition. Critical Reviews in Biochemistry and Molecular Biology 37, 71–119.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Paembonan SA,
Hagihara A, Hozumi K
(1991) Long-term measurement of CO2 release from the above-ground parts of a hinoki forest tree in relation to air temperature. Tree Physiology 8, 399–405.

Pärnik T, Keerberg O
(1995) Decarboxylation of primary and end products of photosynthesis at different oxygen concentrations. Journal of Experimental Botany 46, 1439–1447.

Pisek, A ,
Larcher, W ,
Vegis, A ,
and
Napp-Zinn, K (1973). The normal temperature range. In ‘Temperature and life’. pp. 102–194. (Springer-Verlag: Berlin)
Popov VN,
Simonian RA,
Skulachev VP, Starkov AA
(1997) Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Letters 415, 87–90.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pons TL, Welschen RAM
(2003) Midday depression of net photosynthesis in the tropical rainforest tree Eperua grandiflora: contributions of stomatal and internal conductances, respiration and Rubisco functioning. Tree Physiology 23, 937–947.
| PubMed |

Poorter H,
Remkes C, Lambers H
(1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology 94, 621–627.

Prasad TK,
Anderson MD, Stewart CR
(1994) Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiology 105, 619–627.
| PubMed |

Pregitzer KS,
Kubiske ME,
Yu CK, Hendrick RL
(1997) Root architecture, carbon and nitrogen in four temperate forest species. Oecologia 111, 302–308.
| Crossref | GoogleScholarGoogle Scholar |

Pregitzer KS,
Laskowski MJ,
Burton AJ,
Lessard VC, Zak DR
(1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiology 18, 665–670.
| PubMed |

Purvis AC
(1997) Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiologia Plantarum 100, 165–170.
| Crossref | GoogleScholarGoogle Scholar |

Purvis AC, Shewfelt RL
(1993) Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiologia Plantarum 88, 712–718.
| Crossref | GoogleScholarGoogle Scholar |

Raghavendra AS,
Padmasree K, Saradadevi K
(1994) Interdependence of photosynthesis and respiration in plant cells — interactions between chloroplasts and mitochondria. Plant Science 97, 1–14.
| Crossref | GoogleScholarGoogle Scholar |

Raich JW, Schlesinger WH
(1992) The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus Series–Chem Phys Meteor 44, 81–99.

Reichstein M,
Tenhunen JD,
Roupsard O,
Ourcival J-M,
Rambal S,
Dore S, Valentini R
(2002) Ecosystem respiration in two Mediterranean evergreen Holm Oak forests, drought effects and decomposition dynamics. Functional Ecology 16, 27–39.
| Crossref | GoogleScholarGoogle Scholar |

Ribas-Carbó M,
Aroca R,
Gonzàlez-Meler MA,
Irigoyen JJ, Sanchezdiaz M
(2000) The electron partitioning between the cytochrome and alternative respiratory pathways during chilling recovery in two cultivars of maize differing in chilling sensitivity. Plant Physiology 122, 199–204.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rook DA
(1969) The influence of growing temperature on photosynthesis and respiration of Pinus radiata seedlings. New Zealand Journal of Botany 7, 43–55.

Ryan MG
(1991) Effects of climate change on plant respiration. Ecological Applications 1, 157–167.

Ryan MG,
Hubbard RM,
Pongracic S,
Raison RJ, McMurtrie RE
(1996) Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiology 16, 333–343.
| PubMed |

Saradadevi K, Raghavendra AS
(1992) Dark respiration protects photosynthesis against photoinhibition in mesophyll protoplasts of pea (Pisum sativum). Plant Physiology 99, 1232–1237.

Schimel DS,
Emanuel W,
Rizzo B,
Smith T, Woodward FI , et al.
(1997) Continental scale variability in ecosystem processes, models, data and the role of disturbance. Ecological Monographs 67, 251–271.

Semikhatova, OA ,
Gerasimenko, TV ,
and
Ivanova, TI (1992). Photosynthesis, respiration, and growth of plants in the Soviet Arctic. In ‘Arctic ecosystems in a changing climate’. pp. 169–192. (Academic Press: San Diego)
Shapiro JB,
Griffin KL,
Lewis JD, Tissue DT
(2004) Response of Xanthium strumarium leaf respiration in the light to elevated CO2 concentration, nitrogen availability and temperature. New Phytologist 162, 377–386.
| Crossref | GoogleScholarGoogle Scholar |

Sheen J
(1994) Feedback control of gene expression. Photosynthesis Research 39, 427–438.
| Crossref | GoogleScholarGoogle Scholar |

Shyam R,
Raghavendra AS, Sane PV
(1993) Role of dark respiration in photoinhibition of photosynthesis and its reactivation in the Cyanobacterium anacystis-nidulans. Physiologia Plantarum 88, 446–452.
| Crossref | GoogleScholarGoogle Scholar |

Smakman G, Hofstra RJJ
(1982) Energy metabolism of Plantago lanceolata, as affected by change in root temperature. Physiologia Plantarum 56, 33–37.

