Appropriate time interval of PPFD measurement to estimate daily photosynthetic gain
Keach Murakami A C and Tomohiro Jishi B
A C and Tomohiro Jishi B
A Hokkaido Agricultural Research Center (HARC), National Agriculture and Food Research Organisation (NARO), 062–8555, 1 Hitsujigaoka, Toyohira, Sapporo, Japan.
B Energy Innovation Center, Central Research Institute of Electric Power Industry, 270–1194, 1646 Abiko, Abiko, Chiba, Japan.
C Corresponding author. Email: keach.murakami@affrc.go.jp
Functional Plant Biology - https://doi.org/10.1071/FP20323
Submitted: 16 October 2020 Accepted: 11 January 2021 Published online: 8 February 2021
Abstract
Photosynthetic models sometimes incorporate meteorological elements typically recorded at a time interval of 10 min or 1 h. Because these data are calculated by averaging instantaneous values over time, short-term environmental fluctuations are concealed, which may affect outputs of the model. To assess an appropriate time interval of photosynthetic photon flux density (PPFD) measurement for accurate estimation of photosynthetic gain under open field conditions, we simulated the daily integral net photosynthetic gain using photosynthetic models with or without considering induction kinetics in response to changes in PPFD. Compared with the daily gain calculated from 60-min-interval PPFD data using a steady-state model that ignored the induction kinetics (i.e. a baseline gain), the gains simulated using higher-resolution PPFD data (10-s, 1-min, and 10-min intervals) and using a dynamic model that considered slow induction kinetics were both smaller by ~2%. The gain estimated by the slow dynamic model with 10-s-interval PPFD data was smaller than the baseline gain by more than 5% with a probability of 66%. Thus, the use of low-resolution PPFD data causes overestimation of daily photosynthetic gain in open fields. An appropriate time interval for PPFD measurement is 1 min or shorter to ensure accuracy of the estimates.
Keywords: photosynthetic induction, fluctuating light, photosynthetic dynamics, meteorological observation, PPFD.
References
Acevedo-Siaca LG, Coe R, Wang Y, Kromdijk J, Quick WP, Long SP (2020) Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytologist 227, 1097–1108.| Variation in photosynthetic induction between rice accessions and its potential for improving productivity.Crossref | GoogleScholarGoogle Scholar |
Adachi S, Tanaka Y, Miyagi A, Kashima M, Tezuka A, Toya Y, Kobayashi S, Ohkubo S, Shimizu H, Kawai-Yamada M, Sage RF, Nagano AJ, Yamori W (2019) High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light. Journal of Experimental Botany 70, 5287–5297.
| High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light.Crossref | GoogleScholarGoogle Scholar | 31257443PubMed |
Allen MT, Pearcy RW (2000) Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 122, 470–478.
| Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest.Crossref | GoogleScholarGoogle Scholar | 28308338PubMed |
Anderson JM, Chow WS, Park Y-I (1995) The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynthesis Research 46, 129–139.
| The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues.Crossref | GoogleScholarGoogle Scholar | 24301575PubMed |
Bai K-d, Liao D-b, Jiang D-b, Cao K-f (2008) Photosynthetic induction in leaves of co-occurring Fagus lucida and Castanopsis lamontii saplings grown in contrasting light environments. Trees 22, 449–462.
| Photosynthetic induction in leaves of co-occurring Fagus lucida and Castanopsis lamontii saplings grown in contrasting light environments.Crossref | GoogleScholarGoogle Scholar |
Chazdon RL (1988) Sunflecks and their importance to forest understorey plants. ‘Advances in ecological research’. (Eds M Begon, AH Fitter, M Ford) pp. 1–63. (Academic Press)
Chow W, Hope A (1976) Light-induced pH gradients in isolated spinach chloroplasts. Australian Journal of Plant Physiology 3, 141–152.
