Morulaimus gigas (Nematoda: Belonolaiminae) in southern and eastern Australia
L. Nambiar A C , M. Quader A and J. M. Nobbs BA Department of Primary Industries, Private Bag 15, Ferntree Gully Delivery Centre, Vic. 3156, Australia.
B South Australian Research and Development Institute, Plant and Soil Health, Plant Research Centre, GPO Box 397, Glen Osmond, SA 5064, Australia.
C Corresponding author. Email: lila.nambiar@dpi.vic.gov.au
Australasian Plant Disease Notes 5(1) 39-41 https://doi.org/10.1071/DN10015
Submitted: 20 November 2009 Accepted: 22 April 2010 Published: 5 May 2010
Abstract
This paper reports the first detections of Morulaimus gigas outside Western Australia in turf samples from South Australia, Victoria, New South Wales and Queensland. New infestations were also found in turf samples from Western Australia. Approximately 7% of turf samples tested between 1996 and 2008 contained M. gigas with 87% of these above the damage threshold. M. gigas was associated with turf decline in bent grass, Agrostis tenuis – a new addition to the previously recorded hosts.
The family Belonolaimidae, as defined by Siddiqi (2000), is a group of migratory ectoparasites commonly known as sting nematodes. All four genera in the family have been recorded from Australia (McLeod et al. 1994; Hodda and Nobbs 2008), but Belonolaimus Steiner 1949 has been found mainly in North America, Ibipora Monteiro and Lordello 1977 has been found mainly in Central and South America and Carphodorus Colbran 1965 and Morulaimus Sauer 1966 are generally restricted to Australia and New Zealand (Siddiqi 2000). This group of genera is listed as the subfamily Belonolaiminae by Fortuner and Luc (1987) and Hodda (2007). Morulaimus gigas Nobbs and Eyres 1992 was described in association with declining couch grass turf (Cynodon dactylon) from Western Australia. This paper presents data indicating a widespread distribution of M. gigas in Australia.
From 1996–2008, 3812 turf samples were submitted to Crop Health Services, Department of Primary Industries (DPI) Victoria, Australia by greenkeepers and turf consultants for diagnosis and assessment of plant-parasitic nematodes suspected to be associated with turf decline and/or as part of routine golf course and bowling green management programs. The samples, received throughout each year from coastal and inland areas, exhibited symptoms similar to those caused by other plant-parasitic nematodes, including yellow and brown patches, and reduced vigour. The soils were typically sandy and roots were discoloured, short and stubby with few feeder roots.
Nematodes were extracted from the soil by using the Whitehead tray method (Whitehead and Hemming 1965). Different stages of the nematodes, including juveniles, males and females were recovered from the samples. Nematode specimens were fixed in formalin-acetic fixative 4 : 1 (Hooper 1970) to harden, and then transferred through a series of glycerol-ethanol solutions (Seinhorst 1959) to pure glycerol.
M. gigas was the only species present in the 80% of submitted samples containing plant-parasitic nematodes. Population densities were variable and ranged from 6–2976/200 mL soil. Although other plant-parasitic nematodes were extracted from some samples, symptoms of decline were only associated with population densities of M. gigas exceeding the damage threshold for sting nematodes (20/200 mL soil in couch turf (Robinson et al. 1994)). M. gigas was detected above this damage threshold in 228 of the 262 samples in which it was found.
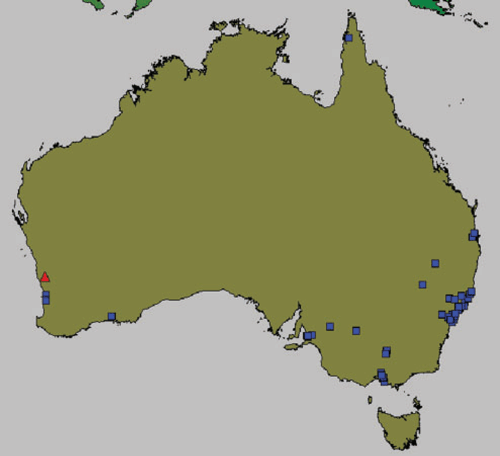
In the present study, turf samples from 48 locations in Australia were found to contain M. gigas (Fig. 3) with new detections in Western Australia, South Australia, Victoria, New South Wales and Queensland. These are the first records of M. gigas in eastern and southern Australia. M. gigas was also found for the first time in bent grass (Agrostis tenuis). It is possible that M. gigas has a wider host range than previously known and this requires further investigation.
A total of 97 permanent microscope slides of M. gigias were deposited in the Victorian Plant Pathology Herbarium (VPRI) DPI Victoria, Australia (Table 1) with 8 from South Australia, 8 from Victoria, 66 from New South Wales, 4 from Queensland and 11 from Western Australia.
Error: Incorrect filename or format (DN10015_T1.gif). Please check out
States
Number of slides
VPRI voucher numbers
South Australia
8
34 295–34 302
Victoria
8
26 008, 34 303–34 309
New South Wales
66
25 743–25 746, 29 225, 29 226, 29 229, 29 230, 29 377–29 381, 29 385–29 388, 29 698–29 703, 29 705, 29 706, 33 651–33 653, 33 658–33 661, 33 681, 33 701–33 703, 33 710, 33 714, 33 717, 33 718, 33 722, 33 735, 33 737, 33 774, 33 826–33 829, 34 089–34 093, 34 096–34 100, 34 024–34 031
Queensland
4
34 290–34 292, 34 310
Western Australia
11
25 765–25 767, 25 780, 25 781, 25 798–25 800, 25 801, 34 293, 34 294
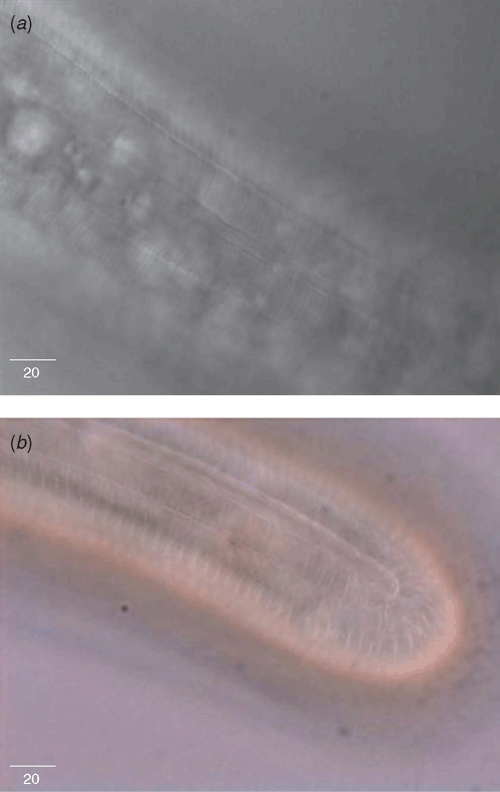
The nematodes were identified as being in the genus Morulaimus by having submedian lobes in the head region not separated by deep grooves; four distinct lines in the lateral field; the outer bands areolated (Fig. 1a and b); conspicuous epiptygma (Fig. 2); an elongated labial disc and an elongated, cylindrical tail with rounded, annulated terminus (Fig. 1b). These are key taxonomic criteria defining the genus Morulaimus (Table 2).
|
Error1: Incorrect filename or format (DN10015_F2.gif). Please check out Fig. 2.
|

