First report of Oidium citri in Bhutan
P. Holford A F , N. J. Donovan B ,A Centre for Plants and the Environment, University of Western Sydney, Locked Bag 1797, Penrith, South DC, NSW 1797, Australia.
B Industry & Investment NSW, Elizabeth Macarthur Agricultural Institute, Private Mail Bag 8, Narellan, NSW 2567, Australia.
C National Plant Protection Centre, Department of Agriculture, Ministry of Agriculture, Thimphu, Bhutan.
D Industry & Investment NSW, Gosford Primary Industries Institute, Locked Bag 26, Gosford, NSW 2250, Australia.
E Horticulture Division, Department of Agriculture, Ministry of Agriculture, PO Box 119, Thimphu, Bhutan.
F Corresponding author. Email: p.holford@uws.edu.au
Australasian Plant Disease Notes 5(1) 55-57 https://doi.org/10.1071/DN10020
Submitted: 25 February 2010 Accepted: 11 May 2010 Published: 7 June 2010
Abstract
Powdery mildew is one of the most important diseases of citrus in Bhutan where it infects new flush growth causing leaf and shoot distortion and twig and branch dieback. It also attacks young fruitlets. This causes premature fruit drop. These symptoms, together with DNA sequence data and the production of single conidia and lobed appressoria, suggest that the disease is caused by Oidium citri (JM Yen) U. Braun. This is the first formal report of this pathogen in Bhutan.
There is uncertainty about the number of species of powdery mildew that attack citrus. According to Fawcett and Lee (1926) and Subramanian (1971; cited in Boesewinkel 1981), only one powdery mildew species, Oidium tingitaninum JC Carter, is found on citrus. However, Yen identified a second species, Oidium citri (JM Yen) U. Braun, in Taiwan. This was initially thought to be a form of O. erysiphoides (Yen 1967) but was later renamed O. citri by Braun (1982). Boesewinkel (1981) proposed that it may be a strain of Microsphaera euonymi-japonici. However, phylogenetic analysis of the internal transcribed spacer (ITS) of rDNA and the D1 and D2 domains of the 28S rDNA clearly place O. citri and M. euonymi-japonici in different species groups (Limkaisang et al. 2006). O. citri has been reported to occur in Sri Lanka (Petch 1915; Boesewinkel 1981) as well as in Java, Sumatra, Honduras and Antigua (Boesewinkel 1981).
The two species of powdery mildew on citrus have distinct morphologies. The mycelium of O. tingitaninum is initially sparse, then becomes denser but never felty. It occurs on the upper surface of young leaves and causes leaf distortions but otherwise little damage (Carter 1915). The mycelium produces rounded appressoria and gives rise to conidiophores that bear chains of 4–8 conidia. The conidia are barrel-shaped and 20–28 × 10–15 μm in size. In contrast, the mycelium of O. citri is dense ‘covering the leaves with a thick, white coating’ (Petch 1915) on both sides of leaves and can cause die-back and defoliation. The pathogen produces lobed appressoria and gives rise to conidiophores that only produce a single conidium that is 30–36 × 16–18 μm (Yen 1967).
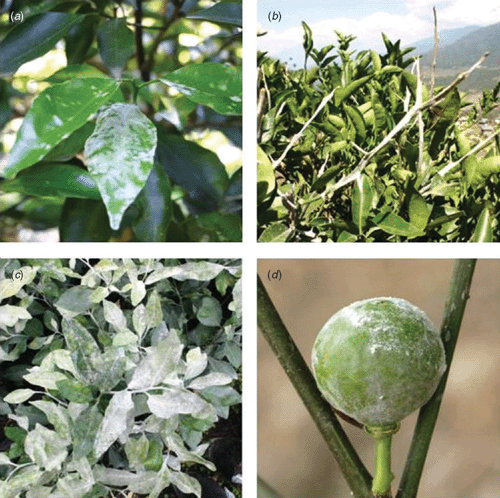
Powdery mildew occurs on mandarin trees (Citrus reticulata Blanco) in nearly all the citrus-growing districts of Bhutan. A survey of citrus farmers in 2007 by Connellan et al. (2007) ranked powdery mildew as one of the most important diseases causing damage to citrus in all of the citrus-growing regions of Bhutan, especially in the Samtse, Pemagatshel, Chhukha, Tsirang and Wangdue Phodrang districts. It is more severe in the humid subtropical zones of southern Bhutan and is particularly prevalent in nursery plantings, shady orchards and poorly ventilated orchards located in valley floors. Powdery mildew reduces tree productivity, fruit yield and quality. The pathogen infects new flush growth causing leaf and shoot distortion and, in severe infections, twig and branch dieback. Depending on orchard location, powdery mildew can be a significant problem in recently pruned trees infecting all the new re-growth. It also attacks young fruitlets causing distortion and premature fruit drop.
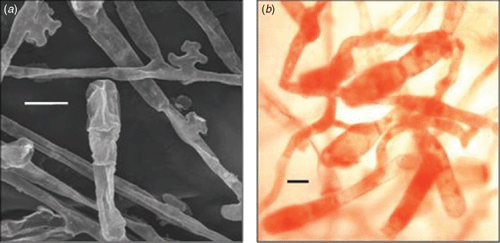
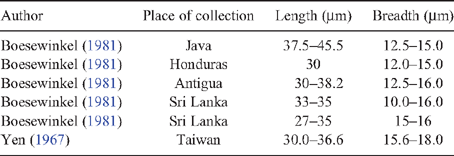
