First record of Itersonilia perplexans on Anethum graveolens (dill) in Australia
R. Aldaoud A B , S. Salib A and J. H. Cunnington AA Biosciences Research Division, Department of Primary Industries, Knoxfield, Private Bag 15, Ferntree Gully Delivery Centre, Vic. 3156, Australia.
B Corresponding author. Email: ramez.aldaoud@dpi.vic.gov.au
Australasian Plant Disease Notes 4(1) 60-61 https://doi.org/10.1071/DN09025
Submitted: 12 May 2009 Accepted: 25 May 2009 Published: 15 June 2009
Abstract
Itersonilia perplexans was found for the first time on hydroponically grown potted plants of dill (Anethum graveolens) in Victoria. All affected plants were destroyed.
Itersonilia perplexans is reported to cause disease in several plants in the families Apiaceae, Compositae and others (Channon 1963). Symptoms may include leaf blight, petal blight and root canker. In Australia, I. perplexans is commonly recorded on parsnip (herbarium ref. no’s.: DPI NSW Plant Pathology Herbarium (DAR) 6523; DPI Victoria Plant Pathology Herbarium (VPRI) 32440; and 40636), and has also been recorded on gerbera (VPRI 17542), endive (VPRI 17984), cineraria (VPRI 18267), coriander (VPRI 18403), umbrella tree (DAR 5016) and rananculus (DAR 24806 and 43046).
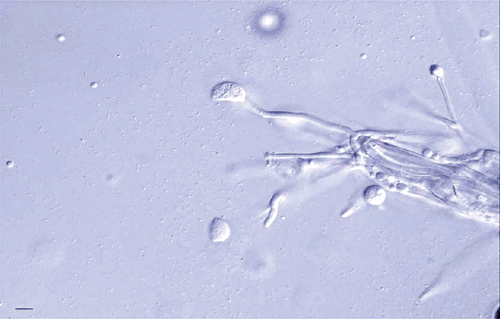
In August 2008, a sample of hydroponically grown dill (Anethum graveolens) plants from Victoria, Australia, was sent to Crop Health Services at the Victorian Department of Primary Industries for diagnosis of pathogens associated with a leaf blight problem (Fig. 1). A basidiomycete fungus, with the morphological characteristics of ballistospores (Fig. 2) and clamp connections that closely fit with those of I. perplexans (Boekhout 1991), was detected on the blight-affected leaves by direct microscopy. Fungal isolation was undertaken using specimens of affected leaf tissues. Specimens were surface-sterilised for 1 min in 0.5% NaOCl solution, rinsed with sterile distilled water, blot-dried on sterile tissue, and placed on tetracycline-amended, 50 mg/L potato dextrose agar (PDAT) plates. Plates were incubated in a cabinet under continuous fluorescent light, at temperatures ranging between 20–24°C, and were examined for fungal growth 2 weeks later. Itersonilia perplexans was consistently found on the PDAT culture plates.

|
|
Pathogenicity testing was undertaken according to a previously described method (Sowell and Korf 1960), where a suspension of ballistospores was prepared and spray-inoculated onto potted dill plants. Sterile water was sprayed on another set of plants as non-inoculated control. Inoculated and control plants were enclosed in plastic bags and kept in the laboratory under constant fluorescent light for 24 h, with temperatures ranging from 17–22°C. After 24 h, plastic bags were removed, and plants were observed for symptom development at weekly intervals over 3 weeks. One week after inoculation, plants developed moderate to severe symptoms of leaf blight (Fig. 3), similar to those observed on the submitted sample. Symptoms became progressively more severe over time, and most of the foliage had completely died by the third week. The control plants remained asymptomatic. Microscopic examination of blight-affected leaves of the artificially inoculated plants consistently detected I. perplexans. The presence of I. perplexans on blight-affected leaves was further confirmed by fungal isolation on PDAT. This confirms I. perplexans as a cause of the leaf blight symptom observed on the submitted dill sample. Infection of dill plants by I. perplexans has been previously reported in California (Koike and Tjosvold 2001), but to our knowledge this is the first record of this fungus on dill plants in Australia. This may have potential implications where dill is grown in rotation with or in close proximity to parsnip crops, which suffer serious losses from this disease in Australia (Smith 1966). A pure culture of the I. perplexans fungus detected here has been deposited with the fungal culture reference collections (herbarium ref no: VPRI 41476) at the Victorian Department of Primary Industries – Knoxfield.

|
Boekhout T
(1991) Systematics of Itersonilia: a comparative phenetic study. Mycological Research 95, 135–146.
| Crossref | GoogleScholarGoogle Scholar |

Channon AG
(1963) Studies on parsnip canker. I. The causes of the diseases. The Annals of Applied Biology 51, 1–15.
| Crossref | GoogleScholarGoogle Scholar |

Koike ST, Tjosvold SA
(2001) A Blight disease of dill in California caused by Itersonilia perplexans. Plant Disease 85, 802.
| Crossref | GoogleScholarGoogle Scholar |

Smith PR
(1966) Seed transmission of Itersonilia pastinacae in parsnip and its elimination by a steam-air treatment. Australian Journal of Experimental Agriculture and Animal Husbandry 6, 441–444.
| Crossref | GoogleScholarGoogle Scholar |

Sowell G, Korf RP
(1960) An emendation of the genus Itersonilia based on studies of morphology and pathogenicity. Mycologia 52, 934–945.
| Crossref | GoogleScholarGoogle Scholar |



