Carbon isotope effect predictions for enzymes involved in the primary carbon metabolism of plant leaves
Guillaume Tcherkez A B and Graham D. Farquhar AA Environmental Biology Group, Research School of Biological Sciences, Institute of Advanced Studies, Australian National University, GPO Box 475, Canberra, ACT 2601, Australia.
B Current address: Laboratoire Structure et Métabolisme des Plantes, Bat. 630, Institut de Biotechnologie des Plantes, Centre Scientifique d’Orsay, Université Paris XI, 91405 Orsay Cedex, France.
Corresponding author. Email: guillaume.tcherkez@ese.u-psud.fr
Functional Plant Biology 32(4) 277-291 https://doi.org/10.1071/FP04211
Submitted: 12 November 2004 Accepted: 7 March 2005 Published: 26 April 2005
Abstract
Carbon isotope effects of enzymes involved in primary carbon metabolism are key parameters in our understanding of plant metabolism. Nevertheless, some of them are poorly known because of the lack of in vitro experimental data on purified enzymes. Some studies have focused on theoretical predictions of isotope effects. Here we show how quantum chemical calculations can be adapted for calculation of isotope effects for the Rubisco-catalysed carboxylation and oxygenation reactions and the citrate synthase reaction. The intrinsic isotope effect of the carboxylation by Rubisco appears to be much smaller than previously thought, being close to the overall isotope effect of the reaction that is, between 25 and 30 per mil. The same applies to the enzyme citrate synthase, that catalyses the first step of the Krebs cycle, with an isotope effect of around 23 per mil. Combined with the isotope effects of equilibrium reactions calculated with β-factors, the Krebs cycle then has an overall isotope effect that depletes organic acids in 13C.
Keywords: carbon isotopes, carbon metabolism, carboxylation, citrate synthase, enzymes, oxygenation, Rubisco.
Acknowledgments
We acknowledge valuable discussions with Dr Jill Gready and Dr J Andrews. GDF also wishes to acknowledge the Australian Research Council for its support through a Discovery Grant.
Abelson H, Hoering TC
(1961) Carbon isotope fractionation in formation of amino-acids by photosynthetic organisms. Proceedings of the National Academy of Sciences USA 47, 623–632.
with CO2 and oxygen (competition between carboxylation and oxygenation). If RuBP is not limiting, the rate is:
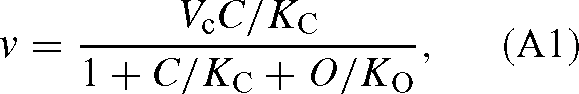
where O is the oxygen concentration, and KC and KO are the apparent Michaelis constants for CO2 and O2. Vc is the maximum velocity of the carboxylase. Similarly, with two 12C and 13C-isotopomers of CO2, we have, neglecting oxygenation:
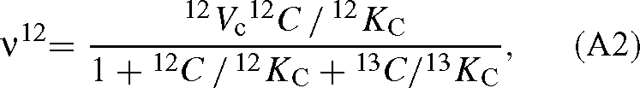
and the symmetrical expression for 13CO2. The ratio of the velocities is then:
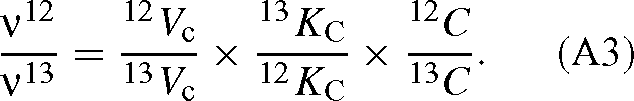
Dividing each side by 12C / 13C, we have the overall isotope effect of the reaction:
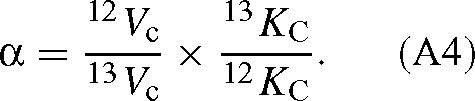
The values of KC and Vc are (derived from the appendix of Farquhar 1979):
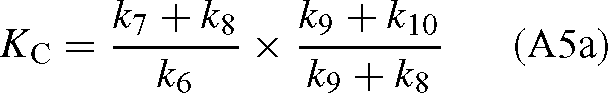
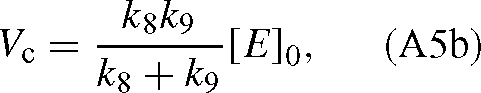
where [E]0 is the total enzyme site concentration. Substituting the Vc and KC values into eqn (A4) gives:
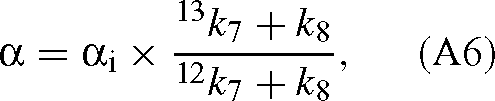
where αi(= 12k6 / 13k6) is the ‘intrinsic’ isotope effect, that is, the isotope effect of the carboxylation step. k8 is assumed to be similar with both isotopes because the corresponding step (hydration and cleavage of the C6 intermediate) does not involve the carbon atom inherited from CO2. This result is not modified when RuBP is limiting or when the oxygenation of RuBP is taken into account. If RuBP is limiting, the velocity is (Farquhar 1979):
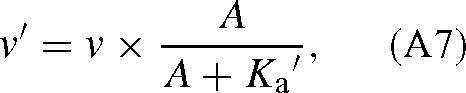
where Ka′ is the apparent Michaelis constant for RuBP and A is the concentration of RuBP. It is the same for 13C and 12C so that it cancels out in eqn (A3). When oxygenation is added, the denominator of v in eqn (A2) has the additional term O / KO, and it has no effect on the ratio of eqn (A4).
