Nandina domestica, a new host of Apple stem grooving virus and Alternanthera mosaic virus
J. Tang A C , J. D. Olson B , F. M. Ochoa-Corona B and G. R. G. Clover AA Plant Health and Environment Laboratory, MAF Biosecurity New Zealand, PO Box 2095, Auckland 1140, New Zealand.
B Oklahoma State University, Department of Entomology and Plant Pathology-PDIDL, NIMFFAB, 127 NRC, Stillwater, OK 74078, USA.
C Corresponding author. Email: joe.tang@maf.govt.nz
Australasian Plant Disease Notes 5(1) 25-27 https://doi.org/10.1071/DN10010
Submitted: 15 October 2009 Accepted: 22 February 2010 Published: 22 March 2010
Abstract
An ornamental plant, Nandina domestica, has been found to be infected for the first time with Apple stem grooving virus (ASGV) in New Zealand and Alternanthera mosaic virus (AltMV) in Oklahoma, USA. ASGV may infect N. domestica latently and the symptoms observed in New Zealand are thought to be induced by the co-infecting Cucumber mosaic virus (CMV). Similarly, Plantago asiatica mosaic virus (PlAMV, syn. Nandina mosaic virus) was found to co-infect with AltMV in the USA, and therefore the observed symptoms may be induced by AltMV and/or PlAMV.
Nandina domestica (heavenly bamboo) is an ornamental shrub and is widely grown as a landscaping plant in many countries including New Zealand and the USA. This species has previously been found to be infected with Cucumber mosaic virus (CMV) in the USA (Barnett and Baxter 1973) and New Zealand (unpublished results), as well as Plantago asiatica mosaic virus (PlAMV, syn. Nandina mosaic virus) and Nandina stem pitting virus (NSPV, a tentative species) in the USA (Ahmed et al. 1983; Hughes et al. 2005).
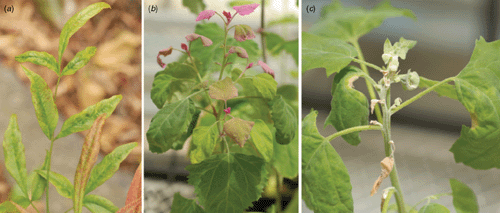
In April 2004, a variety of symptoms including leaf mosaic and rolling (Fig. 1a) were observed on a clump of N. domestica plants in a public garden in Auckland, New Zealand. Chenopodium amaranticolor and C. quinoa inoculated with sap from symptomatic leaves developed small necrotic local lesions, followed by systemic epinasty on new growth (Fig. 1b, c). No systemic symptoms were observed from inoculated plants of Phaseolus vulgaris and Vigna unguiculata. Flexuous, filamentous virus particles ~650 nm long were observed by transmission electron microscopy (TEM) of crude sap preparations from systemically infected C. amaranticolor and C. quinoa. Plants of N. domestica and the two inoculated indicator species containing virus particles tested positive using Apple stem grooving virus (ASGV, Capillovirus, Flexiviridae) polyclonal antiserum in double-antibody sandwich (DAS) – enzyme-linked immunosorbent assay (ELISA) (Loewe Biochemica GmbH, Sauerlach, Germany). Nucleic acid was extracted from leaves of plants of each of the three infected species with an RNeasy Plant Mini Kit (Qiagen, Melbourne, Vic., Australia) and then used in reverse transcription–polymerase chain reaction (RT–PCR) tests using ASGV-specific primers (MacKenzie et al. 1997). PCR products of the expected size (524 bp) were obtained, and an amplicon from N. domestica was cloned using a TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA) and sequenced. The obtained consensus from four clones was deposited in the GenBank database under the accession reference GU126685. A BLAST search in GenBank showed 98% nucleotide sequence identities with several isolates of ASGV (GenBank accessions AB004063, D14455, D16681 and D16368). The ASGV-infected N. domestica plants were found to be co-infected with CMV using a virus-specific RT–PCR (Raj et al. 1998). Subsequent testing of an additional three plants from the site showed that N. domestica plants which were infected with ASGV alone were symptomless. This suggests that the symptoms observed in N. domestica were caused by CMV or a synergistic interaction between CMV and ASGV.
|
In May 2009, a further six samples of N. domestica with chlorotic ringspot and/or distortion symptoms (Fig. 2a) were collected from Oklahoma, USA. Sap from symptomatic leaves induced local and systemic necrotic or chlorotic symptoms on inoculated C. amaranticolor and C. quinoa (Fig. 2b, c). Filamentous particles of ~550 nm were observed by TEM from the N. domestica samples and the two inoculated species. However, these infected plants tested negative for ASGV using the ELISA and RT–PCR methods described above. PCR products of ~740 bp were obtained using generic potexvirus primers (van der Vlugt and Berendsen 2002). One amplicon from N. domestica was cloned and five clones were sequenced in both directions. Two distinct sequences were recovered and BLAST searches showed that sequences had 93% (GenBank Accession number GU126686) and 95% nucleotide sequence identities with Alternanthera mosaic virus (AltMV, Potexvirus, Flexiviridae) (GenBank accession AY863024) and PlAMV (GenBank accession AY800279), respectively. In order to confirm the presence of AltMV in the N. domestica samples, forward (5′-CCACATCCAATCTCCTTCCA-3′) and reverse (5′-AGAGTTGCTGGCGAAGTTGT-3′) primers specific to AltMV were designed to amplify a 495 bp fragment in the coat protein gene of this virus. A single product of the expected size was obtained from all N. domestica samples and the symptomatic indicator plants. The N. domestica plants were shown to be co-infected with PlAMV using RT–PCR with novel PlAMV-specific primers (data not shown) and therefore it is not known whether the observed symptoms were induced by AltMV and/or PlAMV.

