‘Candidatus Phytoplasma cynodontis’ (16SrXIV group) affecting Oplismenus burmannii (Retz.) P. Beauv. and Digitaria sanguinalis (L.) Scop. in India
G. P. Rao A C , S. Mall A and C. Marcone BA Sugarcane Research Station, Kunraghat, Gorakhpur 273 008, UP, India.
B Dipartimento di Scienze Farmaceutiche, Università degli Studi di Salerno, Via Ponte Don Melillo, I-84084 Fisciano (Salerno), Italy.
C Corresponding author. Email: gprao_gor@rediffmail.com
Australasian Plant Disease Notes 5(1) 93-95 https://doi.org/10.1071/DN10033
Submitted: 23 August 2009 Accepted: 18 August 2010 Published: 30 August 2010
Abstract
A phytoplasma has been detected in Oplismenus burmannii (Retz.) P. Beauv. and Digitaria sanguinalis (L.) Scop. plants with symptoms of chlorotic streaks and white leaf disease in India. By sequence and phylogenetic analyses of polymerase chain reaction-amplified rDNA sequences, the detected phytoplasma was identified as ‘Candidatus Phytoplasma cynodontis’, a member of the 16SrXIV group, and the causal agent of Bermuda grass white leaf disease. This is the first report on the occurrence of ‘Ca. Phytoplasma cynodontis’ in O. burmannii and D. sanguinalis grasses.
In India, several weed species have been reported to act as reservoir hosts of many phytoplasmas (Mall 2009). These species include Cynodon dactylon, Parthenium hysterophorus, Cannabis sativa, Achyranthes aspera, Amaranthus spp., Datura inoxia and Dichanthium annulatum (Rao et al. 2007, 2009; Raj et al. 2008). The phytoplasmas infecting the mentioned weeds, belonging mainly to 16SrI, 16SrII and 16SrVI groups, are also known to induce yellow diseases of considerable economic importance in vegetable, ornamental, medicinal, forage and fruit crop plants in India (Mall 2009). Among the most affected cultivated plants are Daucus carota (carrot), Phaseolus vulgaris (French bean), Capsicum annuum (pepper), Solanum melongena (eggplant), Cajanus cajan (pigeon pea), Sesamum indicum (sesame), Gladiolus spp. and Citrus aurantifolia (acid lime) (Ghosh et al. 1999; Khan and Raj 2006; Raj et al. 2006, 2009; Khan et al. 2007; Arocha et al. 2009). During surveys of phytoplasmal diseases of weeds in 2009, symptoms resembling those induced by phytoplasmas were observed on two grasses of the family Poaceae, namely, Oplismenus burmannii (Retz.) P. Beauv. and Digitaria sanguinalis (L.) Scop. The O. burmannii plants showed chlorotic streaks on the leaves when compared with healthy ones (Fig. 1a) at the Sugarcane Research Station campus, Gorakhpur. However, the D. sanguinalis plants exhibited chlorosis of leaves (Fig. 1b) at the Indian Agricultural Research Institute campus, New Delhi. Disease incidence ranged from 2% to 8% at both locations. Symptomatic O. burmannii and D. sanguinalis plants were examined for phytoplasma infections employing highly sensitive polymerase chain reaction (PCR) technology. The detected phytoplasma was identified and characterised using sequence and phylogenetic analyses of PCR-amplified ribosomal DNA (rDNA).

