First report of gray mould caused by Botrytis cinerea on bleeding-hart (Clerodendron splendens) in Brazil
D. M. Macedo A and R. W. Barreto A BA Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000, Viçosa, Minas Gerais, Brasil.
B Corresponding author. Email: rbarreto@ufv.br
Australasian Plant Disease Notes 4(1) 102-104 https://doi.org/10.1071/DN09043
Submitted: 31 August 2009 Accepted: 10 September 2009 Published: 28 September 2009
Abstract
Botrytis cinerea is recorded for the first time causing gray mold on Clerodendron splendens in Brazil; flower blight and leaf spots were observed as a result of attack by the pathogen.
Additional keywords: Botryotinia fuckeliana, flower blight, leaf spot, ornamental plant.
Introduction
Clerodendron (Verbenaceae) is a genus of shrubs and vines mostly from warm regions of Africa and Asia that includes several species and hybrids which are used as ornamentals. Clerodendron splendens G.Don. is a native of tropical Africa, also popularly known as bleeding-hart, flaming glorybower vine (coração sangrento in Brazil) and is widely cultivated on fences or garden walls for its attractive foliage and bright red flowers (Lorenzi and Souza 2001). Relatively few fungal species have been recorded on species of Clerodendron. Farr et al. (2009) listed only 10 fungal species in association with this genus but none on C. splendens. In Brazil four fungal species have been recorded on species of Clerodendron but none of these records was on C. splendens (EMBRAPA Recursos Genéticos 2009). In July 2009, diseased bleeding-hart plants were observed in a private garden (Gávea, Rio de Janeiro, Brazil). Flowers were necrotic and covered by gray mould. The disease progressed and led to the complete necrosis of inflorescences. Additionally, infected flower parts fell and became attached to adjacent leaves and new fungal colonies developed from these fallen residues, leading to the formation of necrotic spots on leaves (Fig. 1).
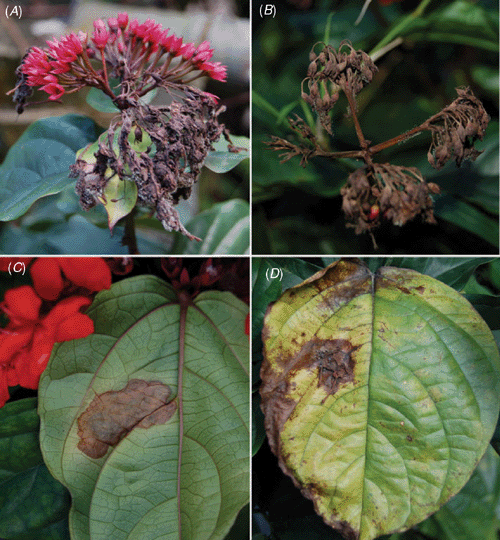
|
Samples of diseased inflorescences and leaves were collected for later observation, dried in a plant press and later deposited in a herbarium (Herbarium of the Universidade Federal de Viçosa) under the accession number Vic. 31212. After observation of the regular presence of fungal structures associated with diseased tissues under a dissecting microscope, microscope slides containing fungal structures scraped from colonised plant parts were mounted in lactophenol and examined with a light microscope (Olympus BX 51) equipped with a digital camera (Olympus evolt E-330). Additionally, conidia were taken from sporulating colonies on infected tissues with the help of a sterile fine-pointed needle and transferred to plates containing vegetable broth agar (VBA) (Pereira et al. 2003). Pure cultures of the fungus were obtained and subcultured onto additional plates containing VBA and left in an incubator for 7 days at 25°C, with a 12-h light regime. Culture discs were taken from those plates and used as inoculum. Three leaves of one 60 cm high healthy plant were inoculated by depositing two culture disks on each. After inoculation plants were left in a humid chamber for 48 h and then transferred to a greenhouse at around 25°C where they were kept and regularly examined for the observation of gray mould symptoms.
The fungus had the typical morphology of the anamorphic stage of Botryotinia fuckeliana (de Bary) Whetzel, Botrytis cinerea Pers. (Ellis and Walker 1974). Lesions on inflorescences led to rapid and complete necrosis of flowers and peduncles that started on portions of inflorescences but spread to the whole inflorescence; plant tissues became brown to grayish brown and covered by intense fungal sporulation; on leaves lesions were circular to irregular straw to pale brown, zonate adaxially, 1.0–4.0 × 1.5–5.0 cm. Internal mycelium intercellular, 3.0–5.0 μm diam., branched, septate, pale brown. Conidiophores isolate, branched and bearing inflated, ampulliform conidiogenous cells at the apices, up to 2 mm long, 12.0–19.0 μm diam., 10–12 septate, dark brown to black, smooth. Conidia ellipsoid to obovoid, formed in groups on the surface of conidiogenous cells, 8.0–17.0 × 6.0–8.0 μm, often with a protruding hillum, 1.0–3.0 μm wide, aseptate, subhyaline, smooth (Fig. 2).
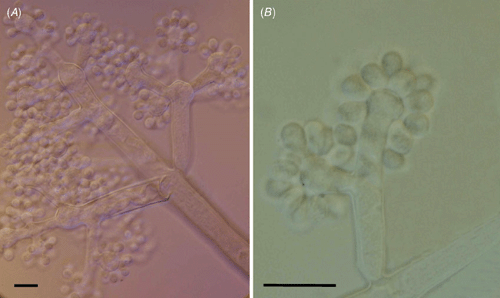
|
Inoculation of healthy leaves led to the emergence of typical gray mould symptoms 2 days after inoculation. Non-inoculated leaves remained healthy. Pathogenicity of B. cinerea to C. splendens was hence demonstrated. It is not uncommon for infected flower parts to serve as an inoculum source for foliage infection in gray mould, as described here (Staats et al. 2005). Botrytis cinerea is one of the fungal pathogens having the widest host range known to plant pathologists (Ngugi and Scherm 2006). According to Ellis and Walker (1974) this fungus is known to be associated with over a thousand host-species. In Brazil more than a hundred hosts for this fungus have been reported, including many important ornamentals (EMBRAPA Recursos Genéticos 2009). This is the first record of B. cinerea as a pathogen of a member of the genus Clerodendron worldwide.
Ellis MB, Walker JM
(1974) Sclerotinia fuckelina (conidial state: Botrytis cinerea). CMI. Descriptions of pathogenic fungi and bacteria. 431, 1–2.
[Verified 13 August 2009].
Ngugi HK, Scherm H
(2006) Biology of flower-infecting fungi. Annual Review of Phytopathology 44, 261–282.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Pereira JM,
Barreto RW,
Ellison CA, Maffia LA
(2003) Corynespora cassiicola f. sp. lantanae: a potential biocontrol agent from Brazil for Lantana camara. Biological Control 26, 21–31.
| Crossref | GoogleScholarGoogle Scholar |

Staats M,
van Baarlen P, van Kan JAL
(2005) Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Molecular Biology and Evolution 22, 333–346.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



