First report of Greeneria uvicola as cause of grapevine dead-arm dieback in Uruguay
F. Navarrete A , E. Abreo A , L. Bettucci A , S. Martínez A and S. Lupo A BA Laboratorio de Micología, Facultad de Ciencias-Facultad de Ingeniería, Universidad de la República, Montevideo, Uruguay.
B Corresponding author. Email: slupo@fing.edu.uy
Australasian Plant Disease Notes 4(1) 117-119 https://doi.org/10.1071/DN09048
Submitted: 16 September 2009 Accepted: 12 November 2009 Published: 30 November 2009
Abstract
This is the first report of Greeneria uvicola isolated from grapevines (Vitis vinifera) showing symptoms of dead-arm dieback and from asymptomatic canes in Uruguay. Greeneria uvicola was identified by morphological characteristics and sequence analysis of the partial LSU DNA. Pathogenicity of isolates from both sources was determined by inoculation of mycelium plugs on 1-year-old canes of cvv. Cabernet Sauvignon and Tannat.
In Uruguay, there are 8000 ha of several cultivars of Vitis vinifera planted in different regions. Symptoms corresponding to trunk diseases have been ascribed to Petri, Esca, dead-arm dieback and black foot (Abreo et al. 2008). Dead-arm dieback occurs on plants older than 6 year, affecting spurs and eventually sections of the cordons and trunks in which Eutypa lata causes brown and wedge-shaped necrosis (Rudelle et al. 2005). Fungi other than E. lata, such as Eutypella vitis, E. leptoplaca, Cryptovalsa ampelina (Mostert et al. 2004; Trouillas and Gubler 2004; Jordan and Schilder 2005) and Botryosphaeria spp. (Amponsah et al. 2009; Úrbez-Torres and Gubler 2009) can cause the same symptoms. In Uruguay, several fungi have been isolated from typical wedge-shaped necrotic tissues in dead arms of grapevines. Among them, Eutypella vitis, Botryosphaeria spp. and Greeneria uvicola have been misidentified as Pilidiella sp. (Abreo et al. 2008). Greeneria uvicola is an Ascomycete (Diaporthales) that causes bitter rot of grape bunches in different countries. This is an anamorphic fungus with unknown teleomorph. The fungus overwinters on stem lesions and in mummified berries (Farr et al. 2001). The objective of this work was to characterise and test the pathogenicity of isolates of G. uvicola on canes. Isolates were obtained from wedge-shaped necrotic tissues, and as an endophyte from healthy canes.
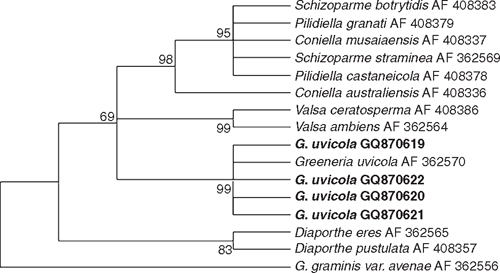
Fungi were isolated from healthy canes of cv. Paulsen rootstock used for propagation in nurseries, and cvv. Ugni Blanc and Cabernet Sauvignon plants showing symptoms of dead-arm dieback, onto potato dextrose agar (PDA) at 25°C. Emerging colonies with morphological characteristics of G. uvicola, such as pycnidia, conidiogenous cell shape, and conidial shape and size (Fig. 1), were grown as pure cultures for DNA isolation and molecular characterisation. Partial DNA amplification of LSU (large subunit) was performed with LR0R and LR7 primers (Vilgalys and Hester 1990) and sequenced by Macrogen Korea (Seoul, South Korea). Four sequences were submitted to GeneBank with accession numbers GQ870619 (FI12007), GQ870620 (FI12008), GQ870622 and GQ870621. Corrected sequences were aligned with G. uvicola (AF362570) and other Diaporthales from GenBank using Clustal W. The phylogenetical analysis was carried out with MEGA 4.0 using the method of Maximum Parsimony with a Boostrap test of 1000 pseudosamples (Fig. 2). Pathogenicity of two isolates of G. uvicola (FI12007 from wedge-shaped tissues and FI12008 from healthy canes) was evaluated on seven canes of cvv. Tannat and Cabernet Sauvignon. In total, 28 canes were inoculated. Discs of agar (5 mm diam.) with mycelia of G. uvicola were obtained from actively growing colonies on PDA and inoculated directly on wounds made with a 5-mm cork borer, and covered with Parafilm (Pechiney Plastic Packaging, Chicago, IL, USA). Control canes were inoculated with agar discs without mycelium. Canes were incubated in the dark under moist conditions in the laboratory at 25°C. Size of lesions was measured 30 days after inoculation, and eight segments (5×3 mm) from each lesion were transferred to fresh PDA medium, incubated at 25°C and 24-h illumination to recover the inoculated fungus. Percentage of canes with discolouration and percentage of recovered fungi was calculated based on total number of inoculated canes.
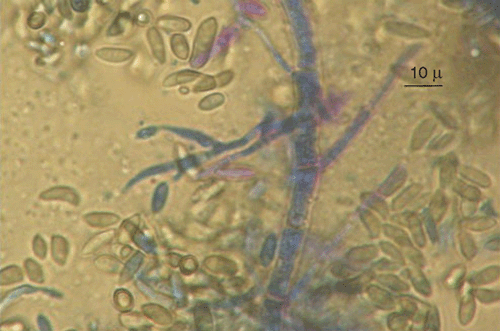
|
|
Phylogenetic analysis confirmed that all isolates could be assigned to G. uvicola as their sequences formed a well-supported clade (99%) with G. uvicola AF362570 from GenBank. (Fig. 2). Isolates FI12007 and FI12008 of G. uvicola were able to produce wood discolouration in xylem tissue of canes of cvv. Tannat and Cabernet Sauvignon (Fig. 3). The size of lesions was variable, and the inoculated fungus was recovered in 86% of the canes inoculated with the strain isolated from diseased tissues (FI12007) and from 71% of the canes inoculated with the strain isolated from non-symptomatic tissues (FI12008) considering both cultivars (Table 1).

