Neonectria fuckeliana is pathogenic to Pinus radiata in New Zealand
M. A. Dick A C and P. E. Crane BA Scion, New Zealand Forest Research Institute Ltd, Private Bag 3020, Rotorua, New Zealand 3046.
B Edmonton, Alberta, Canada T5R 3X1.
C Corresponding author. Email: margaret.dick@scionresearch.com
Australasian Plant Disease Notes 4(1) 12-14 https://doi.org/10.1071/DN09005
Submitted: 6 October 2008 Accepted: 23 December 2008 Published: 16 February 2009
Abstract
Stem malformation associated with stained sapwood, typically developing after pruning, has become a problem in some Pinus radiata (Monterey pine) plantations in southern regions of New Zealand. Neonectria fuckeliana (syn. Nectria fuckeliana) is the most commonly isolated fungus from affected trees. In order to critically assess the pathogenicity of this fungus, Koch’s Postulates were conducted under controlled conditions in the glasshouse and under field conditions in the forest plantation. The pathogen was recovered from diseased tissue; however, there were marked differences in individual tree response to infection as some inoculated trees did not become diseased.
Pinus radiata comprises 90% of the 1.8 million hectares of plantation forests (which cover 7% of the land area) in New Zealand. Pruning within the first 8 years is widely undertaken to produce clear-wood within the short growing cycle of 25–30 years. Any degrading of the butt log may cause relegation from a high return end-use to a lower value product or it may become uneconomic to harvest. The most common agent of degradation has been Diplodia pinea (syn. Sphaeropsis sapinea), which can infect stems through branch stubs after pruning if pruning is conducted while inoculum is present. Diplodia whorl canker symptoms range from minor sapstain confined to the knotty core, through extensive stem cankering (fluting) to wilt and death of the entire crown. Stands in the warmer, drier parts of the country are particularly prone to this disease (Chou and MacKenzie 1988).
In the mid-1990s an increasing incidence of stem malformation after pruning was reported in some Otago and Southland plantations (southern areas of the South Island) which had historically been largely free of Diplodia whorl canker. The percentage of affected trees within stands ranged from 5% to 45%, with symptoms varying from short indentations to long cankers extending several metres. External symptoms were very similar to Diplodia whorl canker, although tree death was rare and only associated with stem breakage at infected whorls. Unlike sapwood infected by D. pinea, which is typically dark blue, the sapwood was discoloured light grey or brown. Culturing indicated that Neonectria fuckeliana was the primary coloniser of this sapwood. This was the first known occurrence of this northern hemisphere fungus in New Zealand (Gadgil et al. 2003).
Neonectria fuckeliana is recorded in the northern hemisphere primarily as a wound invader of conifers. In Europe and Scandinavia it is one of the most common inhabitants of wounded Norway spruce (Picea abies) stems, occurring in both stained and unstained wood (Roll-Hansen 1962; Roll-Hansen and Roll-Hansen 1979; Huse 1981; Vasiliauskas and Stenlid 1998). Other Picea spp. as well as Abies spp. are common hosts in Scandinavia. In North America, N. fuckeliana is known to cause stem cankers of drought-stressed Abies in California (Schultz and Parmeter 1990) and is associated with dieback of Abies and Picea in Quebec (Smerlis 1969). The ability of the fungus to act as a pathogen of several conifer species has been demonstrated in pathogenicity tests (Smerlis 1969). Smerlis (1969) inoculated 3- to 6-year-old branches and stems of 13 species, including five Pinus spp. Neonectria fuckeliana was found to be pathogenic to all conifers tested, but not to non-conifers that were included as controls. The pine species and the percentage of inoculated trees that developed cankers were P. banksiana (60%), P. contorta var. latifolia (80%), P. resinosa (80%), P. strobus (30%) and P. sylvestris (70%).
Neonectria fuckeliana produces characteristic red perithecia that contain the ascospores of the teleomorph and it also has two anamorph stages. The Acremonium anamorph is produced in culture and on the surface of cut, infected wood under conditions of very high humidity. Sporodochia of the synanamorph Cylindrocarpon cylindroides var. tenue are sometimes found associated with perithecia on the dead bark of flutes, but this stage is rare. Ascospores are likely to be the most important in spread of the fungus in nature (Dick et al. unpublished; Vasiliauskas and Stenlid 1997).
