First report of Neofusicoccum australe [Botryosphaeria australis], a cause of grapevine dieback in New Zealand
N. T. Amponsah A B , E. E. Jones A , H. J. Ridgway A and M. V. Jaspers AA Lincoln University, Faculty of Agriculture and Life Sciences, PO Box 84, Lincoln University, Canterbury, New Zealand.
B Corresponding author: Nicholas.Amponsah@lincoln.ac.nz
Australasian Plant Disease Notes 4(1) 6-8 https://doi.org/10.1071/DN09003
Submitted: 21 June 2008 Accepted: 22 January 2009 Published: 9 February 2009
Abstract
This is the first report of Neofusicoccum australe isolated from grapevine (Vitis vinifera) and broom (Cytisus scoparius) in New Zealand. Neofusicoccum australe was identified by morphological characteristics and sequence analysis of the rRNA. Koch’s postulates were confirmed by inoculation of wounded, detached green shoots of pinot noir grapevine, on which N. australe caused dark brown lesions within 10 days.
Grapes for wine production are an economically important crop in New Zealand. However, most vineyards have been affected by grapevine trunk diseases in recent years (Anonymous 2006). These diseases are caused by fungi, including several species in the Botryosphaeriaceae family, Eutypa lata and other Diatrypaceae spp., Phaeomoniella chlamydospora, and Phaeoacremonium spp. Species in the Botryosphaeriaceae have recently gained recognition as major pathogens of grapevines worldwide (Taylor et al. 2005), being cosmopolitan and having wide geographical distribution. They can be saprophytes and endophytes on a wide range of woody angiosperm and gymnosperm hosts (Barr 1972; von Arx 1987; Denman et al. 2000). To determine their significance in New Zealand vineyards, a small survey was carried out to establish the prevalence and identity of species in the Botryosphaeriaceae that are associated with dieback and trunk disease in grapevines (Vitis vinifera) and other woody hosts that commonly surround vineyards.
Samples of shoot dieback and diseased trunks of grapevines and other hosts were collected in November 2006 and May 2007 from 20 selected vineyards in Canterbury, Marlborough, Nelson, Gisborne and Auckland regions. Symptomatic tissues from dieback, cankers and wedge-shaped brown stains in wood or brown necrotic lesions on green shoots were surface-sterilised as described by Amponsah et al. (2007) and the tissues on the margins of the lesions were removed for isolation onto half strength potato dextrose agar (PDA). After incubation at room temperature for 3 days, all fast-growing fungal colonies characteristic of Botryosphaeriaceae were subcultured onto PDA and incubated at 24°C in continuous darkness for 3 days to allow observation of early cultural characteristics. In all, a total of 46 isolates obtained from grapevines and 10 from non-grapevine woody hosts appeared to belong to the Botryosphaeriaceae. Based on initial conidial morphology and mycelial growth characteristics, four groups were identified as being Neofusicoccum luteum [Botryosphaeria lutea] (presence of yellow pigmentation in cultures at 3 days), Neofusicoccum parvum, [Botryosphaeria parva] (similar growth characteristics but with no yellow pigmentation at 3 days), Diplodia mutila [Botryosphaeria stevensii] (dark olive-brown colonies with dense aerial mycelium) and Diplodia seriata [Botryosphaeria obtusa] (greyish-brown colonies with dense aerial mycelium) according to published descriptions (Phillips 2004).
DNA sequence analysis was conducted for representative isolates of all species except D. seriata, whose characteristic conidial morphology was sufficient for identification. The species confirmation with molecular techniques was based on amplification of the internally transcribed spacer region (ITS1, 5.8S and ITS2) of the rRNA and the amplified polymerase chain reaction (PCR) fragment was sequenced directly as described by White et al. (1990), using primer ITS4. Six of the isolates (J-3, Kat-1, Kat-2, Mel-1, Mel-2 and Mel-4) initially identified as N. luteum based on their cultural characteristics on PDA (yellow pigmentation) were thereby re-identified as N. australe because their sequences were 100% identical to the N. australe sequences deposited by Slippers et al. (2004). Sequences from these New Zealand isolates were 99% identical to N. luteum reference cultures (STE-U 4592, STE-U 3085, UCD2118Te) and showed four polymorphisms to two South African isolates (AY343416, AF452554), a USA isolate (DQ233609) and to the ex-type culture, CBS110299, (from AY259091). The New Zealand N. australe sequences have been deposited in GenBank under accession numbers FJ157187 to FJ157192. A representative culture has also been deposited with Landcare Research, New Zealand and assigned to International Collection of Microorganisms from Plants (ICMP) 17597.
