Pratylenchus species in pastures in the South East Region of South Australia
Ian T. Riley A B C , Jackie M. Nobbs A ,A Plant and Soil Health, SARDI, Plant Research Centre, GPO Box 397, Adelaide, SA 5001, Australia.
B School of Agriculture, Food and Wine, University of Adelaide SA 5005, Australia.
C Corresponding author. Email: ian.riley@adelaide.edu.au
Australasian Plant Disease Notes 4(1) 89-90 https://doi.org/10.1071/DN09038
Submitted: 30 June 2009 Accepted: 11 August 2009 Published: 3 September 2009
Abstract
Sixteen pastures in south-eastern South Australia were sampled to gauge the prevalence and diversity of root lesion nematodes (Pratylenchus) and to test the applicability of available DNA assays. Ten sites had Pratylenchus infestations, with three populations identified as P. neglectus or P. thornei by DNA and other morphotypes had affinities with P. penetrans and P. crenatus.
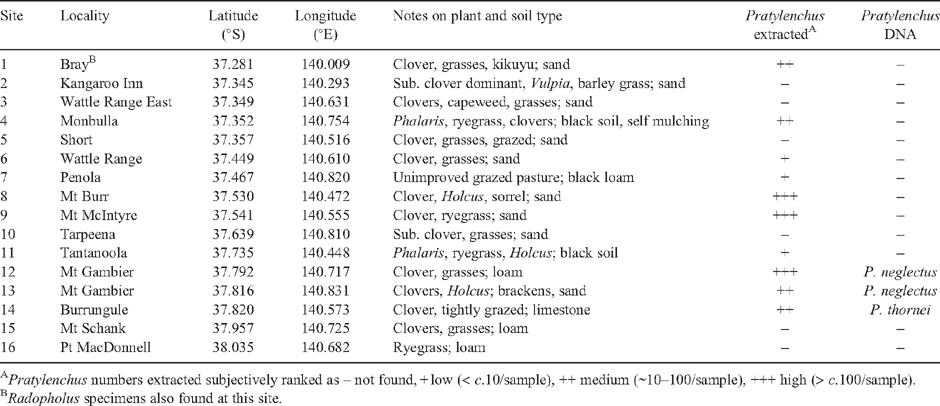
In southern Australia, root lesion nematodes are recognised as important pests in field crops (particularly Pratylenchus neglectus and P. thornei; Vanstone et al. 2008) as well as being the most numerous group of plant parasitic nematodes in pastures (Stirling and Lodge 2005). To assess the risk posed to crops by pre-plant populations of P. neglectus and P. thornei, their population densities can be estimated by real-time polymerase chain reaction (RT-PCR) using DNA extracted from soil (Ophel-Keller et al. 2008). However, when these assays, and an assay being developed for P. penetrans, were applied to 70 pasture soils across southern mainland Australia, few detections were made (I. T. Riley and A. C. McKay, unpubl. data). To reconcile this with the data of Stirling and Lodge (2005), it must be assumed that other species of Pratylenchus predominate in the southern pastures. In order to test this assumption, nematodes were extracted from 16 pasture samples (Fig. 1 and Table 1) collected in the South East Region of South Australia (SA) for species determination by morphology (Loof 1991) and RT-PCR (with TaqMan probes specific for P. neglectus, P. penetrans and P. thornei). Nematodes were extracted from roots (separated from soil and lightly rinsed) in a misting cabinet and from soil by the Whitehead tray method (Hollaway et al. 2003). A representative selection of adults were fixed and mounted for morphological examination. DNA was extracted from 30 individual pratylenchid nematodes from five sites for partial sequencing of the D3 expansion region of the 26S ribosomal gene. Soil samples (~500 g) were submitted to SARDI Diagnostics for soil DNA extraction and assay (Hollaway et al. 2003; Ophel-Keller et al. 2008).
|
Ten of the 16 sites sampled were found to contain Pratylenchus spp. (Table 1). Application of the DNA tests for P. neglectus, P. penetrans and P. thornei indicated that only three samples contained any of these species (two sites with P. neglectus and one with P. thornei). Morphological examination of the Pratylenchus from the other sites identified morphotypes with affinities to P. penetrans (Sites 1, 4, 8 and 9) and P. crenatus (Site 12). Moderate to good D3 sequences (~600 bp) were obtained from eight specimens. Six sequences had 88–99% similarities (BLAST analysis; Altschul et al. 1990) with P. crenatus (4), P. neglectus (1) and P. zeae (1). However, except for the single P. neglectus (99% similarity), these matches were not sufficient to provide confident identification to species level.
The data reconfirm that Pratylenchus is common in high rainfall pastures, as found by Stirling and Lodge (2005). It appears that there is greater species diversity in these pastures than occurs in the northern cropping areas of SA (Riley and Wouts 2001). Consistent with the current results, Riley and Wouts found P. penetrans in the Murray Mallee Region of SA and P. crenatus in western Victoria, and Hollaway and co-workers (2008) found these species in a small proportion of cereal crops at similar latitudes across southern Victoria. However, the negative results for the DNA assay for P. penetrans points to the possibility of cryptic or undescribed species occurring in the study region. Alternatively, that the genetic diversity in P. penetrans was not fully captured by the assay given that P. penetrans has been suggested to be a species complex (De Luca et al. 2004). Either proposition is consistent with the failure of the BLAST analysis to provide convincing species indications. Pratylenchus, even with many species reproducing asexually, appears to be a genetically variable group (Andrés et al. 2000; De Luca et al. 2004). Clearly there is a need to better understand the diversity and impact of Pratylenchus in high rainfall pastures, based on genetic diversity determined by molecular as well as morphological means.
Acknowledgements
Funding was in part provided under the Pasture Soil Biology Initiative of Meat and Livestock Australia Ltd, Grain Research and Development Corporation, and Australian Wool Innovation Ltd.
Altschul SF,
Gish W,
Miller W,
Myers EW, Lipman DJ
(1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410.
|
CAS |
PubMed |