Sowell JB, Spomer GG
(1986) Ecotypic variation in root respiration rate among elevational populations of Abies lasiocarpa and Picea engelmannii. Oecologia 68, 375–379.
| Crossref |

Stockfors J, Linder S
(1998) The effect of nutrition on the seasonal course of needle respiration in Norway spruce stands. Trees 12, 130–138.
| Crossref | GoogleScholarGoogle Scholar |

Strand A,
Hurry V,
Gustafsson P, Gardeström P
(1997) Development of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. The Plant Journal 12, 605–614.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Strand A,
Foyer CH,
Gustafsson P,
Gardeström P, Hurry V
(2003) Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant, Cell and Environment 26, 523–535.
| Crossref |

Stitt M, Hurry VM
(2002) A plant for all seasons: alteration in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology 5, 199–206.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Talts P,
Parnik T,
Gardestrom P, Keerberg O
(2004) Respiratory acclimation in Arabidopsis thaliana leaves at low temperature. Journal of Plant Physiology 161, 573–579.
| PubMed |

Tjoelker MG,
Oleksyn J, Reich PB
(1999a) Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology 5, 679–691.
| Crossref | GoogleScholarGoogle Scholar |

Tjoelker MG,
Oleksyn J, Reich PB
(2001) Modelling respiration of vegetation: evidence for a general temperature-dependent Q10. Global Change Biology 7, 223–230.
| Crossref | GoogleScholarGoogle Scholar |

Tjoelker MG,
Reich PB, Oleksyn J
(1999b) Changes in leaf nitrogen and carbohydrates underlie temperature and CO2 acclimation of dark respiration in five boreal tree species. Plant, Cell and Environment 22, 767–778.
| Crossref | GoogleScholarGoogle Scholar |

Turnbull MH,
Whitehead D,
Tissue DT,
Schuster WSF,
Brown KJ, Griffin KL
(2001) Responses of leaf respiration to temperature and leaf characteristics in three deciduous tree species vary with site water availability. Tree Physiology 21, 571–578.
| PubMed |

Turnbull MH,
Whitehead D,
Tissue DT,
Schuster WSF,
Brown KJ, Griffin KL
(2003) Scaling foliar respiration in two contrasting forest canopies. Functional Ecology 17, 101–114.
| Crossref | GoogleScholarGoogle Scholar |

Vanlerberghe GC, McIntosh L
(1992) Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiology 100, 115–119.

Villar R,
Held AA, Merino J
(1995) Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiology 107, 421–427.
| PubMed |

Wagner AM
(1995) A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Letters 368, 339–342.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wager HG
(1941) On the respiration and carbon assimilation rates of some arctic plants as related to temperature. New Phytologist 40, 1–19.

Warren Wilson J
(1966) An analysis of plant growth and its control in Arctic environments. Annals of Botany 30, 383–402.

Weger HG, Guy RD
(1991) Cytochrome and alternative pathway respiration in white spruce (Picea glauca) roots. Effects of growth and measurement temperature. Physiologia Plantarum 83, 675–681.
| Crossref | GoogleScholarGoogle Scholar |

White A,
Cannell MGR, Friend AD
(2000) The high-latitude terrestrial carbon sink, a model analysis. Global Change Biology 6, 227–245.
| Crossref | GoogleScholarGoogle Scholar |

Will R
(2000) Effect of different daytime and night-time temperature regimes on the foliar respiration of Pinus taeda: predicting the effect of variable temperature on acclimation. Journal of Experimental Botany 51, 1733–1739.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wythers KR,
Reich PB,
Tjoelker MG, Bolstad PB
(2005) Foliar respiration acclimation to temperature and temperature variable Q10 alter ecosystem carbon balance. Global Change Biology (In press) ,

Xiong FS,
Mueller EC, Day TA
(2000) Photosynthetic and respiratory acclimation and growth response of antarctic vascular plants to contrasting temperature regimes. American Journal of Botany 87, 700–710.
| PubMed |

Zha TS,
Ryyppö A,
Wang KY, Kellomäki S
(2001) Effects of elevated carbon dioxide concentration and temperature on needle growth, respiration and carbohydrate status in field-grown Scots pines during the needle expansion period. Tree Physiology 21, 1279–1287.
| PubMed |

Zha TS,
Wang KY,
Ryyppö A, Kellomäki S
(2002) Impact of needle age on the response of respiration in Scots pine to long-term elevation of carbon dioxide concentration and temperature. Tree Physiology 22, 1241–1248.
| PubMed |

Zha TS,
Kellomäki S, Wang KY
(2003) Seasonal variation in respiration of 1-year-old shoots of Scots pine exposed to elevated carbon dioxide and temperature for 4 years. Annals of Botany 92, 89–96.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zha T,
Kellomäki S,
Wang KY,
Ryyppö A, Niinisto S
(2004) Seasonal and annual stem respiration of Scots Pine trees under boreal conditions. Annals of Botany 94, 889–896.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zha TS,
Kellomäki S,
Wang KY, Ryyppö A
(2005) Respiratory responses of Scots pine stems to 5 years of exposure to elevated CO2 concentration and temperature. Tree Physiology 25, 49–56.
| PubMed |

Zimmerman RC,
Smith RD, Alberte RS
(1989) Thermal acclimation and whole-plant carbon balance in Zostera marina L (Eelgrass). Journal of Experimental Marine Biology and Ecology 130, 93–109.
| Crossref | GoogleScholarGoogle Scholar |

Ziska LH, Bunce JA
(1998) The influence of increasing growth temperature and CO2 concentration on the ratio of respiration to photosynthesis in soybean seedlings. Global Change Biology 4, 637–643.
| Crossref | GoogleScholarGoogle Scholar |

Zogg GP,
Zak DR,
Burton AJ, Pregitzer KS
(1996) Fine root respiration in northern hardwood forests in relation to temperature and nitrogen availability. Tree Physiology 16, 719–725.
| PubMed |

Xu M,
Debiase TA,
Qi Y,
Goldstein A, Liu Z
(2001) Ecosystem respiration in a young ponderosa pine plantation in the Sierra Nevada Mountains, California. Tree Physiology 21, 309–318.
| PubMed |