The N. australe conidia obtained from grapevine shoots were smooth, unicellular, hyaline and with granular contents. A septum was rarely formed before germination. The average length of the N. australe isolates (27.0 ± 0.9 µm) was greater than those of N. luteum (23.1 ± 0.6 µm) (Fig. 1). Length/width ratio was also slightly greater (3.9 ± 0.2 vs. 3.6 ± 0.4 µm), although substantially less than the 4.8 previously reported for N. australe (Slippers et al. 2004).
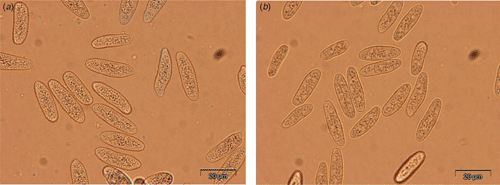
|
The N. australe isolates were from the Canterbury and Nelson regions, five from grapevines showing dieback, reduced vigour and dark-brown necrotic trunk lesions, and one from broom (Cytisus scoparius). Pathogenicity tests were done on detached, green grapevine shoots of cultivar Pinot noir. Mycelium discs from agar cultures or conidia (104/mL) from two N. australe grapevine isolates (Mel-2 and Kat-1) and one isolate from broom (J-3) were inoculated onto wounds created by scratching the surface of the shoot with a sterile scalpel. The bases of the inoculated shoots were placed in Universal bottles containing sterile distilled water and maintained in an enclosed transparent chamber at room temperature (15–23°C), with frequent misting for the first 3 days. Symptom expression began after 5 days, and by day 10 mean lesion length had exceeded 52 mm. The symptoms that developed were dark-brown, dull elliptical lesions (Fig. 2), similar to those observed on green shoots in the field.

|
Inoculation with conidia or plugs cut from actively growing mycelia onto transversely cut ends of 13-month-old canes showed characteristic dark-brown lesions extending across the bark and pith after 3 weeks (Fig. 3). The control shoots, inoculated with sterile PDA plugs, remained healthy. The inoculated fungus was successfully reisolated from all infected parts.
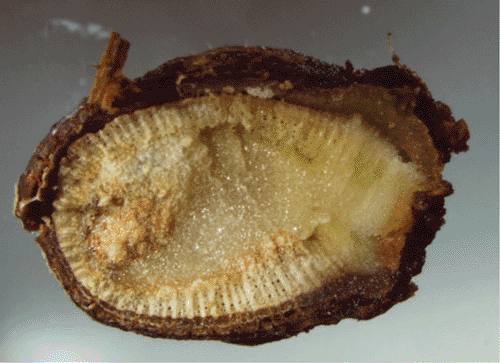
|
The grapevine pathogen, N. australe, recently identified in New Zealand caused many of the same characteristic symptoms described in previous reports, including the wedge-shaped internal lesions in trunk and arms (Taylor et al. 2005). This species appears to be native to the southern hemisphere (Slippers et al. 2004). Its presence in broom is of interest as this is a common weed on uncultivated waste land in New Zealand.
Acknowledgements
The authors are grateful to New Zealand Winegrowers Inc. for providing funding for this work and also to the individual winegrowers who allowed sampling in their vineyards.
Barr ME
(1972) Preliminary studies on the Dothideales in temperate North America. Contributions From the University of Michigan Herbarium 9, 523–638.
(Accessed 20/04/2007).
Slippers B,
Fourie G,
Crous PW,
Coutinho TA,
Wingfield BD, Wingfield MJ
(2004) Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 96(5), 1030–1104.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Taylor A,
Hardy GEStJ,
Wood P, Burgess T
(2005) Identification and pathogenicity of Botryosphaeria species associated with grapevine decline in Western Australia. Australasian Plant Pathology 34, 187–195.
| Crossref | GoogleScholarGoogle Scholar |

von Arx JA
(1987) Plant pathogenic fungi. Nova Hedwigia. Beiheft 87, 1–288.



