First report of powdery mildew on Prunus nepalensis in India
P. Baiswar A B , S. Chandra A , R. K. Patel A and S. V. Ngachan AA ICAR Research Complex for NEH Region, Umiam-793103, Meghalaya, India.
B Corresponding author. Email: pbaiswar@yahoo.com
Australasian Plant Disease Notes 4(1) 131-132 https://doi.org/10.1071/DN09053
Submitted: 3 November 2009 Accepted: 25 November 2009 Published: 14 December 2009
Abstract
Powdery mildew was observed on Prunus nepalensis during July, 2009. This plant is grown as a fruit crop in the north-eastern hilly region of India. Light and scanning electron microscopy revealed the presence of the anamorph of Podosphaera. Conidial germination patterns indicated it belonged to the P. tridactyla complex of species. This is the first report of powdery mildew on P. nepalensis in India.
Prunus nepalensis is a fruit crop grown in the north-eastern hilly region of India (Patel et al. 2008). Powdery mildew infected plants were observed in Barapani, Meghalaya during July 2009. Almost 40% of the plants were infected. Disease symptoms included whitish irregular patches on the lower surface of the leaves with corresponding yellow lesions on the upper surface (Fig. 1). Symptoms were present on young and mature leaves. Heavy infection resulted in severe defoliation. Voucher specimens have been deposited in the herbarium collection of MACS Agharkar Research Institute, Pune and ICAR Research Complex for NEH Region, India (AMH No. 9298, ICARHNEH-92).

|
Light and scanning electron microscopy (SEM) was used for confirmation of the fungus. For light microscopy a strip of clear tape was used to dislodge mycelia and conidia from the infected tissue (Correll et al. 1987). In SEM suitable areas were selected using a dissecting microscope. Leaf areas containing powdery growth were cut into 5-mm pieces and placed on double-sided cellotape then sputter-coated with gold using a fine-coat ion sputter JFC–1100 (JEOL, Tokyo, Japan). Gold-coated samples were then placed on aluminium stubs in a JEOL JSM 6360 SEM (JEOL, Tokyo, Japan). Characters examined were the spore surface pattern, presence or absence of appressoria and type of appressoria.
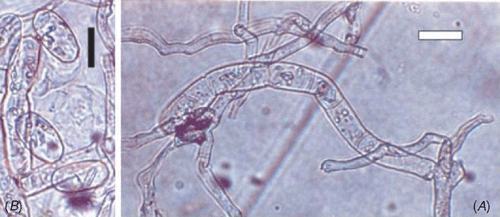
The presence of ectophytic mycelium, conidia in chains, fibrosin bodies and indistinct appressoria indicated Podosphaera and excluded the possibility of Erysiphe, Golovinomyces, Leveillula or Phyllactinia (Braun et al. 2002). SEM studies revealed the presence of smooth wrinkles on the conidial surface and also confirmed the presence of indistinct appressoria (Cook et al. 1997). Conidiophores were mostly erect containing a foot cell (32–46 × 8–11 μm); conidia were ellipsoid-ovoid (27–35 × 14–16 μm) and produced in chains (Fig. 2). The basal septum of the conidiophore was just adjacent to mycelium. Fibrosin bodies were present. Germtubes with simple appressoria were present on the lateral side of the conidia. A perfect stage (chasmothecium) was not found. Podosphaera spp. on Prunus still remain to be resolved using molecular tools since large variations are reported from different species and most of them seem to be paraphyletic (Cunnington et al. 2005). The simple germination pattern indicated that the species belonged to the Podosphaera tridactyla (Wallroth) de Bary complex of species (Cook and Braun 2009). Pathogenicity was confirmed by dusting conidia on healthy plants of P. nepalensis, non-inoculated plants serving as control. Inoculated plants developed symptoms after 8–9 days, whereas control plants remained healthy.
|
To our knowledge, this is the first record of powdery mildew of P. nepalensis caused by Podosphaera sp. in India (Paul and Thakur 2006; Ahmed et al. 2007; Farr and Rossman 2009). This disease is also of regulatory significance since it has not been reported from any other country.
Acknowledgements
Authors would like to thank Head SAIF, Sudeep Dey (Scientific Officer), N. K. Rynjah, R. Charkraborty and D. A. Bareh for scanning electron microscopy (JEOL JSM 6360) at North-eastern Hill University, Shillong, Meghalaya, India.
Cook RTA, Braun U
(2009) Conidial germination patterns in powdery mildews. Mycological Research 113, 616–636.
| Crossref | GoogleScholarGoogle Scholar |

Cook RTA,
Inman AJ, Billings C
(1997) Identification and classification of powdery mildew anamorphs using light and scanning electron microscopy and host range data. Mycological Research 101, 975–1002.
| Crossref | GoogleScholarGoogle Scholar |

Correll JC,
Gordon TR, Elliott VJ
(1987) Host range, specificity and biometrical measurements of Leveillula taurica in California. Plant Disease 71, 248–251.
| Crossref | GoogleScholarGoogle Scholar |

Cunnington JH,
Lawrie AC, Pascoe IG
(2005) Genetic variation within Podosphaera tridactyla reveals a paraphyletic species complex with biological specialization towards specific Prunus subgenera. Mycological Research 109, 357–362.
| Crossref | GoogleScholarGoogle Scholar |
CAS |



