First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia
J. D. Ray A D , T. Burgess B and V. M. Lanoiselet CA Australian Quarantine and Inspection Service, NAQS/OSP, 1 Pederson Road, Marrara, NT 0812, Australia.
B School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, WA 6150, Australia.
C Department of Agriculture and Food, Baron-Hay Court, South Perth, WA 6151, Australia.
D Corresponding author. Email: jane.ray@aqis.gov.au
Australasian Plant Disease Notes 5(1) 48-50 https://doi.org/10.1071/DN10018
Submitted: 12 January 2010 Accepted: 10 May 2010 Published: 27 May 2010
Abstract
Neoscytalidium dimidiatum is reported for the first time in Australia associated with dieback of mango and common fig. Neoscytalidium novaehollandiae is reported for the first time associated with dieback of mango.
Neoscytalidium dimidiatum has a wide geographical and host range. For example, it has been reported on Albizia lebbeck, Delonix regia, Ficus carica, Ficus spp., Peltophorum petrocarpum and Thespesia populena in Oman (Elshafie and Ba-Omar 2001); on Arbutus, Castanea, Citrus, Ficus, Juglans, Musa, Populus, Prunus, Rhus, Sequoiadendron in the USA (Farr et al. 1989); and on Mangifera indica in Niger (Reckhaus 1987). Stress factors such as water stress enhance the severity of disease caused by this fungus and symptoms include branch wilt, dieback, canker, gummosis and tree death (Punithalingam and Waterson 1970; Reckhaus 1987; Elshafie and Ba-Omar 2001). Neoscytalidium novaehollandiae was recently described from north-western Australia as an endophyte of Adansonia gibbosa, Acacia synchronica, Crotalaria medicaginea and Grevillia agrifolia (Pavlic et al. 2008).
A joint Plant Health Survey was carried out by the Australian Quarantine and Inspection Service (AQIS) and the Department of Agriculture and Food Western Australia (DAFWA) in the Ord River Irrigation Area (ORIA) of Western Australia (WA) during August 2008. During this survey a trial planting of >50 common fig shrubs (Ficus carica) and several blocks of commercial mango trees (Mangifera indica) attracted the attention of the plant pathologist. The fig plants were showing symptoms of dieback, root rot, canker and tree decline, whereas the mango trees were showing symptoms of dieback and canker only (Fig. 1). Several fungi were subsequently isolated from a representative symptomatic stem from each of nine different mango trees and the root and stem of a single representative symptomatic fig shrub. Neoscytalidium spp. were isolated from 78% (7/9) of mango trees exhibiting dieback symptoms during this survey. Neoscytalidium spp. were not detected in three asymptomatic mango stems. Neoscytalidium spp. was also isolated from the roots and stems of a single representative fig shrub exhibiting symptoms of dieback.