|
DNA was extracted from leaves of 15 diseased and 10 symptomless plants of each species examined, employing the phytoplasma enrichment procedure described by Ahrens and Seemüller (1992). For PCR amplification, the universal phytoplasma primer pair P1/P6 (Deng and Hiruki 1991) followed by nested primer pair R16F2n/R16R2 (Gundersen and Lee 1996), were used. These primer pairs amplified a 16S rDNA fragment of ~1500 and 1245 bp in length, respectively. PCR conditions and agarose gel electrophoresis analysis of PCR products were used as previously described (Gundersen and Lee 1996). All symptomatic plants of both the grass species tested positive for phytoplasma infections, whereas no PCR products were amplified from DNA extracted from symptomless plants. The R16F2n/R16R2 PCR products were separated by electrophoresis using a 1.5% agarose gel, excised from the gel and eluted using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). The DNA fragments were then cloned and five recombinant clones from each grass species were sequenced. Sequences were then assembled and edited using DNASTAR’s Laser Gene software (DNASTAR) and consensus sequences generated. Sequence alignments were performed by using CLUSTAL version 5, of the same software. The sequences obtained in the present work have been deposited in GenBank database (http://www.ncbi.nlm.nih.gov/Genbank) under the accession numbers GQ403690 (O. burmannii isolate) and GQ403689 (D. sanguinalis isolate).
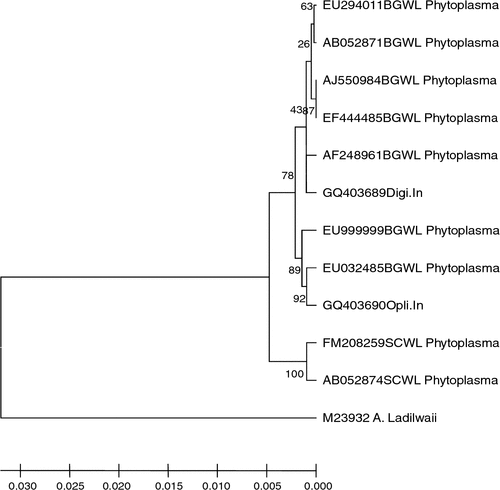
Phylogenetic and molecular evolutionary analyses were conducted using the neighbour-joining program of the genetic analysis software Molecular Evolutionary Genetics Analysis (MEGA), version 4 (Tamura et al. 2007). Acholeplasma laidlawii was included in the analyses as an outgroup. Nucleotide sequence comparisons revealed that the phytoplasmas detected in diseased O. burmannii and D. sanguinalis plants shared 98% 16S rDNA sequence similarities to each other and 99% with members of the Bermuda grass white leaf (BGWL) phytoplasma group or 16SrXIV group which includes ‘Ca. Phytoplasma cynodontis’ (GenBank accession numbers AJ550984 and EF444485), and 97% sequence similarities with sugarcane white leaf (SCWL) phytoplasma (GenBank accession numbers FM208259 and AB052874). Also, the mentioned phytoplasmas clustered together with members of the BGWL group (Fig. 2). Therefore, the phytoplasmas infecting O. burmannii and D. sanguinalis plants in India were identified as isolates of ‘Ca. Phytoplasma cynodontis’ (Marcone et al. 2004). This taxon has previously been reported from India in Bermuda grass (Rao et al. 2007; Snehi et al. 2008) and Dichanthium annulatum (Rao et al. 2009). To our knowledge this is the first report on the occurrence of ‘Ca. Phytoplasma cynodontis’ in O. burmannii and D. sanguinalis grasses. Recognition of weeds as hosts of 16SrXIV group phytoplasmas in India has epidemiological significance and therefore we suggest that emphasis should be given for weed control in and around agricultural fields to minimise phytoplasma disease incidence.
|
Ahrens U, Seemüller E
(1992) Detection of DNA of plant pathogenic mycoplasmalike organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82, 828–832.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Arocha Y,
Singh A,
Pandey M,
Tripathi AN,
Chandra B,
Shukla SK,
Singh Y,
Kumar A,
Srivastava RK,
Zaidi NW,
Arif M,
Narwal S,
Tewari AK,
Gupta AK,
Nath PD,
Rabindran R,
Khirbat SK,
Byadgi AS,
Singh G, Boa E
(2009) New plant hosts for group 16SrII, ‘Candidatus Phytoplasma aurantifolia’, in India. Plant Pathology 58, 391.
| Crossref | GoogleScholarGoogle Scholar |

Deng S, Hiruki C
(1991) Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. Journal of Microbiological Methods 14, 53–61.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Ghosh DK,
Das AK,
Shayam S,
Singh SJ,
Ahlawat YS, Singh S
(1999) Occurrence of witches’ broom, a new phytoplasma disease of acid lime (Citrus aurantifolia) in India. Plant Disease 83, 302.
| Crossref | GoogleScholarGoogle Scholar |

Gundersen DE, Lee IM
(1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea 35, 144–151.
|
CAS |

Khan MS, Raj SK
(2006) First report of molecular detection of an Aster yellows phytoplasma isolate infecting chilli (Capsicum annuum) in India. Plant Pathology 55, 822.
| Crossref | GoogleScholarGoogle Scholar |

Khan MS,
Raj SK, Snehi SK
(2007) First report of molecular identification of ‘Candidatus Phytoplasma asteris’ affecting Sesame (Sesamum indicum) cultivation in India. Plant Pathology 89, 291–295.

Marcone C,
Schneider B, Seemüller E
(2004) ‘Candidatus Phytoplasma cynodontis’, the phytoplasma associated with Bermuda grass white leaf disease. International Journal of Systematic and Evolutionary Microbiology 54, 1077–1082.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Raj SK,
Khan MS,
Snehi SK,
Srivastava S, Singh HB
(2006) ‘Candidatus Phytoplasma asteris’ isolate associated with a little leaf disease of pigeon pea in India. Plant Pathology 55, 823.
| Crossref | GoogleScholarGoogle Scholar |

Raj SK,
Snehi SK,
Khan MS, Kumar S
(2008) ‘Candidatus Phytoplasma asteris’ (group 16SrI) associated with a witches’-broom disease of Cannabis sativa in India. Plant Pathology 57, 1173.
| Crossref | GoogleScholarGoogle Scholar |

Raj SK,
Snehi SK,
Kumar S,
Banjerji BK,
Diwedi AK,
Roy RK, Goel AK
(2009) First report of a ‘Candidatus Phytoplasma asteris’(16SrI group) associated with colour – breaking and mall formation of floral spikes of Gladiolus in India. Plant Pathology 58, 1170.
| Crossref | GoogleScholarGoogle Scholar |

Rao GP,
Raj SK,
Snehi SK,
Mall S,
Singh M, Marcone C
(2007) Molecular evidence for the presence of ‘Candidatus Phytoplasma cynodontis’, the Bermuda grass white leaf agent, in India. Bulletin of Insectology 60(2), 145–146.

Rao GP,
Mall S,
Singh M, Marcone C
(2009) First report of a ‘Candidatus Phytoplasma cynodontis’-related strain (group 16SrXIV) associated with white leaf disease of Dichanthium annulatum in India. Australasian Plant Disease Notes 4(1), 56–58.
|
CAS |

Snehi SK,
Khan MS,
Raj SK,
Mall S,
Singh M, Rao GP
(2008) Molecular identification of Candidatus Phytoplasma cynodontis associated with Bermuda grass white leaf disease in India. Plant Pathology 57, 770.
| Crossref | GoogleScholarGoogle Scholar |

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