Andrés MF,
Pinochet J,
Hernández-Dorrego A, Delibes A
(2000) Detection and analysis of inter- and intraspecific diversity of Pratylenchus spp. using isozyme markers. Plant Pathology 49, 640–649.
| Crossref | GoogleScholarGoogle Scholar |

De Luca F,
Fanelli E,
Di Vito M,
Reyes A, De Giorgi C
(2004) Comparison of the sequences of the D3 expansion of the 26S ribosomal genes reveals different degrees of heterogeneity in different populations and species of Pratylenchus from the Mediterranean region. European Journal of Plant Pathology 110, 949–957.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hollaway GJ,
Ophel-Keller KM,
Taylor SP,
Burns RA, McKay AC
(2003) Effect of soil water content, sampling method and sample storage on the quantification of root lesion nematodes (Pratylenchus spp.) by different methods. Australasian Plant Pathology 32, 73–79.
| Crossref | GoogleScholarGoogle Scholar |

Hollaway GJ,
Vanstone VA,
Nobbs J,
Smith JG, Brown JS
(2008) Pathogenic nematodes of cereal crops in south-west Victoria, Australia. Australasian Plant Pathology 37, 505–510.
| Crossref | GoogleScholarGoogle Scholar |

Ophel-Keller K,
McKay A,
Hartley D,
Herdina
, Curran J
(2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australasian Plant Pathology 37, 243–253.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Riley IT, Wouts WM
(2001) Pratylenchus and Radopholus species in agricultural soils and native vegetation in southern Australia. Transactions of the Royal Society of South Australia 125, 147–153.

Stirling GR, Lodge GM
(2005) A survey of Australian temperate pastures in summer and winter rainfall zones: soil nematodes, chemical, and biochemical properties. Australian Journal of Soil Research 43, 887–904.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Vanstone VA,
Hollaway GJ, Stirling GR
(2008) Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment. Australasian Plant Pathology 37, 220–234.
| Crossref | GoogleScholarGoogle Scholar |