Chow WS, Hope AB (2004) Electron fluxes through photosystem I in cucumber leaf discs probed by far-red light. Photosynthesis Research 81, 77–89.
| Electron fluxes through photosystem I in cucumber leaf discs probed by far-red light.Crossref | GoogleScholarGoogle Scholar | 16328849PubMed |
Chow WS, Melis A, Anderson JM (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proceedings of the National Academy of Sciences of the United States of America 87, 7502–7506.
| Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 11607105PubMed |
De Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP (2020) Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytologist 225, 2498–2512.
| Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light.Crossref | GoogleScholarGoogle Scholar |
Duursma RA (2015) Plantecophys-an R package for analysing and modelling leaf gas exchange data. PLoS One 10, e0143346
| Plantecophys-an R package for analysing and modelling leaf gas exchange data.Crossref | GoogleScholarGoogle Scholar | 26581080PubMed |
Ebenhöh O, Houwaart T, Lokstein H, Schlede S, Tirok K (2011) A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence. Bio Systems 103, 196–204.
| A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence.Crossref | GoogleScholarGoogle Scholar | 21029763PubMed |
Eberhard S, Finazzi G, Wollman F-A (2008) The dynamics of photosynthesis. Annual Review of Genetics 42, 463–515.
| The dynamics of photosynthesis.Crossref | GoogleScholarGoogle Scholar | 18983262PubMed |
Fan D-Y, Nie Q, Hope AB, Hillier W, Pogson BJ, Chow WS (2007) Quantification of cyclic electron flow around Photosystem I in spinach leaves during photosynthetic induction. Photosynthesis Research 94, 347–357.
| Quantification of cyclic electron flow around Photosystem I in spinach leaves during photosynthetic induction.Crossref | GoogleScholarGoogle Scholar | 17211579PubMed |
Faralli M, Cockram J, Ober E, Wall S, Galle A, Van Rie J, Raines C, Lawson T (2019) Genotypic, developmental and environmental effects on the rapidity of gs in wheat: Impacts on carbon gain and water-use efficiency. Frontiers in Plant Science 10, 492
| Genotypic, developmental and environmental effects on the rapidity of gs in wheat: Impacts on carbon gain and water-use efficiency.Crossref | GoogleScholarGoogle Scholar | 31057590PubMed |
Farquhar G, von Caemmerer S, Berry J (1980) A biochemical model of photosynthetic
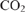 assimilation in leaves of
assimilation in leaves of 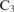 species. Planta 149, 78–90.
species. Planta 149, 78–90.| A biochemical model of photosynthetic
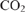 assimilation in leaves of
assimilation in leaves of 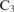 species.Crossref | assimilation in leaves of
species.Crossref | assimilation in leaves of 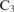 species.&journal=Planta&volume=149&pages=78-90&publication_year=1980&author=G%20Farquhar&hl=en&doi=10.1007/BF00386231" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 24306196PubMed |
species.&journal=Planta&volume=149&pages=78-90&publication_year=1980&author=G%20Farquhar&hl=en&doi=10.1007/BF00386231" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 24306196PubMed | Gross LJ (1982) Photosynthetic dynamics in varying light environments: A model and its application to whole leaf carbon gain. Ecology 63, 84–93.
| Photosynthetic dynamics in varying light environments: A model and its application to whole leaf carbon gain.Crossref | GoogleScholarGoogle Scholar |
Gross L, Kirschbaum M, Pearcy R (1991) A dynamic model of photosynthesis in varying light taking account of stomatal conductance, C3-cycle intermediates, photorespiration and rubisco activation. Plant, Cell & Environment 14, 881–893.
| A dynamic model of photosynthesis in varying light taking account of stomatal conductance, C3-cycle intermediates, photorespiration and rubisco activation.Crossref | GoogleScholarGoogle Scholar |
Han Q, Yamaguchi E, Odaka N, Kakubari Y (1999) Photosynthetic induction responses to variable light under field conditions in three species grown in the gap and understory of a Fagus crenata forest. Tree Physiology 19, 625–634.