Equation (A6) can be re-written with the relationship 12k7 = (1 + ϵ7)13k7 (ϵ7 is the discrimination associated with decarboxylation) and substituting k7 using eqn (A5) as follows:
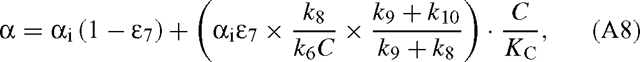
where C is the (overall) carbon dioxide concentration. The concentration C is here added in both the numerator and the denominator in order to recall that k6C has the same dimension as k8, and KC has the dimension of a concentration.
Calculation of the isotope effect during Rubisco ’s reaction with oxygen
The oxygen isotope effect that occurs when the O(O2)–C2(RuBP) is formed, as given by eqn (4) is not equal to the isotope effect that would be measured using the overall isotope ratio in O2 compared with that of the oxygen that is fixed to the C-2 of RuBP. This is because of the symmetry of the O2 molecule. The reaction is described as follows, where for clarity, the oxygen atoms are labelled with numbers:
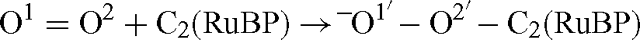
The fractionation given by eqn (4) is:
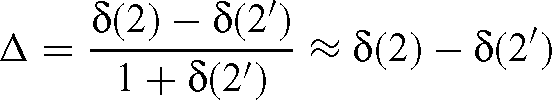
while the isotope fractionation that would be measured is as follows:
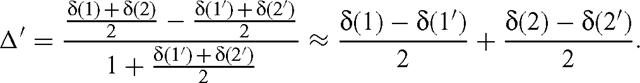
But the oxygen atom number 1 is not involved by the bond considered and so is not subjected to any isotope effect. So we have δ(1) = δ(1′) and the simple relationship: Δ′ = Δ / 2. As the isotope effect is given by α = Δ − 1, we have α′ = 1 + (α − 1) / 2.
Isotope effect of a reversible reaction
We assume here the following reversible reaction, with the reactants A and the product B:
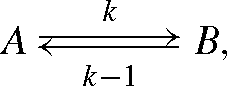
where the rate constants of the forward and backward reactions are k and k–1, respectively. For the 12C and 13C isotopes, we have: k12, k13 and k–112 and k–113. Mass balance equations are such that:
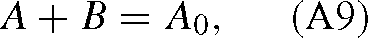
which is true for each isotope: A12 + B12 = A012 and similarly for 13C. With a first order mechanism, we have the following differential equation:
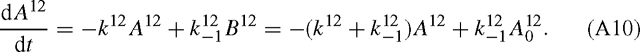
The same applies to 13C:
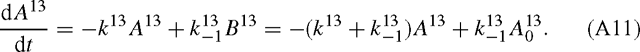
The solutions of (A10) and (A11) are exponential functions. For 12C, we have:
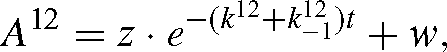
where z and w are obtained at t = 0 so that:
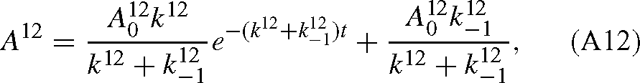
and the same for A13. Combining Eqns (A9) and (A12), we have:
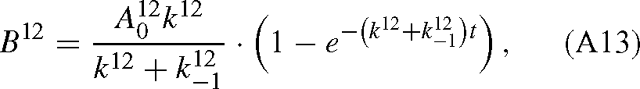
and similarly for B13. The isotope effect is defined by the isotope ratio of the reactants divided by that of the products, that is, with eqns (A12) and (A13):
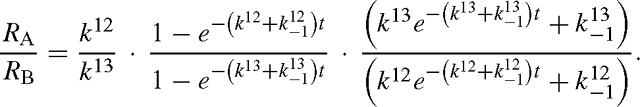
When t → 0, RA / RB tends towards k12 / k13 and when t → +∞, it tends towards:
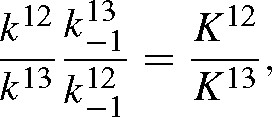
as the exponential term tends to zero.


