First report of Lasiodiplodia theobromae (Pat.) Griffon & Maubl causing root rot and collar rot disease of physic nut (Jatropha curcas L.) in India
P. Latha A , V. Prakasam A , A. Kamalakannan A , C. Gopalakrishnan A C , T. Raguchander A , M. Paramathma B and R. Samiyappan AA Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore 641 003, Tamil Nadu, India.
B Centre of Excellence in Biofuel, Agricultural Engineering College & Research Institute, TNAU, Coimbatore 641 003, Tamil Nadu, India.
C Corresponding author. Email: pc_gopal@yahoo.co.in
Australasian Plant Disease Notes 4(1) 19-20 https://doi.org/10.1071/DN09008
Submitted: 26 August 2008 Accepted: 28 February 2009 Published: 23 March 2009
Abstract
Physic nut (Jatropha curcas), an important bio-fuel crop grown in the state of Tamil Nadu, India suffered heavy losses due to a root disease in 2007. The symptoms observed were yellowing, drooping and shedding of leaves, blackening and decaying of the collar region of the stem and rotting of the roots. Lasiodiplodia theobromae was isolated consistently from the diseased tissues of affected plants. Pathogenicity of L. theobromae was confirmed by artificial inoculations on 1-year-old plants. This is the first report of root rot and collar rot disease of physic nut in India.
Physic nut (Jatropha curcas, Fam. Euphorbiaceae) is a drought-resistant shrub cultivated in Central and South America, South-east Asia, India and Africa (Schmook and Seralta-Peraza 1997). It is a multipurpose crop of significant economic importance as a biofuel. Moreover, parts of the shrub are used in traditional medicine and as raw material for pharmaceutical and cosmetic industries (Paramathma et al. 2006).
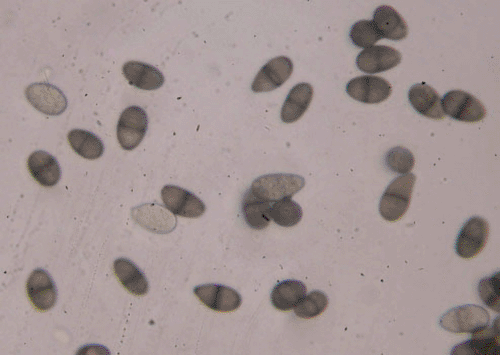
In August–December 2007, physic nut grown in Coimbatore, Erode, Tirunelvelli, Thoothukudi, Virudhunagar, Karur, Dindigul and Thiruvallur districts of Tamil Nadu, India suffered heavy losses. The symptoms observed were yellowing, drooping and shedding of leaves (Fig. 1), blackening and decaying of the collar region of the stem and rotting of roots, followed by death of plants in most severe cases. Small to medium-sized black hard erumpent pycnidia were seen on the collar region. Infected roots and stems exhibited internal discolouration and browning of vascular regions (Fig. 2). Eighteen samples of infected stems and roots were used for isolations. A fungus was consistently isolated on potato dextrose agar (PDA) from the diseased collar and root regions. The same fungus was isolated from all 18 samples, and no other fungus was isolated from the infected roots and stems. After 4–5 days of incubation in PDA at 28 ± 2°C with a 12-h photoperiod, the fungus initially produced white colonies, which later turned black (5–7 days). The mycelium was fast spreading, immersed, branched and septate. Shiny black pycnidia were produced on the medium surface after 7–8 days at the same culture conditions as above. Paraphyses were present interspersed with conidiogenous cells in the pycnidia. Conidia were initially unicellular, ellipsoidal, hyaline, thick-walled with granular content, and measuring 22.5 × 12.5 µm. Mature conidia were one-septate, dark brown with longitudinal striations (Fig. 3). Based on its morphology the fungus was identified as Lasiodiplodia theobromae (Punithalingam 1976). Identification was confirmed by the Indian Type Culture Collection, (Indian Agricultural Research Institute, New Delhi – 110012, India). To confirm fungal pathogenicity, 10 1-year-old healthy plants were inoculated with the fungus. Mycelial plugs (8 mm) were deposited on wounds (3 cm diam.) with a sterilised knife on stems and collar regions and covered with moist cotton, and finally wrapped with Parafilm (Sree Sasthaa Scientific Company, Coimbatore, India). Ten additional control plants were treated similarly but with sterile water (3 mL) instead of the fungal inoculum. Plants were maintained at 28° ± 2°C and 40% relative humidity in a greenhouse. All the inoculated plants produced typical collar rot and root rot symptoms 1 month after inoculation. The fungus was consistently reisolated from all inoculated plants. Control plants did not show any symptom.

|

|
|
Lasiodiplodia theobromae is a ubiquitous pathogen of tropical woody trees, causing shoot blight and dieback of many plant species (Mohali et al. 2005) including: dieback and gummosis of mango (Khanzada et al. 2004); black branch and dieback disease of cashew in Brazil (Cardoso et al. 2002); and collar rot of peanut in Virginia and North Carolina, USA (Phipps and Porter 1998). Lasiodiplodia theobromae has been also reported to cause gummosis of Jatropha podagrica in China (Fu et al. 2007). To the best of our knowledge, this is the first report of collar rot and root rot caused by L. theobromae on physic nut in India. A culture has been deposited in the Indian Type Culture Collection (ITC No. 6969/08).
Cardoso JE,
Vidal JC,
Dos Santos AA,
Freir FCO, Viana FMP
(2002) First report of black branch dieback of cashew caused by Lasiodiplodia theobromae in Brazil. Plant Disease 86, 558.
| Crossref | GoogleScholarGoogle Scholar |

Fu G,
Huang SL,
Wei JG,
Yuan GQ,
Ren JG,
Yan WH, Cen ZL
(2007) First record of Jatropha podagrica gummosis caused by Botryodiplodia theobromae in China. Australasian Plant Disease Notes 2, 75–76.
| Crossref | GoogleScholarGoogle Scholar |

Mohali S,
Burgess TI, Wingfield MJ
(2005) Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. Forest Pathology 35, 385–396.
| Crossref | GoogleScholarGoogle Scholar |

Phipps PM, Porter DM
(1998) Collar rot of peanut caused by Lasiodiplodia theobromae. Plant Disease 82, 1205–1209.
| Crossref | GoogleScholarGoogle Scholar |