| Photosynthetic induction responses to variable light under field conditions in three species grown in the gap and understory of a Fagus crenata forest.Crossref | GoogleScholarGoogle Scholar | 12651318PubMed |
Jishi T, Matsuda R, Fujiwara K (2015) A kinetic model for estimating net photosynthetic rates of cos lettuce leaves under pulsed light. Photosynthesis Research 124, 107–116.
| A kinetic model for estimating net photosynthetic rates of cos lettuce leaves under pulsed light.Crossref | GoogleScholarGoogle Scholar | 25736464PubMed |
Jishi T, Matsuda R, Fujiwara K (2018) Effects of photosynthetic photon flux density, frequency, duty ratio, and their interactions on net photosynthetic rate of cos lettuce leaves under pulsed light: Explanation based on photosynthetic-intermediate pool dynamics. Photosynthesis Research 136, 371–378.
| Effects of photosynthetic photon flux density, frequency, duty ratio, and their interactions on net photosynthetic rate of cos lettuce leaves under pulsed light: Explanation based on photosynthetic-intermediate pool dynamics.Crossref | GoogleScholarGoogle Scholar | 29236208PubMed |
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LF (2015) Dynamic photosynthesis in different environmental conditions. Journal of Experimental Botany 66, 2415–2426.
| Dynamic photosynthesis in different environmental conditions.Crossref | GoogleScholarGoogle Scholar | 25324402PubMed |
Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LF (2017) Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by
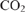 partial pressure, temperature, air humidity and blue irradiance. Annals of Botany 119, 191–205.
partial pressure, temperature, air humidity and blue irradiance. Annals of Botany 119, 191–205.| Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by
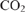 partial pressure, temperature, air humidity and blue irradiance.Crossref | partial pressure, temperature, air humidity and blue irradiance.&journal=Annals of Botany&volume=119&pages=191-205&publication_year=2017&author=E%20Kaiser&hl=en&doi=10.1093/aob/mcw226" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 28025286PubMed |
partial pressure, temperature, air humidity and blue irradiance.Crossref | partial pressure, temperature, air humidity and blue irradiance.&journal=Annals of Botany&volume=119&pages=191-205&publication_year=2017&author=E%20Kaiser&hl=en&doi=10.1093/aob/mcw226" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 28025286PubMed | Kaiser E, Zhou D, Heuvelink E, Harbinson J, Morales A, Marcelis LF (2017) Elevated
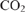 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. Journal of Experimental Botany 68, 5629–5640.
increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. Journal of Experimental Botany 68, 5629–5640.| Elevated
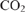 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state.Crossref | increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state.&journal=Journal of Experimental Botany&volume=68&pages=5629-5640&publication_year=2017&author=E%20Kaiser&hl=en&doi=10.1093/jxb/erx357" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 29045757PubMed |
increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state.Crossref | increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state.&journal=Journal of Experimental Botany&volume=68&pages=5629-5640&publication_year=2017&author=E%20Kaiser&hl=en&doi=10.1093/jxb/erx357" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 29045757PubMed | Kaiser E, Morales A, Harbinson J (2018) Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology 176, 977–989.
| Fluctuating light takes crop photosynthesis on a rollercoaster ride.Crossref | GoogleScholarGoogle Scholar | 29046421PubMed |
Kaiser E, Walther D, Armbruster U (2020) Growth under fluctuating light reveals large trait variation in a panel of Arabidopsis accessions. Plants 9, 316
| Growth under fluctuating light reveals large trait variation in a panel of Arabidopsis accessions.Crossref | GoogleScholarGoogle Scholar |
Kimura H, Hashimoto-Sugimoto M, Iba K, Terashima I, Yamori W (2020) Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. Journal of Experimental Botany 71, 2339–2350.
| Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light.Crossref | GoogleScholarGoogle Scholar | 32095822PubMed |
Kirschbaum M, Küppers M, Schneider H, Giersch C, Noe S (1998) Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates. Planta 204, 16–26.
| Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates.Crossref | GoogleScholarGoogle Scholar |
Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and
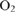 -dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant & Cell Physiology 55, 990–1004.