|
There are eight species in the genus Morulaimus, all described from Australia (Siddiqi 2000). The specimens from the turf samples in the present study had the following diagnostic characters: long body (2180–2479 µm), long stylet (108–113 µm), vagina vera heavily sclerotised (Fig. 2) and tail elongated, cylindrical with rounded, annulated terminus (Fig. 1b). All these characters are diagnostic for M. gigas (Nobbs and Eyres 1992). The measurement ranges found in the different localities are slightly smaller than that recorded in the original description. This variation is expected considering diverse locations from which the specimens were obtained.
Fortuner and Luc (1987) considered the genus Ibipora synonymous with Belonolaimus, rejecting the number of lateral lines as diagnostic and ignoring the other differentiating character (dorsoventral position of dorsal oesophageal gland) (Sauer et al. 1980); without these differentiating characters, M. gigas comes close to B. lolii. However, Siddiqi (2000) considered lateral lines, epiptygma and position of oesophageal glands as valid generic differentiating characters within Belonolaiminae. If these characters are valid, then M. gigas is not synonymous with B. lolii. In this paper the latest view that M. gigas is distinct is accepted, but note that the possible synonymy is being investigated, with results presented elsewhere.
Acknowledgements
The authors wish to thank Sportsturf Consultants Pty Ltd and Australian Golf Course Superintendents Association, Victoria for submitting turf samples from bowling and golf greens for diagnosis of plant-parasitic nematodes.
Colbran RC
(1965) Studies of plant and soil nematodes. 11. Carphodorus bilineatus gen. nov., sp. nov. (Nematoda: Dolichodorinae) from eucalyptus forest in Queensland. Queensland Journal of Agricultural Animal Sciences 22, 481–484.

Fortuner R, Luc M
(1987) A reappraisal of Tylenchina (Nemata). 6. The family Belonolaimidae Whithead, 1960. Revue de Nematologie 10, 183–202.

Hodda M
(2007) Comprehensive keys to nematodes at species and genus levels using INTKEY. Phytopathology 97, S157.

Hodda M, Nobbs J
(2008) A review of current knowledge on particular taxonomic features of the Australian nematode fauna, with special emphasis on plant feeders. Australasian Plant Pathology 37, 308–317.
| Crossref | GoogleScholarGoogle Scholar |

Monteiro AR, Lordello LGE
(1977) Dois novos nematoides encontrados associados á cana de acuar. Revista de Agricultura. Piracicaba, Brazil 52, 5–11.

Nobbs JM, Eyres M
(1992)
Morulaimus gigas sp. nov. from Western Australia. Afro-Asian Journal of Nematology 2, 115–117.

Sauer MR
(1966)
Morulaimus, A new genus of the Belonolaiminae. Nematologica 11, 609–618.

Sauer MR,
Brzeski MW, Chapman RN
(1980) Observations on the morphology of Belonolaiminae. Nematologia Mediterranea 8, 121–129.

Seinhorst JW
(1959) A rapid method for the transfer of nematodes from fixatives to anhydrous glycerine. Nematologica 4, 67–69.

Steiner G
(1949) Plant nematodes the grower should know. Proceedings – Soil Science Society of Florida 4-B, 72–117.

Whitehead AG, Hemming JR
(1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. The Annals of Applied Biology 55, 25–38.
| Crossref | GoogleScholarGoogle Scholar |