To determine whether N. fuckeliana could be the causal agent of the fluting disease of P. radiata in the South Island, inoculations were carried out on 4-year-old cuttings in a glasshouse (in 2003) and 6-year-old trees in a forest plantation (2005). In the glasshouse, a small flap of bark and cambium (10 × 5 mm) was peeled from a branch internode of each of 11 cuttings to expose the sapwood. A 10 × 5 mm section of 2% malt extract agar (MEA) colonised by the Acremonium anamorph (NZFS 982, isolated from a P. radiata stem canker in Otago in 2002) was placed mycelium-side down on the exposed cambium. The bark was replaced and the inoculation point was then moistened with water and wrapped with parafilm to seal the inoculation site. The control was conducted by wounding a different branch of the same tree followed by inoculation with sterile MEA. There was no evidence of infection 3 months after inoculation. After 4 months, sunken discoloured bark above and below the inoculation point was recorded on 6 of the 11 trees. Beads of resin exudate were associated with the lesions. The inoculation wounds on the remaining five trees had healed, as had all of the wounds that were treated with the sterile media control. Trees with lesions were harvested 8 months after inoculation. Lesions ranged from 20 to 92 mm in length and averaged 68 mm. Neonectria fuckeliana, identity confirmed by morphological and colony characteristics, was reisolated from the full length of the lesions on all symptomatic trees but not from the healed control wounds on these trees. The five trees on which the disease did not develop were harvested at 28 months. The wounds made during inoculation, and the control wounds, were still just visible within the wood but there was no discolouration or cankering in the sapwood beyond the initial inoculation point. For all these trees wood chips from around the wound and at 2, 4, 6, 8 and 10 cm distances above and below the inoculation point were placed on agar plates. Neonectria fuckeliana was not recovered from any of these trees.
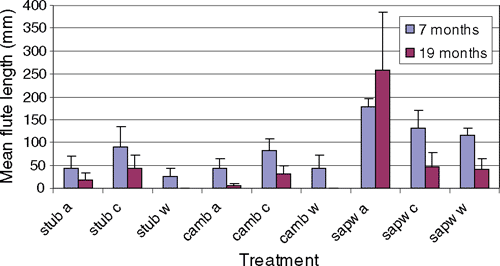
A second inoculation trial was initiated in late April (autumn) in Flagstaff Forest, near Dunedin, New Zealand, to compare infections produced by N. fuckeliana ascospores and the conidia of the Acremonium anamorph. Forty-five 6-year-old P. radiata were inoculated with conidia, ascospores or water (control). The inoculum was applied to either: (i) a surface wound, where a 10-mm diameter piece of bark was removed to expose the cambium; (ii) a 10-mm-deep wound into sapwood; or (iii) a hole drilled into the stub of a recently pruned branch. There were 15 replicates of each wound type, and of these, five trees were inoculated with ascospores, five with Acremonium conidia and five with sterile water. Two hundred microlitres of liquid inoculum was delivered into each wound using a pipetter. Perithecia were crushed and ascospores released into water immediately before inoculation and their concentration was therefore unknown. Conidial inoculum (of NZFS 982) was prepared in shake culture (0.5% malt extract for 7 days at ambient temperature) and concentration adjusted with sterile distilled water to 104 viable spores/mL (2000 spores per inoculation site). Germinability of the conidial inoculum was verified in the laboratory before field inoculation. Viability of the ascospore inoculum was not tested but laboratory tests of similar material on several other occasions has consistently shown germination levels between 50 and 90 percent. Disease was assessed by measuring the length of flutes formed on the stem 7 and 19 months after inoculation. At 24 months, all trees were felled, and isolations made from the cambium and the sapwood in the manner described for the glasshouse tests.
After 7 months, small depressions were recorded on the stems of at least two trees from each of the nine treatments, including the water controls. Depressions observed in the sapwood wound treatments (e.g. Fig. 1) were longer and deeper than the inoculations applied to the cambium or stub wounds. After 19 months, mean flute length had decreased for all treatments with the exception of the ascospore inoculation of the sapwood (Fig. 2). Flutes were no longer visible in almost all trees in the cambium or stub control treatments. It was apparent that in these trees the cankers were not active and that further cambial death had not occurred. Reduction in size of the flutes was the result of the rapid diameter growth, which is characteristic of P. radiata in the region, and thus the gradual occlusion of the affected area. At 24 months, there was no evidence of disease caused by any of the inoculations to the cambium, and only one tree inoculated through a hole drilled in a branch stub had a flute present. Three of the five trees that were inoculated at the sapwood with ascospores still had visible flutes, and one of these had an extensive canker with clusters of perithecia on the surface. Neonectria fuckeliana was reisolated from the margins of discoloured sapwood from all stem cankers. Differences in host response to inoculation with either ascospores or conidia need to be explored further as the level of replication and the application of unknown concentrations of ascospores in this study do not permit conclusions to be drawn about comparative infectivity of these different spore types.