A second survey of citrus orchards conducted in the districts of Phunakha, Wangdue Phodrang and Tsirang by the authors of this study commonly found powdery mildew on mandarin seedlings, trees and fruitlets (Fig. 1) of the one local variety grown. Powdery mildew was also found on Rangpur lime (Citrus × limon (L.) Osbeck), Carrizo citrange (Citrus × insitorum Mabb.) and Tsunokaori tangor (Citrus × aurantium L.). Severe symptoms were observed including leaf defoliation and twig dieback. Samples were taken and preserved in either double aldehyde fixative (1.5% paraformaldehyde, 2.5% glutaraldehyde in 0.05 M phosphate buffer) (Spence 2001) or 70% ethanol. Samples placed into 70% ethanol were lodged with the Plant Pathology Herbarium, Industry and Investment New South Wales under accession numbers DAR 80519–23. For light microscopy, samples were washed or rehydrated in phosphate buffer before staining with Congo red (~5%). For electron microscopy, samples that were killed and fixed in double aldehyde fixative were rinsed in phosphate buffer (0.1 M, pH 6.9) then dehydrated in an ethanol series (10–100% for 30 min per step): samples that were placed directly into 70% ethanol were further dehydrated (80, 90, 95, 100% as previous). After critical point drying and coating with gold, the specimens were examined by scanning electron microscopy (JEOL JSM-6510 LV, JEOL, Tokyo, Japan). Light and electron microscopy showed that the appressoria (Fig. 2a) produced on all samples collected were lobed and that conidiophores produce single conidia: no multiple conidia were observed. The conidia were 31.3 (±0.4) × 15.3 (±0.2) μm in size (Fig. 2b). Table 1 outlines the conidial sizes found in other studies of powdery mildew attacking Citrus spp., where the conidiophores bore single conidia. The conidial size found in this study falls within the ranges reported in these studies.
|
|
|
DNA was extracted from all samples using the RedExtract-N-Amp kit (Sigma-Aldrich, Sydney, NSW, Australia) and then used in a nested polymerase chain reaction to amplify the ITS region using the primers (PMITS1 and PMITS2, first reaction; PMITS1 and ITS4, second reaction) and cycling conditions detailed in Cunnington et al. (2003). The resulting amplicons were sequenced at the Australian Genome Research Facility Ltd. All sequences were identical to each other and were also identical with all published sequences (AB237791–3) for O. citri. The longest sequence was lodged with GenBank under accession number HM164136.
Powdery mildew has been previously reported on citrus in Bhutan, although the causal organism was identified as O. tingitaninum (Firman et al. 1988) (IMI 290379). Nevertheless, given that the symptoms of powdery mildew we observed in Bhutan are consistent with those reported for O. citri, that the pathogen produces single conidia and lobed appressoria and that the sequence of its ITS region was identical with published sequences for O. citri, we propose that this is the main species attacking citrus in Bhutan. The identification of O. citri in Bhutan supports Boesewinkel’s (1981) contention that this pathogen, rather than O. tingitaninum, is the major cause of powdery mildew worldwide and further survey work should be carried out to determine if O. tingitaninum does indeed occur in Bhutan.
Acknowledgements
We thank Michael Priest for his valuable taxonomic advice. This work was part-funded through a grant (Hort/2005/142) from the Australian Centre for International Agricultural Research.
Boesewinkel HJ
(1981) The identity of powdery mildew of citrus. Nova Hedwigia 34, 731–741.

Braun U
(1982) Morphological studies on the genus Oidium III. Zentralblatt fur Mikrobiologie 137, 314–324.

Carter CN
(1915) A powdery mildew on citrus. Phytopathology 5, 193–196.

Cunnington JH,
Takamatsu S,
Lawrie AC, Pascoe IG
(2003) Molecular identification of anamorphic powdery mildews (Erysiphales). Australasian Plant Pathology 32, 421–428.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Limkaisang S,
Cunnington JH,
Liew KW,
Salleh B,
Sato Y,
Divarangkoon R,
Fangfuk W,
To-anun C, Takamatsu S
(2006) Molecular phylogenetic analyses reveal a close relationship between powdery mildew fungi on some tropical trees and Erysiphe alphitoides, an oak powdery mildew. Mycoscience 47, 327–335.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Petch T
(1915) Citrus mildew. Phytopathology 5, 350–352.

Yen JM
(1967) Quelques espéces d’Oidium de Formose. Cahiers du Pacifique 11, 75–115.