-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant & Cell Physiology 55, 990–1004.| Roles of the cyclic electron flow around PSI (CEF-PSI) and
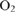 -dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.Crossref | -dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.&journal=Plant & Cell Physiology&volume=55&pages=990-1004&publication_year=2014&author=M%20Kono&hl=en&doi=10.1093/pcp/pcu033" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |
-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.Crossref | -dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.&journal=Plant & Cell Physiology&volume=55&pages=990-1004&publication_year=2014&author=M%20Kono&hl=en&doi=10.1093/pcp/pcu033" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | Kou J, Takahashi S, Fan D-Y, Badger MR, Chow WS (2015) Partially dissecting the steady-state electron fluxes in Photosystem I in wild-type and pgr5 and ndh mutants of Arabidopsis. Frontiers in Plant Science 6, 758
| Partially dissecting the steady-state electron fluxes in Photosystem I in wild-type and pgr5 and ndh mutants of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 26442071PubMed |
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861.
| Improving photosynthesis and crop productivity by accelerating recovery from photoprotection.Crossref | GoogleScholarGoogle Scholar | 27856901PubMed |
Lawson T, Vialet-Chabrand S (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist 221, 93–98.
| Speedy stomata, photosynthesis and plant water use efficiency.Crossref | GoogleScholarGoogle Scholar |
Medlyn B, Dreyer E, Ellsworth D, Forstreuter M, Harley P, Kirschbaum M, Le Roux X, Montpied P, Strassemeyer J, Walcroft A, Wang K, Loustau D (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell & Environment 25, 1167–1179.
| Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data.Crossref | GoogleScholarGoogle Scholar |
Medlyn BE, Duursma RA, Eamus D, Ellsworth DS, Prentice IC, Barton CV, Crous KY, De Angelis P, Freeman M, Wingate L (2011) Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biology 17, 2134–2144.
| Reconciling the optimal and empirical approaches to modelling stomatal conductance.Crossref | GoogleScholarGoogle Scholar |
Naumburg E, Ellsworth DS (2000) Photosynthetic sunfleck utilization potential of understory saplings growing under elevated
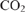 in FACE. Oecologia 122, 163–174.
in FACE. Oecologia 122, 163–174.| Photosynthetic sunfleck utilization potential of understory saplings growing under elevated
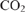 in FACE.Crossref | in FACE.&journal=Oecologia&volume=122&pages=163-174&publication_year=2000&author=E%20Naumburg&hl=en&doi=10.1007/PL00008844" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 28308370PubMed |
in FACE.Crossref | in FACE.&journal=Oecologia&volume=122&pages=163-174&publication_year=2000&author=E%20Naumburg&hl=en&doi=10.1007/PL00008844" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | 28308370PubMed | Naumburg E, Ellsworth DS (2002) Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species. Tree Physiology 22, 393–401.
| Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species.Crossref | GoogleScholarGoogle Scholar | 11960764PubMed |
Ögren E, Sundin U (1996) Photosynthetic responses to variable light: A comparison of species from contrasting habitats. Oecologia 106, 18–27.
| Photosynthetic responses to variable light: A comparison of species from contrasting habitats.Crossref | GoogleScholarGoogle Scholar | 28307153PubMed |
Osmond B, Chow WS, Wyber R, Zavafer A, Keller B, Pogson B, Robinson SA (2017) Relative functional and optical absorption cross-sections of PSII and other photosynthetic parameters monitored in situ, at a distance with a time resolution of a few seconds, using a prototype light induced fluorescence transient (LIFT) device. Functional Plant Biology 44, 985–1006.