|
|
We interpreted the small depressions that were initially recorded in the water control treatments of the stub and cambium as a host response to wounding. Discoloured or dead sapwood was not associated with these flutes.
These results demonstrated that N. fuckeliana is capable of causing flute cankers of P. radiata, albeit in a limited proportion of trees. Field observations of disease development in other inoculated trees have revealed a similar range of individual tree response to that found in the pathogenicity tests reported here. In a mixed genotype P. radiata stand, active persistent cankers formed on only a small proportion of the trees that exhibit flutes following infection (unpubl. data). These trees develop seriously malformed stems and will be culled when the stand is felled. The remainder appear to contain the infection, and the flute will gradually occlude over the following two to five growing seasons. The end result of these ‘contained’ infections is likely to be a merchantable log, albeit with a larger than normal defect core. The effect of weather conditions, wound type, and host genotype on the formation of persistent damaging stem cankers is currently under further investigation.
Chou CKC, MacKenzie M
(1988) Effect of pruning intensity and season on Diplodia pinea infection of Pinus radiata stems through pruning wounds. European Journal of Forest Pathology 18, 437–444.
| Crossref | GoogleScholarGoogle Scholar |

Gadgil PD,
Dick MA, Dobbie K
(2003) Fungi Silvicolae Novaezelandiae: 4. New Zealand Journal of Forestry Science 33, 265–272.

Huse KJ
(1981) The distribution of fungi in sound-looking stems of Picea abies in Norway. European Journal of Forest Pathology 11, 1–6.
| Crossref | GoogleScholarGoogle Scholar |

Roll-Hansen F
(1962)
Nectria cucurbitula sensu Wollenweber, its Cephalosporium state, and some other Cephalosporium species from stems of conifers. Meddelelser Norske Skogforsoksv. 17(61), 289–312.

Roll-Hansen F, Roll-Hansen H
(1979) Microflora of sound-looking wood in Picea abies stems. European Journal of Forest Pathology 9, 308–316.
| Crossref | GoogleScholarGoogle Scholar |

Schultz ME, Parmeter JR
(1990) A canker disease of Abies concolor caused by Nectria fuckeliana. Plant Disease 74, 178–180.
| Crossref | GoogleScholarGoogle Scholar |

Smerlis E
(1969) Pathogenicity tests of four Pyrenomycetes in Quebec. Plant Disease Reporter 53, 979–981.

Vasiliauskas R, Stenlid J
(1997) Population structure and genetic variation in Nectria fuckeliana. Canadian Journal of Botany 75, 1707–1713.
| Crossref | GoogleScholarGoogle Scholar |

Vasiliauskas R, Stenlid J
(1998) Fungi inhabiting stems of Picea abies in a managed stand in Lithuania. Forest Ecology and Management 109, 119–126.
| Crossref | GoogleScholarGoogle Scholar |