|
ASGV has been reported to infect several important horticultural crops, including Actinidia, Citrus, Malus, Lilium, Prunus and Pyrus (Clover et al. 2003). In New Zealand, some apple (Malus domestica) cultivars are commonly but latently infected with ASGV (Wood 1996). A BLAST Align comparison of the partial coat protein gene of the Nandina isolate of ASGV with an isolate from M. domestica collected in New Zealand (GenBank accession GU250886) showed 90% and 97% nucleotide and amino acid identities, respectively. These two isolates of ASGV also showed similar reactions in ELISA using an ASGV antiserum but were found to have different experimental host ranges. The Malus ASGV isolate induced symptoms on C. quinoa, P. vulgaris and V. unguiculata, but not on C. amaranticolor whereas the Nandina isolate induced symptoms on both Chenopodium species but not on P. vulgaris or V. unguiculata.
AltMV was first described in Alternanthera pungens in 1999 (Geering and Thomas 1999) and was subsequently reported in Angelonia, Crossandra, Phlox, Portulaca, Scutellaria and Torenia (Baker et al. 2006; Hammond et al. 2006; Duarte et al. 2008; Lockhart and Daughtrey 2008). This is the first report of ASGV and AltMV infecting N. domestica.
Ahmed NA,
Christie SR, Zettler FW
(1983) Identification and partial characterization of a closterovirus infecting Nandina domestica. Phytopathology 73, 470–475.
| Crossref | GoogleScholarGoogle Scholar |

Baker CA,
Breman L, Jones L
(2006) Alternanthera mosaic virus found in Scutellaria, Crossandra, and Portulaca spp. in Florida. Plant Disease 90, 833.
| Crossref | GoogleScholarGoogle Scholar |

Barnett OW, Baxter LW
(1973) Cucumber mosaic virus on Nandina domestica in South Carolina. Plant Disease Reporter 57, 917–920.

Clover GRG,
Pearson MN,
Elliott DR,
Tang Z,
Smales TE, Alexander BJR
(2003) Characterization of a strain of Apple stem grooving virus in Actinidia chinensis from China. Plant Pathology 52, 371–378.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Duarte LML,
Toscano AN,
Alexandre MAV,
Rivas EB, Harakava R
(2008) Identification and control of Alternanthera mosaic virus isolated from Torenia sp. (Scrophulariaceae). Revista Brasileira de Horticultura Ornamental 14, 59–66.

Geering ADW, Thomas JE
(1999) Characterisation of a virus from Australia that is closely related to Papaya mosaic potexvirus. Archives of Virology 144, 577–592.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hughes PL,
Harper F,
Zimmerman MT, Scott SW
(2005) Nandina mosaic virus is an isolate of Plantago asiatica mosaic virus. European Journal of Plant Pathology 113, 309–313.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lockhart BE, Daughtrey ML
(2008) First report of Alternanthera mosaic virus infection in angelonia in the United States. Plant Disease 92, 1473.
| Crossref | GoogleScholarGoogle Scholar |

MacKenzie DJ,
McLean MA,
Mukerji S, Green M
(1997) Improved RNA extraction from woody plants for the detection of viral pathogens by reverse transcription-polymerase chain reaction. Plant Disease 81, 222–226.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Raj SK,
Saxena S,
Hallan V, Singh BP
(1998) Reverse transcription-polymerase chain reaction (RT-PCR) for direct detection of Cucumber mosaic virus (CMV) in gladiolus. IUBMB Life 44, 89–95.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

van der Vlugt RAA, Berendsen M
(2002) Development of a general potexvirus detection method. European Journal of Plant Pathology 108, 367–371.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Wood GA
(1996) Past and present status of virus and phytoplasma diseases in apple rootstocks in New Zealand. New Zealand Journal of Crop and Horticultural Science 24, 133–141.