|
The Neoscytalidium spp. isolates were further identified as Neoscytalidium dimidiatum (Syn: Fusicoccum dimidiatum, Torula dimidiata, Scytalidium dimidiatum, Hendersonula toruloidea) (Crous et al. 2006) and N. novaehollandiae by sequencing of part of the internal transcribed spacer (ITS) region of the rDNA operon and part of the elongation factor 1α gene (EF-1α) as described by Burgess et al. (2005) followed by a phylogenetic analysis where sequences obtained in this study were compared with those of known isolates (Fig. 2). Neoscytalidium dimidiatum was isolated from fig and mango stems while N. novaehollandiae was only isolated from mango stems.
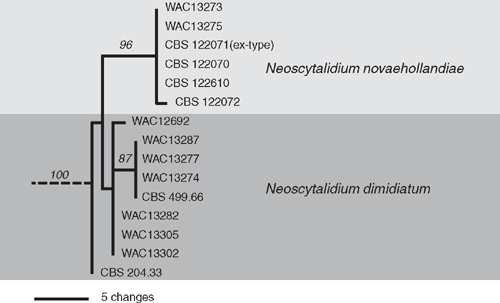
|
During subsequent AQIS Plant Health Surveys, N. dimidiatum and N. novaehollandiae were again isolated from symptomatic mango branches sampled at Derby, WA in September 2008. Neoscytalidium dimidiatum was also isolated from symptomatic mango branches sampled at Broome in September 2008 and Bathurst Island, Northern Territory in October 2008. All cultures have been deposited at the West Australian Culture Collection (Table 1).
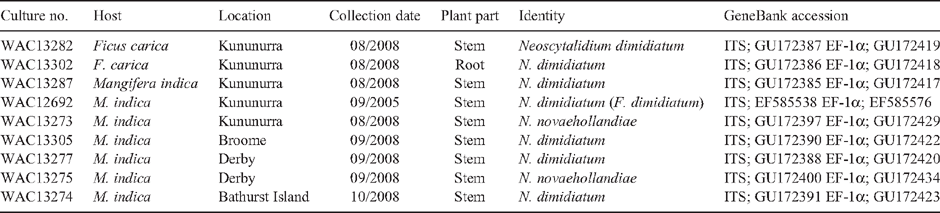
|
Excised mango stems were inoculated to prove isolate pathogenicity and fulfil Koch’s postulates. Field-collected mango stems were ~25 cm long, the ends were dipped in wax and assigned randomly to replicates. Eleven replicates of representative isolates of N. novaehollandiae and N. dimidiatum were under-bark inoculated. Under-bark inoculation was performed by using a sterile scalpel to cut a flap into the bark layer, an agar plug colonised by mycelia was placed under the flap; mycelium faced toward the stem. The control with 16 replicates was non-colonised agar. The inoculation site was wrapped with parafilm and replicates of inoculated stems were placed in plastic bags and incubated at ~22°C. After 14 days, lesions were measured. Both N. novaehollandiae caused lesions and N. dimidiatum caused lesions (27.4 ± 3.5 mm and 26.2 ± 5.2 mm, respectively) on the excised mango stems in comparison to the control (5.8 ± 3.2 mm). Koch’s postulates were tested by re-isolation from the inoculated material.
Re-examination of isolates collected during previous surveys of the ORIA in 2005 from symptomatic mango trees revealed that N. dimidiatum has been present in the region for some time. These isolates were previously identified as Fusicoccum dimidiatum. Similarly, records from the Australian Plant Pest Database (APPD 2010) suggest that the pathogen may have been present in other parts of Australia for a long time. Indeed, the APPD records include a report of Torula dimidiata found on Citrus aurantium in 1914 and several reports of Hendersonula sp. from various hosts including Mangifera indica and Citrus limon. Molecular diagnostics are recommended to confirm the identity of these isolates from Queensland and other parts of Australia to conclusively determine if the pathogen is widespread in Australia.
The extreme climate of the north-west regions of WA and site stress factors at Bathurst Island, NT, may contribute to the susceptibility of common fig and mango to the dieback disease associated with Neoscytalidium. This is the first report of N. novaehollandiae associated with mango dieback. This is also the first report of N. dimidiatum associated with dieback of mango and common fig in Australia.
Acknowledgements
We would like to thank Monique Sakalidis for assistance with pathogenicity testing at Murdoch University.
Burgess TI,
Barber PA, Hardy GESJ
(2005)
Botryosphaeria spp. associated with eucalypts in Western Australia including description of Fusicoccum macroclavatum sp. nov. Australasian Plant Pathology 34, 557–567.
| Crossref | GoogleScholarGoogle Scholar |

Crous PW,
Slippers B,
Wingfield MJ,
Rheeder J,
Marasas WFO,
Philips AJL,
Alves A,
Burgess T,
Barber P, Groenewald JZ
(2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55, 235–253.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Elshafie AE, Ba-Omar T
(2001) First report of Albizia lebbeck dieback caused by Scytalidium dimidiatum in Oman. Mycopathologia 154, 37–40.
| Crossref | GoogleScholarGoogle Scholar |

Pavlic D,
Wingfield MJ,
Barber P,
Slippers B,
Hardy GESJ, Burgess TI
(2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100, 851–866.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Punithalingam E, Waterson JM
(1970) Hendersonula toruloidea. CMI Descriptions of Pathogenic Fungi and Bacteria 28, 274.
| Crossref |

Reckhaus P
(1987) Hendersonula Dieback of Mango in Niger. Plant Disease 71, 1045.
| Crossref | GoogleScholarGoogle Scholar |



