Efficacy of fungicides against damping-off in papaya seedlings caused by Pythium aphanidermatum
M. F. Male A B and L. L. Vawdrey AA Plant Pathology, Department of Employment, Economic Development and Innovation, Centre for Wet Tropics Agriculture, South Johnstone, 4859 Qld, Australia.
B Corresponding author. Email: mike.male@deedi.qld.gov.au
Australasian Plant Disease Notes 5(1) 103-104 https://doi.org/10.1071/DN10037
Submitted: 7 September 2010 Accepted: 13 September 2010 Published: 27 September 2010
Abstract
Various chemical and non-chemical treatments were tested for their efficacy against damping-off in papaya seedlings caused by Pythium aphanidermatum. Three-week-old papaya seedlings were placed in a climate controlled experimental chamber and inoculated with macerated mycelium of P. aphanidermatum. Propamocarb as Previcur was found to be most effective at managing damping-off in papaya seedlings.
Pythium aphanidermatum is an aggressive, cosmopolitan soil-borne fungal pathogen with a wide host range. It is known to cause damping-off, root and stem rots and blights of fruit and grasses (van der Plaats-Niterink 1981). In northern Queensland, it is economically important as it causes damping-off in papaya (Carica papaya) (Teakle 1960). The establishment of new papaya plantings during winter requires that containerised seedlings are grown during the warm and often wet autumn months. These conditions favour the development of damping-off of young seedlings (Adams 1971). At present, limiting infection by P. aphanidermatum is based on cultural controls such as growing seedlings on raised benches in pasteurised media free of soil and plant material, and regulating irrigation. There are no chemicals registered for the control of damping-off in containerised papaya seedlings (Infopest 2003). Previous unpublished studies examining chemical control options for damping-off and other oomycete fungal pathogens of papaya identified several possible candidate compounds. This paper reports on the results of two in vivo experiments which evaluated the efficacy of a range of chemicals for the control of damping-off caused by P. aphanidermatum.
Papaya seeds (cultivar 1B) were sown into 100 mm diameter squat pots and thinned to eight plants per pot following germination. At 3 weeks of age, seedlings were placed in a climate-controlled experimental chamber and maintained at 30°C, >90% relative humidity and exposed to 14 h of light per day, in a completely randomised design. An isolate (BRIP 53639) of P. aphanidermatum (L. Tesoriero, pers. comm.) previously stored under sterile water was revived on culture plates of 2% potato dextrose agar amended with streptomycin sulfate and incubated in the dark at 27°C. Axenic broth cultures were produced by transferring 5 mm plugs from the margin of the fungal colony to potato dextrose broth and then placed in an incubator in the dark at 27°C for 5 days. Mycelial mats were then rinsed with distilled water, weighed and macerated in distilled water in a Waring blender (Torrington, CT, US) for 20 s. In each experiment, the equivalent of 1 g of mycelium suspended in 50 mL of distilled water was used to inoculate each pot 48 h after placement in the climate control chamber.
All treatments (Table 1) except Bacillus+silica as Parkway Blend + Autofert (Barmac Pty. Ltd., Blackstone, Qld, Australia), metalaxyl-M as Ridomil Gold 25G (Syngenta Crop Protection Pty. Ltd., Macquarie Park, NSW, Australia) and acibenzolar-s-methyl as Boost 500SC (Syngenta) were applied as a pot drench with a watering can several hours after inoculation with P. aphanidermatum. In experiment 1, Bacillus+silica was applied as a drench 24 h before inoculation and acibenzolar-s-methyl was applied as a 24 h seed soak before sowing. In experiment 2, Bacillus+silica was applied as a drench 0, 12 and 28 days after sowing. Granules of metalaxyl-M as Ridomil Gold 25G were thoroughly incorporated into the potting media before sowing. In preliminary research, metalaxyl-M as Ridomil Gold 480EC was found to be phytotoxic to papaya leaves, so plants receiving this treatment had their leaves rinsed with a small quantity of water immediately after the application. Untreated controls were treated with distilled water only. Plant mortality was recorded daily from the first day of symptom expression until no further plants died. Data was analysed with one-way analysis of variance (ANOVA) and pair-wise testing was performed between treatment means using the protected least significance difference test.
|
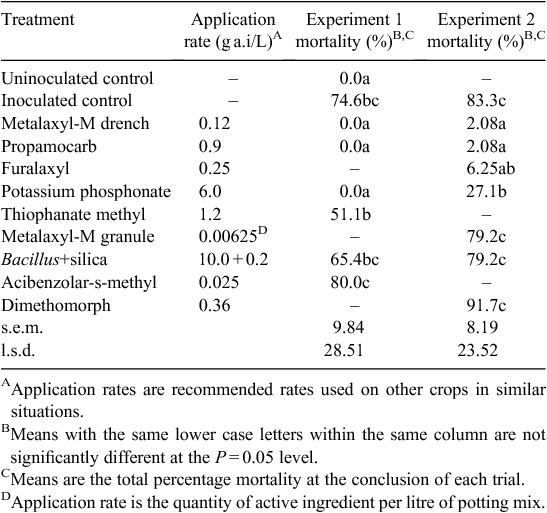
Results from each experiment (Table 2) showed that propamocarb as Previcur (Bayer CropScience Pty. Ltd., Hawthorn, Vic., Australia), furalaxyl as Fongarid 250WP (Garden King Products Pty. Ltd., Parramatta, NSW, Australia) and metalaxyl-M as Ridomil Gold 480EC (Syngenta) provided an acceptable level of control of damping-off caused by P. aphanidermatum. Potassium phosphonate provided a variable level of control and the remainder of the treatments were ineffective.
|
The systemic fungicides furalaxyl and metalaxyl-M have been shown to be prone to resistance in Pythium aphanidermatum (Sanders and Soika 1988) and Phytophthora palmivora (Lucas et al. 1990). Metalaxyl-M is currently used in papaya at transplanting for the control of Phytophthora root rot. Due to the potential risk of biodegradation and fungicide resistance with the additional use of these chemicals, propamocarb was recommended to the Australian Pesticides and Veterinary Medicines Authority for registration as a chemical control for damping-off in papaya seedlings.
Adams PB
(1971)
Pythium aphanidermatum oospore germination as affected by time, temperature and pH. Phytopathology 61, 1149–1150.
| Crossref | GoogleScholarGoogle Scholar |

Lucas JA,
Bower LA, Coffey MD
(1990) Fungicide resistance in soil-borne Phytophthora species. EPPO Bulletin 20, 199–206.
| Crossref | GoogleScholarGoogle Scholar |

Sanders PL, Soika MD
(1988) Metalaxyl resistance frequency in overwintering populations of Pythium aphanidermatum from metalaxyl control failure sites. Phytopathology 78, 1510.

Teakle DS
(1960) Species of Pythium in Queensland. Queensland Journal of Agricultural Science 17, 15–31.

van der Plaats-Niterink AJ
(1981) Monograph of the genus Pythium. Studies in Mycology 21, 1–24.