|

|
The t-test analysis of length of discolouration showed that FI12008 produced more extended wood discolouration than FI12007 for both varieties (P = 0.07). When Cabernet Sauvignon was analysed separately, the statistical difference between fungal strains was evident (P = 0.0005). When cultivar sensitivity was assessed, there was no difference in length of discolouration between cvv. Tannat and Cabernet Sauvignon (P = 0.88). Finally, the fungus could be recovered in some of the canes that did not show discolouration. (Table 1).
This is the first report that G. uvicola isolated from healthy and dead-arm affected grapevines, able to produce wood discolouration similar to that caused by E. lata and Botryosphaeria spp.
Acknowledgements
The authors are grateful to ANII (Agencia Nacional de Investigación e Innovación, Uruguay).
Abreo E,
Lupo S,
Martinez S, Bettucci L
(2008) Fungal species associated to grapevine trunk diseases in Uruguay. Journal of Plant Pathology 90, 591.

Amponsah NT,
Jones EE,
Ridgway HJ, Jaspers MV
(2009) First report of Neofusiccum australe (Botryosphaeria australis), a cause of grapevine dieback in New Zealand. Australasian Plant Disease Notes 4, 6–8.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Farr DF,
Castlebury LA,
Rossman AY, Erincik O
(2001) Greeneria uvicola, cause of bitter rot of grapes, belongs to the Diaporthales. Sydowia 53, 185–199.

Jordan S, Schilder A
(2005) Eutypella vitis, a potential pathogen of grapevines in Michigan. (Abstr.). Phytopathology 95, S51.

Mostert L,
Halleen F,
Crous PW, Creaser ML
(2004) Cryptovalsa ampelina, a forgotten shoot and cane pathogen of grapevines. Australasian Plant Pathology 33, 295–299.
| Crossref | GoogleScholarGoogle Scholar |

Rudelle J,
Octave S,
Kaid-Harche M,
Roblin G, Fleurat-Lessard P
(2005) Structural modifications induced by Eutypa lata in the xylem of trunk and canes of Vitis vinifera. Functional Plant Biology 32, 537–547.
| Crossref | GoogleScholarGoogle Scholar |

Trouillas FP, Gubler WD
(2004) Identification and characterization of Eutypa lepoplaca, a new pathogen of grapevine in northen California. Mycological Research 108, 1195–1204.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Úrbez-Torres J R, Gubler WD
(2009) Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Disease 93, 584–592.
| Crossref | GoogleScholarGoogle Scholar |

Vilgalys R, Hester M
(1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172, 4238–4246.
|
CAS |
PubMed |



