First report of Pyrenochaeta lycopersici, causal agent of tomato corky root rot in Australia
H. GolzarDepartment of Agriculture and Food Western Australia, Bentley Delivery Centre, WA 6983, Australia. Email: hgolzar@agric.wa.gov.au
Australasian Plant Disease Notes 4(1) 126-128 https://doi.org/10.1071/DN09051
Submitted: 28 August 2009 Accepted: 20 November 2009 Published: 2 December 2009
Abstract
Corky root rot, chlorosis of foliage, necrosis of tip branches and poor fruit setting were observed in field and glasshouse tomatoes (Lycopersicon esculentum L.) grown north of Perth during the summer of 2008. Based on morphological characteristics of the isolated fungus, disease symptoms and a pathogenicity test, Pyrenochaeta lycopersici was identified as the causal agent of corky root rot of tomato. Pyrenochaeta lycopersici has not previously been reported on tomato in Australia.
Corky root rot of tomato is an important soil-borne disease of field- and glasshouse-grown tomatoes (Campbell et al. 1982; McGrath and Campbell 1983; Kim et al. 2003).
The causal agent of tomato corky root rot was originally known as the grey sterile fungus (GSF) and later described as P. lycopersici based on the morphological characteristics of the pycnidia, conidiophores and conidia (Schneider and Gerlach 1973). Pyrenochaeta lycopersici attacks the root system of infected plants, and causes rotting of the smaller feeder roots, brown lesions on medium size roots and typical corky lesions on larger roots (Pohronezny and Volin 1997). The disease affects nutrient uptake and leads to damage to the root system (Goodenough and Maw 1973). Yield loss has been reported up to 40–70% (Workneh et al. 1993).
The pathogen survives in the soil as either microsclerotia that are formed on the surface of roots or as mycelium in residues from infected roots. Many genera and species in the Solanaceae and Cucurbitaceae families have been reported to be susceptible to P. lycopersici. Pepper, eggplant, cucumber, melon and some symptomless hosts that occur in rotations with tomato may increase inoculum in soil (Grove and Campbell 1987). There has been no published report, in the scientific literature, of the occurrence of P. lycopersici in Australia, although the Australian Plant Pest Database (Plant Health Australia) includes a record from Rochester in Victoria in 1987.
During the summer of 2008, tomato plants with chlorosis of foliage, lack of vigour, poor fruit setting and necrosis of tip branches were collected from the field-grown and glasshouse-grown tomatoes, 30 km north of Perth.
Roots were carefully washed under running tap water. Symptoms on the root system appeared as rot of the fine and medium-sized roots, brown swollen lesions on the larger roots with typical corky rot on the bark almost cracked along the length of the roots.
The roots and stem base tissues of diseased and asymptomatic plants were surface-sterilised by immersion in a 1.25% aqueous solution of sodium hypochlorite for 1 min, rinsed in sterile water and dried in a laminar flow cabinet. Out of 25 diseased and asymptomatic plants, 250 root and stem base segments with equal numbers of 10 pieces per plant were selected randomly. Segments were separately placed on potato dextrose agar (PDA), then incubated at 22 ± 3°C. Fungal colonies which developed from the root and stem base segments were sub-cultured on water agar for 3–4 days and then sub-cultured on double-strength V8 juice agar (V8A) and incubated at 25°C with a 12-h dark and light cycle (McGrath and Campbell 1983).
In addition, pieces of infected roots were surface-sterilised as described above and placed on moist filter paper trays and incubated at 25°C with a 12-h dark and light cycle.
The growth rate, colony morphology and micro-morphological characteristics of the isolated fungus were determined as described by Schneider and Gerlach (1973). Pyrenochaeta lycopersici was consistently isolated from diseased root and stem base tissues.
Pyrenochaeta lycopersici is a slow-growing fungus and its growth rate on PDA (pH 5.5) was ~1.5 mm per day. The fungus formed microsclerotia and pycnidia on V8A; however, some of the isolates failed to form pycnidia.
Pycnidia were solitary, globose to subglobose, 145–290 μm in diameter, and ornamented with septate setae around the neck. Conidiophores were mostly branched, 15–35 μm long and 3 μm wide and formed on the hyaline cells lining the inside of the pycnidial cavity. Conidia were hyaline, unicellular cylindrical to allantoid and 5–8 × 1.5–2 μm diameter (Fig. 1).
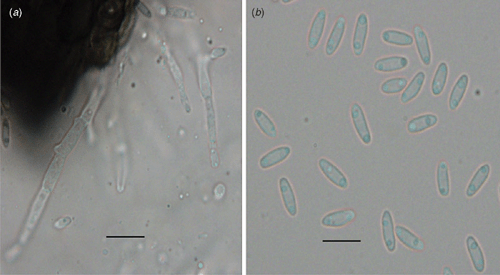
|
In this study both microsclerotia and pycnidia were observed on infected roots in moist trays that had been incubated for 3 weeks. Pycnidia have not previously been found in the natural state on infected tomato (Schneider and Gerlach 1973; Grove and Campbell 1987).
A pathogenicity test was conducted using 4-week-old seedlings of tomato (cv. Swanson) grown in a potting mix consisting of coarse river sand and peat (2 : 1) amended with nitrogen, phosphorus and potassium (NPK) and trace elements in a growth cabinet at 22°C ± 2°C with an 8-h dark and 16-h light cycle. The seedlings were inoculated with two of the fungal isolates by dipping the roots in a spore suspension (106 spores/mL) before planting in the pots. Controls were dipped in tap water. Symptoms on inoculated plants appeared as poor growth, chlorosis and then necrosis of the tip branches, by maturity. Typical symptoms on the infected root were brown areas that became swollen with cracks along the infected tissues consistent with both those that were observed in the field, and those reported for this disease (Fig. 2). Koch’s Postulates were fulfilled by reisloation of P. lycopersici. A culture of P. lycopersici was deposited in the WA Plant Pathogen Collection (WAC 13264).

|
To our knowledge, this is the first published report of corky root rot of tomato in Australia.
Acknowledgements
The author would like to thank Ms Paula Mather for her technical assistance.
Campbell RN,
Schweers VH, Hall DH
(1982) Corky root of tomato in California caused by Pyrenochaeta lycopersici and control by soil fumigation. Plant Disease 66, 657–660.

Goodenough PW, Maw GA
(1973) Effects of Pyrenochaeta lycopersici infection on nutrient uptake by tomato plants. The Annals of Applied Biology 73, 339–347.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Grove GG, Campbell RN
(1987) Host range and survival in soil of Pyrenochaeta lycopersici. Plant Disease 71, 806–809.
| Crossref | GoogleScholarGoogle Scholar |

Kim JT,
Park IH,
Ryu KY,
Cheon JU, Yu SH
(2003) Corky root of tomato caused by Pyrenochaeta lycopersici in Korea. The Plant Pathology Journal 19, 181–183.

McGrath DM, Campbell RN
(1983) Improved methods for inducing sporulation of Pyrenochaeta lycopersici. Plant Disease 67, 1245–1248.
| Crossref | GoogleScholarGoogle Scholar |

Workneh F,
van Bruggen AHC,
Drinkwater LE, Shennan C
(1993) Variables associated with corky root and Phytophthora root rot of tomatoes in organic and conventional farms. Phytopathology 83, 581–589.
| Crossref | GoogleScholarGoogle Scholar |