| Relative functional and optical absorption cross-sections of PSII and other photosynthetic parameters monitored in situ, at a distance with a time resolution of a few seconds, using a prototype light induced fluorescence transient (LIFT) device.Crossref | GoogleScholarGoogle Scholar | 32480627PubMed |
Papanatsiou M, Petersen J, Henderson L, Wang Y, Christie J, Blatt M (2019) Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363, 1456–1459.
| Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth.Crossref | GoogleScholarGoogle Scholar | 30923223PubMed |
Pastorello G, Trotta C, Canfora E, Chu H, Christianson D, Cheah Y-W, Poindexter C, Chen J, Elbashandy A, Humphrey M, et al. (2020) The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Scientific Data 7, 1–27.
| The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data.Crossref | GoogleScholarGoogle Scholar |
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annual Review of Plant Biology 41, 421–453.
| Sunflecks and photosynthesis in plant canopies.Crossref | GoogleScholarGoogle Scholar |
Pearcy RW, Krall JP, Sassenrath-Cole GF (1996) Photosynthesis in fluctuating light environments. In‘Photosynthesis and the environment’. pp. 321–346. (Springer)
Pearcy R, Gross L, He D (1997) An improved dynamic model of photosynthesis for estimation of carbon gain in sunfleck light regimes. Plant, Cell & Environment 20, 411–424.
| An improved dynamic model of photosynthesis for estimation of carbon gain in sunfleck light regimes.Crossref | GoogleScholarGoogle Scholar |
Pfitsch WA, Pearcy RW (1989) Daily carbon gain by Adenocaulon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment. Oecologia 80, 465–470.
| Daily carbon gain by Adenocaulon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment.Crossref | GoogleScholarGoogle Scholar | 28312829PubMed |
Pons T, Pearcy R, Seemann J (1992) Photosynthesis in flashing light in soybean leaves grown in different conditions. I. Photosynthetic induction state and regulation of ribulose-1, 5-bisphosphate carboxylase activity. Plant, Cell & Environment 15, 569–576.
| Photosynthesis in flashing light in soybean leaves grown in different conditions. I. Photosynthetic induction state and regulation of ribulose-1, 5-bisphosphate carboxylase activity.Crossref | GoogleScholarGoogle Scholar |
Porcar-Castell A, Bäck J, Juurola E, Hari P (2006) Dynamics of the energy flow through photosystem II under changing light conditions: A model approach. Functional Plant Biology 33, 229–239.
| Dynamics of the energy flow through photosystem II under changing light conditions: A model approach.Crossref | GoogleScholarGoogle Scholar | 32689230PubMed |
Portes MT, Alves TH, Souza GM (2006) Water deficit affects photosynthetic induction in Bauhinia forficata Link (Fabaceae) and Esenbeckia leiocarpa Engl. (Rutaceae) growing in understorey and gap conditions. Brazilian Journal of Plant Physiology 18, 491–502.
| Water deficit affects photosynthetic induction in Bauhinia forficata Link (Fabaceae) and Esenbeckia leiocarpa Engl. (Rutaceae) growing in understorey and gap conditions.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rabinowitch EI (1956) Time effects. II. Photosynthesis in intermittent light. A. altering light. In ‘Photosynthesis and related processes. Vol. II. Part 2’. (Ed. EI Rabinowitch) pp. 1435–1447. (Interscience Publishers Inc.: NY, USA)
Šafránek D, Červen? J, Klement M, Pospíšilová J, Brim L, Lazár D, Nedbal L (2011) E-photosynthesis: Web-based platform for modeling of complex photosynthetic processes. Bio Systems 103, 115–124.
| E-photosynthesis: Web-based platform for modeling of complex photosynthetic processes.Crossref | GoogleScholarGoogle Scholar | 21073914PubMed |
Salter WT, Merchant AM, Richards RA, Trethowan R, Buckley TN (2019) Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat. Journal of Experimental Botany 70, 2787–2796.
| Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat.Crossref | GoogleScholarGoogle Scholar | 30821324PubMed |
Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant & Cell Physiology 55, 1184–1193.
| Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves.Crossref | GoogleScholarGoogle Scholar |
Slattery RA, Walker BJ, Weber AP, Ort DR (2018) The impacts of fluctuating light on crop performance. Plant Physiology 176, 990–1003.
| The impacts of fluctuating light on crop performance.Crossref | GoogleScholarGoogle Scholar | 29192028PubMed |
Soleh MA, Tanaka Y, Nomoto Y, Iwahashi Y, Nakashima K, Fukuda Y, Long SP, Shiraiwa T (2016) Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr.]. Plant, Cell & Environment 39, 685–693.
| Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr.].Crossref | GoogleScholarGoogle Scholar |
Sukhova E, Khlopkov A, Vodeneev V, Sukhov V (2020) Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1861, 148138
| Simulation of a nonphotochemical quenching in plant leaf under different light intensities.Crossref | GoogleScholarGoogle Scholar |
Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro E-M (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. The Plant Cell 24, 2934–2948.
| PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions.Crossref | GoogleScholarGoogle Scholar | 22822205PubMed |
Tanaka Y, Adachi S, Yamori W (2019) Natural genetic variation of the photosynthetic induction response to fluctuating light environment. Current Opinion in Plant Biology 49, 52–59.
| Natural genetic variation of the photosynthetic induction response to fluctuating light environment.Crossref | GoogleScholarGoogle Scholar | 31202005PubMed |
Taniyoshi K, Tanaka Y, Shiraiwa T (2020) Genetic variation in the photosynthetic induction response in rice (Oryza sativa L.). Photochemical & Photobiological Sciences 23, 513–521.
Taylor SH, Long SP (2017) Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 372, 20160543
| Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity.Crossref | GoogleScholarGoogle Scholar | 28808109PubMed |
von Caemmerer S, Farquhar G (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387.
| Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves.Crossref | GoogleScholarGoogle Scholar | 24276943PubMed |
Woodrow IE, Mott KA (1989) Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach. Australian Journal of Plant Physiology 16, 487–500.
| Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach.Crossref | GoogleScholarGoogle Scholar |
Yamori W, Kusumi K, Iba K, Terashima I (2020) Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant, Cell & Environment 43, 1230–1240.
| Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice.Crossref | GoogleScholarGoogle Scholar |
Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012) A kinetic model of rapidly reversible nonphotochemical quenching. Proceedings of the National Academy of Sciences of the United States of America 109, 15757–15762.
| A kinetic model of rapidly reversible nonphotochemical quenching.Crossref | GoogleScholarGoogle Scholar | 22891305PubMed |
Zhang Y, Kaiser E, Zhang Y, Yang Q, Li T (2018) Short-term salt stress strongly affects dynamic photosynthesis, but not steady-state photosynthesis, in tomato (Solanum lycopersicum). Environmental and Experimental Botany 149, 109–119.
| Short-term salt stress strongly affects dynamic photosynthesis, but not steady-state photosynthesis, in tomato (Solanum lycopersicum).Crossref | GoogleScholarGoogle Scholar |
Zhang M-M, Fan D-Y, Murakami K, Badger MR, Sun G-Y, Chow WS (2019) Partially dissecting electron fluxes in both photosystems in spinach leaf discs during photosynthetic induction. Plant & Cell Physiology 60, 2206–2219.
| Partially dissecting electron fluxes in both photosystems in spinach leaf discs during photosynthetic induction.Crossref | GoogleScholarGoogle Scholar |
Zhu X-G, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis. Journal of Experimental Botany 55, 1167–1175.
| The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis.Crossref | GoogleScholarGoogle Scholar | 15133059PubMed |


